Abstract
Background
Ischemic heart disease (IHD) is a major cause of death globally. Countries vary in their rates, and changes have occurred over time. Nowadays, developing countries pose new public health challenges.
Objectives
The objective of the present study was to appraise the alterations in the levels of serum Zn, Cu, Fe, and Mn that occur in patients with ischemic heart disease and to depict the correlations of the effects of these changes that lead to the pathogenesis of IHD.
Methods
Zn, Cu, Fe, and Mn in the IHD patients were determined by Atomic Absorption Spectroscopy (AAS).
Results
This study evaluated 52 patients with IHD, and 61 healthy volunteers served as controls. The primary outcomes of interest were explored regarding the correlations of the serum levels of these trace elements in patients with IHD. The secondary outcomes were explored in terms of inter-element relations to connect them with the pathogenesis of IHD. Our study found significantly reduced levels of Zn and Cu (2.50 ± 0.19 mg/L and 2.52 ± 0.17 mg/L, respectively) and an elevated level of Fe (148.97 ± 17.25 mg/L) in the patient group with IHD. The level of Mn (7.32 ± 1.23 mg/L) was elevated in the sera of the patients with ischemic heart disease (IHD) compared to healthy control subjects.
Conclusion
Our results indicate strong associations of the pathogenesis of IHD with depleted serum levels of Zn and Cu and elevated Fe and Mn levels, which may provide a prognostic tool for the treatment of this concerning the disease.
Keywords: Cardiology, Biochemistry, Molecular biology, Pathophysiology, Public health, Pharmacology, Clinical research, Ischemic heart disease, Case-control study, Trace elements, Pearson's correlation, Bangladesh
Cardiology; Biochemistry; Molecular biology; Pathophysiology; Public health; Pharmacology; Clinical research; Ischemic heart disease, Case-control study, Trace elements, Pearson's correlation, Bangladesh.
1. Introduction
IHD, also known as coronary artery disease (CAD) or coronary heart disease, is a condition of recurring chest pain or discomfort that occurs most often during exertion or excitement and develops due to the accumulation of blood cholesterol particles as well as plaques on the walls of the arteries that lead to the eventual blocking of blood flow [1]. Consecutive risk factors for IHD or CAD, such as hypertension, smoking, and primarily hypercholesterolemia, only account for ≤50% of the relative mortality [2, 3]. IHD is based on decreases in the diameters of arteries due to the development of fatty streaks. These fatty streaks are purely inflammatory lesions that consist of macrophages and T-lymphocytes, and the latter contains fat [4]. Endothelial cell dysfunction is an essential initial event in IHD that is caused by elevated blood lipids, particularly low-density lipoproteins (LDLs) [5], and other considered pretexts are hypertension [6], smoking [7], diabetes [8] and genetic predisposition [9]. LDL is trancytosed and trapped in the intima and modified by oxidation [5]. After being recognized by scavenger receptors, LDL is endocytosed by the macrophages, which results in the lipid-filled foam cells that are the hallmark cells of atherosclerotic lesions [5]. The role of oxidized LDL in the propagation of IHD through the formation of atheromas is the topic of continuing research [10, 11].
Trace elements are the metals that are essential in tissue, plant cells, and also animal cells in very small quantities, even at molecular levels. These metals, or a group of them, exist in the blood at micro volumes and include both transitional and heavy elements. There are two groups of these metals; some are crucially fundamental, and some have unknown biological functions for living healthy. Naturally existing essential metals in trace amounts play important roles in human health, and at altered levels, they may also produce some diseases [12].
Zinc is an important component of copper-zinc superoxide dismutase (Cu-Zn SOD), which plays an emergent role in CHD. Zn deficiency can cause an increase in tissue oxidation damage. The antagonistic relationship between these metals is also associated with increases in Cu and Fe. Zinc may be an essential element in the cause of IHD due to imbalances between Cu and Zn [13]. The effects of several pro-oxidant metals, including copper, on cardiovascular disease, have come under investigation because metals can produce oxidative modifications of low-density lipoprotein (LDL) cholesterol and result in the formation of free radicals. Various results from ecologic, angiographic, cross-sectional, case-control, nested case-control, and cohort studies reinforce the possibility that elevated Cu concentrations may enhance cardiovascular disease risk [14, 15, 16]. Manganese (Mn) is an important trace element in human organic systems. Enzymes dependent on Mn are diversely located in a number of cell components, including the mitochondria, cytoplasm and Golgi bodies. However, high levels of manganese are conceivably harmful. Particularly in individuals with hypoglycemia, manganese is an essential mineral for balancing the blood sugar level and decreasing total cholesterol (drugs that lower cholesterol effectively increases manganese) [13, 17, 18]. Formerly alluded to observations suggest the possibilities that alterations in the discussed essential trace elements (Cu, Zn, Mn, and Fe) play roles in the pathogenesis of IHD. Several works have investigated the role and importance of trace elements in health and disease [12, 19].
Although a few studies have separately reported on the levels of these trace elements though, no work has yet been performed regarding the correlations of the serum levels of these trace elements in patients with IHD in relation to the pathogenesis of this disease. Therefore, this study attempted to evaluate and correlate the changes in the serum levels of Cu, Fe, Mn, and Zn in IHD patients relative to normal individuals, to hypothesize about their changes, and to relate their altered statuses to the pathogenesis and progression of IHD.
2. Materials and methods
2.1. Materials and chemicals
Analytical grade reagents from commercial companies were used for this study. Standards of Zn, Cu, Fe, and Mn were purchased from Buck Scientific, USA. Hydrochloric acid (37%) and nitric acid were purchased from Merck, Germany. Other supportive chemicals of the recommended grade were supplied by the Department of Pharmacy, Noakhali Science and Technology University.
2.2. Selection of study subjects
The Pharmacy Department of Noakhali Science and Technology University, Bangladesh performed this study in collaboration with the Department of Cardiology, Cumilla Sadar Hospital & Lakshmipur Sadar Hospital, Bangladesh. This study took duration of eight months from July 2015 to March 2016. This study was performed after randomly recruiting 52 patients with IHD and 61 healthy volunteers for the control group. The diagnosis of IHD was made by a specialized cardiologist. The diagnosis of ischemic heart disease patient was based on ICD-9 (International Classification of Diseases, 9th Revision). Patients with angina pectoris or unstable angina, coronary artery surgery, acute myocardial infarction, and were taking nitrates were included in this study. Patients with alcoholic, psychosis, neoplasia, liver disorder, chronic illness, non-cooperative patients, and patients below eighteen years old were excluded from the study [20, 21]. The purpose of this study was explained to each patient, and written consent was obtained from each patient prior to data collection. The total study workout, the protocol of the study and the consent forms of the volunteers were approved by the Ethical Review Committees of both Cumilla Sadar Hospital and Lakshmipur Sadar Hospital.
2.3. Blood sample collection and storage
Venous blood (10 ml in total) was withdrawn from each patient with IHD and each of the control subjects using a plastic syringe fitted with a stainless steel needle. A metal-free plastic tube was used to collect the blood sample, and the sample was placed at room temperature for 30 min to allow for the formation of a clot. The collected clot subsequently underwent centrifugation for 15 min at 3000 rpm in a dust-free room. The serum samples were stored at −80 °C and protected from light until the study day. All tubes used were polypropylene; no glass material was used to prevent Al and Si contamination. To eliminate metal contamination during blood collection and storage, the National precaution criteria from the Committee of Clinical Laboratory Standards (NCCLS) were followed [22].
2.4. Instrumentation and elementary analysis
Determination of the trace elements was performed by flame atomic absorption spectrometry (FAAS; Varian SpectraAA 220) as well as graphite furnace atomic absorption spectrometry (GFAAS) following the method of Czupryn et al. [23, 24, 25, 26, 27]. The serum samples were diluted using ultra-deionized water with a dilution factor of 10. Different concentrations (0.5, 1.0, 2.0, 5.0, and 10.0 mg/L) of trace elements were used for the calibration of the standard graphs. Absorbance was collected at 213.9, 224.8, 248.3, and 279.8 nm for zinc, copper, iron and manganese, respectively, using an atomic absorption spectrophotometer. To verify the assay accuracy and to maintain the quality, the standard solutions were run concomitantly with each of the test samples. A software package (Spectra Software) was used to calculate the concentrations of zinc, copper, iron, and manganese. The contingent elemental interrelations (positive or/and negative) in the serum samples from the IHD and control participants were examined.
2.5. Statistical analysis
The elemental concentrations were represented in mean ± SEM, with their corresponding p values. Statistical analysis was performed using the statistical software package SPSS, version 18.0 (SPSS Inc., Chicago, IL). The mole percentages {concentration of element in mol% = concentration of element × 100/total concentration of analyzed elements in each sample} were calculated and correlative distributions of each of these elements was computed based on this percentage. Arithmetical calculations of this attained percentage are vital parameters to understand the relative elemental ordination among each other in matrix level of the biological system. Mole percentage also directs to the enumeration of elemental correlations and element-to-element ratios to uncover contingent elemental interrelations (positive or/and negative) in serum samples of IHD and control. Independent sample t-tests used to understand whether difference (set at p < 0.05) between patient and control groups is statistically significant or not. Pearson's correlation analysis was used to find the correlation among the various study parameters.
3. Results
This study involved a sample size 113. Fifty-two volunteers were suffering with ischemic heart disease, and 61 healthy volunteers were in normal healthy condition. Table 1 depicts the demographic profile of the study population.
Table 1.
Demographic profile of the study population.
| Parameter | IHD Patients Group | Control group | p value |
|---|---|---|---|
| No. of subjects | 52 | 61 | |
| Age (Years) |
43.83 ± 1.22 |
40.98 ± 1.32 |
0.062NS |
| Gender |
0.840NS |
||
| Male | 28 (53.85) | 34 (55.74) | |
| Female | 24 (46.15) | 27 (44.26) | |
| BMI (kg/m2) | 27.53 ± 0.55 | 24.51 ± 0.35 | 0.000∗∗ |
Values are expressed as Mean ± SEM, NS: Not significant, BMI: Body mass index, ∗∗p < 0.05 (Significant difference patient group and control groups at 95% confidence interval).
3.1. Elemental concentrations
Table 2 depicts the serum trace element concentrations (mg/L) of the IHD cases and the control subjects. Statistical evaluation revealed that the mean values of the serum concentrations (mg/L) of Zn, Cu, Fe and Mn were 2.50 ± 0.19 mg/L, 2.52 ± 0.17 mg/L, 148.97 ± 17.25 mg/L and 7.32 ± 1.23 mg/L for the IHD patient group and 4.02 ± 0.29 mg/L, 3.63 ± 0.32 mg/L, 59.94 ± 7.28 mg/L and 7.05 ± 1.55 mg/L for the healthy participants, respectively. Significantly reduced levels of Zn and Cu were found in the patient group compared with the control subjects (p < 0.05). A statistically significantly increased concentration of Fe and a dramatically increased concentration of Mn were found in the IHD case group compared with the control group (p < 0.05). This procedure was performed following the postulates of Muniz et al [28]. Further, the same parameters of the IHD patient and healthy control groups were used to perform the congruous analysis.
Table 2.
Serum level of Zn, Cu, Fe and Mn in study population.
| Parameters | Values (Mean ± SEM) |
||
|---|---|---|---|
| IHD Patients Group | Control group | p-value | |
| Zn (mg/L) | 2.5 ± 0.19∗∗ | 4.02 ± 0.29 | 0.002 |
| Cu (mg/L) | 2.52 ± 0.17∗∗ | 3.63 ± 0.32 | 0.001 |
| Fe (mg/L) | 148.97 ± 1.25∗∗ | 59.94 ± 7.28 | 0.002 |
| Mn (mg/L) | 7.32 ± 1.23 | 7.32 ± 1.55 | 0.215 |
∗∗p < 0.05 (Significant difference between patient and control groups at 95% confidence interval).
3.2. Inter-element correlations
All these positive and inverse correlations were found to be statistically insignificant (p < 0.05), except for the positive correlation between Zn and Cu (R = 0.348, p = 0.021) and the inverse relationship between Fe and Mn (R = 0.493, p = 0.006), which were statistically significant (p < 0.05) in the IHD patient group (Table 3).
Table 3.
Comparison of inter-element-relationships between patient and control groups.
| Correlation parameters | IHD Patient group |
Control group |
||
|---|---|---|---|---|
| r | p | r | p | |
| Zn and Cu | 0.348∗ | 0.021 | 0.194 | 0.113 |
| Zn and Fe | 0.055 | 0.764 | 0.246 | 0.310 |
| Zn and Mn | 0.234 | 0.213 | -0.334 | 0.058 |
| Cu and Fe | -0.259 | 0.152 | 0.024 | 0.922 |
| Cu and Mn | -0.095 | 0.619 | -0.208 | 0.246 |
| Fe and Mn | -0,493∗∗ | 0.006 | -0.122 | 0.619 |
Here, r = Correlation co-efficient; p = Significance; Values with negative sign indicate an inverse correlation; ∗p < 0.05, Correlation is significant at 0.05 level (two-tailed).
In the control group, Zn was directly correlated with Cu (R = 0.194, p = 0.113) and Fe (R = 0.246, p = 0.310), and Cu and Fe (R = 0.024, p = 0.922) also exhibited a tendency toward a positive relationship. Moreover, Mn was found to exhibit tendencies toward negative relationships with Zn (R = 0.334, p = 0.058), Cu (R = 0.208, p = 0.246) and Fe (R = 0.122, p = 0.619), but none of these values were found to be statistically significant.
3.3. Relative mole percentages
For the investigated elements the relative mole percentage was measured which is important for understanding the relative distribution of each element in relation to other elements in the biological matrix. Relative mole percentages were enumerated for the investigated elements, and the results are provided in Figure 1. The concentrations of trace elements (mg/L) were normalized to illustrate the inter-element relationships among the data sets of the controls and patients by assessing the mole percentage of every element present in the samples. This study found that the relative mole percentages of Zn, Cu, and Mn were comparatively low in the IHD study subjects relative to the controls, whereas the percentages of Fe were found to be higher in the same comparison.
Figure 1.
Relative mole percentages of elements in the serum samples of patients and controls.
3.4. Mole percent ratios
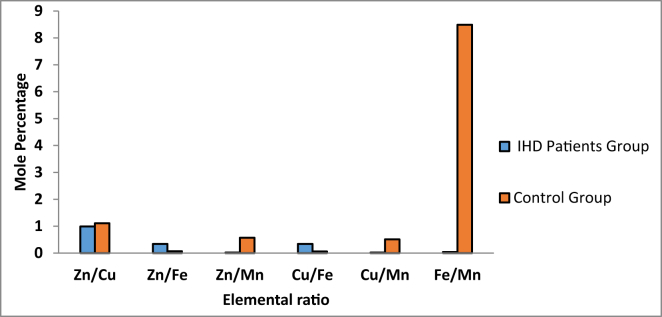
Subsequently, the relative mole percentages were analyzed to determine the element-to-element mole percent ratios between the IHD patient and the healthy controls. The resolved ratios serve the cognition of the elemental interrelations in human biological systems. The present data sets revealed that the elemental ratios of mole percentages for each component, namely, Zn/Cu, Zn/Mn, Cu/Mn, and Fe/Mn were decreased, and the Zn/Fe and Cu/Fe ratios were increased in the samples from the sera of IHD patients compared with the controls (Figure 2).
Figure 2.
Element-to-element mole percentage ratio in the serum samples of patients and controls.
4. Discussion
This study analyzed the serum trace elements (Zn, Cu, Fe, and Mn) levels in patients with IHD. Our study found significantly reduced levels of Zn, and Cu, and significantly increased levels of Fe as well as insignificantly higher concentrations of Mn in the IHD patients compared with the healthy controls.
The importance of trace elements is indisputable when the compositions of complex carbohydrates, proteins, and enzymes and the participation of trace elements in biochemical reactions are considered. It is apparently hypothesized that metabolic imbalances regarding zinc and copper are a major factor in the etiology of ischemic heart disease based on consistent epidemiologic and metabolic data. Metabolic imbalances are either relative or absolute deficiencies of Cu or Zn that can be characterized by the ratio of Zn to Cu [28, 29, 30]. Zn as a vital trace element that functions such as a co-factor in several synthetic enzymes and is also required for the proper antioxidant functioning to protect against free radical injury [30]. Detrimental effects on cellular function are caused by oxidative damage and result in myriad disease conditions. Thus, IHD or CHD also includes the involvement of oxygen radicals, and the zinc status has been demonstrated to have a significant effect on the metabolism of cholesterol and HDL, which are two important molecules that are involved in the disease [31]. Because Zn is a fundamental component of Cu-Zn SOD, Zn deficiency could induce an increase in oxidative tissue damage. A study also reported that in the case of CHD with systemic conditions like periodontitis observed higher concentration of malondialdehyde which suggests the oxidative stress in CHD. Increased oxidative stress further causes an increased level of CRP (C-reactive protein) [14, 32]. Deficiency in Zn is associated with an increase in Fe due to the antagonistic relationship (inter-elemental and relative mole percentages) between them, and a decrease in Cu may also result from the parallelism of Cu and Zn. Such imbalances between Cu and Zn have been suggested to be a factor in the etiology of CHD. Moreover, deficiency of this element may decrease the antioxidant potential of cells and lead to an increase in blood pressure [33]. This study demonstrated a significant decrease in the serum zinc concentration in the IHD patients compared with the controls (p < 0.05), and this result also supports the reports of others [34]. Cu is a constituent of Cu-Zn SOD, which is involved in the prevention of oxidative injury and is assumed to be a potential inciter of LDL oxidation because it has the aptitude to oxidize LDL [35, 36]. Conversely, a multifunctional protein called ceruloplasmin contains most of the Cu in the blood and also possesses antioxidant functions that reduce LDL oxidation [37, 38]. This study revealed a significantly decreased concentration of Cu in the sera of the IHD patients, which is supported by a few other studies, although some authors have hypothesized to the contrary [39, 40]. The increased susceptibility of LDL to peroxidation in conditions of Cu deficiency is due to a combination of two factors: an inadequate antioxidant defense mechanism due to a reduction in Cu-Zn SOD and an increase in body Fe stores due to the negative relationship between Cu and Fe [41]. Decreases in Cu elicit increases in serum cholesterol. It has been conceived that an asymmetrical relationship between Cu and Zn may be a factor in the etiology of CHD [42]. Although the role of Cu in atherosclerosis remains controversial according to prior studies [30, 43, 44, 45], the assumption from this and other studies may not be inconsistent with the postulations of Klevay [43]. Epidemiological experiments examining Fe stores in the body and IHD risk have provided results to the contrary [30, 46, 47]. Thereafter, the strongest supporting studies regarding the association of Fe with IHD conditions have also proposed that a reduction in the risk of fatal IHD is associated with Fe depletion [38, 46, 47, 48]. Thus, Fe may contribute to the leading cause of death in western countries through two possible mechanisms, i.e., reperfusion and atherogenesis. Atherogenesis may be the result of LDL oxidation in vitro that is catalyzed by Fe because it is a strong oxidant. Ischemic events result in free radicals of oxygen upon reflow in cases of reperfusion injury, whereas macrophages are only attracted toward oxidatively modified artery walls that provide the ground floor for atherosclerotic lesions, as brought to light by previous studies [49, 50]. Reactive oxygen species (ROS) also appear, in suppressed antioxidant conditions, to contribute to the development of atherosclerosis, but an increased Fe store or an inadequate antioxidant defense system alone cannot promote atherosclerotic conditions [51, 52]. Therefore, the combination of deficient Cu and an elevated Fe store is responsible for increased blood cholesterol and free radical production [53, 54]. An insufficient number of reports in the literature have examined the effects of excessive oral exposure to Mn in humans, considering that Mn is an essential trace element. The urine of IHD patients also exhibits increased Mn concentrations compared with the urine of healthy controls [38]. Several standard lines of evidence have related lipid peroxidation, free radical processes, and oxidatively modified LDL to atherosclerosis. The protective mechanisms of the body include antioxidants (a-tocopherol, b-carotene, transferring, ceruloplasmin, etc.) and enzymes, such as copper-zinc superoxide dismutase, manganese superoxide dismutase, and the iron-containing enzyme catalase [55, 56]. Manganese acts as a cofactor for several enzymatic systems, such as like transferases, lyases, isomerases, ligases, oxido-reductases, lectins, and a component of Mn-SOD, that also addresses the toxic effects of superoxide, and this issue is a matter of concern here. The level of Mn tends to be low in the heart and aorta, and higher concentrations are present in the plasma in atherosclerotic subjects compared with healthy controls. Therefore, no studies have confirmed the serum manganese levels in IHD patients and evaluated their role in the prognosis or pathogenesis of ischemic patients. Thus, the insignificant increase in Mn (p < 0.05) in the sera of the patient group reported in the current investigation may have a role in ischemic heart disease, although further study is recommended for pathophysiological confirmation. The data summarized here ultimately illustrate the possibility that a reduction in Cu-Zn SOD, an elevation in Fe stores, and an imbalance in Mn-SOD may lead to two pathways, i.e., inadequate antioxidant ability and decreased immune efficiency. This assumption is based on our findings of significant reductions in serum Zn and Cu, an increase in Fe, and an increase in Mn as well as an imbalance in the latter. The inadequate antioxidant activity follows the accumulation of oxidation products in the artery from LDL, whereas ineffective immune function drives the reduction in macrophages that apparently results in increased production of oxidized LDL. The subsequent outcomes of the oxidized products of both pathways are foam cells [57, 58]. The further association of foam cells with atherosclerosis through the formation of atherogenic plaques, which is the pathological basis of IHD, is well established by physiological investigations [59]. Therefore, it seems reasonable to state that the findings of the current study may play a significant role as the pathogenic basis of the formation as well as the development of IHD.
5. Limitations of the study
Apart from the significance of our current study, we should note some limitations. This study performed only the analysis of trace elements qualitatively. We have not identified supplementation of the nutritional effect in our study conditions; thus, more studies might be necessary to assess whether or not nutritional supplements will benefit the IHD patient's condition. Also, the research was performed on a few subjects. Hence, a large-scale study with more samples from various regions of Bangladesh can depict the actual scenario. Although this study still has some shortcomings, we hope that our study will play a significant role in providing a new pathological resource for IHD patients in Bangladesh.
6. Conclusion
Our study assumed that reduced concentrations of Zn and Cu and elevated concentrations of Fe and Mn may play combinatorial roles in the gradual development of IHD. Therefore, depleted serum levels of Zn and Cu and elevated Fe and Mn levels may provide a prognostic indicator for managing the disease. Furthermore, extensive studies with related clinicopathological data to replicate the results are needed.
Declarations
Author contribution statement
S. Anonna: Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Sa. Ahamed and M Uddin: Performed the experiments; Contributed reagents, materials, analysis tools or data.
M. Adnan and S. Uddin: Contributed reagents, materials, analysis tools or data.
M. Hussain and M. Millat, M Sarwar and M. Rashid: Analyzed and interpreted the data; Wrote the paper.
L. Bulbul and J. Chowdhury: Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data.
R. Bhatta: Performed the experiments; Wrote the paper.
M. Islam:Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
The authors are grateful to the Department of Pharmacy, Noakhali Science and Technology University for providing partial financial support, lab facilities and instrumental support to conduct this research work. The authors are also so much grateful to the Department of Cardiology, Comilla & Lakshmipur Sadar Hospital, Lakshmipur, Bangladesh, for their co-operation and ethical support during sample collection. The authors are also shown their gratitude to the American journal expert agency for correcting the language of this manuscript.
References
- 1.Varbo A., Benn M., Smith G.D., Timpson N.J., Tybjærg-Hansen A., Nordestgaard B.G. Remnant cholesterol, low-density lipoprotein cholesterol, and blood pressure as mediators from obesity to ischemic heart disease. Circ. Res. 2015;116:665–673. doi: 10.1161/CIRCRESAHA.116.304846. [DOI] [PubMed] [Google Scholar]
- 2.Dahlöf B. Cardiovascular disease risk factors: epidemiology and risk assessment. Am. J. Cardiol. 2010;105:3A–9A. doi: 10.1016/j.amjcard.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 3.Essien O.E., Andy J., Ansa V., Otu A.A., Udoh A. Coronary artery disease and the profile of cardiovascular risk factors in South Nigeria: a clinical and autopsy study. Cardiol. Res. Pract. 2014;2014 doi: 10.1155/2014/804751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Calder P.C. The role of marine omega-3 (n-3) fatty acids in inflammatory processes, atherosclerosis and plaque stability. Mol. Nutr. Food Res. 2012;56:1073–1080. doi: 10.1002/mnfr.201100710. [DOI] [PubMed] [Google Scholar]
- 5.Helkin A., Stein J.J., Lin S., Siddiqui S., Maier K.G., Gahtan V. Dyslipidemia part 1 - review of lipid metabolism and vascular cell physiology. Vasc. Endovasc. Surg. 2016;50:107–118. doi: 10.1177/1538574416628654. [DOI] [PubMed] [Google Scholar]
- 6.Rosendorff C., Black H.R., Cannon C.P., Gersh B.J., Gore J., Izzo J.L., Kaplan N.M., O’Connor C.M., O’Gara P.T., Oparil S. Treatment of hypertension in the prevention and management of ischemic heart disease: a scientific statement from the American Heart Association council for high blood pressure research and the councils on clinical cardiology and epidemiology and preventi. Circulation. 2007;115:2761–2788. doi: 10.1161/CIRCULATIONAHA.107.183885. [DOI] [PubMed] [Google Scholar]
- 7.Franssen F.M.E., Soriano J.B., Roche N., Bloomfield P.H., Brusselle G., Fabbri L.M., García-Rio F., Kearney M.T., Kwon N., Lundbäck B., Rabe K.F., Raillard A., Muellerova H., Cockcroft J.R. Lung function abnormalities in smokers with ischemic heart disease. Am. J. Respir. Crit. Care Med. 2016;194:568–576. doi: 10.1164/rccm.201512-2480OC. [DOI] [PubMed] [Google Scholar]
- 8.Thomsen S.B., Rathcke C.N., Zerahn B., Vestergaard H. Increased levels of the calcification marker Matrix Gla Protein and the inflammatory markers YKL-40 and CRP in patients with type 2 diabetes and ischemic heart disease. Cardiovasc. Diabetol. 2010;9:1–7. doi: 10.1186/1475-2840-9-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nordlie M.A., Wold L.E., Kloner R.A. Genetic contributors toward increased risk for ischemic heart disease. J. Mol. Cell. Cardiol. 2005;39:667–679. doi: 10.1016/j.yjmcc.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 10.Mitra S., Deshmukh A., Sachdeva R., Lu J., Mehta J.L. Oxidized low-density lipoprotein and atherosclerosis implications in antioxidant therapy. Am. J. Med. Sci. 2011:135–142. doi: 10.1097/MAJ.0b013e318224a147. Lippincott Williams and Wilkins. [DOI] [PubMed] [Google Scholar]
- 11.Duarte M.M.M.F., Rocha J.B.T., Moresco R.N., Duarte T., Da Cruz I.B.M., Loro V.L., Schetinger M.R.C. Association between ischemia-modified albumin, lipids and inflammation biomarkers in patients with hypercholesterolemia. Clin. Biochem. 2009;42:666–671. doi: 10.1016/j.clinbiochem.2009.01.010. [DOI] [PubMed] [Google Scholar]
- 12.Tang Y.R., Zhang S.Q., Xiong Y., Zhao Y., Fu H., Zhang H.P., Xiong K.M. Studies of five microelement contents in human serum, hair, and fingernails correlated with aged hypertension and coronary heart disease. Biol. Trace Elem. Res. 2003;92:97–103. doi: 10.1385/BTER:92:2:97. [DOI] [PubMed] [Google Scholar]
- 13.Zatta P., Lucchini R., Van Rensburg S.J., Taylor A. The role of metals in neurodegenerative processes: aluminum, manganese, and zinc. Brain Res. Bull. 2003;62:15–28. doi: 10.1016/s0361-9230(03)00182-5. [DOI] [PubMed] [Google Scholar]
- 14.Isola G., Polizzi A., Santonocito S., Alibrandi A., Ferlito S. Expression of salivary and serum malondialdehyde and lipid profile of patients with periodontitis and coronary heart disease. Int. J. Mol. Sci. 2019;20 doi: 10.3390/ijms20236061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Qu X., Jin F., Hao Y., Li H., Tang T., Wang H., Yan W., Dai K. Magnesium and the risk of cardiovascular events: a meta-analysis of prospective cohort studies. PloS One. 2013;8 doi: 10.1371/journal.pone.0057720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Singh R.B., Gupta U.C., Mittal N., Niaz M.A., Ghosh S., Rastogi V. Epidemiologic study of trace elements and magnesium on risk of coronary artery disease in rural and urban Indian populations. J. Am. Coll. Nutr. 1997;16:62–67. doi: 10.1080/07315724.1997.10718650. [DOI] [PubMed] [Google Scholar]
- 17.Bae Y.J., Choi M.K., Kim M.H. Manganese supplementation reduces the blood cholesterol levels in Ca-deficient ovariectomized rats. Biol. Trace Elem. Res. 2011;141:224–231. doi: 10.1007/s12011-010-8714-1. [DOI] [PubMed] [Google Scholar]
- 18.Niskanen J., Marniemi J., Piironen O., Maatela J., Mäki J., Vuori I., Seppänen A., Kallio V., Aromaa A. Trace element levels in serum and urine of subjects died of coronary heart disease. Acta Pharmacol. Toxicol. (Copenh). 1986;59:340–343. doi: 10.1111/j.1600-0773.1986.tb02775.x. [DOI] [PubMed] [Google Scholar]
- 19.Sullivan J.F., Blotcky A.J., Jetton M.M., Hahn H.K. Serum levels of selenium, calcium, copper magnesium, manganese and zinc in various human diseases. J. Nutr. 1979;109:1432–1437. doi: 10.1093/jn/109.8.1432. [DOI] [PubMed] [Google Scholar]
- 20.Hippisley-Cox J., Pringle M., Hammersley V., Crown N., Wynn A., Meal A., Coupland C. Antidepressants as risk factor for ischaemic heart disease: case-control study in primary care. Br. Med. J. 2001;323:666–669. doi: 10.1136/bmj.323.7314.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ho P.M., Magid D.J., Masoudi F.A., McClure D.L., Rumsfeld J.S. Adherence to cardioprotective medications and mortality among patients with diabetes and ischemic heart disease. BMC Cardiovasc. Disord. 2006;6:48. doi: 10.1186/1471-2261-6-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.NCCLS Control of pre-analytical variation in trace element determination. Nat. Committ. Clin. Lab. Stand. Appr. Guid. 1997;17:1–30. [Google Scholar]
- 23.Amin M.N., Siddiqui S.A., Uddin M.G., Ibrahim M., Uddin S.M.N., Adnan M.T., Rahaman M.Z., Kar A., Islam M.S. Increased oxidative stress, altered trace elements, and macro-minerals are associated with female obesity. Biol. Trace Elem. Res. 2020;197:384–393. doi: 10.1007/s12011-019-02002-z. [DOI] [PubMed] [Google Scholar]
- 24.Uddin M.G., Hossain M.S., Rahman M.A., Uddin A.H.M.M., Bhuiyan M.S. Elemental zinc is inversely associated with C-reactive protein and oxidative stress in chronic liver disease. Biol. Trace Elem. Res. 2017;178:189–193. doi: 10.1007/s12011-016-0919-5. [DOI] [PubMed] [Google Scholar]
- 25.Adnan M.T., Amin M.N., Uddin M.G., Hussain M.S., Sarwar M.S., Hossain M.K., Uddin S.M.N., Islam M.S. Increased concentration of serum MDA, decreased antioxidants and altered trace elements and macro-minerals are linked to obesity among Bangladeshi population. Diabetes Metab. Syndr. Clin. Res. Rev. 2019;13:933–938. doi: 10.1016/j.dsx.2018.12.022. [DOI] [PubMed] [Google Scholar]
- 26.Das D.C., Jahan I., Uddin M.G., Hossain M.M., Chowdhury M.A.Z., Fardous Z., Rahman M.M., Kabir A.K.M.H., Deb S.R., Siddique M.A.B., Das A. Serum CRP, MDA, vitamin C, and trace elements in Bangladeshi patients with rheumatoid arthritis. Biol. Trace Elem. Res. 2020:1–9. doi: 10.1007/s12011-020-02142-7. [DOI] [PubMed] [Google Scholar]
- 27.Czupryn M., Falchuk K.H., Stankiewicz A., Vallee B.L. A Euglena gracilis zinc endonuclease. Biochemistry. 1993;32:1204–1211. doi: 10.1021/bi00056a002. [DOI] [PubMed] [Google Scholar]
- 28.Sariego Muñiz C., Marchante-Gayón J.M., García Alonso J.I., Sanz-Medel A. Multi-elemental trace analysis of human serum by double-focusing ICP-MS. J. Anal. At. Spectrom. 1999;14:193–198. [Google Scholar]
- 29.Klevay L.M. Coronary heart disease: the zinc/copper hypothesis. Am. J. Clin. Nutr. 1975;28:764–774. doi: 10.1093/ajcn/28.7.764. [DOI] [PubMed] [Google Scholar]
- 30.Ford E.S. Serum copper concentration and coronary heart disease among US adults. Am. J. Epidemiol. 2000;151:1182–1188. doi: 10.1093/oxfordjournals.aje.a010168. [DOI] [PubMed] [Google Scholar]
- 31.Kumru S., Aydin S., Simsek M., Sahin K., Yaman M., Ay G. Comparison of serum copper, zinc, calcium, and magnesium levels in preeclamptic and healthy pregnant women. Biol. Trace Elem. Res. 2003;94:105–112. doi: 10.1385/BTER:94:2:105. [DOI] [PubMed] [Google Scholar]
- 32.Isola G., Lo Giudice A., Polizzi A., Alibrandi A., Patini R., Ferlito S. Periodontitis and tooth loss have negative systemic impact on circulating progenitor cell levels: a clinical study. Genes (Basel) 2019;10 doi: 10.3390/genes10121022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jain S., Sharma P., Kulshreshtha S., Mohan G., Singh S. The role of calcium, magnesium, and zinc in pre-eclampsia. Biol. Trace Elem. Res. 2010;133:162–170. doi: 10.1007/s12011-009-8423-9. [DOI] [PubMed] [Google Scholar]
- 34.Al-Araji S.M., Abbase A.H., Hassan Z.F. Homocystein and trace elements levels in patient with ischemic heart disease and some associated Diseases. Int. Conf. Appl. Life Sci. 2012 https://ideas.repec.org/h/ito/pchaps/93312.html [Google Scholar]
- 35.Burkitt M.J. A critical overview of the chemistry of copper-dependent low density lipoprotein oxidation: roles of lipid hydroperoxides, α-tocopherol, thiols, and ceruloplasmin. Arch. Biochem. Biophys. 2001;394:117–135. doi: 10.1006/abbi.2001.2509. [DOI] [PubMed] [Google Scholar]
- 36.Natalia Giurgea A.M., Constantinescu M.I., Stanciu R., Suciu S. Ceruloplasmin - acute-phase reactant or endogenous antioxidant? The case of cardiovascular disease. Med. Sci. Monit. 2005;11:RA48–51. https://pubmed.ncbi.nlm.nih.gov/15668644/ [PubMed] [Google Scholar]
- 37.Ehrenwald E., Chisolm G.M., Fox P.L. Intact human ceruloplasmin oxidatively modifies low density lipoprotein. J. Clin. Invest. 1994;93:1493–1501. doi: 10.1172/JCI117127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fox P.L., Mazumder B., Ehrenwald E., Mukhopadhyay C.K. Ceruloplasmin and cardiovascular disease. Free Radic. Biol. Med. 2000;28:1735–1744. doi: 10.1016/s0891-5849(00)00231-8. [DOI] [PubMed] [Google Scholar]
- 39.Cebi A., Kaya Y., Gungor H., Demir H., Yoruk I.H., Soylemez N., Gunes Y., Tuncer M. Trace elements, heavy metals and vitamin levels in patients with coronary artery disease. Int. J. Med. Sci. 2011;8:456–460. doi: 10.7150/ijms.8.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Altekin E., Çoker C., Şişman A.R., Önvural B., Kuralay F., Kirimli Ö. The relationship between trace elements and cardiac markers in acute coronary syndromes. J. Trace Elem. Med. Biol. 2005;18:235–242. doi: 10.1016/j.jtemb.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 41.Owen C.A. Effects of iron on copper metabolism and copper on iron metabolism in rats. Am. J. Physiol. 1973;224:514–518. doi: 10.1152/ajplegacy.1973.224.3.514. [DOI] [PubMed] [Google Scholar]
- 42.Virtamo J., Huttunen J.K. Minerals, trace elements and cardiovascular disease. An overview. Ann. Clin. Res. 1988;20:102–113. https://pubmed.ncbi.nlm.nih.gov/3044250/ [PubMed] [Google Scholar]
- 43.Klevay L.M. Ischemic heart disease as copper deficiency. Adv. Exp. Med. Biol., Adv. Exp. Med. Biol. 1989:197–208. doi: 10.1007/978-1-4613-0537-8_17. [DOI] [PubMed] [Google Scholar]
- 44.Klevay L.M. Re: “ serum copper and the risk of acute myocardial infarction: a prospective population study in men in eastern Finland. Am. J. Epidemiol. 1992;135:832–833. doi: 10.1093/oxfordjournals.aje.a116370. [DOI] [PubMed] [Google Scholar]
- 45.Tiber A.M., Sakhaii M., David Joffe C., Ratnaparkhi M.V. Relative value of plasma copper, zinc, lipids and lipoproteins as markers for coronary artery disease. Atherosclerosis. 1986;62:105–110. doi: 10.1016/0021-9150(86)90054-7. [DOI] [PubMed] [Google Scholar]
- 46.Sempos C.T., Looker A.C., Gillum R.F., Makuc D.M. Body iron stores and the risk of coronary heart disease. N. Engl. J. Med. 1994;330:1119–1124. doi: 10.1056/NEJM199404213301604. [DOI] [PubMed] [Google Scholar]
- 47.Ascherio A., Willett W.C., Rimm E.B., Giovannucci E.L., Stampfer M.J. Dietary iron intake and risk of coronary disease among men. Circulation. 1994;89:969–974. doi: 10.1161/01.cir.89.3.969. [DOI] [PubMed] [Google Scholar]
- 48.Sullivan J.L. Iron and the sex difference in heart disease risk. Lancet. 1981;317 doi: 10.1016/s0140-6736(81)92463-6. [DOI] [PubMed] [Google Scholar]
- 49.Parthasarathy Sampath, Steinberg Daniel, Witztum J.L. The role of oxidized low-density lipoproteins in the pathogenesis of atherosclerosis. Annu. Rev. Med. 1992;43:219–225. doi: 10.1146/annurev.me.43.020192.001251. [DOI] [PubMed] [Google Scholar]
- 50.Price K.D., Price C.S.C., Reynolds R.D. Hyperglycemia-induced ascorbic acid deficiency promotes endothelial dysfunction and the development of atherosclerosis. Atherosclerosis. 2001;158:1–12. doi: 10.1016/s0021-9150(01)00569-x. [DOI] [PubMed] [Google Scholar]
- 51.Harrison D., Griendling K.K., Landmesser U., Hornig B., Drexler H. Role of oxidative stress in atherosclerosis. Am. J. Cardiol. 2003:7–11. doi: 10.1016/s0002-9149(02)03144-2. Elsevier Inc. [DOI] [PubMed] [Google Scholar]
- 52.Fields M., Lewis C.G., Lure M.D., Burns W.A., Antholine W.E. Low dietary iron prevents free radical formation and heart pathology of copper-deficient rats fed fructose. Proc. Soc. Exp. Biol. Med. 1993;202:225–232. doi: 10.3181/00379727-202-43531. [DOI] [PubMed] [Google Scholar]
- 53.Stadler N., Lindner R.A., Davies M.J. Direct detection and quantification of transition metal ions in human atherosclerotic plaques: evidence for the presence of elevated levels of iron and copper. Arterioscler. Thromb. Vasc. Biol. 2004;24:949–954. doi: 10.1161/01.ATV.0000124892.90999.cb. [DOI] [PubMed] [Google Scholar]
- 54.Johnson M.A., Murphy C.L. Adverse effects of high dietary iron and ascorbic acid on copper status in copper-deficient and copper-adequate rats. Am. J. Clin. Nutr. 1988;47:96–101. doi: 10.1093/ajcn/47.1.96. [DOI] [PubMed] [Google Scholar]
- 55.Niall Martin C.D. Protective mechanisms of the body. Anaesth. Intensive Care Med. 2006;7:459–461. [Google Scholar]
- 56.Wong G.H.W., Goeddel D.V. Induction of manganous superoxide dismutase by tumor necrosis factor: possible protective mechanism. Science (80-) 1988;242:941–944. doi: 10.1126/science.3263703. [DOI] [PubMed] [Google Scholar]
- 57.Rose N., Afanasyeva M. Autoimmunity: busting the atherosclerotic plaque. Nat. Med. 2003;9:641–642. doi: 10.1038/nm0603-641. [DOI] [PubMed] [Google Scholar]
- 58.Xu S., Huang Y., Xie Y., Lan T., Le K., Chen J., Chen S., Gao S., Xu X., Shen X., Huang H., Liu P. Evaluation of foam cell formation in cultured macrophages: an improved method with Oil Red O staining and DiI-oxLDL uptake. Cytotechnology. 2010;62:473–481. doi: 10.1007/s10616-010-9290-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Xiao-Hua Yua C.-K.T., Fuc Yu-Chang, Zhangd Da-Wei, Yinb Kai. Foam cells in atherosclerosis. Clin. Chim. Acta. 2013;424:245–252. doi: 10.1016/j.cca.2013.06.006. [DOI] [PubMed] [Google Scholar]