Abstract
Introduction
To assess the safety and efficacy of selective laser trabeculoplasty (SLT) for ocular hypertension (OHT) induced by a dexamethasone (DEX) intravitreal implant.
Materials and Methods
We performed a retrospective study of patients who underwent an SLT procedure for ocular hypertension induced by injection of a DEX intravitreal implant. Patients had, at least, one injection of the DEX-implant for symptomatic macular edema. SLT was delivered to 360° of the trabecular meshwork in two sessions. The primary outcome was a decrease in IOP, evaluated at one, three, and six months after the SLT procedure.
Results
Twenty-six eyes of 22 patients were included. The mean intraocular pressure (IOP) measured after DEX-implant injection was 25.4 ± 5.4 mmHg, and the mean increase in IOP was 35.8 ± 14.6%. The mean follow-up after SLT was 18.3 ± 7.7 months. After SLT, the mean IOP dropped by 30.9% at one month (16.9 ± 4.5 mmHg, p=0.01), 33.6% at three months (16.0 ± 2.7 mmHg, p < 0.01), and 34.9% at six months (15.6 ± 2.1 mmHg, p < 0.01). Each patient had a minimum follow-up of 6 months after SLT. Eight eyes (31%) received a second DEX-implant injection after the SLT procedure without experiencing an increase in the IOP above 21 mmHg or >20%. No glaucoma surgery was required during the follow-up. The mean number of medications (1.65 ± 1.36) was significantly reduced at one (1.19 ± 1.20, p=0.04), three (0.96 ± 1.03, p < 0.01), and six months (0.77 ± 0.95, p < 0.01) after SLT.
Conclusion
SLT is an effective and safe procedure to control OHT following DEX-implant intravitreal injection.
1. Introduction
Dexamethasone intravitreal implant 0.7 mg (Ozurdex®, Allergan, Dublin, Ireland) (DEX-implant) is a sustained-release corticosteroid device that is effective in the management of diabetic macular edema [1], age-related macular degeneration [2], retinal vein occlusions [3], noninfectious posterior uveitis [4], and resistant Irvine–Gass syndrome [5]. After DEX-implant injection, an increase in intraocular pressure (IOP) may be observed after 1.5 to 2.5 months [6] in 12.6% [7] to 36.0% [8, 9] of patients. Rajesh et al. reported that antiglaucoma medication or filtering surgery was needed for 91.8% and 3.1% of patients exhibiting a postinjection increase in intraocular pressure (IOP), respectively [10]. Similarly, 3% of patients treated with a DEX-implant for Irvine–Gass syndrome were eventually injected with anti-VEGF because of ocular hypertension (OHT), despite maximal medical antiglaucoma treatment [5]. After three or more injections, the frequency of mild OHT increased to 53% versus 31% after the first injection [11]. Moreover, the risk of a large increase in IOP is greater for patients with primary open-angle glaucoma (POAG) or suspected glaucoma at the time of DEX implantation [12].
The physiopathology of glucocorticoid-induced OHT and glaucoma may be related to the impairment of outflow through the trabecular meshwork (TM) via an enzymatic pathway responsible for mucopolysaccharide accumulation in the iridocorneal angle [13]. A mouse model has suggested a role for glucocorticoid receptor transactivation in regulating glucocorticoid-mediated gene expression in the TM [14]. Steroids may also have an effect on the TM extracellular matrix due to altered rates of protein synthesis or degradation or a combination of the two [12].
Selective laser trabeculoplasty (SLT) uses a 532 nm Q-switched, frequency-doubled Nd:YAG laser that delivers a short pulse (3 nanoseconds) [15]. It prevents heat dissipation outside of pigmented TM cells and does not cause collateral damage [15]. Selective laser trabeculoplasty is effective in lowering IOP in open-angle glaucoma [16–18] and can be offered as a first-line treatment for POAG or OHT [19]. SLT induces biological changes that modulate increased aqueous outflow through the TM, including changes in cytokine and interleukin-8 (IL-8) secretion and TM remodeling, which are affected by glucocorticoids [20]. Selective laser trabeculoplasty is also effective in treating OHT after intravitreal triamcinolone acetonide injection [21], as well as in preventing an increase in IOP when performed before injection [22].
Various guidelines establishing the management of OHT after intravitreal injection have been published [23]. Topical treatment is the first approach proposed, followed by surgical treatment in case of refractory OHT. The exact role of SLT in the algorithm of treatment of OHT after DEX-implant injection has not been yet determined. Moreover, no studies have evaluated the effect of SLT in patients with DEX-implant-induced OHT. Thus, the main objective of the present study was to evaluate the effectiveness and safety of the SLT procedure on increased IOP after DEX-implant injection.
2. Patients and Methods
This study was conducted at the Quinze-Vingts National Ophthalmology Hospital, Paris, France, in accordance with the tenets of the Declaration of Helsinki. We retrospectively reviewed the medical records of all patients who underwent an SLT procedure for uncontrolled OHT induced by DEX-implant injection between November 2017 and October 2018.
The study included patients who had, at least, one injection of the DEX-implant for symptomatic macular edema. All patients were symptomatic, and diagnosis of macular edema was established based on examination of the fundus and macular optical coherence tomography (OCT) showing a central macular thickness (CMT) of >300 μm (Spectralis HRA + OCT, Heidelberg engineering Inc, Heidelberg®, Germany). Patients with uncontrolled IOP (according to their target IOP) despite maximal tolerated topical treatment three months after DEX-implant injection underwent the SLT procedure.
2.1. Injection Technique
An implant of 0.7 mg dexamethasone (Ozurdex®, Allergan, Irvine, CA) was inserted into the vitreous cavity through the pars plana at a distance of 3.5 mm from the limbus in pseudophakic eyes and 4 mm in phakic eyes after topical anesthesia (oxybuprocaine 1.6 mg/0.4 ml, Thea®, Clermont-Ferrand, France) and sterilization of the ocular and periocular surface with 5% povidone iodine. Patients were seen, at least, one, three, and six months after the injections, allowing measurement of the best corrected visual acuity (BCVA), IOP, CMT, and examination of the fundus.
2.2. SLT Procedure
All SLT procedures were performed, after topical anesthesia (oxybuprocaine), by trained physicians of our group with the same machine (TangoTM, EllexInc, Adelaide, Australia) using a Latina SLT lens (Ocular Instruments, Bellevue, Washington, USA) to visualize the TM. Selective laser trabeculoplasty was delivered to 360° of the trabecular meshwork in two sessions, one week apart, with the total number of nonoverlapping impacts varying from 80 to 110. The energy setting was from 0.8 to 1.2 mJ. One drop of 0.1% Indocollyre (Indometacine, Bausch & Lomb, Berlin, Germany) and 0.5% Iopidine (Alcon Laboratories, Inc., Ft. Worth, TX) were immediately administered after the procedure and for five days, three times a day, for Indocollyre.
2.3. Study Population
Each patient underwent a standardized examination, performed in our retinal disease department, with measurement of the BCVA in Early Treatment Diabetic Retinopathy Study (ETDRS) letters converted to LogMAR (Minimum Angle of Resolution) [24], IOP measurement using a Goldman applanation tonometer, examination of the fundus, and macular OCT. Every patient had an open angle on the Schaffer scale at gonioscopy examination. Open-angle glaucoma risk factors and the date and number of DEX-implant injections, the date, number, and characteristics of the SLT procedures, number and type of antiglaucoma medications, and surgical history were recorded.
2.4. Statistical Assessment
All statistical analyses were performed using GraphPad Prism 7® for Windows® (GraphPad Software, La Jolla, CA, USA). The primary outcome was a decrease in IOP evaluated at one, three, and six months after the SLT procedure. Quantitative variables were compared using the Student t-test. Survival curves were generated using the Kaplan–Meier method. Double-sided p values <0.05 were considered statistically significant.
3. Results
The study included 26 eyes of 22 patients. Baseline demographics and ocular parameters are presented in Table 1.
Table 1.
Baseline demographic characteristics of the 22 patients who underwent SLT after DEX-implant injection.
| Patient characteristics (n = 22) | |
| Male | 12 (55) |
| Female | 10 (45) |
| Caucasian | 18 (82) |
| African | 4 (18) |
| Age, mean ± SD | 69.6 ± 15.4 |
| Family history of glaucoma | 2 (9) |
|
| |
| Eyes characteristics (n = 26) | |
| Retinal disease | |
| Diabetic macular edema | 15 (57) |
| Irvine–Gass syndrome | 8 (31) |
| Branch retinal vein occlusion | 3 (12) |
| Cup-to-disc ratio ± SD | 0.38 ± 0.19 |
| Pseudophakic | 19 (73) |
| PPV | 5 (19) |
| Retinal laser | 8 (31) |
| PPRP | 7 (27) |
| Focal laser | 1 (4) |
The results are presented as n (%) for categorical variables. SD, standard deviation; PPV, pars plana vitrectomy; PPRP, peripherical pan-retical photocoagulation.
Best corrected visual acuity significantly improved following DEX-implant injection from 0.58 ± 0.42 to 0.32 ± 0.39 at six months after injection (p=0.01). The mean time between DEX-implant injection and the diagnosis of OHT was 55.8 ± 27.9 days. The SLT procedure was performed at a mean of 96.71 ± 14.73 days after DEX-implant injection. The mean duration of follow-up after SLT was 18.3 ± 7.7 months. The maximal IOP measured after DEX-implant injection was 25.4 ± 5.4 mmHg, with an increase in IOP of 35.8 ± 14.6%. After SLT, the mean IOP dropped by 30.9% at one month (16.9 ± 4.5 mmHg), 33.6% at three months (16.0 ± 2.7 mmHg), and 34.9% at six months (15.6 ± 2.1 mmHg) after SLT, showing a persistent and significant decrease in IOP (p < 0.01 compared to the pre-SLT IOP at each visit) (Figure 1). The SLT procedures are described in Table 2.
Figure 1.
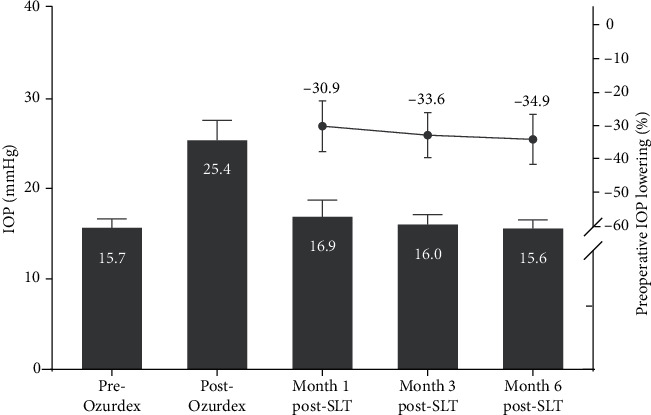
Intraocular pressure lowering after the SLT procedure with a follow-up of 6 months.
Table 2.
Characteristics of the SLT procedures.
| Parameters of the SLT procedures | |
|---|---|
| Total number of SLT procedures | 26 |
| Right eyes | 10 |
| Left eyes | 16 |
| Mean number of spots ± SD | 91.6 ± 20.2 |
| Total energy (mJ) ± SD | 75.9 ± 24.4 |
| New DEX-implant after SLT | 8 |
Eight eyes (31%) underwent a second DEX-implant injection after the SLT procedure without experiencing an increase in IOP (>20%) after one week or one, three, or six months of follow-up. Seven eyes did not require additional antiglaucoma drugs. The mean peak IOP after reinjection for patients who had SLT was 18.8 ± 1.5 mmHg, lower than the peak IOP after the first injection for patients requiring reinjection (27.3 ± 1.8 mmHg, p=0.01). The mean IOP values for the eyes that underwent SLT versus those of the contralateral eyes during the follow-up period are shown in Figure 2. During follow-up, 96.2%, 80.8%, and 69.2% of the treated eyes showed an IOP below 25, 21, and 18 mmHg, respectively (Figure 3).
Figure 2.
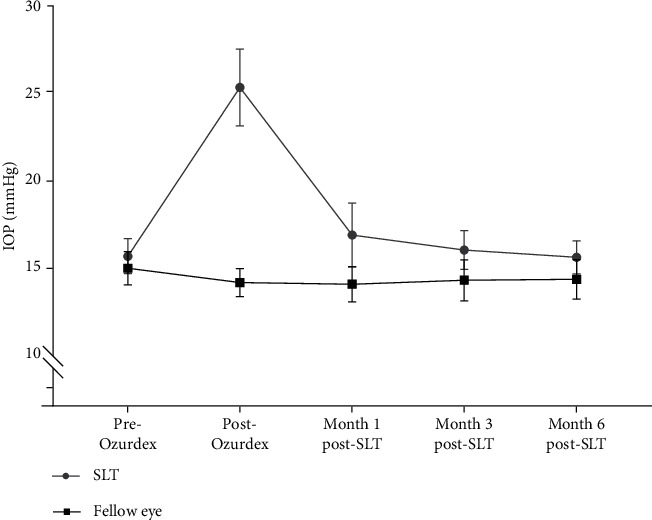
Evolution of mean intraocular pressure (IOP) before and after a DEX-implant injection in eyes that underwent selective laser trabeculoplasty versus contralateral eyes at one, three, and six months after treatment.
Figure 3.
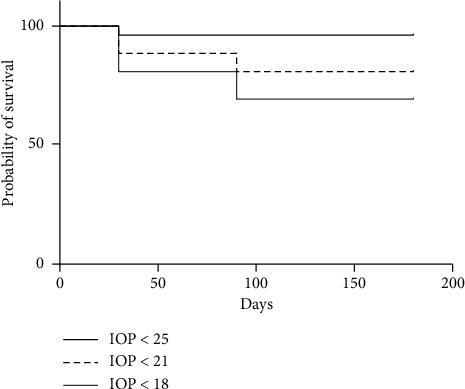
Kaplan–Meier survival curves plotting the cumulative probabilities that the IOP remains below 25 mmHg, 21 mmHg, and 18 mmHg, respectively, following the SLT procedure.
The mean number of medications (1.65 ± 1.36) was significantly lower at one (1.19 ± 1.20, p=0.04), three (0.96 ± 1.03, p < 0.01), and six months (0.77 ± 0.95, p < 0.01) after trabeculoplasty. No oral treatment or surgery was required during the follow-up. Six of the 26 patients (23%) required antiglaucoma eye drops after six months, with a mean number of 0.5 ± 0.88 topical treatments, without oral medication or surgery.
Adverse effects after SLT included a moderate transient anterior chamber reaction for one patient (4%) and traumatic keratitis for another (4%), which healed after one-week of topical treatment (Tobradex® (0.3% tobramycin, 0.1% dexamethasone; Alcon Laboratories Inc., Fort Worth, Texas) three times a day and vitamin A ointment associated with artificial tears, respectively).
4. Discussion
The SLT procedure appears to be a valuable and safe tool to manage increased IOP after DEX-implant injection. The exact mechanism by which SLT decreases IOP has not been yet fully elucidated. It may involve macrophage activation, resulting from increased chemokine production, allowing TM clearing. The SLT procedure may also allow the TM to release factors that regulate the permeability of Schlemm canal endothelial cells [25]. This effect mirrors the physiopathology of steroid-induced OHT, which involves increased responsiveness of the membranes of goniocytes to steroids, leading to increased production of fibroblasts in the TM and resulting in aqueous retention [12]. The TM extracellular matrix may be remodeled by the expression of stromelysin-1 (MMP-3), resulting in an increase in aqueous outflow [26]. Selective laser trabeculoplasty appears to have some clinical efficacy in secondary glaucoma patients, especially when dysfunction of the TM is involved, such as in DEX-implant-induced OHT. Here, we report a larger decrease in IOP after SLT than previously reported after SLT performed for pigmentary glaucoma (14.5%) or pseudoexfoliation glaucoma (16.6%) [27].
This study highlights the potential interest of SLT after steroid-induced OHT. This procedure may be an alternative to the usual treatment, which involves topical ocular antiglaucoma medications. Two studies have already reported efficacy of the SLT procedure in steroid-induced OHT. Rubin et al. reported the efficacy of SLT, with a significative decrease of IOP (p < 0.007) in five of seven patients [21]. Bozkurt et al., showed that the increase in IOP after intravitreal triamcinolone acetonide injection may be prevented by performing SLT if the baseline IOP is >21 mmHg [22]. The SAFODEX study demonstrated that OHT can be observed for 28.5% of DEX-implant injected eyes [28]. A patient who experiences OHT after the first injection has a significant risk of experiencing an increase in IOP after reinjection. The frequency of OHT (>23 mmHg) following one, two, or three reinjections is 31%, 26%, and 53%, respectively [11]. In the present study, none of the eight eyes that underwent DEX-implant reinjection after the SLT procedure experienced a major peak of IOP after SLT. These results suggest that the SLT procedure may also be useful in preventing new episodes of OHT after reinjection of the DEX-implant in patients who already experienced steroid-induced OHT.
Filtering surgery is more often needed to control corticosteroid-induced OHT in patients with branch or central retinal vein occlusion (RVO). Ocular hypertension in these patients may be multifactorial and the associated retinal ischemia may be responsible for persistent OHT in RVO [6]. Of note, in our study, the SLT procedure was also effective in controlling corticosteroid-induced OHT for patients with RVO, as the IOP of treated eyes tended to decrease to the same level as that of the adelphic eyes after three to six months.
Our study had several limitations, notably its being a retrospective study with a limited number of patients. Nonetheless, SLT can be considered to lower DEX-implant-induced increases in IOP. Selective laser trabeculoplasty may be a safe and effective alternative to antiglaucoma eye drops as a first-line treatment and probably as a prophylactic procedure to avoid peak IOP in patients presenting corticosteroid-induced OHT.
Data Availability
The paper data used to support the findings of this study are available from the corresponding author upon request.
Ethical Approval
All procedures performed in these studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- 1.Iglicki M., Busch C., Zur D., et al. Dexamethasone implant for diabetic macular edema in naive compared with refractory eyes. Retina. 2019;39(1):44–51. doi: 10.1097/IAE.0000000000002196. [DOI] [PubMed] [Google Scholar]
- 2.Calvo P., Ferreras A., Al Adel F., Wang Y., Brent M. H. Dexamethasone intravitreal implant as adjunct therapy for patients with wet age-related macular degeneration with incomplete response to ranibizumab. British Journal of Ophthalmology. 2015;99(6):723–726. doi: 10.1136/bjophthalmol-2014-305684. [DOI] [PubMed] [Google Scholar]
- 3.Ozkok A., Saleh O. A., Sigford D. K., Heroman J. W., Schaal S. The omar study. Retina. 2015;35(7):1393–1400. doi: 10.1097/iae.0000000000000475. [DOI] [PubMed] [Google Scholar]
- 4.London N. J. S., Chiang A., Haller J. A. The dexamethasone drug delivery system: indications and evidence. Advances in Therapy. 2011;28(5):351–366. doi: 10.1007/s12325-011-0019-z. [DOI] [PubMed] [Google Scholar]
- 5.Bellocq D., Pierre-Kahn V., Matonti F., et al. Effectiveness and safety of dexamethasone implants for postsurgical macular oedema including Irvine-Gass syndrome: the EPISODIC-2 study. British Journal of Ophthalmology. 2017;101(3):333–341. doi: 10.1136/bjophthalmol-2016-308544. [DOI] [PubMed] [Google Scholar]
- 6.Chin E. K., Almeida D. R. P., Velez G., et al. Ocular hypertension after intravitreal dexamethasone (OZURDEX) sustained-release implant. Retina. 2017;37(7):1345–1351. doi: 10.1097/IAE.0000000000001364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haller J. A., Bandello F., Belfort R., et al. Dexamethasone intravitreal implant in patients with macular edema related to branch or central retinal vein occlusion. Ophthalmology. 2011;118(12):2453–2460. doi: 10.1016/j.ophtha.2011.05.014. [DOI] [PubMed] [Google Scholar]
- 8.Boyer D. S., Yoon Y. H., Belfort R., et al. Three-year, randomized, sham-controlled trial of dexamethasone intravitreal implant in patients with diabetic macular edema. Ophthalmology. 2014;121(10):1904–1914. doi: 10.1016/j.ophtha.2014.04.024. [DOI] [PubMed] [Google Scholar]
- 9.Maturi R. K., Pollack A., Uy H. S., et al. Intraocular pressure in patients with diabetic macular edema treated with dexamethasone intravitreal implant in the 3-year mead study. Retina. 2016;36(6):1143–1152. doi: 10.1097/IAE.0000000000001004. [DOI] [PubMed] [Google Scholar]
- 10.Rajesh B., Zarranz-Ventura J., Fung A. T., et al. Safety of 6000 intravitreal dexamethasone implants. British Journal of Ophthalmology. 2019;104(1):39–46. doi: 10.1136/bjophthalmol-2019-313991. [DOI] [PubMed] [Google Scholar]
- 11.Bahadorani S., Krambeer C., Wannamaker K., et al. The effects of repeated Ozurdex injections on ocular hypertension. Clinical Ophthalmology. 2018;12:639–642. doi: 10.2147/OPTH.S148990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Razeghinejad M. R., Katz L. J. Steroid-induced iatrogenic glaucoma. Ophthalmic Research. 2012;47(2):66–80. doi: 10.1159/000328630. [DOI] [PubMed] [Google Scholar]
- 13.François J. Corticosteroid glaucoma. Ophthalmologica. 1984;188(2):76–81. doi: 10.1159/000309345. [DOI] [PubMed] [Google Scholar]
- 14.Patel G. C., Millar J. C., Clark A. F. Glucocorticoid receptor transactivation is required for glucocorticoid-induced ocular hypertension and glaucoma. Investigative Opthalmology & Visual Science. 2019;60(6):1967–1978. doi: 10.1167/iovs.18-26383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Latina M. A., Sibayan S. A., Shin D. H., Noecker R. J., Marcellino G. Q-switched 532-nm Nd:YAG laser trabeculoplasty (selective laser trabeculoplasty) Ophthalmology. 1998;105(11):2082–2090. doi: 10.1016/S0161-6420(98)91129-0. [DOI] [PubMed] [Google Scholar]
- 16.Stein J. D., Challa P. Mechanisms of action and efficacy of argon laser trabeculoplasty and selective laser trabeculoplasty. Current Opinion in Ophthalmology. 2007;18(2):140–145. doi: 10.1097/ICU.0b013e328086aebf. [DOI] [PubMed] [Google Scholar]
- 17.Latina M., Deleon J. Selective laser trabeculoplasty. Ophthalmology Clinics of North America. 2005;18(3):409–419. doi: 10.1016/j.ohc.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 18.Acott T. S., Samples J. R., Bradley J. M., Bacon D. R, Bylsma S. S, van Buskirk E. M. Trabecular repopulation by anterior trabecular meshwork cells after laser trabeculoplasty. American Journal of Ophthalmology. 1989;107(1):1–6. doi: 10.1016/0002-9394(89)90805-2. [DOI] [PubMed] [Google Scholar]
- 19.Gazzard G., Konstantakopoulou E., Garway-Heath D., et al. Selective laser trabeculoplasty versus eye drops for first-line treatment of ocular hypertension and glaucoma (LiGHT): a multicentre randomised controlled trial. The Lancet. 2019;393(10180):1505–1516. doi: 10.1016/S0140-6736(18)32213-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kagan D. B., Gorfinkel N. S., Hutnik C. M. Mechanisms of selective laser trabeculoplasty: a review. Clinical & Experimental Ophthalmology. 2014;42(7):675–681. doi: 10.1111/ceo.12281. [DOI] [PubMed] [Google Scholar]
- 21.Rubin B., Taglienti A., Rothman R. F., Marcus C. H., Serle J. B. The effect of selective laser trabeculoplasty on intraocular pressure in patients with intravitreal steroid-induced elevated intraocular pressure. Journal of Glaucoma. 2008;17(4):287–292. doi: 10.1097/IJG.0b013e318031676c. [DOI] [PubMed] [Google Scholar]
- 22.Bozkurt E., Kara N., Yazici A. T., et al. Prophylactic selective laser trabeculoplasty in the prevention of intraocular pressure elevation after intravitreal triamcinolone acetonide injection. American Journal of Ophthalmology. 2011;152(6):976–981. doi: 10.1016/j.ajo.2011.05.027. [DOI] [PubMed] [Google Scholar]
- 23.Poli M., Denis P., Dot C., Nordmann J.-P. Conduite à tenir face au risque d’hypertonie oculaire après injection intra-vitréenne. Journal Français d’Ophtalmologie. 2017;40(3):e77–e82. doi: 10.1016/j.jfo.2017.01.003. [DOI] [PubMed] [Google Scholar]
- 24.Ferris F. L., Kassoff A., Bresnick G. H., Bailey I. New visual acuity charts for clinical research. American Journal of Ophthalmology. 1982;94(1):91–96. doi: 10.1016/0002-9394(82)90197-0. [DOI] [PubMed] [Google Scholar]
- 25.Alvarado J. A., Yeh R. F., Franse-Carman L., Marcellino G., Brownstein M. J. Interactions between endothelia of the trabecular meshwork and of Schlemm’s canal: a new insight into the regulation of aqueous outflow in the eye. Transactions of the American Ophthalmological Society. 2005;103:148–163. [PMC free article] [PubMed] [Google Scholar]
- 26.Lee J. Y., Kagan D. B., Roumeliotis G., Liu H., Hutnik C. M. Secretion of matrix metalloproteinase-3 by co-cultured pigmented and non-pigmented human trabecular meshwork cells following selective laser trabeculoplasty. Clinical & Experimental Ophthalmology. 2016;44(1):33–42. doi: 10.1111/ceo.12591. [DOI] [PubMed] [Google Scholar]
- 27.Koucheki B., Hashemi H. Selective laser trabeculoplasty in the treatment of open-angle glaucoma. Journal of Glaucoma. 2012;21(1):65–70. doi: 10.1097/IJG.0b013e3182027596. [DOI] [PubMed] [Google Scholar]
- 28.Malclès A., Dot C., Voirin N., et al. Safety of intravitreal dexamethasone implant (ozurdex) Retina. 2017;37(7):1352–1359. doi: 10.1097/IAE.0000000000001369. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The paper data used to support the findings of this study are available from the corresponding author upon request.


