Abstract
Aortic dissection (AD) is the rupture of the aortic intima, causing the blood in the cavity to enter the middle of the arterial wall. Without urgent and proper treatment, the mortality rate increases to 50% within 48 hours. Most patients present with acute onset of symptoms, including sudden severe pain and complex and variable clinical manifestations, which can be easily misdiagnosed. Despite this, the molecular mechanisms underlying AD are still unknown. Recently, non‐coding RNAs have emerged as novel regulators of gene expression. Previous studies have proven that ncRNAs can regulate several cardiovascular diseases; therefore, their potential as clinical biomarkers and novel therapeutic targets for AD has aroused widespread interest. To date, several studies have reported that microRNAs are crucially involved in AD progression. Additionally, several long non‐coding RNAs and circular RNAs have been found to be differentially expressed in AD samples, suggesting their potential roles in vascular physiology and disease. In this review, we discuss the functions of ncRNAs in AD pathophysiology and highlight their potential as biomarkers and therapeutic targets for AD. Meanwhile, we present the animal models previously used for AD research, as well as the specific methods for constructing mouse or rat AD models.
Keywords: aortic dissection, non‐coding RNAs, potential biomarkers, therapeutic targets
1. INTRODUCTION
Aortic dissection (AD) is a fatal vascular disease defined as the intimal tear of the aorta, which causes the blood in the cavity to pass into the middle of the artery wall, and eventually forms the dissected haematoma and the true and false lumen expanding along the long axis of the artery. 1 Accumulating evidence indicates that the annual incidence of AD is 4.586 per 100 000 persons aged 65‐75 years, with more than 35 deaths per 100 000 persons. 2 If left untreated, about 24% of the patients die within the first 24 hours and 50% die within 48 hours. 3 In short, AD is an acute and severe disease with a high mortality rate. There are two classification systems for AD: Stanford, which is the most commonly used, and DeBakey. The Stanford classification is further divided into two types—A and B (Figure 1). Stanford type A AD originates from the ascending aorta and is divided into two subtypes according to the involvement of the abdominal aorta or the limitation to the ascending aorta. It is usually treated by sternotomy, especially for emergency surgical intervention, whereas Stanford type B AD does not involve the ascending aorta and is usually treated using drugs, unless the condition is complex. 4 AD can also be categorized as acute, subacute and chronic, specifically at two time‐points (2 weeks and 3 months). 3
FIGURE 1.
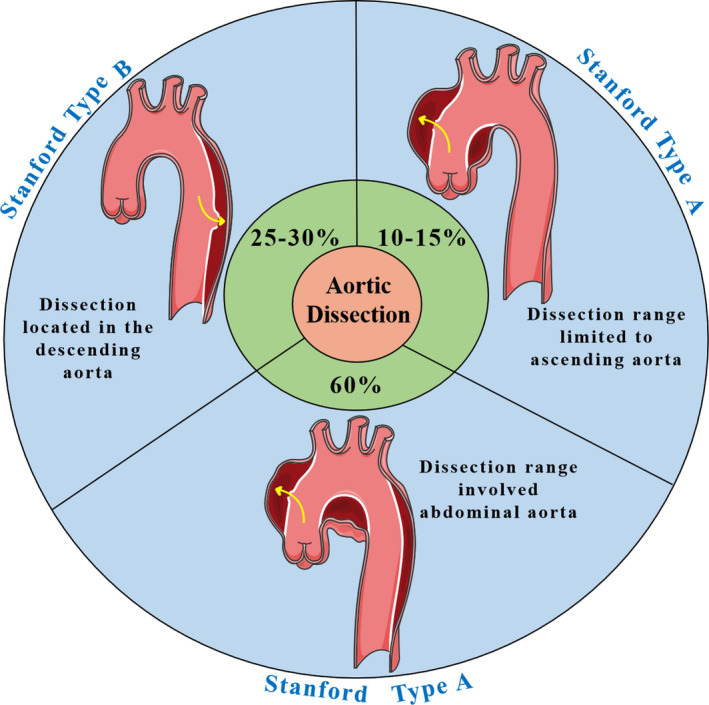
The Stanford classification of aortic dissection. There are mainly two types: Stanford A with dissection range limited to ascending aorta or dissection range involved abdominal aorta, and Stanford B with dissection located in the descending aorta
Studies have demonstrated that several factors can influence AD. For example, various genetic mutations were reported to lead to the occurrence and progression of ADs, such as Marfan syndrome (FBN1 mutations), 5 Ehlers‐Danlos syndrome (COL3A1 mutations) 6 and Loeys‐Dietz syndrome (TGFBR1 or TGFBR2 mutations). 7 Moreover, infections and vascular inflammation, which involves some inflammatory factors including matrix metalloproteinases (MMPs) and vascular endothelial growth factor (VEGF), and vascular smooth muscle cell (VSMC) phenotypic transition are also significantly involved in AD. Additionally, bicuspid aortic valve, one of the most common cardiovascular malformations with an incidence of 1%‐2% in the general population, is also reported to be closely related to AD development 8 mainly owing to lack of elastic fibre components and increased release of matrix metalloproteinases. 9 Recently, increased studies focus on the participation of non‐coding RNAs (ncRNAs) in various diseases. 10 , 11 , 12 , 13 ncRNAs are mainly divided into two main categories according to their length: small ncRNA and long ncRNA. They normally cannot encode proteins, which were regarded as ‘junk’ transcriptional products. However, recent studies have found that ncRNAs are functional regulatory molecules that can regulate gene expression at the transcription and post‐transcription levels. Particularly, numerous studies have reported that non‐coding RNAs (ncRNAs) play a significant role in the development of cardiovascular diseases, such as atherosclerosis, restenosis after stent and aneurysm. 14 , 15 , 16 , 17 , 18 Likewise, several reports have suggested that ncRNAs also play a crucial regulatory role in the occurrence and development of AD. 19 , 20 , 21 In this review, we discuss the role of ncRNAs and their target genes, as well as their regulatory mechanisms in AD. In this review, we highlight some previously identified ncRNAs that are involved in multiple processes that lead to AD. In‐depth research of these ncRNAs can provide new insights into the treatment and prevention of AD and serve as potentially effective biomarkers and therapeutic targets for predicting the risk of AD and evaluating prognosis in clinical settings. Furthermore, smoking, hypertension, atherosclerosis, hyperlipidaemia and age are certain risk factors that contribute to the occurrence of AD. 22
2. THE PATHOGENESIS OF AD
The pathogenesis of AD includes vascular inflammation, activity of MMPs and phenotype switching of VSMCs. 23 Damage to the endothelial cells (ECs) triggers vascular inflammation, which is regulated by the immune response mechanism, and it subsequently activates MMP, an enzyme used to degrade the extracellular matrix (ECM). The imbalance between MMPs and TIMPs directly contributes to the remodelling of the aortic wall, which is a central link in the formation of AD. 24 It has also been reported that the macrophages and their related products localized within the walls of the aorta can trigger and maintain the thoracic aortic dissection (TAD) inflammation and matrix degradation. 25 Collagen synthesis and MMP‐2 (gelatinase A) production are elevated in the synthetic phenotype of VSMCs and can promote collagen deposition and type IV collagen and elastin degradation. 26 These evidences suggested that AD is a very complex process involving inflammation and matrix degradation.
Media degradation is a major histopathological feature of AD, which includes the severe degradation of the ECM that is associated with the depletion of the smooth muscle cells (SMCs), rupture of elastic fibres and collagen degradation, consequently weakening the artery wall and eventually leading to the formation of AD. 27 The VSMCs in the middle layer of the aorta play an important role in maintaining aortic wall homeostasis. Notably, dysfunction in the proliferation and migration of VSMCs has been reported to be associated with vascular diseases. 28 Therefore, VSMCs are also believed to be responsible for the formation of AD. During AD pathogenesis, the dysfunctional VSMCs are considered to be one of the most important factors, mainly including apoptosis and phenotype switching. 29 VSMC phenotypic transformation is a biological process which is characterized by the transformation of a contractile (differentiated) phenotype into a synthetic (dedifferentiated) phenotype that could occur under the influence of the environment including signal transduction, gene transcription and epigenetic modification. 30 Several molecular mechanisms are involved in this process, such as serum response factor (SRF), Krüppel‐like factor 4 (KLF4), forkhead box O 4 (FOXO4), microRNAs, ten‐eleven‐translocation 2 (TET2), Rho‐actin and transforming growth factor‐β (TGF‐β). 31 Two VSMC‐derived ECM proteins, collagen and elastin, have been implicated with apoptosis and phenotype switching during AD pathogenesis. Apoptosis of VSMCs and elastin fibre fragmentation can lead to the degeneration of the ECM, thereby weakening the strength and elasticity of the vessel wall, 9 whereas abnormal phenotypic switching can promote collagen deposition and elastin degradation. 26 These two processes directly influence the development of AD; however, their underlying molecular mechanisms remain to be elucidated. Different stimuli, such as growth factors, chemical factors, cell adhesion molecules, ECM enzymes and damage‐stimulating signals, can also result in the occurrence of AD. 32
Hypertension has also been confirmed as a risk factor that contributes to the occurrence of AD, which represents an important haemodynamic basis. 9 For example, miR‐21 has been shown to be involved in the development of hypertension and target organ damage (TOD) by regulating renin‐angiotensin‐aldosterone system (RAAS) and peripheral blood mononuclear cells. 33 miR‐505 promotes the development of hypertension by affecting the function of endothelial cells. 34 Particularly, vascular endothelial cells and haemodynamics were reported to be significant for vascular homeostasis and hypertension. 35 Shear stress is a force per unit area, which is generated when tangential force (blood flow) acts on the inner membrane of vessel (endothelial cells), and plays an important role in determining the pathological origin of arterial disease. 36 In addition, shear stress is also associated with endothelial phenotype changes, which are related to atherosclerosis. 37 Recently, a large number of literature reported that a series of microRNAs can regulate haemodynamics and endothelial cell behaviour, thus participating in the occurrence of aortic diseases. microRNA‐126 was shown to be involved in the signal transmission from endothelial cells to SMC, thereby inducing SMC turnover which was affected by haemodynamic shear stress. 38 It was also reported that Krüppel‐like factor 2 (KLF2) can regulate the gene expression pattern of endothelial cells under the stimulation of atheroprotective flow. KLF2 transduction or shear stress stimulation can induce endothelial cells to secrete extracellular vesicles to enrich miR‐143/145 and control the target gene expression of co‐cultured SMC, which is of great significance in combating atherosclerosis. 39 Moreover, haemodynamic changes and endothelial cell dysfunction are also crucially involved in the pathogenesis of AD. Recent reports demonstrated that EC dysfunction participates in the development of AD through endoplasmic reticulum (ER) stress depending on microparticles derived from SMCs, and eventually leads to EC apoptosis and inflammation. 40 The modification of protein S‐nitrosylation (SNO) in endothelial cells can result in the destruction of the endothelial barrier, which ultimately leads to the formation of TAD. 41 Currently, some studies revealed the regulatory roles of microRNAs on endothelial cells behaviour during aortic dissection. For example, miR‐27a promotes the activation of apoptotic pathway by targeting the expression of fas‐associated protein with death domain (FADD), thereby promoting the apoptosis of EC, whereas the treatment of miR‐27a activator targeting ECs apoptosis can significantly reduce the incidence of AD. 42 Together, these evidences provide us with new insights into the occurrence and development of AD, and suggest more comprehensive prevention and diagnostic methods.
3. CLINICAL EVALUATION OF MIRNAS IN AD PATIENTS
As mentioned above, AD is an acute and severe disease with a high mortality rate. The annual incidence of AD is 4.586 per 100 000 persons aged 65‐75 years, with more than 35 deaths per 100 000 persons. 2 Without urgent and proper treatment, about 24% of the patients die within the first 24 hours and 50% die within 48 hours. 3 Therefore, early diagnosis of AD is particularly important for reducing mortality and improving prognosis. Given their significant regulatory roles, ncRNAs, especially miRNAs, have already been used for clinical testing. 43 Several ncRNAs have been identified as critical regulators of AD; hence, we reviewed the pathophysiology of AD regulated by ncRNAs including miRNAs (Table 1), lncRNAs (Table 2) and circRNAs (Table 3), and the diagnostic and therapeutic potential of some statistically different ncRNAs detected in the tissue or blood samples of AD patients and healthy people. We also summarized the information regarding the identified ncRNAs and their diagnostic values, particularly their sensitivity and specificity, and the four miRNAs (miR‐25, miR‐29a, miR‐155 and miR‐26b) showed more reliable diagnostic value with a sensitivity of 92%, a specificity of 93.33% and area under curves (AUC) of 0.973 44 , 45 (Table 4). And we listed novel potential biomarkers that need to be further validated in Table 5. 46 , 47 , 48 , 49 , 50 The expression of these non‐coding RNAs in the peripheral blood or aortic tissues of AD patients and healthy people was obviously different (fold change > 2), which has potential clinical significance as early diagnostic biomarkers. However, their regulatory function and underlying mechanisms in the occurrence and development of AD need to be further explored.
Table 1.
Representative miRNAs regulating pathophysiology of AD
| miRNA | Sample type | Cell | Regulation | Target | Function | Mechanism | Ref. |
|---|---|---|---|---|---|---|---|
| miR‐21 | NA | VSMC | Increased | SMAD7 | Promote phenotypic transition | TGF‐β signalling pathway | 63 |
| miR‐134‐5p | Thoracic aorta | VSMC | Decreased | STAT5B/ITGB1 | Inhibit phenotypic transition/apoptosis | ITGB1/CRE signalling pathway | 19 |
| miR‐145 | Ascending aorta | VSMC | Decreased | CTGF | Inhibit apoptosis | TGF‐β/SMAD3 signalling pathway | 20, 67 |
| miR‐320 | Peripheral blood | Monocytes, macrophage | Decreased | MMP2/9 | Inhibit degradation/remodelling of the ECM | Post‐transcriptional control | 69 |
| miR‐320d/582 | Thoracic aorta | VSMC | Decreased |
TRIAP1/NET1, COLIA1/SPP1 |
Inhibit apoptosis | Apoptotic pathway | 21 |
| miR‐144‐3p | Dissection specimens | VSMC | Increased | TE | Reduce elastin | Protein translation | 73 |
| miR‐146b | Peripheral blood | VSMC, EC, MPh | Increased | NF‐κB1/TRAF6/MMP6/ACTA2 | Promote inflammation/apoptosis/ECM degradation | TLR/TGF‐β signalling pathway | 76 |
| miR‐146a‐5p | TAAD plasma sample and tissue | VSMC | Increased | SMAD4 | Promote the proliferation and migration of VSMC | TGF‐β signalling pathway | 82 |
| miR‐30a | Ascending aorta | VSMC | Increased | LOX | Inhibit cross‐link collagen and elastin | Inhibit the protein abundance of LOX | 83 |
| miR‐143/145 | Ascending aorta | VSMC | Decreased | TGF‐β1/P38‐MAPK | Inhibit phenotypic transition | TGF‐β1 signalling pathway | 12, 52 |
| miR‐4787‐5p/4306 | Peripheral blood | VSMC | Increased | PKD1/TGF‐β1 | Damage cell‐cell adhesion/inflammation | TGF‐β1 signalling pathway | 90 |
| miR‐26b | Ascending aorta | VSMC | Increased | HMGA2 | Promote apoptosis EMT | TGF‐β signalling pathway | 94 |
Abbreviations: EC, endothelial cell; ECM, extracellular matrix; EMT, endothelial‐mesenchymal transition; MPh, macrophages; TAAD, thoracic aortic aneurysm and dissection; TE, tropoelastin; VSMC, vascular smooth muscle cell.
Table 2.
Summary of lncRNAs regulating pathophysiology of AD
| lncRNA | Sample type | Cell | Regulation | Target | Function | Potential biomarker or target | Ref. |
|---|---|---|---|---|---|---|---|
| lncP2RX7 | TAD ascending aorta | VSMC | Increased | P2RX7 | Promote inflammation | Yes | 113 |
| HIF1A‐AS2 | VSMC | Increased | HIF1A | Inhibit the proliferation and migration of AoSMCs | Yes | ||
| AX746823 | VSMC | Increased | RUNX1 | Promote inflammation | Yes | ||
| RP11‐69I8.3 | VSMC | Increased | CTGF | Promote apoptosis | Yes | ||
| RP11‐536K7.5 | VSMC | Increased | IL2RA | Promote inflammation | Yes | ||
| CDKN2B‐AS1 | VSMC | Increased | CDKN2B | Promote apoptosis | Yes | ||
| ENSG00000269936 | TAD ascending aorta | VSMC | Increased | MAP2K6 | Inhibit collagen synthesis | Yes | 97 |
| lncRNA‐1421 | VSMC | Decreased | ACTA2/FBLN5/TIMP3 | Decrease SMC contraction/inhibit VSMC proliferation and migration. | Yes | ||
| lncRNA‐XIST | VSMC | Increased | P21 | Inhibit VSMC proliferation | Yes | ||
| ENSG00000248508 | VSMC | Increased | Up‐regulated by RUNX1 | Promote inflammation | Yes | ||
| ENSG00000226530 | VSMC | Increased | Promote inflammation | Yes | |||
| EG00000259719 | VSMC | Increased | Promote inflammation | Yes | |||
| PTENP1 | AD tissues and adjacent aortic tissue specimens | VSMC | Increased | miR‐21 | Promote apoptosis | Yes | 107 |
Abbreviations: AoSMCs, aortic smooth muscle cells; TAD, thoracic aortic dissectionVSMC, vascular smooth muscle cell.
Table 3.
Summary of circRNAs regulating pathophysiology of AD
| circRNAs | Sample type | Cell | Regulation | Target | Function | Potential diagnostic biomarker | Ref. |
|---|---|---|---|---|---|---|---|
| circMARK3 | AAAD ascending aortic specimens | VSMC | Increased | miR‐1273 | Promote inflammatory | Yes | 47 |
| circRNA‐101238 | Type A TAD aortic specimens | VSMC | Increased | miR‐320a | Increase apoptosis | Yes | 119 |
| circRNA‐104634 | VSMC | Increased | miR‐145‐3p | Promote phenotype switching | Yes | ||
| circRNA‐104349 | VSMC | Increased | miR‐26a‐3p | Increase apoptosis | Yes | ||
| circRNA‐102683 | VSMC | Decreased | miR‐29b‐1‐5p | Promote apoptosis/ECM degradation | Yes | ||
| circRNA‐104033 | VSMC | Decreased | miR‐195‐3p | Stimulate collagen remodelling | Yes |
Abbreviations: AAAD, Stanford type A aortic dissection; ECM, extracellular matrix; TAD, thoracic aortic dissection; VSMC, vascular smooth muscle cell.
Table 4.
Representative miRNAs for diagnosing acute aortic dissection with risk score analysis
| miRNA | Sample type | Result | Sensitivity | Specificity | AUC (95% CI) | Ref. |
|---|---|---|---|---|---|---|
| miR‐25 | AAAD peripheral blood | Up‐regulated | 92.00% | 76.67% | 0.881 | 44 |
| miR‐29a | Up‐regulated |
80.00% |
93.33% | 0.899 | ||
| miR‐155 | Up‐regulated |
84.00% |
83.33% | 0.863 | ||
| miR‐26b | Down‐regulated |
88.00% |
90.00% | 0.911 | ||
| miR‐15a | Peripheral blood | Up‐regulated | 75.7% | 100% | 0.855 | 45 |
| miR‐23a | Up‐regulated | 91.9% | 85.7% | 0.925 | ||
| let‐7b | Up‐regulated | 79.4% | 92.9% | 0.887 | ||
| hcmv‐miR‐US33‐5p | Up‐regulated | 73.5% | 85.7% | 0.815 |
Abbreviations: AAAD, Stanford type A aortic dissection; AUC, area under curves; CI, confidence interval.
Table 5.
Novel potential biomarkers for diagnosing acute aortic dissection in patients (fold change > 2, q‐value ≤ 0.05)
| Up‐regulated ncRNAs | Sample type | Reference | Down‐regulated miRNAs | Sample type | Ref. |
|---|---|---|---|---|---|
| hsa‐miR‐93 | TAD aorta segments | 48 | hsa‐miR‐1268 | Aortic dissection tissue | 49 |
| hsa‐miR‐485‐3p | hsa‐miR‐939 | ||||
| hsa‐miR‐146b‐5p | Aortic dissection tissue | 49 | miR‐29a | TAD ascending aorta segments | 46 |
| hsa‐miR‐19a | miR‐29c | ||||
| hsa‐miR‐505 | miR‐30 family except miR‐30c | ||||
| miR‐518e | AAD peripheral blood | 50 | miR‐3682 | AAD peripheral blood | 50 |
| miR‐16 | miR‐3196 | ||||
| miR‐451 | miR‐3162 | ||||
| miR‐663 | miR‐3131 | ||||
| circUBA2 | AAAD ascending aorta | 47 | circCEP70 | AAAD ascending aorta | 47 |
| circARHGAP26 | circFAM120B | ||||
| circCHSY1 |
Abbreviations: AAAD, Stanford type A aortic dissection; AAD: acute aortic dissection; TAD, thoracic aortic dissection.
We believe that these ncRNAs can be developed into reliable molecular targets for the clinical diagnosis and treatment of AD in the future. Although there are limitations and challenges in the clinical application of ncRNAs, such as off‐target metabolism and delivery, the examples that we presented in this review highlight their potential utilization in creating strategies for the treatment of AD in the future.
4. MIRNAS IN AORTIC DISSECTION
miRNAs are a class of endogenous ncRNAs approximately 20‐25 nucleotides (nt) long that play an essential part in modulating pathophysiological functions, such as differentiation, proliferation, migration and apoptosis. Mature miRNAs recognize the 3′‐untranslated region (3′‐UTR) of the target mRNA through base complementary pairing and either guide the degradation or repress translation of the target mRNA depending on degree of complementation. miRNAs are abundant and exist in a stable form in the plasma/serum. 51 Changes in miRNA expression levels were observed in association with the occurrence of different diseases, such as miR‐183/96/182 cluster in retinal disorder, 10 miR‐133b in neurodegenerative diseases 11 and miR‐143/145 in cardiovascular diseases 52 ; these changes can be detected by several methods, and the most common one is quantitative real‐time PCR (qRT‐PCR) assays. 53 Therefore, we can diagnose certain diseases including cancers, such as pancreatic cancer, 54 cholangiocarcinoma 55 and breast cancer, 56 cardiovascular diseases including heart failure, 57 acute myocardial infarction 58 and arrhythmias, 59 by measuring the expression levels of circulating miRNAs, particularly, cardiovascular diseases, were significantly involved. Interestingly, under pathophysiological conditions, miRNAs have also been discovered to regulate VSMC phenotypic transition and consequently result in the development of AD. 60 Here, we introduce several specific miRNAs that have been reported to play definite roles in the development of AD, and could be considered as potential diagnostic biomarkers therapeutic targets of AD (Figure 2) (Table 6).
FIGURE 2.
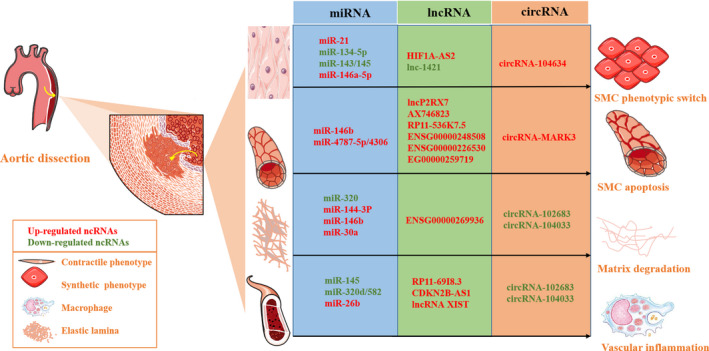
The function of non‐coding RNAs in aortic dissection. miRNAs, lncRNAs and circRNAs are all critically involved in biological regulation, including phenotypic transformation and apoptosis of VSMCs, matrix degradation and vascular inflammation
Table 6.
Representative animal models in AD studies
| microRNA | Targets | Model | Sample type | Parameter measured | Ref. |
|---|---|---|---|---|---|
| miR‐21 | SMAD7 | S3± mice infused with AngII | NA |
Echocardiography, histological examination |
63 |
| miR‐134‐5p | STAT5B/ITGB1 | Mice infused with AngII and high‐fat diet | TAD thoracic aorta |
Ultrasonic histological examination |
19 |
| miR‐144‐3p | TE | BAPN solution dissolved in the drinking water | Dissection specimens |
Blood pressure, histological examination |
73 |
| miR‐30a | Lysyl oxidase | Mice infused with AngII | AAD ascending aorta |
Immunohistochemical staining |
83 |
Abbreviations: AAD, acute aortic dissection; AngII, angiotensin II; BAPN, β‐aminopropionitrile monofumarate; TAD, thoracic aortic dissection; TE, tropoelastin.
4.1. miRNA
4.1.1. miRNA‐21 (miR‐21)
VSMC phenotypic switching and ECM degradation directly affect AD. 23 Phenotypic transformation from the contractile to the synthetic type can be induced by an abnormality in the TGF‐β signalling pathway, which further promotes the activation of the nuclear factor κ‐light‐chain‐enhancer of activated B cells (NF‐κB) and chemokine expression. Furthermore, the up‐regulation of the NF‐κB signalling pathway promotes chemokine expression, leading to VSMC inflammation 61 and ultimately facilitating the occurrence of AD. The expression of miR‐21 increased in a mouse AD model, which corroborates previously reported data in ascending thoracic aortic aneurysm (TAA) samples from patients. 62 TAA is a pathological dilatation of the intima, media and adventitia of the aorta, although the final result of TAA expansion is to progress to TAAD, most TAA patients do not develop to TAAD, and the pathogenesis of TAA and TAAD is different. Moreover, miR‐21 knockout (miR‐21−/−) can aggravate the formation of SMAD3± (mothers against decapentaplegic homolog 3) heterozygous (S3+/−) mice infused with AngII‐induced thoracic aortic aneurysm and dissection (TAAD) which occurs mainly in the ascending aorta. 63 Mechanistically, miR‐21−/− promoted SMAD7 expression and inhibited canonical TGF‐β signalling. SMAD7 overexpression further promoted the silencing of the TGF‐β signalling pathway in miR‐21−/− mice, ultimately inducing the phenotypic transformation of VSMCs. At the same time, further experiments prove the lentiviral‐mediated silencing of SMAD7 reversed the phenotype switching of VSMCs in S3±21−/− mice. The proliferation and apoptosis rates of VSMCs, as well as the MMP9 expression, were elevated in miR‐21−/− mice. In contrast, collagen fibrillation was inhibited in miR‐21−/− mice. Collectively, these results suggest that miR‐21 is a potential therapeutic target for TAAD.
4.1.2. microRNA‐134‐5p (miR‐134‐5p)
miR‐134‐5p was previously implicated in the growth and migration of endothelial cells (ECs) and tuber formation during atherosclerosis. 64 Recently, miR‐134‐5p was found to be highly down‐regulated in TAD thoracic aorta tissues via miRNA microarray screening assay. The overexpression of miR‐134‐5p in aortic smooth muscle cells (AoSMCs) significantly increased the expression of contractility markers and the formation of migration‐related stress fibres and significantly decreased the expression of ADAMTS‐1 and ADAMTS‐7 metalloproteinases. Furthermore, dual‐luciferase reporter assay confirmed that the signal transducer and activator of transcription 5B (STAT5B) and integrin beta‐1 (ITGB1) were direct downstream targets of miR‐134‐5p. STAT5B, which promotes growth factor expression and cell proliferation, is found to be activated during vascular injury, whereas ITGB1 can inhibit the expression of VSMC contractile genes and the proliferation of AoSMCs. 65 , 66 Notably, miR‐134‐5p was also confirmed to inhibit tunica media degeneration and TAD evolution in vivo. 19 In an AngII‐induced and high‐fat diet TAD model, Ad‐miR‐134‐5p significantly inhibited aortic dilatation and vascular media degeneration, thereby reducing the incidence of AD by 39%. These results suggest that the miR‐134‐5p, STAT5B and ITGB1 in VSMCs may be potential therapeutic targets for AD.
4.1.3. microRNA‐145 (miR‐145)
It has been proven that there is decreased miR‐145 expression in AAD ascending aorta samples. 52 Subsequently, Huang et al confirmed that miR‐145 induces the proliferation, migration and apoptosis of VSMCs by targeting SMAD family member 3 (SMAD3) during AD. 67 Another study reported that the miR‐145 expression level is consistent with the mortality of patients with AD. Increased miR‐145 expression can accelerate VSMC proliferation and ameliorate cell apoptosis by binding to SMAD3 and connective tissue growth factor (CTGF), which can reduce the effect of miR‐145 in the progression of AAD. 20 CTGF, a matricellular protein, can ameliorate aortic remodelling in the rat AD model and participate in the proliferation and apoptosis of VSMCs. 68 In summary, studies on the miR‐145‐CTGF/SMAD3 axis may provide potential therapeutic targets for AAD.
4.1.4. microRNA‐320 (miR‐320), microRNA‐320d (miR‐320d) and microRNA‐582 (miR‐582)
The expression of miR‐320 was found to be highly down‐regulated in peripheral blood of AD patients and confirmed to be negatively correlated with the expression of MMP, which is produced by monocytes or macrophages. 69 MMPs, which are enzymes with similar structures but different functions, are classified into five groups: collagenases, gelatinases, stromelysins, matrilysins and membrane type. 70 Specific types of MMPs can be produced by different cells, whereas activated monocytes and macrophages can produce multiple MMPs. 71 Physiological remodelling of the ECM and vascular system requires MMP expression; however, its overexpression has adverse effects. For example, MMP‐2 is related to the increased risk of AD. 26 Collectively, results suggest that miR‐320 can regulate MMP expression post‐transcriptionally. In addition, the overexpression of MMP contributes to the ECM degradation, thereby influencing the progression of AD. However, further studies are required to determine whether miR‐320 can also regulate the function of VSMCs, ECs and other upstream elements.
Hsa‐miR‐320d and hsa‐miR‐582 expression detected by next‐generation sequencing (NGS) and gene expression microarrays had >10‐fold reduction in human AD thoracic aortic fragments. Transfecting miR‐320d mimic can alter the expression of 577 genes, whereas transfection with miR‐582 altered the expression of 203 genes. Of the 20,972 genes listed in the gene ontology database, only 2.7% (miR‐320d) and 1% (miR‐582) had altered expression levels. Interestingly, several genes were discovered to be related to the apoptotic pathway. In particular, TRIAP1 and NET1 may be downstream targets of miR‐320d, whereas COL1A1 and SPP1, which are related to extracellular matrix degradation, may be downstream targets of miR‐582. 21 The apoptotic pathways in VSMCs play an essential role in the development of AD. Das et al confirmed that the enrichment of S100A12, which is a pro‐inflammatory protein, can activate caspase 3 (CASP3) and promote apoptosis in human AD tissues. 72 In summary, miR‐320d and miR‐582 can regulate the progression of AD by participating in the apoptotic pathway and serve as potential biomarkers for the diagnosis and treatment of different diseases. However, this hypothesis needs further validation in human or mouse models.
4.1.5. microRNA‐144‐3p (miR‐144‐3p)
miR‐144‐3p has increased expression levels in AoSMCs derived from AD patients dissection specimens. Notably, the down‐regulation of miR‐144‐3p expression through adeno‐associated viruses can reduce the incidence and severity of AD. Bioinformatic analysis and dual‐luciferase reporter assay confirmed that miR‐144‐3p binds to the 3′‐UTR of the tropoelastin (TE) mRNA to inhibit protein translation. 73 TE is a soluble monomer of elastin, a polymeric extracellular protein that determines the ductility and elastic rebound of many cells, accounting for more than 50% of the AoSMC dry weight. The polymerization of TE and other protein components forms the extracellular elastic matrix, which determines the mechanical stability and physical properties of tissues. 74 Moreover, TE polymorphism defects greatly contribute to the initiation of AD. 75 In a BAPN‐induced mouse AD model, the inhibition of miR‐144‐3p expression resulted in the reduced incidence of AD from 90% to 50%. This indicates that miR‐144‐3p plays a vital role in the pathogenesis of AD and may serve as a potential therapeutic target.
4.1.6. microRNA‐146b (miR‐146b) and microRNA‐146a‐5p (miR‐146a‐5p)
It was reported that miR‐146b expression was significantly increased in peripheral blood and aortic wall tissues of the TAAD group compared with the control group (P < .001). In addition, the differential expression of miR‐146b positively correlated with the high risk of AD. Many ways were used to predict miR‐146b–related target genes, identifying NF‐κB1, tumour necrosis factor receptor‐associated factor 6 (TRAF6), MMP16 and actin alpha 2 (ACTA2). 76 It has been previously confirmed that atherosclerosis and autoimmune inflammation are associated with the excessive activation of NF‐κB1. 77 Therefore, NF‐κB1 may also be involved in the occurrence of AD through the immune‐inflammatory response. miR‐146b can also target the 3′‐UTR of TRAF6 and promote vascular EC inflammation by regulating the Toll‐like receptor (TLR) immune signalling pathway. Hence, these predicted gene targets may also be involved in the formation of AD. 78 MMPs, which can degrade the ECM and regulate immune‐inflammatory responses, are associated with apoptosis and inflammatory signalling pathways, and therefore, MMPs play an important role in vascular remodelling. 24 Mutations in ACTA2 have been reported to regulate the TGF‐β signalling pathway; thus, miR‐146b can participate in the development of AD by regulating the TGF‐β signalling pathway. 79 In summary, the increased expression of miR‐146b may be related to the incidence and severity of TAAD. Therefore, miR‐146b may be a potential biomarker that can predict the risk and severity of TAAD rupture and become a basis for selecting appropriate treatment methods.
miR‐146a‐5p was discovered to participate in the regulation of the immune system and myeloid tumorigenesis, 80 as well as cell proliferation and migration. 81 SMAD4‐mediated regulation resulted in significantly increased miR‐146a‐5p expression (P < .05) and elevated VSMC proliferation and migration rates via in Stanford type A AD patients plasma samples and tissue specimens. 82 However, the small sample size and unknown association between circulating miR‐146a‐5p levels and AD severity limit the potential of miR‐146a‐5p as a biomarker for predicting prognosis.
4.1.7. microRNA‐30a (miR‐30a)
miR‐30a expression was found to be significantly up‐regulated in human AD ascending aorta specimens, consequently reducing the lysyl oxidase (LOX) expression via translation inhibition in the aortic wall. 83 LOX is an extracellular, copper‐dependent enzyme that cross‐links collagen and elastin. LOX inactivation inhibits the cross‐linking of collagen and elastin, leading to aortic aneurysm (AA) in inbred mottled mice. 84 In addition, LOX knockout or reduced LOX expression of in mice, turkeys and rats was associated with AD. 85 In AngII‐induced AAD and aortic angiography animal model, the rats pre‐treated with agomiR‐30a have a significantly higher probability of developing AD. Collectively, these results provide new insights into the molecular mechanism of AD formation and highlight the potential of miR‐30a as a potential therapeutic target for AD.
4.1.8. microRNA‐143/145 (miR‐143/145) gene cluster
In AngII‐induced AD model, the down‐regulated expression of the miRNA‐143/145 gene cluster and the switch of VSMCs from a contractile to a synthetic phenotype were associated with the P38‐MAPK signalling pathway, and in AD ascending aorta tissues, the expression of phosphorylated p38‐MAPK increased obviously. 52 Additionally, the down‐regulation of the miR‐143/145 gene cluster during the pathogenesis of AD can promote VSMC phenotypic switching and aortic media degeneration through the TGF‐β1 signalling pathway. 12 The miR‐143/145 gene cluster, including miR‐143 and miR‐145, is limited to the adult SMC lineages during development. 86 Notably, AngII regulates the expression of miR143/145 and promotes the transition of murine VSMCs from a synthetic to a contractile phenotype during the formation of AD. 87 It was reported that AngII has elevated levels in the serum of AD patients and the overexpression of AngII can induce aortic atherosclerosis and AA by modulating the proliferation, differentiation, apoptosis and hypertrophy of VSMCs. 88 Recently, it was confirmed that AngII can also activate MAPKs, including signal‐regulated kinase (ERK1/2), JNK and p38, by activating the signalling cascades specifically related to the proliferation, migration, differentiation and fibrosis of VSMC. 89 However, further studies are needed to verify whether the down‐regulation of the miR‐143/145 gene cluster activates p38 signals before or after the onset of AD.
4.1.9. microRNA‐4787‐5p (miR‐4787‐5p) and microRNA‐4306 (miR‐4306)
miRNA microarray analysis revealed that the expression levels of circulating miR‐4787‐5p and miR‐4306 were >2‐fold higher in AAD patients. 90 Furthermore, the combination value of miR‐4787‐5p and miR‐4306 was equal or greater than D‐dimer during the early diagnosis of AAD. Bioinformatic analysis predicted PKD1 and TGF‐β1 as potential targets of miR‐4787‐5p and miR‐4306, respectively. The results of dual‐luciferase reporter assay validated this prediction. PKD1 and TGF‐β1 were suggested to be associated with the development of aortic diseases, and these two were observed to have decreased expression levels in AAD patients. 90 , 91 In summary, miR‐4787‐5p and miR‐4306 may play important roles in the early diagnosis of AAD. However, more samples need to be studied to verify the diagnostic value of miR‐4787‐5p and miR‐4306 as potential biomarkers in AAD.
4.1.10. microRNA‐26b (miR‐26b)
High‐mobility group AT‐hook 2 (HMGA2) plays an essential role in cellular proliferation and differentiation during embryonic development and is involved in the development of cardiovascular diseases, including atherosclerosis and cardiac lipomas. 92 Furthermore, HMGA2 overexpression in AAD occurs in a let‐7d–independent manner, which influences AAD through epithelial‐mesenchymal transition (EMT). 93 It was confirmed that the miR‐26b/HMGA2 axis contributes to TAAD development through the TGF‐β signalling pathway. miR‐26b regulates the expression of HMGA2, thereby indirectly regulating VSMC proliferation and apoptosis, and activates the TGF‐β/SMAD3 signalling pathway to promote VSMC proliferation. 94 miR‐26b was found to be negatively correlated with the risk and severity of TAAD, and miR‐26b expression was decreased in ascending aorta tissue of TAAD patients. It was confirmed as a biomarker for TAAD diagnosis, which can predict the risk of AD, assess the prognosis and serve as a basis for choosing the timing of surgery. 44
5. LONG NON‐CODING RNAS (LNCRNAS) IN AORTIC DISSECTION
Long non‐coding RNAs (lncRNAs), generally 200 nt long, are reported to be three‐dimensional (3D) regulators of transcription and translation by acting as molecular decoys and scaffolds or by binding the guide ribonucleoprotein complexes to their targets. The cellular expression of lncRNAs is tissue‐specific; however, some are only expressed at specific stages of eukaryotic development. Moreover, these can be host genes for the transcription of miRNAs in the nucleus or act as miRNA sponges in the cytoplasm. 95 In recent years, lncRNAs were found to play an important role in the occurrence and development of cardiovascular diseases. 96 Most lncRNAs have significant spatiotemporal expression and specificity during tissue differentiation and development, making them better biomarkers for the diagnosis of AD (Figure 2).
High‐throughput sequencing (HTS) technology was previously used to study the expression profile of lncRNAs in human TAD tissues. Several genes were found to be differentially expressed in human TAD, including 269 lncRNAs (110 down‐regulated and 159 up‐regulated) and 2255 mRNAs (961 down‐regulated and 1,294 up‐regulated). Results also demonstrated the positive correlations between lncRNAs (ENSG00000269936, lncRNA‐1421) and their adjacent mRNAs (MAP2K6, FBLN5, ACTA2, TIMP3). The ENSG00000248508, ENSG00000226530 and EG00000259719 lncRNAs and their upstream target, RUNX1, were also up‐regulated. 97 Previous studies have reported that RUNX1 can regulate MMP9 expression and their increased expression contributes to the immune response in abdominal AA. 98 Therefore, the identified lncRNAs that are regulated by RUNX1 may have important functions in the development of TAD.
5.1. lncRNA‐XIST, ENSG00000269936 and lncRNA‐1421
The lncRNA‐miRNA‐mRNA network revealed that lncRNA‐XIST directly binds to miR‐17‐5p to regulate p21 expression. lncRNA‐XIST and p21 were up‐regulated, whereas has‐miR‐17‐5p was down‐regulated in TAD tissues. miR‐17‐5p can regulate the expression of p21, PKD2 and SOD2, which are all involved in the regulation of aortic diseases. 99 , 100 , 101 Therefore, lncRNA‐XIST can indirectly regulate the expression of p21 through sponging miR‐17‐5p and ultimately participate in the development of TAD. In addition, ENSG00000269936 mediates AD development by cis‐targeting its adjacent gene, MAP2K6, a member of the MAPK signalling pathway. Notably, the p38 MAPK signalling pathway was reported to regulate the factors associated with AD progression. 102 lncRNA‐1421 regulates TAD development by trans‐regulating the expression of TIMP3, FBLN5 and ACTA2, which participate in AD development through different ways. 103 , 104 , 105 Collectively, these studies provide new insights into the molecular mechanisms of AD and may reveal the important function of lncRNAs in creating future prevention strategies.
5.2. PTENP1
The lncRNA PTENP1 is a pseudogene of the tumour suppressor gene phosphatase and tensin homologue deleted on chromosome ten (PTEN). 106 PTENP1 and PTEN have an endogenous competitive relationship. PTENP1 can act as a molecular sponge of miR‐21 to bind with miR‐21, release the expression of PTEN, further inhibit downstream threonine kinase (Akt) signal transduction and finally reduce the expression of cyclin D1 and cyclin E. Increased PTEN expression further promotes apoptosis and inhibits the proliferation of human aortic smooth muscle cells (HASMCs). In contrast, PTENP1 silencing inhibits the H2O2‐induced HASMC apoptosis. In AngII‐induced mouse AA model, PTENP1 overexpression enhanced the apoptosis of AoSMCs and aggravated the formation of aneurysms. Therefore, PTENP1 plays an important role in maintaining HASMC homeostasis and may serve as a potential target for therapeutic intervention of AD or AA. 107
In TAD, a total of 765 lncRNAs and 619 mRNAs have been identified to be aberrantly expressed. Further, 16 lncRNAs and their target genes may be associated with TAD pathogenesis. The qRT‐PCR results showed that the expression levels of five lncRNAs (RP11‐536K7.5, AX746823, lncP2RX7, HIF1A‐AS2 and RP11‐69I8.3) and 6 mRNAs (CTGF, IL2RA, HIF‐1A, P2RX7, CDKN2B and RUNX1) significantly increased in TAD, suggesting that these are potentially involved in TAD occurrence and development. In addition, RP11‐69I8.3, AX746823, RP11‐536K7.5 and lncP2RX7 were discovered to be related to the activation of several protein‐coding genes involved with connective tissue development (CTGF), nuclear transcription (RUNX1), inflammation (IL2RA) and nuclear receptors (P2RX7). Previous studies demonstrated that HIF‐1A, CTGF, CDKN2B, P2X7, RUNX1 and IL2RA can regulate specific factors that are involved in the formation and development of AD. 98 , 108 , 109 , 110 , 111 , 112 However, further studies are needed to determine whether these genes are also TAD‐related and what cell types the proteins are expressed in Ref. 113
6. CIRCULAR RNAS (CIRCRNAS) IN AORTIC DISSECTION
circRNAs are a special group of novel endogenous lncRNAs that can bind to and interact with miRNAs and play an important regulatory role at the transcriptional or post‐transcriptional level. 114 The circRNA expression profiles are usually analysed by high‐throughput RNA sequencing (RNA‐Seq) technology and computational analysis, revealing numerous circRNAs with differential expression. 115 Unlike miRNAs and lncRNAs, circRNAs have closed‐ring structures and therefore have no 5′–3′ polarity and polyA tail; however, circRNAs do retain the sequence direction according to their mRNA of origin. circRNAs are more stable than linear RNAs and not easily degraded by RNA exonuclease or RNase R, and thus, the expression of circRNA in various human cells and tissues is detectable. The biological functions of circRNAs include the following: (a) sponge adsorbents of miRNAs as competitors of endogenous mRNAs; (b) regulate variable shear or transcriptional processes; and (b) regulate the expression of parental genes. 116 These characteristics determine the important functions of circRNAs in transcription and post‐transcription, suggesting that these may be ideal biomarkers for disease diagnosis (Figure 2).
6.1. circRNA‐101238
Zou et al found 8,173 circRNAs that are differentially expressed in human type A TAD. The qRT‐PCR results showed that hsa‐circRNA‐101238, hsa‐circRNA‐002271, hsa‐circRNA‐104634, hsa‐circRNA‐104349, hsa‐circRNA‐102771, COL1A1 and COL6A3 were up‐regulated, whereas FLNA, hsa‐circRNA‐102683, hsa‐circRNA‐103458 and hsa‐circRNA‐005525 were down‐regulated. Some TAD‐associated miRNAs can be regulated by several circRNAs. For example, miRNA expression profile analysis revealed that the expression of hsa‐miR‐320a, hsa‐miR‐320b and hsa‐miR‐320c was inhibited by hsa‐circRNA‐101238 in TAD aortic specimens. 85 Therefore, the higher the expression level of circRNA‐101238 in human TAD tissues, the lower the expression level of the downstream target miR‐320a, leading to increased MMP9 expression. SMC phenotypic transformation or apoptosis was promoted by the up‐regulation of hsa‐circRNA‐104349 and hsa‐circRNA‐104634, which interacted with hsa‐miR‐26a‐3p and hsa‐miR‐145‐3p, respectively. 117 hsa‐circRNA‐102683 and hsa‐circRNA‐104033 can inhibit the expression of hsa‐miR‐29b‐1‐5p and hsa‐miR‐195‐3p, respectively, thereby promoting apoptosis and ECM degradation in the aortic wall, as well as stimulating collagen remodelling. 118 Thus, the identified differentially expressed circRNAs may function in the development of TAD through multiple biological processes. 119
6.2. circ‐MARK3
Tian et al also performed RNA‐Seq on human acute Stanford type A aortic dissection (AAAD) diseased ascending aortic specimens and discovered that 506 circRNAs were significantly differentially expressed. 47 The top 10 highly differentially expressed circRNAs, with 2‐ to 5‐fold change in expression, included the up‐regulated circARHGAP26, circUBA2, circCHSY1, circIQGAP1, circMBNL1, circMED13, circRAB7A and circMYH10 and the down‐regulated circCEP70 and circFAM120B. The circRNA‐miRNA‐mRNA network analysis results suggested that circMARK3 could regulate the expression of Fgr, a tyrosine‐protein kinase. Fgr participates in cellular signal transmission and regulates the immune response and release of inflammatory factors. 120 Additionally, the diagnostic value of serum circMARK3 was further validated by ROC analysis, revealing 0.9344 AUC, 90.0% sensitivity and 86.7% specificity. The RNAhybrid programme predicted the miR‐1273g‐3p‐circRNA interaction pair, suggesting that the combination of serum miR‐1273‐3p and circMARK3 can greatly improve their diagnostic efficiency as biomarkers of AD. In summary, the interaction of circMARK3‐miR‐1273‐Fgr was believed to have certain clinical significance and combining circRNAs with other biomarkers can provide higher diagnostic value. However, the direct interaction between circMARK3‐miR‐1273‐Fgr needs to be verified by further biological experiments.
7. DISCUSSION
Previous studies have identified many ncRNAs, such as miRNAs, lncRNAs and circRNAs, which are involved in the pathogenesis of AD. Several miRNAs with therapeutic potential, such as miR‐21, miR‐134‐5p, miR‐144‐3p and miR‐30a, have been reported in animal AD models and cross‐validated in human AD tissue samples. In contrast, although several lncRNAs have been implicated in certain pathophysiological processes during AD development, only lncRNAs‐XIST, PTENP1, lncP2RX7 and ENSG00000269936 have been validated. In addition, circ‐101238 and circ‐MARK3 may have potential diagnostic value. As mentioned above, some miRNAs that are involved in the development of AD have already been studied using animal models. Currently, scientific research is more advanced and clinical applications are continuously being developed; these will complement the in‐depth research on the potential applications of miRNAs in clinical settings. Likewise, numerous lncRNAs and circRNAs that can potentially regulate AD have been detected through sequencing and microarray experiments. However, the exact functions and mechanisms of these ncRNAs are unknown and require further investigations.
To understand the biological and pathophysiological mechanisms associated with AD occurrence and progression, the abnormally expressed miRNAs, lncRNAs and circRNAs must be identified and validated in related animal models or human AD tissues. Extracting the RNA from key cell types, such as ECs, SMCs and immune cells, will help obtain specific insights into the cell‐type specific changes during AD development. After verifying their differential expression, the molecular mechanisms through which these ncRNAs regulate AD development must also be tested in vivo and in vitro.
In most cases, AD has very poor prognosis; however, the development of interventional therapy has markedly improved the current therapeutic efficacy. Recently, a new method for AD treatment previously used for the nucleic acid therapy of thoracic aortic dissection (TAD) through a multifunctional cationic nanoparticle system has been reported. 121 This system has no therapeutic effect on its own and is only a microRNA (miRNA) carrier that can be stored in the blood and will not damage the organs. This treatment strategy is suitable for people with TAD susceptibility, such as patients with Marfan syndrome. However, this delivery system has low specificity because its target is exposed to the pathological tissue of type IV collagen. Currently, an effective treatment for AD is still unavailable. Therefore, in‐depth studies focusing on the pathogenesis, new interventions and treatment strategies are required.
The rapid growth of miRNA research provides new insights for future studies on the identified potential biomarkers and therapeutic targets. However, some research gaps still need to be addressed. First, ncRNA biology research focuses on the effects of miRNAs, lncRNAs or circRNAs on VSMCs. This needs to be extended to the effect of miRNAs or lncRNAs on other cellular components, such as ECs, fibroblasts and macrophages. Second, besides miRNAs, lncRNAs and circRNAs, other ncRNAs such as repeat‐associated small interfering RNAs (rasiRNAs) and Piwi‐interacting RNAs (piRNAs) may also be involved in the development of cardiovascular pathology, and thus, these need to be investigated. Third, additional studies on miRNA medicinal chemistry and delivery methods are needed to create strategies for the local, systemic and safe delivery of ncRNA‐based therapeutics, as well as subsequent research in clinically relevant animal models and corresponding in vivo imaging systems.
In conclusion, in‐depth research of ncRNAs will provide potential therapeutic targets and biomarkers to monitor the progress and severity of AD in patients. Early and correct diagnosis of AD can significantly reduce mortality in human patients. Therefore, further efforts to identify other candidate biomarkers and to elucidate their underlying mechanisms, as well as their diagnostic and therapeutic potentials, are required.
CONFLICT OF INTERESTS
None.
AUTHOR CONTRIBUTIONS
Mengdie Cheng: Data curation (lead); formal analysis (lead); resources (lead); writing‐original draft (lead). Yanyan Yang: Conceptualization (equal); funding acquisition (equal); resources (equal); writing‐review and editing (equal). Hai Xin: Conceptualization (supporting); resources (supporting). Tingyu Zong: Data curation (supporting). Xingqiang He: Data curation (supporting); resources (supporting). Min Li: Formal analysis (supporting); validation (supporting). Tao Yu: Conceptualization (lead); funding acquisition (equal); project administration (lead); supervision (equal); validation (lead); visualization (lead); writing‐review and editing (lead). Hui Xin: Funding acquisition (equal); project administration (equal); supervision (equal).
ACKNOWLEDGEMENT
This work was supported by the National Natural Science Foundation of China (grant no. 81870331, 31701208), the Natural Science Foundation of Shandong Province (grant no. ZR2017MC067) and the Qingdao municipal science and technology bureau project (grant no. 18‐2‐2‐65‐jch,19‐6‐1‐7‐nsh).
Cheng M, Yang Y, Xin H, et al. Non‐coding RNAs in aortic dissection: From biomarkers to therapeutic targets. J Cell Mol Med. 2020;24:11622–11637. 10.1111/jcmm.15802
Cheng and Yang contributed equally to this paper.
Contributor Information
Tao Yu, Email: yutao0112@qdu.edu.cn.
Hui Xin, Email: xinhuiqy@163.com.
REFERENCES
- 1. Mussa FF, Horton JD, Moridzadeh R, Nicholson J, Trimarchi S, Eagle KA. Acute aortic dissection and intramural hematoma: a systematic review. JAMA. 2016;316(7):754‐763. [DOI] [PubMed] [Google Scholar]
- 2. Howard DPJ, Banerjee A, Fairhead JF, Perkins J, Silver LE, Rothwell PM. Population‐based study of incidence and outcome of acute aortic dissection and premorbid risk factor control: 10‐year results from the Oxford Vascular Study. Circulation. 2013;127(20):2031‐2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Erbel R, Aboyans V, Boileau C, et al. 2014 ESC Guidelines on the diagnosis and treatment of aortic diseases: Document covering acute and chronic aortic diseases of the thoracic and abdominal aorta of the adult. The Task Force for the Diagnosis and Treatment of Aortic Diseases of the European Society of Cardiology (ESC). Eur Heart J. 2014;35(41):2873‐2926. [DOI] [PubMed] [Google Scholar]
- 4. Nienaber CA, Clough RE. Management of acute aortic dissection. Lancet (London, England). 2015;385(9970):800‐811. [DOI] [PubMed] [Google Scholar]
- 5. Dietz HC, Cutting CR, Pyeritz RE, et al. Marfan syndrome caused by a recurrent de novo missense mutation in the fibrillin gene. Nature. 1991;352(6333):337‐339. [DOI] [PubMed] [Google Scholar]
- 6. Superti‐Furga A, Gugler E, Gitzelmann R, Steinmann B. Ehlers‐Danlos syndrome type IV: a multi‐exon deletion in one of the two COL3A1 alleles affecting structure, stability, and processing of type III procollagen. J Biol Chem. 1988;263(13):6226‐6232. [PubMed] [Google Scholar]
- 7. Loeys BL, Chen J, Neptune ER, et al. A syndrome of altered cardiovascular, craniofacial, neurocognitive and skeletal development caused by mutations in TGFBR1 or TGFBR2. Nat Genet. 2005;37(3):275‐281. [DOI] [PubMed] [Google Scholar]
- 8. Hoffman JIE, Kaplan S. The incidence of congenital heart disease. J Am Coll Cardiol. 2002;39(12):1890‐1900. [DOI] [PubMed] [Google Scholar]
- 9. Gawinecka J, Schönrath F, von Eckardstein A. Acute aortic dissection: pathogenesis, risk factors and diagnosis. Swiss Med Wkly. 2017;147:w14489. [DOI] [PubMed] [Google Scholar]
- 10. Lumayag S, Haldin CE, Corbett NJ, et al. Inactivation of the microRNA‐183/96/182 cluster results in syndromic retinal degeneration. Proc Natl Acad Sci USA. 2013;110(6):E507‐E516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Xin H, Li YI, Buller B, et al. Exosome‐mediated transfer of miR‐133b from multipotent mesenchymal stromal cells to neural cells contributes to neurite outgrowth. Stem cells (Dayton, Ohio). 2012;30(7):1556‐1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhang M, Wang Z. Downregulation of miR143/145 gene cluster expression promotes the aortic media degeneration process via the TGF‐β1 signaling pathway. Am J Transl Res. 2019;11(1):370‐378. [PMC free article] [PubMed] [Google Scholar]
- 13. Yu T, Wang Z, Jie W, et al. The kinase inhibitor BX795 suppresses the inflammatory response via multiple kinases. Biochem Pharmacol. 2020;174:113797. [DOI] [PubMed] [Google Scholar]
- 14. Yang Y, Yu T, Jiang S, et al. miRNAs as potential therapeutic targets and diagnostic biomarkers for cardiovascular disease with a particular focus on WO2010091204. Expert Opin Ther Pat. 2017;27(9):1021‐1029. [DOI] [PubMed] [Google Scholar]
- 15. Liu S, Yang Y, Jiang S, et al. Understanding the role of non‐coding RNA (ncRNA) in stent restenosis. Atherosclerosis. 2018;272:153‐161. [DOI] [PubMed] [Google Scholar]
- 16. Tang N, Jiang S, Yang Y, et al. Noncoding RNAs as therapeutic targets in atherosclerosis with diabetes mellitus. Cardiovasc Ther. 2018;36(4):e12436. [DOI] [PubMed] [Google Scholar]
- 17. Liu Y, Yang Y, Wang Z, et al. Insights into the regulatory role of circRNA in angiogenesis and clinical implications. Atherosclerosis. 2020;298:14‐26. [DOI] [PubMed] [Google Scholar]
- 18. Wang QI, Yang Y, Fu X, et al. Long noncoding RNA XXYLT1‐AS2 regulates proliferation and adhesion by targeting the RNA binding protein FUS in HUVEC. Atherosclerosis. 2020;298:58‐69. [DOI] [PubMed] [Google Scholar]
- 19. Wang Y, Dong C‐Q, Peng G‐Y, et al. MicroRNA‐134‐5p regulates media degeneration through inhibiting VSMC phenotypic switch and migration in thoracic aortic dissection. Mol Ther Nucl Acids. 2019;16:284‐294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Li T, Liu C, Liu L, et al. Regulatory mechanism of MicroRNA‐145 in the pathogenesis of acute aortic dissection. Yonsei Med J. 2019;60(4):352‐359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Shen H, Lu S, Dong L, et al. hsa‐miR‐320d and hsa‐miR‐582, miRNA biomarkers of aortic dissection, regulate apoptosis of vascular smooth muscle cells. J Cardiovasc Pharmacol. 2018;71(5):275‐282. [DOI] [PubMed] [Google Scholar]
- 22. Coady MA, Davies RR, Roberts M, et al. Familial patterns of thoracic aortic aneurysms. Arch Surg, 1999;134(4):361‐367. [DOI] [PubMed] [Google Scholar]
- 23. Humphrey JD, Schwartz MA, Tellides G, Milewicz DM. Role of mechanotransduction in vascular biology: focus on thoracic aortic aneurysms and dissections. Circ Res. 2015;116(8):1448‐1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Koullias GJ, Ravichandran P, Korkolis DP, Rimm DL, Elefteriades JA. Increased tissue microarray matrix metalloproteinase expression favors proteolysis in thoracic aortic aneurysms and dissections. Annals Thoracic Surg. 2004;78(6). [DOI] [PubMed] [Google Scholar]
- 25. Del Porto F, di Gioia C, Tritapepe L, et al. The multitasking role of macrophages in Stanford type A acute aortic dissection. Cardiology. 2014;127(2):123‐129. [DOI] [PubMed] [Google Scholar]
- 26. Wang L, Zhang J, Fu W, Guo D, Jiang J, Wang Y. Association of smooth muscle cell phenotypes with extracellular matrix disorders in thoracic aortic dissection. J Vasc Surg. 2012;56(6):1698‐1709.e1. [DOI] [PubMed] [Google Scholar]
- 27. Didangelos A, Yin X, Mandal K, et al. Extracellular matrix composition and remodeling in human abdominal aortic aneurysms: a proteomics approach. Mol Cell Proteomics. 2011;10(8):M111.008128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hopkins PN. Molecular biology of atherosclerosis. Physiol Rev. 2013;93(3):1317‐1542. [DOI] [PubMed] [Google Scholar]
- 29. Ignatieva E, Kostina D, Irtyuga O, et al. Mechanisms of smooth muscle cell differentiation are distinctly altered in thoracic aortic aneurysms associated with bicuspid or tricuspid aortic valves. Front Physiol. 2017;8:536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Owens GK. Regulation of differentiation of vascular smooth muscle cells. Physiol Rev. 1995;75(3):487‐517. [DOI] [PubMed] [Google Scholar]
- 31. Frismantiene A, Philippova M, Erne P, Resink TJ. Smooth muscle cell‐driven vascular diseases and molecular mechanisms of VSMC plasticity. Cell Signal. 2018;52:48‐64. [DOI] [PubMed] [Google Scholar]
- 32. Owens GK, Kumar MS, Wamhoff BR. Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol Rev. 2004;84(3):767‐801. [DOI] [PubMed] [Google Scholar]
- 33. Li X, Wei Y, Wang Z. microRNA‐21 and hypertension. Hypertension Res. 2018;41(9):649‐661. [DOI] [PubMed] [Google Scholar]
- 34. Yang Q, Jia C, Wang P, et al. MicroRNA‐505 identified from patients with essential hypertension impairs endothelial cell migration and tube formation. Int J Cardiol. 2014;177(3):925‐934. [DOI] [PubMed] [Google Scholar]
- 35. Davies PF. Hemodynamic shear stress and the endothelium in cardiovascular pathophysiology. Nat Clin Pract Cardiovasc Med. 2009;6(1):16‐26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Glagov S, Zarins C, Giddens DP, Ku DN. Hemodynamics and atherosclerosis. Insights and perspectives gained from studies of human arteries. Arch Pathol Lab Med. 1988;112(10):1018‐1031. [PubMed] [Google Scholar]
- 37. Passerini AG, Polacek DC, Shi C, et al. Coexisting proinflammatory and antioxidative endothelial transcription profiles in a disturbed flow region of the adult porcine aorta. Proc Natl Acad Sci USA. 2004;101(8):2482‐2487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zhou J, Li Y‐S, Nguyen P, et al. Regulation of vascular smooth muscle cell turnover by endothelial cell‐secreted microRNA‐126: role of shear stress. Circ Res. 2013;113(1):40‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hergenreider E, Heydt S, Tréguer K, et al. Atheroprotective communication between endothelial cells and smooth muscle cells through miRNAs. Nat Cell Biol. 2012;14(3):249‐256. [DOI] [PubMed] [Google Scholar]
- 40. Jia L‐X, Zhang W‐M, Li T‐T, et al. ER stress dependent microparticles derived from smooth muscle cells promote endothelial dysfunction during thoracic aortic aneurysm and dissection. Clinical Sci. 2017;131(12):1287‐1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Pan L, Lin Z, Tang X, et al. S‐Nitrosylation of Plastin‐3 exacerbates thoracic aortic dissection formation via endothelial barrier dysfunction. Arterioscler Thromb Vasc Biol. 2020;40(1):175‐188. [DOI] [PubMed] [Google Scholar]
- 42. Sun Y, Xiao YU, Sun H, et al. miR‐27a regulates vascular remodeling by targeting endothelial cells' apoptosis and interaction with vascular smooth muscle cells in aortic dissection. Theranostics. 2019;9(25):7961‐7975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bouchie A. First microRNA mimic enters clinic. Nat Biotechnol. 2013;31(7):577. [DOI] [PubMed] [Google Scholar]
- 44. Xu Z, Wang Q, Pan J, et al. Characterization of serum miRNAs as molecular biomarkers for acute Stanford type A aortic dissection diagnosis. Sci Rep. 2017;7(1):13659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Dong J, Bao J, Feng R, et al. Circulating microRNAs: a novel potential biomarker for diagnosing acute aortic dissection. Sci Rep. 2017;7(1):12784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Liao M, Zou S, Weng J, et al. A microRNA profile comparison between thoracic aortic dissection and normal thoracic aorta indicates the potential role of microRNAs in contributing to thoracic aortic dissection pathogenesis. J Vasc Surg. 2011;53(5). [DOI] [PubMed] [Google Scholar]
- 47. Tian C, Tang X, Zhu X, et al. Expression profiles of circRNAs and the potential diagnostic value of serum circMARK3 in human acute Stanford type A aortic dissection. PLoS One. 2019;14(6):e0219013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Su Y, Li Q, Zheng Z, Wei X, Hou P. Integrative bioinformatics analysis of miRNA and mRNA expression profiles and identification of associated miRNA‐mRNA network in aortic dissection. Medicine. 2019;98(24):e16013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Hu Z‐Y, Luo JF, Zhong SL, Xue L, Chen YF, Fan RX. MicroRNAs expression in normal and dissected aortic tissue. Zhonghua xin xue guan bing za zhi. 2012;40(5):406‐410. [PubMed] [Google Scholar]
- 50. Hiraya D, Sato A, Aonuma K. Circulating microRNAs as an emerging biomarker for acute aortic dissection diagnosis‐comparing with prior biomarkers. J Thorac Dis. 2018;10(3):1186‐1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116(2):281‐297. [DOI] [PubMed] [Google Scholar]
- 52. Li B, Wang Z, Hu Z, et al. P38 MAPK Signaling Pathway Mediates Angiotensin II‐Induced miR143/145 Gene Cluster Downregulation during Aortic Dissection Formation. Ann Vasc Surg. 2017;40:262‐273. [DOI] [PubMed] [Google Scholar]
- 53. Kroh EM, Parkin RK, Mitchell PS, Tewari M. Analysis of circulating microRNA biomarkers in plasma and serum using quantitative reverse transcription‐PCR (qRT‐PCR). Methods. 2010;50(4):298‐301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Qadir MI, Faheem A. miRNA: a diagnostic and therapeutic tool for pancreatic cancer. Crit Rev Eukaryot Gene Expr. 2017;27(3):197‐204. [DOI] [PubMed] [Google Scholar]
- 55. Puik JR, Meijer LL, Le Large TYS, et al. miRNA profiling for diagnosis, prognosis and stratification of cancer treatment in cholangiocarcinoma. Pharmacogenomics. 2017;18(14):1343‐1358. [DOI] [PubMed] [Google Scholar]
- 56. McGuire A, Brown JAL, Kerin MJ. Metastatic breast cancer: the potential of miRNA for diagnosis and treatment monitoring. Cancer Metastasis Rev. 2015;34(1):145‐155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Ovchinnikova ES, Schmitter D, Vegter EL, et al. Signature of circulating microRNAs in patients with acute heart failure. Eur J Heart Fail. 2016;18(4):414‐423. [DOI] [PubMed] [Google Scholar]
- 58. Recchioni R, Marcheselli F, Olivieri F, Ricci S, Procopio AD, Antonicelli R. Conventional and novel diagnostic biomarkers of acute myocardial infarction: a promising role for circulating microRNAs. Biomarkers. 2013;18(7):547‐558. [DOI] [PubMed] [Google Scholar]
- 59. Harling L, Lambert J, Ashrafian H, Darzi A, Gooderham NJ, Athanasiou T. Elevated serum microRNA 483–5p levels may predict patients at risk of post‐operative atrial fibrillation. Eur J Cardio‐Thoracic Surg. 2017;51(1):73‐78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Zhang J, Wang L, Fu W, et al. Smooth muscle cell phenotypic diversity between dissected and unaffected thoracic aortic media. J Cardiovasc Surg. 2013;54(4):511‐521. [PubMed] [Google Scholar]
- 61. Shen J, Yang M, Ju D, et al. Disruption of SM22 promotes inflammation after artery injury via nuclear factor kappaB activation. Circ Res. 2010;106(8):1351‐1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Patuzzo C, Pasquali A, Trabetti E, et al. A preliminary microRNA analysis of non syndromic thoracic aortic aneurysms. Balkan J Med Genetics: BJMG. 2012;15(Suppl):51‐55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Huang X, Yue Z, Wu J, et al. MicroRNA‐21 knockout exacerbates angiotensin II–induced thoracic aortic aneurysm and dissection in mice with abnormal transforming growth factor‐β–SMAD3 signaling. Arterioscler Thromb Vasc Biol. 2018;38(5):1086‐1101. [DOI] [PubMed] [Google Scholar]
- 64. Miao C, Cao H, Zhang Y, Guo X, Wang Z, Wang J. LncRNA DIGIT accelerates tube formation of vascular endothelial cells by sponging miR‐134. Int Heart J. 2018;59(5):1086‐1095. [DOI] [PubMed] [Google Scholar]
- 65. Zhang Y, Liu K, Zhang Y, et al. ABL‐N may induce apoptosis of human prostate cancer cells through suppression of KLF5, ICAM‐1 and Stat5b, and upregulation of Bax/Bcl‐2 ratio: An in vitro and in vivo study. Oncol Rep. 2015;34(6):2953‐2960. [DOI] [PubMed] [Google Scholar]
- 66. Janmaat ML, Heerkens JLT, de Bruin AM, Klous A, de Waard V, de Vries CJM. Erythropoietin accelerates smooth muscle cell‐rich vascular lesion formation in mice through endothelial cell activation involving enhanced PDGF‐BB release. Blood. 2010;115(7):1453‐1460. [DOI] [PubMed] [Google Scholar]
- 67. Huang W, Huang C, Ding H, et al. Involvement of miR‐145 in the development of aortic dissection via inducing proliferation, migration, and apoptosis of vascular smooth muscle cells. J Clin Lab Anal. 2020;34(1):e23028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Zhan B, Hu Z, Chen J, et al. KLF15 overexpression protects β‐aminopropionitrile‐induced aortic rupture in rodent model via inhibiting connective tissue growth factor. Thorac Cardiovasc Surg. 2017;65(2):120‐125. [DOI] [PubMed] [Google Scholar]
- 69. Liao M, Zou S, Bao Y, et al. Matrix metalloproteinases are regulated by MicroRNA 320 in macrophages and are associated with aortic dissection. Exp Cell Res. 2018;370(1). [DOI] [PubMed] [Google Scholar]
- 70. Parks WC, Wilson CL, López‐Boado YS. Matrix metalloproteinases as modulators of inflammation and innate immunity. Nat Rev Immunol. 2004;4(8):617‐629. [DOI] [PubMed] [Google Scholar]
- 71. Newby AC. Metalloproteinase expression in monocytes and macrophages and its relationship to atherosclerotic plaque instability. Arterioscler Thromb Vasc Biol. 2008;28(12):2108‐2114. [DOI] [PubMed] [Google Scholar]
- 72. Das D, Gawdzik J, Dellefave‐Castillo L, et al. S100A12 expression in thoracic aortic aneurysm is associated with increased risk of dissection and perioperative complications. J Am Coll Cardiol. 2012;60(8):775‐785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Qi Y‐F, Shu C, Xiao ZX, et al. Post‐transcriptional control of tropoelastin in aortic smooth muscle cells affects aortic dissection onset. Mol Cells. 2018;41(3):198‐206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Morello F, Piler P, Novak M, , Kruzliak P. Biomarkers for diagnosis and prognostic stratification of aortic dissection: challenges and perspectives. Biomarkers in medicine. 2014;8(7):931‐941. [DOI] [PubMed] [Google Scholar]
- 75. Sato F, Seino‐Sudo R, Okada M, Sakai H, Yumoto T, Wachi H. Lysyl oxidase enhances the deposition of tropoelastin through the catalysis of tropoelastin molecules on the cell surface. Biol Pharm Bull. 2017;40(10):1646‐1653. [DOI] [PubMed] [Google Scholar]
- 76. Li J, Zhou Q, He X, Cheng Y, Wang D. Expression of miR‐146b in peripheral blood serum and aortic tissues in patients with acute type Stanford A aortic dissection and its clinical significance. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2017;42(10):1136‐1142. [DOI] [PubMed] [Google Scholar]
- 77. Wullaert A. Role of NF‐kappaB activation in intestinal immune homeostasis. Int J Med Microbiol. 2010;300(1):49‐56. [DOI] [PubMed] [Google Scholar]
- 78. Taganov KD, Boldin MP, Chang KJ, Baltimore D. NF‐kappaB‐dependent induction of microRNA miR‐146, an inhibitor targeted to signaling proteins of innate immune responses. Proc Natl Acad Sci USA. 2006;103(33):12481‐12486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Renard M, Callewaert B, Baetens M, et al. Novel MYH11 and ACTA2 mutations reveal a role for enhanced TGFβ signaling in FTAAD. Int J Cardiol. 2013;165(2):314‐321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Testa U, Pelosi E, Castelli G, Labbaye C. miR‐146 and miR‐155: two key modulators of immune response and tumor development. Non‐Coding RNA. 2017;3(3):22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Xiao W‐Z, Lu AQ, Liu XW, Li Z, Zi Y, Wang ZW. Role of miRNA‐146 in proliferation and differentiation of mouse neural stem cells. Biosci Rep. 2015;35(5):e00245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Xue L, Luo S, Ding H, et al. Upregulation of miR‐146a‐5p is associated with increased proliferation and migration of vascular smooth muscle cells in aortic dissection. J Clin Lab Anal. 2019;33(4):e22843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Si M‐S. MicroRNA‐30a‐lysyl oxidase axis in aortic dissection pathogenesis. J Thorac Cardiovasc Surg. 2017;154(6):1870‐1871. [DOI] [PubMed] [Google Scholar]
- 84. Rowe DW, McGoodwin EB, Martin GR, Grahn DOUGLAS. Decreased lysyl oxidase activity in the aneurysm‐prone, mottled mouse. J Biol Chem. 1977;252(3):939‐942. [PubMed] [Google Scholar]
- 85. Guo D‐C, Regalado ES, Gong L, et al. LOX mutations predispose to thoracic aortic aneurysms and dissections. Circ Res. 2016;118(6):928‐934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Cordes KR, Sheehy NT, White MP, et al. miR‐145 and miR‐143 regulate smooth muscle cell fate and plasticity. Nature. 2009;460(7256):705‐710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Boettger T, Beetz N, Kostin S, et al. Acquisition of the contractile phenotype by murine arterial smooth muscle cells depends on the Mir143/145 gene cluster. J Clin Investig. 2009;119(9):2634‐2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Daugherty A, Manning MW, Cassis LA. Angiotensin II promotes atherosclerotic lesions and aneurysms in apolipoprotein E‐deficient mice. J Clin Investig. 2000;105(11):1605‐1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Taniyama Y, Ushio‐Fukai M, Hitomi H, et al. Role of p38 MAPK and MAPKAPK‐2 in angiotensin II‐induced Akt activation in vascular smooth muscle cells. Am J Physiol Cell Physiol. 2004;287(2):C494‐C499. [DOI] [PubMed] [Google Scholar]
- 90. Wang L, Zhang S, Xu Z, Zhang J, Li L, Zhao G. The diagnostic value of microRNA‐4787‐5p and microRNA‐4306 in patients with acute aortic dissection. Am J Transl Res. 2017;9(11):5138‐5149. [PMC free article] [PubMed] [Google Scholar]
- 91. Huang C‐K, Luo J, Lai KP, et al. Androgen receptor promotes abdominal aortic aneurysm development via modulating inflammatory interleukin‐1α and transforming growth factor‐β1 expression. Hypertension. 2015;66(4):881‐891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Vaughan CJ, Weremowicz S, Goldstein MM, et al. A t(2;19)(p13;p13.2) in a giant invasive cardiac lipoma from a patient with multiple lipomatosis. Genes Chromosom Cancer. 2000;28(2):133‐137. [DOI] [PubMed] [Google Scholar]
- 93. Belge G, Radtke A, Meyer A, et al. Upregulation of the high mobility group AT‐hook 2 gene in acute aortic dissection is potentially associated with endothelial‐mesenchymal transition. Histol Histopathol. 2011;26(8):1029‐1037. [DOI] [PubMed] [Google Scholar]
- 94. Yang P, Wu P, Liu X, et al. MiR‐26b suppresses the development of stanford type A aortic dissection by regulating HMGA2 and TGF‐β/Smad3 signaling pathway. Ann Thorac Cardiovasc Surg. 2020;26:140‐150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Ørom UA, Shiekhattar R. Long noncoding RNAs usher in a new era in the biology of enhancers. Cell. 2013;154(6):1190‐1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Batista PJ, Chang HY. Long noncoding RNAs: cellular address codes in development and disease. Cell. 2013;152(6):1298‐1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Sun J, Chen G, Jing Y, et al. LncRNA expression profile of human thoracic aortic dissection by high‐throughput sequencing. Cell Physiol Biochem. 2018;46(3):1027‐1041. [DOI] [PubMed] [Google Scholar]
- 98. Pahl M, Erdman R, Kuivaniemi H, Lillvis J, Elmore J, Tromp G. Transcriptional (ChIP‐Chip) analysis of ELF1, ETS2, RUNX1 and STAT5 in human abdominal aortic aneurysm. Int J Mol Sci. 2015;16(5):11229‐11258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Kavurma MM, Khachigian LM. Vascular smooth muscle cell‐specific regulation of cyclin‐dependent kinase inhibitor p21(WAF1/Cip1) transcription by Sp1 is mediated via distinct cis‐acting positive and negative regulatory elements in the proximal p21(WAF1/Cip1) promoter. J Cell Biochem. 2004;93(5):904‐916. [DOI] [PubMed] [Google Scholar]
- 100. Hao F, Wu DD, Xu X, Cui M‐Z. Histamine induces activation of protein kinase D that mediates tissue factor expression and activity in human aortic smooth muscle cells. Am J Physiol Heart Circ Physiol. 2012;303(11):H1344‐H1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Zhou R‐H, Vendrov AE, Tchivilev I, et al. Mitochondrial oxidative stress in aortic stiffening with age: the role of smooth muscle cell function. Arterioscler Thromb Vasc Biol. 2012;32(3):745‐755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Beltrán AE, Briones AM, García‐Redondo AB, et al. p38 MAPK contributes to angiotensin II‐induced COX‐2 expression in aortic fibroblasts from normotensive and hypertensive rats. J Hypertens. 2009;27(1):142‐154. [DOI] [PubMed] [Google Scholar]
- 103. Zhang X, Shen YH, LeMaire SA. Thoracic aortic dissection: are matrix metalloproteinases involved? Vascular. 2009;17(3):147‐157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Spencer JA, Hacker SL, Davis EC, et al. Altered vascular remodeling in fibulin‐5‐deficient mice reveals a role of fibulin‐5 in smooth muscle cell proliferation and migration. Proc Natl Acad Sci USA. 2005;102(8):2946‐2951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Guo D‐C, Pannu H, Tran‐Fadulu V, et al. Mutations in smooth muscle alpha‐actin (ACTA2) lead to thoracic aortic aneurysms and dissections. Nat Genet. 2007;39(12):1488‐1493. [DOI] [PubMed] [Google Scholar]
- 106. Poliseno L, Haimovic A, Christos PJ, et al. Deletion of PTENP1 pseudogene in human melanoma. J Invest Dermatol. 2011;131(12):2497‐2500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Lai Y, Li J, Zhong L, et al. The pseudogene PTENP1 regulates smooth muscle cells as a competing endogenous RNA. Clinical Sci. 2019;133(13):1439‐1455. [DOI] [PubMed] [Google Scholar]
- 108. Liu K, Fang C, Shen Y, et al. Hypoxia‐inducible factor 1a induces phenotype switch of human aortic vascular smooth muscle cell through PI3K/AKT/AEG‐1 signaling. Oncotarget. 2017;8(20):33343‐33352. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 109. Chiao C‐W, da Silva‐Santos JE, Giachini FR, Tostes RC, Su M‐J, Webb RC. P2X7 receptor activation contributes to an initial upstream mechanism of lipopolysaccharide‐induced vascular dysfunction. Clinical Sci, 2013;125(3):131‐141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Chiao C‐W, Tostes RC, Webb RC. P2X7 receptor activation amplifies lipopolysaccharide‐induced vascular hyporeactivity via interleukin‐1 beta release. J Pharmacol Exp Therapeutics. 2008;326(3):864‐870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Li L, Yang S‐H, Yao Y, et al. Block of both TGF‐β and IL‐2 signaling impedes Neurophilin‐1 regulatory T cell and follicular regulatory T cell development. Cell Death Dis. 2016;7(10):e2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Sachdeva J, Mahajan A, Cheng J, et al. Smooth muscle cell‐specific Notch1 haploinsufficiency restricts the progression of abdominal aortic aneurysm by modulating CTGF expression. PLoS One. 2017;12(5):e0178538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Li Y, Yang N, Zhou X, et al. LncRNA and mRNA interaction study based on transcriptome profiles reveals potential core genes in the pathogenesis of human thoracic aortic dissection. Mol Med Rep. 2018;18(3):3167‐3176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Memczak S, Jens M, Elefsinioti A, et al. Circular RNAs are a large class of animal RNAs with regulatory potency. Nature. 2013;495(7441):333‐338. [DOI] [PubMed] [Google Scholar]
- 115. Szabo L, Salzman J. Detecting circular RNAs: bioinformatic and experimental challenges. Nat Rev Genet. 2016;17(11):679‐692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Han B, Chao J, Yao H. Circular RNA and its mechanisms in disease: From the bench to the clinic. Pharmacol Ther. 2018;187:31‐44. [DOI] [PubMed] [Google Scholar]
- 117. Boeckel J‐N, Jaé N, Heumüller AW, et al. Identification and characterization of hypoxia‐regulated endothelial circular RNA. Circ Res. 2015;117(10):884‐890. [DOI] [PubMed] [Google Scholar]
- 118. Holdt LM, Stahringer A, Sass K, et al. Circular non‐coding RNA ANRIL modulates ribosomal RNA maturation and atherosclerosis in humans. Nat Commun. 2016;7:12429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Zou M, Huang C, Li X, et al. Circular RNA expression profile and potential function of hsa_circRNA_101238 in human thoracic aortic dissection. Oncotarget. 2017;8(47):81825‐81837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Liu Y, Bezverbnaya K, Zhao T, et al. Involvement of the HCK and FGR src‐family kinases in FCRL4‐mediated immune regulation. J Immunol. 2015;194(12)5851‐5860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Xu C, Zhang Y, Xu KE, et al. Multifunctional cationic nanosystems for nucleic acid therapy of thoracic aortic dissection. Nat Commun. 2019;10(1):3184. [DOI] [PMC free article] [PubMed] [Google Scholar]


