Abstract
There has been longstanding interest in virtual care in oncology, but outdated reimbursement structures and a paradoxical lack of agility within electronic systems limited widespread adoption. Through the example of the Province of Ontario, Canada and the Princess Margaret Cancer Centre, we describe how a collective sense of action from COVID-19, a system of distributed leadership and decision-making, and the use of a Service Design process to map the ambulatory encounter onto a digital workflow were critical enablers of a large-scale virtual transition. Rigorous evaluation of virtual care models will be essential to maintain integration of virtual care post-pandemic.
Keywords: Telemedicine, Cancer, Ambulatory care, Virtual care, COVID-19
1. Background
There has been growing interest in virtual care solutions in clinical medicine, but until the onset of COVID-19, traditional in-person visits have remained the primary method of delivering outpatient services. The lack of telehealth has been a common focus of discussions on improving the delivery of value-based and patient-centered care.1 , 2 Outdated reimbursement structures, inertia, a lack of value assigned to patients’ time, and the paucity of quantitative data on novel clinical models have all contributed to this stagnation.3 , 4 Further, a paradoxical lack of agility within electronic systems to encompass new modes of care delivery have contributed to the reluctance of many physicians, hospitals, and health systems to fully embrace virtual care.5 A national Canadian task force on virtual care released their recommendations in February 2020 and identified current electronic ecosystems as a major impediment to virtual care and provided a set of recommendations on how these may be addressed.6 With the widespread imposition of social distancing laws just one month later to combat the spread of COVID-19, payers, providers, and patients have been forced to challenge the status quo and adapt to new models of virtual care to meet the demands of the moment.7 , 8
Within oncology, there have been several calls that the current model of traditional in-person care delivery by the specialist team is unsustainable as the population of cancer patients and survivors grows.9 Further, accessing both acute care and survivorship services for cancer patients in rural and remote areas has been a longstanding challenge.10 Existing evidence suggests that cancer follow-up care can be delivered effectively using novel technological solutions while maintaining both patient safety and satisfaction.9 However, this evidence has been limited to very specific clinical scenarios and has not been focused on large-scale institutional shifts toward virtual care provision.9 , 11 With COVID-19, several high-volume cancer centres made rapid shifts toward virtual care delivery, although many published reports of effective transitions were in centres with pre-existing and well-established telehealth programs. Further, these transitions were often accompanied by a reduction in overall outpatient volumes.12 , 13 Other health systems relied on a variety of technological platforms that were not all HIPPAA-compliant and were implemented in the absence of institutional strategies for effective post-pandemic virtual care delivery.14 Taken together, the rapid and vast transition to virtual cancer care during the pandemic offers a demonstration of feasibility and poses the question: how might virtual care become a valuable fixture of health systems beyond COVID-19?
2. Organizational context
Over 10 years ago, the province of Ontario, Canada, which has a population of over 14.5 million, launched the Ontario Telemedicine Network (OTN) to provide medical care to rural and remote communities. The provincially-funded OTN system is the largest telemedicine service provider in Canada and one of the largest in the world.15 Between 2008 and 2013, OTN had an average annual utilization growth rate of 51%, driven by more physicians using this service and by existing OTN physicians seeing more patients through the platform.16 However, family practice, internal medicine, and psychiatry accounted for 82% of visits (72%, 6%, and 4%, respectively), with most visits dedicated to mental health and addiction.15 By 2016–2018, 20% of Ontario's medical and radiation oncologists had used telemedicine,17 but these visits were ad-hoc, with no systematic, institutional commitment to patient scheduling, registration, billing, or follow-up.
Until November 2019, OTN-based provision of services had lower reimbursement rates and could be solely delivered from designated telemedicine studios within healthcare facilities. Phone calls remained unbillable under Ontario's fee-for-service reimbursement structure, with limited exceptions. This lack of payment parity between telemedicine and in-person physician services was not unique to Ontario. In the United States, only 16 states had laws addressing telemedicine reimbursement and only 10 offered payment parity with in-person physician services.18 Ontario aimed to address the underutilization of OTN by enabling direct-to-patient video visits, so that patients could receive care from home on their own device, and by aligning compensation of OTN videoconferencing to that of in-person care. Nevertheless, its use in oncology remained siloed and limited to very specific scenarios.19
3. Problem
Princess Margaret (PM) Cancer Centre, a large academic comprehensive cancer program within the University Health Network (UHN) in Toronto (Ontario, Canada), has over 1000 daily ambulatory patient visits and another 1000 daily visits for chemotherapy, radiotherapy, imaging, and laboratory testing. In February 2020, however, only an average of 0.8% of daily visits (range: 0.5–2.8%) were conducted virtually. With the outbreak of the COVID-19 pandemic and new directives to keep people at home and out of healthcare facilities, PM was faced with the dual challenge of minimizing exposure risk to an immunocompromised population of cancer patients while minimizing disruptions in the delivery of critical ambulatory services. To protect both patients and staff from exposure, the PM executive team instructed the front-line providers to reduce in-person ambulatory clinic visits by 50%.
Within three days of the World Health Organization declaring the COVID-19 outbreak a pandemic, the provincial government and the Ontario Medical Association, the membership organization representing the province's physicians, came to an agreement on new temporary telemedicine fee codes.20 In alignment with regulations on social distancing, all direct-to-patient telephone and video calling platforms were approved for reimbursement within a new structure offering the same rate as in-person visits. As compared to the United States' multi-payer system, the Ontario Health Insurance Plan facilitated these universal, province-wide changes in virtual care reimbursement. However, while these new billing codes addressed the issue of payment parity, they did not address the larger technological limitations that had prevented the more widespread uptake of virtual care across the cancer centre prior to the pandemic. Prior to COVID-19, the ambulatory encounter was centered around paper orders that lacked standardization across clinics.
4. Solution
In response to the provincial directive on virtual care during COVID-19, PM aimed to shift a minimum of 50% of in-person visits to virtual care. To achieve this, a novel digital solution called the Virtual Care Management System (VCMS) was developed and deployed, facilitating the widespread adoption of virtual care across PM. The 50% target was achieved within 4 days of VCMS launch, increasing to 66.8% by week 3 after deployment on April 10, 2020; of these, 79.8% were carried out by phone and 20.2% videoconference. There was no standardized protocol for assigning patients to video or phone visits and the decision was based on the preference of the patient and/or provider on a case-by-case basis. Although the rapid adoption of telemedicine in disasters and emergency settings is not a new phenomenon,5 the structural changes that facilitated its adoption, particularly in the setting of cancer care, where there are a high number of ambulatory visits in a commonly elderly and/or immunocompromised population, have provided significant insights and a proof-of-concept for its permanent integration into health care delivery.
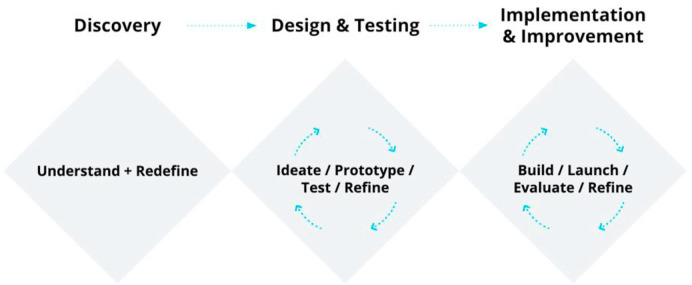
This rapid transfer of care from traditional to digital platforms was catalyzed by the collective sense of action to address COVID-19, which, in turn, enabled a number of other important facilitators to become embedded within PM's permanent workflow. One of the historical concerns about transitioning to virtual care was the potential loss of coordination and direct interaction between members of the healthcare and administrative teams, thus creating new inefficiencies and potential risks.21 To address these concerns at the outset, a small multidisciplinary team of physicians, service designers, health informatics and information technology experts, administrative assistants, and members from the legal department and hospital executive undertook a rapid Service Design process (Fig. 1 ). Service Design is a human-centered approach to innovation, which emphasizes solving core needs of end-users, in contrast to common technology- and business-centric approaches.22 It aims to balance desirability and utility for the users, technical feasibility, and financial viability.
Fig. 1.
“Triple Diamond” – an iterative, three-phased design framework for service innovation used in the creation of the Virtual Care Management System at Princess Margaret. The Discovery phase (~4-days) was followed by the Design and Testing phase (~7-days), and ended with the Implementation (~7-days) & Improvement phase (continuous improvements to the product, launched every 2-weeks). Adapted from, What is the framework for innovation? Design Council's evolved Double Diamond.23
The Service Design framework used in the development of VCMS was adapted from the “Double Diamond”, described by Richard Eisermann's team at the Design Council, which is an interdependent and iterative design process that first focuses on exploring an issue (divergent thinking) followed by focused action (convergent thinking).23 The original version of this framework was developed in the context of a design consultancy, in which the end-point is the completion of a new product (for example, manufacturing for a physical product, or publishing to the app store for a digital product). In the healthcare context, however, the implementation of a finished product and the continuous improvement of a new intervention is critical to its long-term success. As a result, the original Double Diamond was modified into a “Triple Diamond” to emphasize the importance of the Implementation & Improvement phase.
The Triple Diamond process begins with the addition of an initial Discovery phase, which focuses on understanding the problem by engaging with the people directly affected; in this case, front-line healthcare staff. This research data is analyzed to produce current-state process maps and prioritized problem-statements to be tackled by the design team. At PM, the VCMS team mapped and analyzed each step of the traditional ambulatory workflow to identify critical bottlenecks that could impede the delivery of care virtually.22 The first critical bottleneck identified was the lack of a centralized, digital communication platform between members of the health care team. Prior to April 2020, orders at PM were traditionally processed using a combination of direct entry to EMR, paper notes, in-person conversations, email, and phone, whereas the new, integrated VMCS allowed for rapid online information exchange and digital order sets. This process also uncovered other bottlenecks in the system, including the lack of automated safety checks (e.g. allergies) built into physician order sets.
After prioritization of the core challenges, the Design & Testing phase involved rapid ideation and exploration of many different ways to solve the problems. Software prototypes are rapidly designed and tested with core representatives from each role, followed by revisions and more testing, before committing to full development of the new technology. This process led to the decision to integrate existing infrastructure (OTN platform, scheduling, and electronic medical record (EMR) software) into the VCMS as-is to minimize adoption barriers and time to delivery. It also led to the inclusion of safety checks in the VCMS through mandatory order fields, as well as a tracking system to monitor deferrals of patient treatment due to COVID-19.
The final Implementation & Improvement phase involved technical development and quality assurance, traditional implementation launching to the field (communications strategy, education for users, and training support staff), and feedback mechanisms to continuously collect, analyze program data, and refine the intervention. Virtual training sessions were offered to designated “super-users” across the hospital, who championed the system amongst the rest of the staff. There were also regular meetings with site and clinic managers to identify any new challenges that were arising in real-time. This process of distributed leadership and decision-making fostered direct input from the front-line and allowed strengths of the existing electronic infrastructure to be leveraged to accelerate the digital transition. The alignment amongst the different stakeholders and actors, as well as between different digital tools within a single institutional platform, was essential in this process, and promoted a new institutional willingness to work around bureaucratic hurdles to adoption.
The development of digital order entry forms was an example of both the challenge of rapid implementation as well as the agility of the VCMS Implementation & Improvement process to respond. For example, the paper order forms at PM were not standardized across clinics or providers and demonstrated large variability. Given the short timeframe available, it was not possible to assemble expert panels to reach a consensus on the design of new digital order entry forms. Instead, a service designer and oncologist from the VCMS team visited each clinic over the course of a day to obtain input from the frontline administrative staff on the most common orders. Following the VCMS launch, the forms underwent continuous revision every 2 weeks based on user feedback. Currently, steering committees of clinical experts are being struck to perform a review of all new changes proposed to the forms.
4.1. Unresolved questions and Lessons for the field
The PM experience has provided an important opportunity to demonstrate a new mechanism for the delivery of care to patients with cancer and other chronic diseases, as well as to study the impact of this change. The goal of a 50% transition from in-person to virtual care was achieved within four days, thereby minimizing interruptions to in-person treatment and avoiding an enormous backlog of ambulatory services. This rapid scale-up demonstrated the acceptability and feasibility of a virtual approach to care delivery by both patients and care providers in the oncologic setting. Such improvements, however, cannot be taken for granted and new telemedicine platforms must be subject to rigorous evaluative processes like other new health care interventions. An evaluation system has been embedded into the VCMS to study the impact of this initiative on patient and provider experience through voluntary and confidential surveys on an ongoing basis. Additional information on demographics and other quality-of-care indicators are being routinely collected to quantify the impact of the virtual care roll-out on the quality, safety and equity of this evolving care paradigm within our cancer system. Moreover, this information will enable ongoing improvements to care delivery and will facilitate effective virtual care post-pandemic.
The circumstances around COVID-19 that fueled the migration to virtual care were unique and were predominantly focused on the safety of patients and healthcare workers, while minimizing potential lost continuity of care. This experience, however, demonstrated the possibilities for system-wide change and the opportunity to think about novel care models such as asynchronous messaging, nurse-lead virtual clinics, and virtual peer-navigation in post-Covid-19 cancer care. However, robustly advocating for its incorporation into the standard-of-care armamentarium will require attention to other critical elements of the Institute of Medicine's quality dimensions,24 such as value, efficiency, and cost of telemedicine platforms. Temporary billing codes for phone calls in Ontario facilitated the uptake of telemedicine by physicians, but these easy billing encounters could encourage overuse in Ontario's fee-for-service system. It could also further de-incentivize physicians to assess patients in person, when such interactions are indeed required, and could risk exacerbating inequalities when less affluent patients cannot access necessary infrastructure (i.e. devices, internet) or private space to engage virtually.7
While the COVID-19 pandemic has caused enormous disruption in health care and significant harm across all levels of society, it has also motivated nimble and rapid change and an opportunity for disruptive innovation, rare to healthcare. The adaptation of a process mapping approach to the ambulatory cancer context facilitated the scale-up of the VCMS amongst patients and providers who had not previously been accustomed to a virtual care workflow. Ultimately, the ideal system will likely not be an either/or approach for in-person and virtual encounters, but rather, the thoughtful integration of these paradigms. Indeed, there are dimensions of care that are enhanced by face-to-face human interaction. As new virtual models of care are rolled-out during pandemic times, we must build on this momentum of deep and rapid multidisciplinary-collaboration, while continuously collecting data on cost and quality, to ensure that virtual care remains integrated in the post-pandemic landscape.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Duffy S., Lee T.H. In-person health care as option B. N Engl J Med. 2018;378(2):104–106. doi: 10.1056/NEJMp1710735. [DOI] [PubMed] [Google Scholar]
- 2.Kvedar J., Coye M.J., Everett W. Connected health: a review of technologies and strategies to improve patient care with telemedicine and telehealth. Health Aff. 2014;33(2):194–199. doi: 10.1377/hlthaff.2013.0992. [DOI] [PubMed] [Google Scholar]
- 3.Dinesen B., Nonnecke B., Lindeman D. Personalized telehealth in the future: a global research agenda. J Med Internet Res. 2016;18(3):e53. doi: 10.2196/jmir.5257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tuckson R.V., Edmunds M., Hodgkins M.L. Telehealth. N Engl J Med. 2017;377(16):1585–1592. doi: 10.1056/NEJMsr1503323. [DOI] [PubMed] [Google Scholar]
- 5.Vogel L. Canada has long way to go on virtual care. CMAJ News. 2020:2020. doi: 10.1503/cmaj.1095851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Virtual Care Recommendations for scaling up virtual medical services. February. 2020;11 https://www.cma.ca/sites/default/files/pdf/virtual-care/ReportoftheVirtualCareTaskForce.pdf [Google Scholar]
- 7.Smith W.R., Atala A.J., Terlecki R.P., Kelly E.E., Matthews C.A. Implementation guide for rapid integration of an outpatient telemedicine program during the COVID-19 pandemic. J Am Coll Surg. 2020;231(2):216–222. doi: 10.1016/j.jamcollsurg.2020.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mann D.M., Chen J., Chunara R., Testa P.A., Nov O. COVID-19 transforms health care through telemedicine: evidence from the field. J Am Med Inf Assoc. 2020;27(7):1132–1135. doi: 10.1093/jamia/ocaa072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dickinson R., Hall S., Sinclair J.E., Bond C., Murchie P. Using technology to deliver cancer follow-up: a systematic review. BMC Canc. 2014;14:311. doi: 10.1186/1471-2407-14-311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weaver K.E., Geiger A.M., Lu L., Case L.D. Rural-urban disparities in health status among US cancer survivors. Cancer. 2013;119(5):1050–1057. doi: 10.1002/cncr.27840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cox A., Lucas G., Marcu A. Cancer survivors' experience with telehealth: a systematic review and thematic synthesis. J Med Internet Res. 2017;19(1):e11. doi: 10.2196/jmir.6575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lonergan P.E., Washington S.L., Iii, Branagan L. Rapid utilization of telehealth in a comprehensive cancer center as a response to COVID-19. J Med Internet Res. 2020;22(7):e19322. doi: 10.2196/19322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu R., Sundaresan T., Reed M.E., Trosman J.R., Weldon C.B., Kolevska T. Telehealth in oncology during the COVID-19 outbreak: bringing the house call back virtually. JCO Oncol Pract. 2020;16(6):289–293. doi: 10.1200/OP.20.00199. [DOI] [PubMed] [Google Scholar]
- 14.Mehrotra A., Ray K., Brockmeyer D.M., Barnett M.L., Bender J.A. Rapidly converting to “virtual practices”: outpatient care in the era of covid-19. https://catalyst.nejm.org/doi/full/10.1056/CAT.20.0091 April 1, 2020.
- 15.O'Gorman L.D., Hogenbirk J.C., Warry W. Clinical telemedicine utilization in Ontario over the Ontario telemedicine Network. Telemed J e Health. 2016;22(6):473–479. doi: 10.1089/tmj.2015.0166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Whitten P., Holtz B. Provider utilization of telemedicine: the elephant in the room. Telemed J e Health. 2008;14(9):995–997. doi: 10.1089/tmj.2008.0126. [DOI] [PubMed] [Google Scholar]
- 17.Williams R., Aspden M., Stetson P.D., Otto C. American Society of Clinical Oncology; Chicago: 2020. Utilization of Telemedicine at Two High-Volume Cancer Care Organizations. [Google Scholar]
- 18.Lacktman N.M., Acosta J.N., Levine S.J. Foley & Lardner LLP; 2019. 50-state Survey of Telehealth Commerical Payer Statutes. [Google Scholar]
- 19.Dorsey E.R., Topol E.J. State of telehealth. N Engl J Med. 2016;375(2):154–161. doi: 10.1056/NEJMra1601705. [DOI] [PubMed] [Google Scholar]
- 20.Virtual care and the 2019 novel coronavirus (COVID-19) https://ontariomd.news/
- 21.Schwamm L.H. Telehealth: seven strategies to successfully implement disruptive technology and transform health care. Health Aff. 2014;33(2):200–206. doi: 10.1377/hlthaff.2013.1021. [DOI] [PubMed] [Google Scholar]
- 22.Stickdorn M., Lawrence A., Hormess M.E., Schneider J. O'Reilly Media; Sebastopol, California: 2018. This Is Service Design Doing: Applying Service Design Thinking in the Real world. [Google Scholar]
- 23.What is the framework for innovation? Design Council's evolved Double Diamond. 2020. https://www.designcouncil.org.uk/news-opinion/what-framework-innovation-design-councils-evolved-double-diamond
- 24.Institute of Medicine Committee on Quality of Health Care in A . National Academies Press (US); Washington (DC): 2001. Crossing the Quality Chasm: A New Health System for the 21st Century. [Google Scholar]