Coronavirus disease 2019 (Covid-19), caused by severe acute respiratory syndrome coronavirus (SARS-CoV)-2, exerts far-reaching effects on public health and socio-economic welfare. The majority of infected individuals have mild to moderate symptoms, but a significant proportion develops respiratory failure due to pneumonia. Thrombosis is another frequent manifestation of Covid-19 that contributes to poor outcomes. Vitamin K plays a crucial role in the activation of both pro- and anticlotting factors in the liver and the activation of extrahepatically synthesised protein S which seems to be important in local thrombosis prevention. However, the role of vitamin K extends beyond coagulation. Matrix Gla protein (MGP) is a vitamin K-dependent inhibitor of soft tissue calcification and elastic fibre degradation. Severe extrahepatic vitamin K insufficiency was recently demonstrated in Covid-19 patients, with high inactive MGP levels correlating with elastic fibre degradation rates. This suggests that insufficient vitamin K-dependent MGP activation leaves elastic fibres unprotected against SARS-CoV-2-induced proteolysis. In contrast to MGP, Covid-19 patients have normal levels of activated factor II, in line with previous observations that vitamin K is preferentially transported to the liver for activation of procoagulant factors. We therefore expect that vitamin K-dependent endothelial protein S activation is also compromised, which would be compatible with enhanced thrombogenicity. Taking these data together, we propose a mechanism of pneumonia-induced vitamin K depletion, leading to a decrease in activated MGP and protein S, aggravating pulmonary damage and coagulopathy, respectively. Intervention trials should be conducted to assess whether vitamin K administration plays a role in the prevention and treatment of severe Covid-19.
Key words: Vitamin K, Covid-19, Matrix Gla protein, Protein S, Prothrombin
Coronavirus disease 2019 (Covid-19) is an infectious disorder caused by the severe acute respiratory syndrome coronavirus (SARS-CoV)-2 that emerged from the Chinese city of Wuhan at the end of 2019 and has since then relentlessly spread across the globe(1). The Covid-19 pandemic is causing a worldwide medical and socio-economic crisis of unprecedented proportions in modern times. The vast majority of individuals who contract SARS-CoV-2 have mild to moderate symptoms. However, a significant minority develops respiratory failure due to pneumonia and/or acute respiratory distress syndrome(1).
Particularly, SARS-CoV-2-infected individuals suffering from certain premorbid conditions, such as hypertension, diabetes, CVD and obesity, are at increased risk of complicated disease course(1). Although these conditions are also associated with poor outcomes due to other infectious diseases, precisely why these groups have high morbidity and mortality is currently unknown. We made the observation that these disorders are associated with elastic fibre pathologies as well as vitamin K insufficiency.
Thromboembolism and generalised microvascular thrombosis are also prevalent in severe Covid-19(2,3). The mechanisms leading from pulmonary infection to systemic coagulopathy in Covid-19 have not yet been entirely elucidated. It has previously been shown that severe vitamin K deficiency in critically ill patients can be misdiagnosed as disseminated intravascular coagulation(4). Given the importance of vitamin K-dependent proteins in coagulation as well as elastic fibre metabolism, we recently hypothesised that vitamin K is implicated in Covid-19 pathogenesis and could represent the missing link between pulmonary damage and thrombogenicity.
Vitamin K metabolism
Vitamin K is a monofunctional nutrient from a biochemical perspective as its only well-described function is facilitating γ-carboxylation. However, it can be regarded as pleiotropic because it activates proteins with distinct, opposing and not yet fully unravelled functions.
Vitamin K catalyses the carboxylation reaction that transforms glutamic acid into γ-carboxyglutamic (Gla) residues and is well known as an activator of hepatic procoagulant factors II (prothrombin), VII, IX and X. However, vitamin K also activates anticoagulant proteins C and S as well as a number of extrahepatic proteins not involved in blood coagulation.
Endothelial protein S
Contrary to other vitamin K-dependent procoagulant factors and protein C, which are almost exclusively hepatic proteins, about 50 % of anticoagulant protein S is produced outside the liver(5). This part of protein S is mainly synthesised in endothelial cells and thought to play an important role in the local prevention of thrombosis(5–7). Endothelium-produced protein S has the ability to associate with the cell surface and promote procoagulant factor V inactivation in the presence of activated protein C(7).
Matrix Gla protein
Vitamin K-dependent matrix Gla protein (MGP) has been extensively studied as an inhibitor of vascular mineralisation(8); however, its role in the pulmonary compartment seems to be comparable(9). Besides preventing soft tissue calcification, MGP also protects against elastic fibre degradation. This was demonstrated in MGP knockout mice, which developed severely mineralised as well as fragmented elastic fibres(10).
Elastic fibres are critical components in the extracellular matrix of dynamic tissues(11). They provide deformability to lungs and arteries, which facilitates respiration and circulation(11). Initial elastic fibre development is almost exclusively restricted to the perinatal period(11). Elastic fibre degradation and repair, however, are continuous processes(11). The balance between the two is delicate and of vital importance for cardiovascular and pulmonary health(12). The rate of proteolytic elastic fibre degradation increases during ageing(13). This age-related acceleration of elastolysis is enhanced in certain pulmonary conditions such as chronic obstructive pulmonary disease and idiopathic pulmonary fibrosis(13,14).
Affinity of elastic fibres for Ca is high(15). Critically, elastic fibre calcification and proteolytic degradation processes are closely related. Partially degraded elastic fibres are more negatively charged, attracting positively charged Ca(15). As elastic fibre Ca content increases, the synthesis of matrix metalloproteinases, proteolytic enzymes that degrade elastin fibres, is also up-regulated(16). Peri-arterial application of Ca on rat abdominal aortas induces both calcification and proteolytic degradation of elastic fibres(17). Subdermal implantation of elastin in rats results in significant calcification, but local application of a protease inhibitor attenuates this mineralisation(18,19). MGP plays a critical role in the protection of elastic tissues against mineralisation(10), most likely because other proteins that inhibit calcification (e.g. fetuin-A) are too large to enter the lumen of the fibres(8,20).
Vitamin K recycling
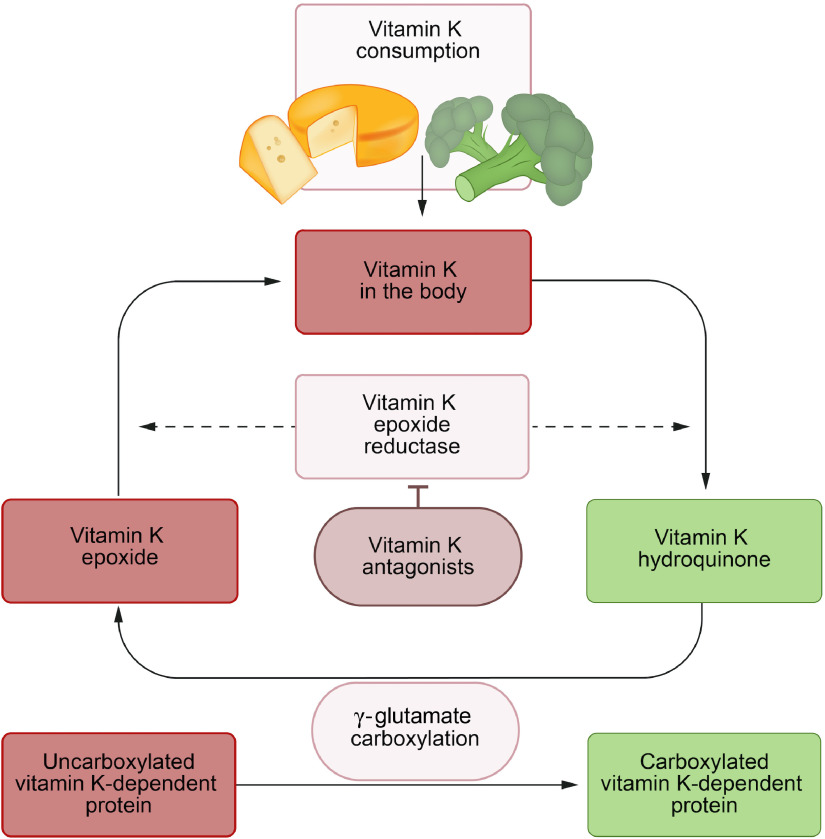
Storage capacity of vitamin K is limited, and therefore, its metabolism must be very efficient. After being oxidised during the carboxylation reaction, vitamin K is reactivated repeatedly by the enzyme vitamin K epoxide reductase in the vitamin K cycle (Fig. 1)(21). Nevertheless, insufficiency may develop within days of poor intake, particularly in pathological states of increased vitamin K utilisation(4,9,22).
Fig. 1.
Vitamin K cycle. The vitamin K cycle and the effects of vitamin K antagonists.  , Active;
, Active;  , inactive.
, inactive.
Triage-based distribution
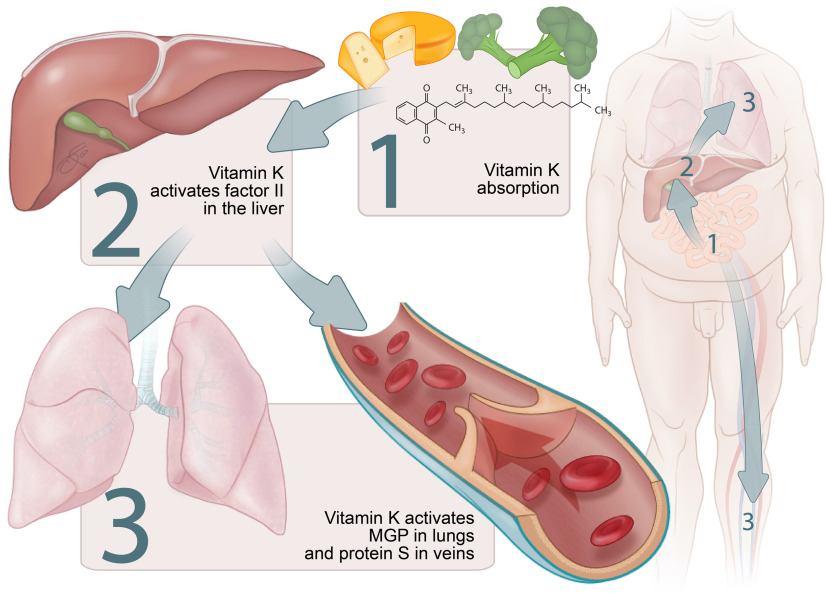
The triage theory posits that during times of scarcity, micronutrients are reserved for use in processes that form the greatest threat to short-term survival if not properly executed(23). This implies that in case of vitamin K insufficiency, the vitamin is preferentially transported to the liver for the activation of the above-mentioned procoagulant factors at the expense of extrahepatic vitamin K-dependent proteins such as MGP (Fig. 2).
Fig. 2.
Micronutrient triage theory with regard to vitamin K. Particularly vitamin K1 is preferentially transported to the liver. This implies that the grade of carboxylation in a state of vitamin K deficiency is usually higher for hepatic procoagulant factors, such as factor II, than for endothelial protein S as well as for pulmonary matrix Gla protein (MGP).
This was demonstrated in women between 60 and 80 years old who consumed a vitamin K1-deficient diet for 28 d. Undercarboxylated osteocalcin, a vitamin K-dependent bone protein, increased almost immediately, whereas undercarboxylated factor II increased more slowly(24). Moreover, when patients using vitamin K antagonists (VKA) as anticoagulants steadily increased their dietary intake of vitamin K1, a significant decrease in undercarboxylated factor II was seen at 150 μg/d, while a significant decrease in undercarboxylated osteocalcin was only seen at an intake of 300 μg/d(25).
Similar to osteocalcin and MGP, vitamin K insufficiency would result in deficient activation of endothelial protein S before causing a decrease in carboxylated procoagulant factors (Fig. 2)(26). This could explain the seemingly paradoxical increase of thrombosis risk in the first week of treatment with VKA(27).
Although the biological function of vitamins K1 and K2 is similar, there are differences with regard to bioavailability and tissue distribution. Half-life times of most K2 vitamins are longer than that of K1, and vitamin K2 may have more extrahepatic potential than K1 (28). Vitamin K1 is found in green vegetables such as broccoli, spinach and kale. Certain bacteria have the ability to produce vitamin K2. It is therefore present in fermented food products such as cheese, curd and sauerkraut as well as in certain fishes.
Assessment of vitamin K status
Measuring circulating levels of the two naturally occurring forms of vitamin K – vitamin K1 (phylloquinone) and K2 (the group of menaquinones) – is technically feasible(29). However, the value of such measurements for assessing general vitamin K status is limited. Quantification of vitamin K-dependent proteins that have not been carboxylated, on the other hand, is a valuable method reflecting the combined functional deficit of vitamin K1 and K2 (29). Determination of desphospho-uncarboxylated (dp-uc; i.e. inactive) MGP levels and the ratio between uncarboxylated and carboxylated osteocalcin are validated assays of extrahepatic vitamin K status(29).
High dp-ucMGP reflects low vitamin K status and vice versa. Although increasing vitamin K consumption decreases dp-ucMGP(30–32), its levels are not simply a biomarker of vitamin K intake but depend on other factors as well. Circulating dp-ucMGP concentration can best be regarded as a reflection of the total extrahepatic vitamin K deficit, that is, the amount of vitamin K that is needed to carboxylate all the uncarboxylated vitamin K-dependent proteins in the body(33).
Hepatic vitamin K status is usually quantified by measuring levels of protein induced by vitamin K absence (PIVKA)-II (i.e. uncarboxylated prothrombin)(24).
Vitamin K metabolism in Coronavirus disease 2019
Extrahepatic vitamin K status is severely reduced in Covid-19 patients, reflected by elevated dp-ucMGP levels(34). Reasons for this could include premorbid low vitamin K status in combination with accelerated utilisation during infection.
Vitamin K status in co-morbidities associated with poor Coronavirus disease 2019 outcomes
dp-ucMGP levels are elevated in various diseases that are associated with elastic fibre calcification and degradation such as diabetes(35), hypertension(36), CVD(37), chronic kidney disease(35,38) and obesity(39). It is possible that reduced vitamin K intake increases the risk of developing these conditions(40). However, increased vitamin K demand due to enhanced utilisation may be another important cause of high dp-ucMGP(41). Partially degraded and mineralised elastic fibres – which are prevalent in diabetic, hypertensive, renal and cardiovascular patients – are more vulnerable to further proteolysis and calcification(17,42). This increases the need for MGP synthesis to protect elastic fibres(9), draining vitamin K stores for MGP carboxylation and leading to higher dp-ucMGP levels.
In vascular diseases(40), as well as in the general population(43,44), increased dp-ucMGP levels associate with higher all-cause mortality. Vitamin K supplementation reduces dp-ucMGP levels and has a favourable effect on progression of clinically relevant endpoints, including aortic valve calcification, arterial stiffness and bone loss(30–32).
Vitamin K insufficiency in the pathogenesis of Coronavirus disease 2019
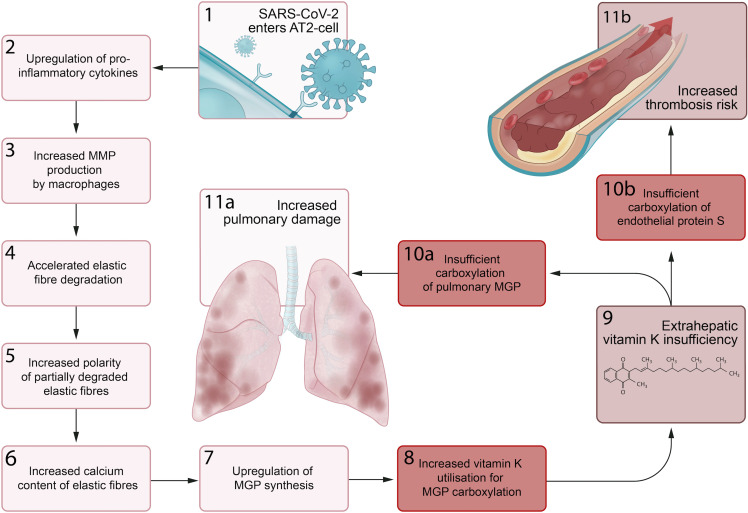
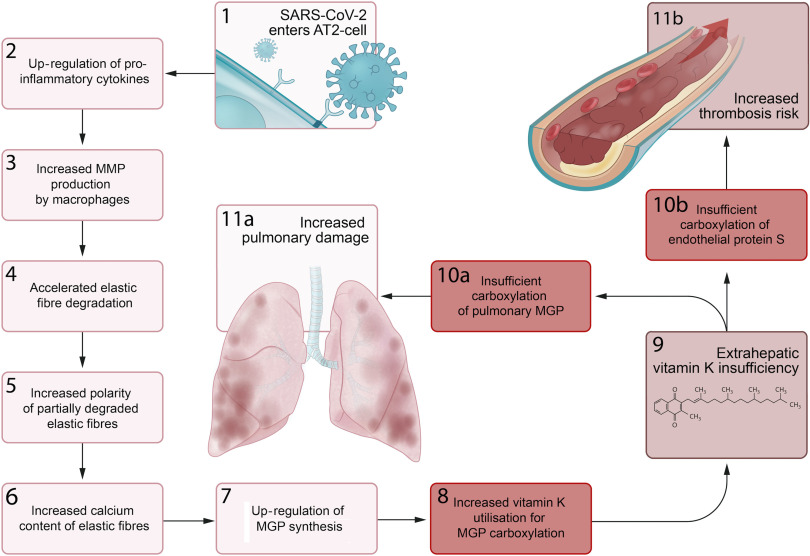
We propose a series of sequential pathological steps occurring in response to SARS-CoV-2 infection that are responsible for up-regulation of MGP expression and extrahepatic vitamin K depletion, leading to pulmonary damage and thrombosis in Covid-19 (Fig. 3).
Fig. 3.
Proposed sequential steps linking severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) pneumonia to vitamin K insufficiency, pulmonary damage and thrombogenecity. (1) SARS-CoV-2 enters alveolar type II (ATII) cells. (2) Infected AT2 cells response by up-regulating synthesis of proinflammatory cytokines, including IL-6. (3) This leads to an increase in the number and activation of alveolar macrophages (4) that produce matrix metalloproteinases (MMP), which accelerates degradation of elastic fibres. (5) The increased polarity of partially degraded elastic fibres (6) enhances their affinity for calcium and leads to increased elastic fibre calcium content. (7) Matrix Gla protein (MGP) synthesis is up-regulated in an attempt to protect elastic fibres from calcification and degradation, (8) and the need for vitamin K to carboxylate additional MGP increases. (9) This increased utilisation of vitamin K may induce extrahepatic vitamin K insufficiency, (10a) leading to insufficient carboxylation of pulmonary MGP and (11a) increased pulmonary damage. (10b) The second consequence of extrahepatic vitamin K insufficiency is decreased carboxylation of endothelial protein S, (11b) which increases thrombosis risk.
Infection primarily begins through the inhalation of SARS-CoV-2-containing aerosols. The virus’ so-called ‘spike proteins’ have the ability of binding to angiotensin-converting enzyme 2 receptors, which enables viral entry into human cells, including alveolar type II cells. These proteins are also the most immunogenic part of the virus(45).
Although Covid-19 is a communicable disease, morbidity and mortality are mainly attributable to immunological rather than direct infectious complications. SARS-CoV-2 has the ability to put the immune system of infected individuals into overdrive resulting in a hyperinflammatory pulmonary state(46). Data from SARS infections suggest that the synthesis of proinflammatory cytokines begins in infected alveolar type II cells(47). Increased levels of IL-6 and TNF-α are associated with poor Covid-19 outcome(48). Autopsy studies consistently implicate infiltrating lymphocytes and macrophages as further drivers of pulmonary inflammation during Covid-19(49,50). Importantly, a specific subset of macrophages appears in the lungs of Covid-19 patients(51), which has previously been demonstrated to produce matrix metalloproteinases-9 in patients with idiopathic pulmonary fibrosis(52).
Upon SARS-CoV-2 infection, elastic fibre breakdown accelerates compared with degradation rates in age-matched controls(34). High pulmonary concentrations of matrix metalloproteinases-9 or other proteases produced either by infiltrating or locally proliferating macrophages could be an explanation for this(51,52). A significant correlation between dp-ucMGP and the rate of elastic fibre degradation was observed in Covid-19 patients(34). We suspect that accelerated elastic fibre degradation due to enhanced proteolytic activity in SARS-CoV-2-infected lungs increases elastic fibre vulnerability to Ca, leading to an up-regulation of MGP synthesis and depletion of extrahepatic vitamin K stores. This shortage impairs MGP activation, presumably causing further elastic fibre damage, and elevation of circulating dp-ucMGP. The rate of elastic fibre degradation associates with poor outcome in patients with SARS-CoV-2 pneumonia(34), as it does in other pulmonary diseases such as chronic obstructive pulmonary disease, cystic fibrosis and bronchiectasis(13,53,54).
Conditions associated with chronic elastic fibre pathology, including diabetes, hypertension and CVD, are also related to worse prognosis of SARS-CoV-2 infection. Recent data demonstrated that Covid-19 patients with poor outcomes had increased thoracic aortic and coronary artery calcification on computed tomography scan, though these analyses lost significance after correction for age and sex(34). Nevertheless, pre-existing elastic fibre damage predisposes to enhanced proteolytic degradation during inflammation(42), potentially explaining the increased severity of Covid-19 in those populations.
We further theorise that vitamin K depletion also has a key effect on another characteristic disease manifestation of Covid-19. Though dp-ucMGP is elevated in Covid-19, hepatic procoagulant vitamin K status, quantified by measuring PIVKA-II, was hardly affected(34). According to the micronutrient triage theory(23), the preferential activation of hepatic over extrahepatic proteins and the fact that about 50 % of protein S synthesis occurs in endothelial cells imply that the uncarboxylated protein S fraction would also be increased(6). This could increase the risk of thrombosis. Consumption of clotting factors during thrombosis puts a further burden on vitamin K stores by increasing demand for activation of newly synthesised coagulation factors to replace used ones(55). With preference given to the carboxylation of procoagulant factors, progressive depletion of active endothelial protein S increasingly skews the balance towards coagulation.
Interaction between vitamins D and K
Vitamin D is both endogenously produced in the skin and exogenously acquired from food. Intake of vitamins D and K is correlated due to their co-presence in various food sources. Both vitamin D and K deficiencies are prevalent around the world. Contrary to vitamin K, however, assessment of vitamin D status and propagation of vitamin D supplementation are widespread.
A meta-analysis conducted prior to the emergence of SARS-CoV-2 demonstrated that daily or weekly vitamin D supplementation reduced the risk of acute respiratory tract infection(56). The role of vitamin D in susceptibility to SARS-CoV-2 infection has been assessed by various groups, and to date, results appear to be conflicting(57–59). Studies evaluating the modulatory role of vitamin D on disease severity in Covid-19 have not yet been reported. Vitamin D has anti-inflammatory and anti-proteolytic properties(60–62), which may potentially be favourable in Covid-19. Increasing vitamin D intake is generally regarded to be safe(63), although clinical data are limited. Due to tight hormonal regulation, serum Ca levels are hardly and at most transiently increased even after high-dose vitamin D administration(64). However, short-term hypercalcaemia may induce deposition of Ca on elastic fibres, which is not necessarily released from fibres after normalisation of systemic Ca levels(64). High-dose vitamin D administration in rats depletes extrahepatic vitamin K stores by strongly up-regulating MGP synthesis leading to acceleration of elastic fibre calcification and degradation(9,64). Vitamin D administration in a state of vitamin K deficiency may thereby endanger pulmonary and vascular health. There is also human data that raised these concerns. Vitamin D supplementation was associated with premature mortality in vitamin K-insufficient stable kidney transplant recipients(65). It may therefore be prudent to first supplement vitamin K in invariably vitamin K-insufficient Covid-19 hospitalised patients and to start vitamin D supplementation in those who are vitamin D-deficient only when extrahepatic vitamin K status has been restored(34).
Furthermore, vitamin K might be a useful additive to vitamin D because there is some evidence that it can act as an anti-inflammatory agent by suppressing NF-κB signal transduction. It may also exert a protective effect against oxidative stress by blocking the generation of reactive oxygen species(66).
Vitamin K antagonists
Although progressively substituted by direct oral anticoagulants, VKA remain important drugs for the prevention of venous and arterial thrombosis. VKA exert their antithrombotic function through inhibition of vitamin K 2,3-epoxide reductase complex 1, thereby interrupting the vitamin K cycle and inducing vitamin K deficiency (Fig. 1). This obstructs carboxylation of hepatic procoagulant factors, which delays blood clotting.
Remarkably, it has been reported that within the epicentre of the Covid-19 outbreak in the UK, the OR of having a supra-therapeutic anticoagulation with VKA (i.e. international normalised ratio > 8·0) was 6·3 around the lockdown date compared with the same period in the year before(67). Root cause analysis suggested that at least 50 % of these elevations were related to Covid-19(67). Although the majority of possible/confirmed Covid-19 cases had used antibiotics which may influence INR(68), we speculate that enhanced pulmonary vitamin K utilisation during SARS-CoV-2 pneumonia could also disturb the narrow therapeutic balance between VKA dosage and vitamin K intake levels.
VKA use in idiopathic pulmonary fibrosis patients associates with reduced survival(69), and it has been suggested that this effect of VKA may be very acute(70). There are reasons to suspect that vitamin K-dependent MGP activation is already compromised in both animals and humans with fibrotic lung disease and that this is further compromised by VKA administration(71,72). Other potential mechanisms by which VKA could exacerbate lung fibrosis may be via preventing anticoagulant protein C and S activation, which both have antifibrotic properties(73,74). Through these mechanisms, VKA may also have an unfavourable effect on pneumonia severity in Covid-19 patients; however, this has not yet been evaluated.
VKA may also have potentially favourable effects on disease course of Covid-19 by prevention of thrombosis, as has been shown for heparins(75). However, considering the consequences of vitamin K insufficiency for pulmonary disease(69,70), it may be worthwhile to conduct a study comparing the risk of severe Covid-19 in patients on VKA with those using other classes of anticoagulant medications, provided the availability of a sufficiently large cohort to correct for confounding factors.
Future perspective
There is a need for further experimental evidence to link vitamin K deficiency with the pathology of Covid-19 and determine whether vitamin K supplementation has a place in treatment protocols.
First, there is need for lung-specific data. The current data on vitamin K status in Covid-19 are confined to measurement of circulating parameters, and we were unable to distinguish pulmonary from systemic elastic fibre degradation(34). Autopsy studies performed on Covid-19 patients could shed light on the presence of carboxylated and uncarboxylated vitamin K-dependent proteins at sites of SARS-CoV-2-related lung disease. This could give support to our hypothesis of increased pulmonary MGP expression and enhanced vitamin K utilisation. Animal models may also be used to elucidate the effect of vitamin K insufficiency, administration and antagonism specifically on pathologies of pulmonary elastic fibres.
Second, it is important to confirm that protein S activity is decreased during vitamin K insufficiency in Covid-19. This could be explored by measuring protein S activity, but this method has potential confounders(76). An alternative would be to quantify undercarboxylated protein S either with targeted antibodies or using liquid chromatography-tandem MS; however, to our knowledge, such assays have yet to be developed.
Finally, there is need for human intervention studies to determine whether vitamin K supplementation has a place in the prevention and treatment of severe Covid-19. Clinical trials assessing vitamin K administration in hospitalised populations are needed to evaluate both safety and efficacy. The safety of even high doses of vitamin K has been established in healthy persons(77) but remains to be assessed in severely ill Covid-19 patients. The potential role of vitamin K supplementation to prevent development of severe Covid-19 in subjects who have not yet contracted SARS-CoV-2, but are at risk for the infection, is also very relevant to assess.
In conclusion, the potential role of vitamin K supplementation to prevent the development and progression of severe Covid-19 remains largely unexplored. We would argue that the impact of the current crisis warrants thorough evaluation of the therapeutic potential of vitamin K in Covid-19 pathogenesis for two key reasons. Unlike other treatment strategies currently under development for Covid-19 such as dexamethasone, vitamin K does not have any known unfavourable effects in those who do not use VKA. Furthermore, it is relatively simple and inexpensive to manufacture contrary to other therapies like remdesivir or convalescent plasma. Taken together this means that effectiveness can be rapidly and cheaply evaluated in clinical trials and easily implemented if proven successful.
Acknowledgements
This work was funded by Kappa Bioscience AS, a manufacturer of vitamin K2 (MK-7).
R. J. developed the theory. R. J., M. P. J. V. and J. W. wrote the first draft of the manuscript. A. S. M. D., C. V. and W. J. critically revised the manuscript.
R. J. discloses application of a patent on vitamin K in Covid-19. R. J. and J. W. have a scientific collaboration with Kappa Bioscience AS, a manufacturer of vitamin K2 (MK-7). M. P. J. V., A. S. M. D., C. V. and W. J. declare no competing interests.
References
- 1. Huang C, Wang Y, Li X, et al. (2020) Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 395, 497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Tang N, Li D, Wang X, et al. (2020) Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost 18, 844–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cui S, Chen S, Li X, et al. (2020) Prevalence of venous thromboembolism in patients with severe novel coronavirus pneumonia. J Thromb Haemost 18, 1421–1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Alperin JB (1987) Coagulopathy caused by vitamin K deficiency in critically ill, hospitalized patients. JAMA 258, 1916–1919. [PubMed] [Google Scholar]
- 5. Burstyn-Cohen T, Heeb MJ & Lemke G (2009) Lack of protein S in mice causes embryonic lethal coagulopathy and vascular dysgenesis. J Clin Invest 119, 2942–2953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fair DS, Marlar RA & Levin EG (1986) Human endothelial cells synthesize protein S. Blood 67, 1168–1171. [PubMed] [Google Scholar]
- 7. Stern D, Brett J, Harris K, et al. (1986) Participation of endothelial cells in the protein C-protein S anticoagulant pathway: the synthesis and release of protein S. J Cell Biol 102, 1971–1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Schurgers LJ, Uitto J & Reutelingsperger CP (2013) Vitamin K-dependent carboxylation of matrix Gla-protein: a crucial switch to control ectopic mineralization. Trends Mol Med 19, 217–226. [DOI] [PubMed] [Google Scholar]
- 9. Price PA, Buckley JR & Williamson MK (2001) The amino bisphosphonate ibandronate prevents vitamin D toxicity and inhibits vitamin D-induced calcification of arteries, cartilage, lungs and kidneys in rats. J Nutr 131, 2910–2915. [DOI] [PubMed] [Google Scholar]
- 10. Luo G, Ducy P, McKee MD, et al. (1997) Spontaneous calcification of arteries and cartilage in mice lacking matrix GLA protein. Nature 386, 78–81. [DOI] [PubMed] [Google Scholar]
- 11. Mithieux SM & Weiss AS (2005) Elastin. Adv Protein Chem 70, 437–461. [DOI] [PubMed] [Google Scholar]
- 12. Liu X, Zhao Y, Gao J, et al. (2004) Elastic fiber homeostasis requires lysyl oxidase-like 1 protein. Nat Genet 36, 178–182. [DOI] [PubMed] [Google Scholar]
- 13. Rabinovich RA, Miller BE, Wrobel K, et al. (2016) Circulating desmosine levels do not predict emphysema progression but are associated with cardiovascular risk and mortality in COPD. Eur Respir J 47, 1365–1373. [DOI] [PubMed] [Google Scholar]
- 14. Huang JT, Bolton CE, Miller BE, et al. (2016) Age-dependent elastin degradation is enhanced in chronic obstructive pulmonary disease. Eur Respir J 48, 1215–1218. [DOI] [PubMed] [Google Scholar]
- 15. Rucker RB (1974) Calcium binding to elastin. Adv Exp Med Biol 48, 185–209. [DOI] [PubMed] [Google Scholar]
- 16. Lee JS, Basalyga DM, Simionescu A, et al. (2006) Elastin calcification in the rat subdermal model is accompanied by up-regulation of degradative and osteogenic cellular responses. Am J Pathol 168, 490–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Basalyga DM, Simionescu DT, Xiong W, et al. (2004) Elastin degradation and calcification in an abdominal aorta injury model: role of matrix metalloproteinases. Circulation 110, 3480–3487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Vyavahare N, Jones PL, Tallapragada S, et al. (2000) Inhibition of matrix metalloproteinase activity attenuates tenascin-C production and calcification of implanted purified elastin in rats. Am J Pathol 157, 885–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bailey M, Xiao H, Ogle M, et al. (2001) Aluminum chloride pretreatment of elastin inhibits elastolysis by matrix metalloproteinases and leads to inhibition of elastin-oriented calcification. Am J Pathol 159, 1981–1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Price PA, Toroian D & Lim JE (2009) Mineralization by inhibitor exclusion: the calcification of collagen with fetuin. J Biol Chem 284, 17092–17101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Shearer MJ & Newman P (2014) Recent trends in the metabolism and cell biology of vitamin K with special reference to vitamin K cycling and MK-4 biosynthesis. J Lipid Res 55, 345–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Usui Y, Tanimura H, Nishimura N, et al. (1990) Vitamin K concentrations in the plasma and liver of surgical patients. Am J Clin Nutr 51, 846–852. [DOI] [PubMed] [Google Scholar]
- 23. Ames BN (2006) Low micronutrient intake may accelerate the degenerative diseases of aging through allocation of scarce micronutrients by triage. Proc Natl Acad Sci U S A 103, 17589–17594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Booth SL, Martini L, Peterson JW, et al. (2003) Dietary phylloquinone depletion and repletion in older women. J Nutr 133, 2565–2569. [DOI] [PubMed] [Google Scholar]
- 25. Schurgers LJ, Shearer MJ, Hamulyák K, et al. (2004) Effect of vitamin K intake on the stability of oral anticoagulant treatment: dose-response relationships in healthy subjects. Blood 104, 2682–2689. [DOI] [PubMed] [Google Scholar]
- 26. McCann JC & Ames BN (2009) Vitamin K, an example of triage theory: is micronutrient inadequacy linked to diseases of aging? Am J Clin Nutr 90, 889–907. [DOI] [PubMed] [Google Scholar]
- 27. Azoulay L, Dell’Aniello S, Simon TA, et al. (2014) Initiation of warfarin in patients with atrial fibrillation: early effects on ischaemic strokes. Eur Heart J 35, 1881–1887. [DOI] [PubMed] [Google Scholar]
- 28. Akbulut AC, Pavlic A, Petsophonsakul P, et al. (2020) Vitamin K2 needs an RDI separate from vitamin K1 . Nutrients 12, E1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Shea MK & Booth SL (2016) Concepts and controversies in evaluating vitamin K status in population-based studies. Nutrients 8, E8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Brandenburg VM, Reinartz S, Kaesler N, et al. (2017) Slower progress of aortic valve calcification with vitamin K supplementation: results from a prospective interventional proof-of-concept study. Circulation 135, 2081–2083. [DOI] [PubMed] [Google Scholar]
- 31. Knapen MH, Braam LA, Drummen NE, et al. (2015) Menaquinone-7 supplementation improves arterial stiffness in healthy postmenopausal women. A double-blind randomised clinical trial. Thromb Haemost 113, 1135–1144. [DOI] [PubMed] [Google Scholar]
- 32. Knapen MH, Drummen NE, Smit E, et al. (2013) Three-year low-dose menaquinone-7 supplementation helps decrease bone loss in healthy postmenopausal women. Osteoporos Int 24, 2499–2507. [DOI] [PubMed] [Google Scholar]
- 33. Janssen R & Vermeer C (2017) Vitamin K deficit and elastolysis theory in pulmonary elasto-degenerative diseases. Med Hypotheses 108, 38–41. [DOI] [PubMed] [Google Scholar]
- 34. Dofferhoff ASM, Piscaer I, Schurgers LJ, et al. (2020) Reduced vitamin K status as a potentially modifiable prognostic risk factor in COVID-19. Clin Infect Dis (Epublication ahead of print version 27 August 2020). [Google Scholar]
- 35. Griffin TP, Islam MN, Wall D, et al. (2019) Plasma dephosphorylated-uncarboxylated Matrix Gla-Protein (dp-ucMGP): reference intervals in Caucasian adults and diabetic kidney disease biomarker potential. Sci Rep 9, 18452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Chirinos JA, Sardana M, Syed AA, et al. (2018) Aldosterone, inactive matrix gla-protein, and large artery stiffness in hypertension. J Am Soc Hypertens 12, 681–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mayer O Jr, Seidlerová J, Bruthans J, et al. (2014) Desphospho-uncarboxylated matrix Gla-protein is associated with mortality risk in patients with chronic stable vascular disease. Atherosclerosis 235, 162–168. [DOI] [PubMed] [Google Scholar]
- 38. Schurgers LJ, Barreto DV, Barreto FC, et al. (2010) The circulating inactive form of matrix gla protein is a surrogate marker for vascular calcification in chronic kidney disease: a preliminary report. Clin J Am Soc Nephrol 5, 568–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Jespersen T, Møllehave LT, Thuesen BH, et al. (2020) Uncarboxylated matrix Gla-protein: a biomarker of vitamin K status and cardiovascular risk. Clin Biochem 83, 49–56. [DOI] [PubMed] [Google Scholar]
- 40. Chen HG, Sheng LT, Zhang YB, et al. (2019) Association of vitamin K with cardiovascular events and all-cause mortality: a systematic review and meta-analysis. Eur J Nutr 58, 2191–2205. [DOI] [PubMed] [Google Scholar]
- 41. Parker BD, Ix JH, Cranenburg EC, et al. (2009) Association of kidney function and uncarboxylated matrix Gla protein: data from the Heart and Soul Study. Nephrol Dial Transplant 24, 2095–2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Umeda H, Aikawa M & Libby P (2011) Liberation of desmosine and isodesmosine as amino acids from insoluble elastin by elastolytic proteases. Biochem Biophys Res Commun 411, 281–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Liu YP, Gu YM, Thijs L, et al. (2015) Inactive matrix Gla protein is causally related to adverse health outcomes: a Mendelian randomization study in a Flemish population. Hypertension 65, 463–470. [DOI] [PubMed] [Google Scholar]
- 44. Riphagen IJ, Keyzer CA, Drummen NEA, et al. (2017) Prevalence, effects of functional vitamin K insufficiency: the PREVEND Study. Nutrients 9, E1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wan Y, Shang J, Graham R, et al. (2020) Receptor recognition by the novel coronavirus from Wuhan: an analysis based on decade-long structural studies of SARS coronavirus. J Virol 94, e00127–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Mehta P, McAuley DF, Brown M, et al. (2020) COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet 395, 1033–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. He L, Ding Y, Zhang Q, et al. (2006) Expression of elevated levels of pro-inflammatory cytokines in SARS-CoV-infected ACE2+ cells in SARS patients: relation to the acute lung injury and pathogenesis of SARS. J Pathol 210, 288–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Qin C, Zhou L, Hu Z, et al. (2020) Dysregulation of immune response in patients with COVID-19 in Wuhan, China. Clin Infect Dis 71, 762–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Carsana L, Sonzogni A, Nasr A, et al. (2020) Pulmonary post-mortem findings in a series of COVID-19 cases from northern Italy: a two-centre descriptive study. Lancet Infect Dis 20, 1135–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Beigmohammadi MT, Jahanbin B, Safaei M, et al. (2020) Pathological findings of postmortem biopsies from lung, heart, and liver of 7 deceased COVID-19 patients. Int J Surg Pathol (Epublication ahead of print version 19 June 2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Liao M, Liu Y, Yuan J, et al. (2020) Single-cell landscape of bronchoalveolar immune cells in patients with COVID-19. Nat Med 26, 842–844. [DOI] [PubMed] [Google Scholar]
- 52. Morse C, Tabib T, Sembrat J, et al. (2019) Proliferating SPP1/MERTK-expressing macrophages in idiopathic pulmonary fibrosis. Eur Respir J 54, 1802441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Downey DG, Martin SL, Dempster M, et al. (2007) The relationship of clinical and inflammatory markers to outcome in stable patients with cystic fibrosis. Pediatr Pulmonol 42, 216–220. [DOI] [PubMed] [Google Scholar]
- 54. Huang JT, Kuzmanova E, Dicker AJ, et al. (2020) Serum desmosine is associated with long-term all-cause, cardiovascular mortality in bronchiectasis. Am J Respir Crit Care Med 202, 897–899. [DOI] [PubMed] [Google Scholar]
- 55. Iba T & Levy JH (2020) Sepsis-induced coagulopathy and disseminated intravascular coagulation. Anesthesiology 132, 1238–1245. [DOI] [PubMed] [Google Scholar]
- 56. Martineau AR, Jolliffe DA, Hooper RL, et al. (2017) Vitamin D supplementation to prevent acute respiratory tract infections: systematic review and meta-analysis of individual participant data. BMJ 356, i6583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Hastie CE, Mackay DF, Ho F, et al. (2020) Vitamin D concentrations and COVID-19 infection in UK Biobank. Diabetes Metab Syndr 14, 561–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Meltzer DO, Best TJ, Zhang H, et al. (2020) Association of vitamin D deficiency and treatment with COVID-19 incidence. JAMA Netw Open 3, e2019722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. D’Avolio A, Avataneo V, Manca A, et al. (2020) 25-Hydroxyvitamin D concentrations are lower in patients with positive PCR for SARS-CoV-2. Nutrients 12, E1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Janssens W, Decramer M, Mathieu C, et al. (2013) Vitamin D and chronic obstructive pulmonary disease: hype or reality? Lancet Respir Med 1, 804–812. [DOI] [PubMed] [Google Scholar]
- 61. Song Y, Qi H & Wu C (2007) Effect of 1,25-(OH)2D3 (a vitamin D analogue) on passively sensitized human airway smooth muscle cells. Respirology 12, 486–494. [DOI] [PubMed] [Google Scholar]
- 62. Heulens N, Korf H, Cielen N, et al. (2015) Vitamin D deficiency exacerbates COPD-like characteristics in the lungs of cigarette smoke-exposed mice. Respir Res 16, 110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Mitchell F (2020) Vitamin-D and COVID-19: do deficient risk a poorer outcome? Lancet Diabetes Endocrinol 8, 570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Niederhoffer N, Bobryshev YV, Lartaud-Idjouadiene I, et al. (1997) Aortic calcification produced by vitamin D3 plus nicotine. J Vasc Res 34, 386–398. [DOI] [PubMed] [Google Scholar]
- 65. van Ballegooijen AJ, Beulens JWJ, Keyzer CA, et al. (2020) Joint association of vitamins D and K status with long-term outcomes in stable kidney transplant recipients. Nephrol Dial Transplant 35, 706–714. [DOI] [PubMed] [Google Scholar]
- 66. Simes DC, Viegas CSB, Araújo N, et al. (2019) Vitamin K as a powerful micronutrient in aging and age-related diseases: pros and cons from clinical studies. Int J Mol Sci 20, 4150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Speed V, Patel RK, Byrne R, et al. (2020) A perfect storm: Root cause analysis of supra-therapeutic anticoagulation with vitamin K antagonists during the COVID-19 pandemic. Thromb Res 192, 73–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Clark TR & Burns S (2011) Elevated international normalized ratio values associated with concomitant use of warfarin and ceftriaxone. Am J Health Syst Pharm 68, 1603–1605. [DOI] [PubMed] [Google Scholar]
- 69. Noth I, Anstrom KJ, Calvert SB, et al. (2012) A placebo-controlled randomized trial of warfarin in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 186, 88–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Alagha K, Secq V, Pahus L, et al. (2015) We should prohibit warfarin in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 191, 958–960. [DOI] [PubMed] [Google Scholar]
- 71. Hardie WD, Korfhagen TR, Sartor MA, et al. (2007) Genomic profile of matrix and vasculature remodeling in TGF-alpha induced pulmonary fibrosis. Am J Respir Cell Mol Biol 37, 309–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Booth AJ, Hadley R, Cornett AM, et al. (2012) Acellular normal and fibrotic human lung matrices as a culture system for in vitro investigation. Am J Respir Crit Care Med 186, 866–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Urawa M, Kobayashi T, D’Alessandro-Gabazza CN, et al. (2016) Protein S is protective in pulmonary fibrosis. J Thromb Haemost 14, 1588–1599. [DOI] [PubMed] [Google Scholar]
- 74. Lin C, von der Thüsen J, Isermann B, et al. (2016) High endogenous activated protein C levels attenuates bleomycin-induced pulmonary fibrosis. J Cell Mol Med 20, 2029–2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Tang N, Bai H, Chen X, et al. (2020) Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost 18, 1094–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Marlar RA & Gausman JN (2011) Protein S abnormalities: a diagnostic nightmare. Am J Hematol 86, 418–421. [DOI] [PubMed] [Google Scholar]
- 77. Institute of Medicine (US) Panel on Micronutrients (2001) Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc. Washington, DC: National Academies Press. [PubMed] [Google Scholar]