Abstract
Exosomes are extracellular vesicles secreted by donor cells, and one of the important roles of exosomes is intercellular communication. Exosomes contain proteins, lipids, DNA and RNA. The components exert their functions by modulating the cellular processes of recipient cells. Exosomal long non‐coding RNAs (lncRNAs) are important components and play multiple roles in tumorigenesis and tumour development. In this review, we summarize the biological functions and clinical applications of exosomal lncRNAs in cancer. Exosomal lncRNAs regulate cell proliferation, metastasis, drug resistance and angiogenesis in human cancers. Since exosomal lncRNAs are associated with clinicopathological characteristics of cancer, these might be potentially useful biomarkers for diagnosis and prognosis of cancer. Exosomal lncRNAs participate in multiple processes of cancer progression, which makes them promising therapeutic targets for cancer treatment.
Keywords: cancer, exosomal lncRNA, exosome
1. INTRODUCTION
Exosomes are microvesicles that are derived from multivesicular bodies (MVBs) and released into the extracellular space upon fusion of MVBs with the plasma membrane. 1 Exosomes contain multiple components including lipids, proteins, RNA and DNA. Exosomes take part in the intercellular communication by transferring cargoes from donor cells to recipient cells.
One of the cargoes of exosomes is long non‐coding RNA (lncRNA). LncRNAs are RNA transcripts longer than 200 nt and have limited protein‐coding potential. 2 LncRNAs are involved in numerous cellular processes. LncRNAs participate in the pathogenesis of many diseases, including cancer. 3 Lots of studies have demonstrated that lncRNAs regulate the malignant characteristics of cancer such as metastasis and drug resistance. Exosomal lncRNAs are RNA molecules, and exosomal lncRNAs acquired by recipient cells will exert their cancer‐related roles in the recipient cells to regulate cancer progression. In this review, we summarize recent research regarding exosomal lncRNAs in cancers. We describe the biological roles of exosomal lncRNAs in cancer and discuss the potential clinical applications of exosomal lncRNAs in the future.
2. EXOSOMES
Exosomes are extracellular vesicles with a diameter of 30‐100 nm and are released by multiple types of cells. 4 , 5 , 6 In the 1980s, exosomes were observed during reticulocyte maturation. 7 , 8 The production of exosomes begins with a process called endocytosis. 9
Exosomes are derived from inward budding of the plasma membrane. The inward budding of the plasma membrane forms an endosome. Further inward budding of the membrane results in the formation of intraluminal vesicles (ILVs) inside the MVB. Then, the MVB fuses with the plasma membrane and releases the ILVs called exosomes to the extracellular milieu (Figure 1).
Figure 1.
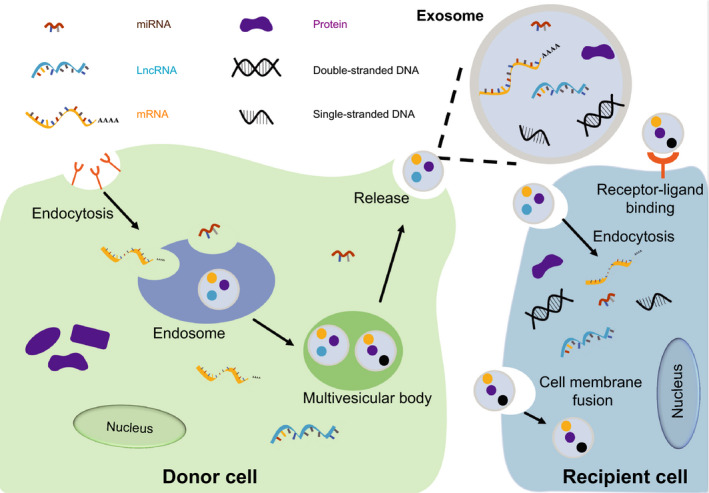
The intercellular communication performed by exosomes. The inward budding of cell membrane results in the formation of endosome. The further inward budding of endosome membrane results in multivesicular body (MVB) formation, then MVBs fuse with cell membrane and release exosomes to extracellular space. The exosomes are received by recipient cells, and the cargoes (DNAs, RNAs, proteins) contained in exosome exert function in recipient cells
Various factors take part in the formation of exosomes, such as proteins and lncRNAs. 10 , 11
Rab GTPases regulate the biogenesis and secretion of exosomes. 12 Rab5b plays a role in the motility and fusion of early endosomes. 13 Rab35 regulates MVB transport and controls the docking process. Rab35 depletion increases intracellular accumulation of endosomal vesicles and decreases exosome secretion. 14 Soluble N‐ethylmaleimide‐sensitive factor attachment protein receptors (SNAREs) are trans‐membrane proteins and SNARE complexes mediate membrane fusion and regulate the release of exosomes. Ternary SNARE complexes consist of a SNARE on vesicle membrane (v‐SNARE) and two SNAREs on target membrane (t‐SNARE). 15 , 16 Synaptosomal‐associated protein (SNAP) such as SNAP23 is t‐SNAREs and vesicle‐associated membrane protein (VAMP) such as VAMP3 and VAMP8 are v‐SNAREs. 17 , 18 , 19 The phosphorylation of SNAP23 enhanced the stability of the SNARE complex and promoted the secretion of exosomes. 20 , 21
LncRNA‐APC1 regulates the production of exosomes by interacting with Rab5b mRNA. 22 The interplay of lncRNA‐APC and Rab5b mRNA reduces the stability of Rab5b mRNA and inhibits Rab5b expression, leading to a reduction in exosomes. On the contrary, HOTAIR enhances the release of exosomes by modulation of several processes. 23 It regulates the docking process by modulating Rab35 expression and localization. In addition, HOTAIR facilitates the fusion process by controlling the colocalization of VAMP3 and SNAP23. HOTAIR also enhances the release of exosomes via phosphorylation of SNAP23.
Exosomes contain multiple bioactive molecules, including lipids, proteins, RNA and DNA. 24 , 25 , 26 , 27 The components of plasma membranes such as cholesterol, sphingomyelin, hexosylceramides, phosphatidylserine and saturated fatty acids are also present in the exosomes. 28 Rab GTPases and annexins, the proteins associated with membrane transport and fusion, are found abundantly in the exosomes. ESCRT components, ALIX and TSG101 are consistently detected in exosomes. Moreover, exosomes are enriched in heat‐shock proteins, HSP70 and HSP90; tetraspanins, including CD9, CD63, CD81 and CD82; MHC class II proteins; members of the human epidermal receptor family; and epithelial cell adhesion molecules. 29 , 30 , 31 , 32 , 33 LncRNAs lncARSR and LNMAT2 have been reported in the exosomes derived from cancer cells. 34 , 35 Various investigations have revealed the presence of miRNAs, such as miR‐21 and miR‐221 in the exosomes. 36 , 37 Single‐stranded DNA and double‐stranded DNA are also found in the exosomes. 38 , 39
Earlier, exosomes were regarded as cellular garbage bags with non‐functional cellular molecules or excess constituents. Emerging studies demonstrated the various functional roles of exosomes in physiological and pathological processes. 40 , 41
As exosomes released from donor cells are accepted by recipient cells, one of the most important roles of exosomes is intercellular communication. Exosomes contain multiple biological active molecules and the message included in cargoes was transferred from one cell to another. Exosomal components, such as proteins, miRNAs and lncRNAs regulate the biological processes of recipient cells.
The cargoes inside the exosomes reflect the pathophysiological state of the donor cells. Exosomal constitution may vary depending on the donor cells. 25 , 27 , 42 , 43 Moreover, exosomes are detected in multiple physiological fluids, including plasma or serum, saliva, amniotic fluid, breast milk, urine, nasal secretion, cerebrospinal fluid, semen and pathological fluids such as ascitic fluid. 44 The wide existence of exosomes makes it convenient to employ the exosomes into the non‐invasive diagnosis of diseases, including human cancers. 27 , 45 , 46 , 47 , 48
3. LNCRNAS
LncRNAs are a type of transcripts with a length >200nt and limited protein‐coding potential. LncRNAs are reported to take part in multiple cellular processes through various mechanisms. LncRNAs interact with miRNA, mRNA, protein and genomic DNA, and subsequently regulate gene transcription, translation, mRNA stability, protein modification and the interplay of protein with other factors 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 (Figure 2). LncRNAs inhibit the degradation of miRNA target genes by interacting with miRNAs. The expression of ZEB1, a target gene of miR‐200 was found to be enhanced through the interaction of LncRNA‐ATB with miR‐200. 52 LncRNAs recruit transcription factors or epigenetic modulators to the chromatin region or genomic DNA to regulate gene expression. In cancer cells, the oncogene EPIC1 interacts with MYC and regulates the occupancy of MYC on target promoters. 57
Figure 2.
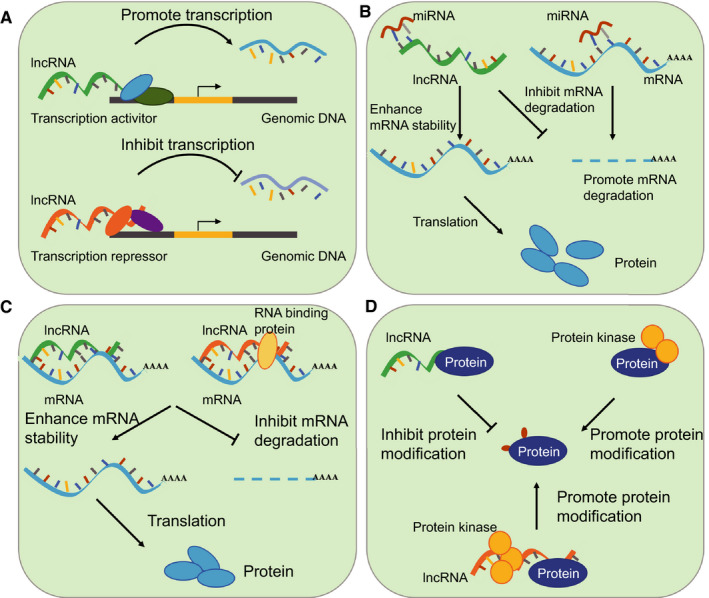
Regulatory roles of lncRNAs. A, LncRNAs recruit transcript factors or epigenetic regulators to gene promoter region and regulate gene transcription. B, LncRNAs competitively bind to miRNAs and inhibit the degradation of miRNAs target genes. C, LncRNAs interact with mRNA directly or enhance the interplay of mRNA and protein, which promotes the mRNA stability and increases gene expression. D, LncRNAs interact with protein and inhibit protein modification. LncRNAs interact with protein kinase and enhance protein modification
LncRNAs also play important roles in disease occurrence and tumour progression. They are found to regulate cell proliferation, migration and invasion of cancer cells, and modulate cell cycle, apoptosis, lymphatic metastasis and drug sensitivity. 58 , 59 , 60 , 61 VEGF‐C is a critical factor in lymphatic metastasis of human cancers. 62 , 63 It is overexpressed in many types of cancers and associated with regional lymph node metastasis and poor survival. LncRNA BLACAT2 has been reported to promote lymphatic metastasis of bladder cancer via regulating VEGF‐C expression. 64 WDR5 is a core subunit of H3K4 methyltransferase complexes and a binding protein of BLACAT2. BLACAT2 interacts with WDR5 and enhances the H3K4me3 levels of VEGF‐C promoter thereby up‐regulating VEGF‐C expression. Another lncRNA in bladder cancer, LNMAT1, promotes lymphatic metastasis through modulation of tumour microenvironment. 65 LNMAT1 overexpression is seen in bladder cancer with lymph node metastasis and associated with poor survival. In addition, LNMAT1 can recruit hnRNPL to CCL2 promoter and up‐regulate the levels of H3K4me3 of this region, thereby increasing the expression of CCL2. Increased CCL2 recruits macrophages to the tumour which promotes lymphatic metastasis via VEGF‐C secretion. Furthermore, the up‐regulation of LncRNA GMAN is associated with metastasis in gastric cancer. Thus, high levels of GMAN predict poor survival in patients. Previous studies have found that GMAN enhances the translation of Ephrin A1 via interaction with GMAN‐AS. 66
4. EXOSOMAL LNCRNAS IN CANCER—BIOLOGICAL FUNCTIONS
LncRNAs play important roles in cancer progression through multiple mechanisms. Exosomal lncRNAs are transferred to recipient cells and exert oncogenic roles or tumour suppressive roles in the recipient cells (Table 1). 67 , 68 , 69 , 70 , 71
Table 1.
The function and application of exosomal lncRNAs in cancer
| LncRNA | Type of cancer | Donor cell/recipient cell | Biological function | Clinical application | Reference |
|---|---|---|---|---|---|
| lncARSR | Renal cancer | Drug‐resistant cells/drug‐sensitive cells | Promote sunitinib resistance | Therapeutic target | 24 |
| LNMAT2 | Bladder cancer | Cancer cells/HLECs | Promote lymphatic metastasis | Therapeutic target | 25 |
| MALAT1 | Colorectal cancer | Metastatic cancer cells/primary cancer cells | Promote metastasis | Therapeutic target | 67 |
| RPPH1 | Colorectal cancer | Cancer cells/macrophage M2 | Promote metastasis | Therapeutic and diagnostic target | 68 |
| GAS5 | Lung Cancer | Cancer cells/HUVECs | Promotes angiogenesis | Therapeutic target | 82 |
| AFAP1‐AS1 | Breast cancer | Drug‐resistant cells/drug‐sensitive cells | Promote trastuzumab resistance | Therapeutic target | 75 |
| SNHG14 | Breast cancer | Drug‐resistant cells/drug‐sensitive cells | Promote trastuzumab resistance | Therapeutic target | 76 |
| LINC00461 | Multiple myeloma cell | Mesenchymal stromal cells/multiple myeloma cells | Promote proliferation and suppress apoptosis | Therapeutic target | 63 |
| PART1 | ESCC | Drug‐resistant cells/drug‐sensitive cells | Promote gefitinib resistance | Therapeutic target | 73 |
| PCAT1 | ESCC | Cancer cells/immortalized normal oesophageal epithelial cells | Promote proliferation | Biomarker | 64 |
| TIRY | Oral squamous cell carcinoma | Cancer‐associated fibroblast/cancer cells | Promote metastasis | Therapeutic target | 70 |
| SOX2OT | Pancreatic ductal adenocarcinoma | Cancer cells/cancer cells | Promote metastasis | Biomarker for prognosis | 66 |
| UCF1 | NSCLC | Cancer cells/cancer cells | Promote proliferation, migration and invasion | Biomarker for diagnosis | 60 |
| UCA1 | NSCLC | Drug‐resistant cells/drug‐sensitive cells | Promote gefitinib resistance | Therapeutic target | 71 |
| H19 | NSCLC | Drug‐resistant cells/drug‐sensitive cells | Promote gefitinib resistance | Therapeutic target | 72 |
| HOTTIP | Gastric cancer | Drug‐resistant cells/drug‐sensitive cells | Promote cisplatin resistance | Therapeutic target | 78 |
| TUG1 | Cervical cancer | Cancer cells/HUVECs | Promote angiogenesis | Target for early diagnosis | 81 |
| CCAT2 | Glioma | Cancer cells/HUVECs | Promote angiogenesis | Therapeutic target | 83 |
| LINC‐POU3F3 | Glioma | Cancer cells/human brain microvascular endothelial cells | Promote angiogenesis | Therapeutic target | 84 |
4.1. Proliferation
Excessive growth of cancer cells is a fundamental characteristic of human cancers. Cancer‐associated genes typically participate in cell proliferation to regulate cell growth. Exosomal lncRNAs are involved in this process.
LncRNA UFC1 is up‐regulated in non‐small cell lung cancer (NSCLC) tissues and serum exosomes of NSCLC patients. It has been found that UFC1 promotes the proliferation of NSCLC cells through modulating the expression of PTEN. It interacts with EZH2 to regulate the H3K27me3 levels of PTEN promoter region. Exosomal UFC1 is transferred to receptor cells and induces their growth. 72
MALAT1 from exosomes promotes the proliferation of breast cancer cells and NSCLC cells. 73 , 74 In multiple myeloma, exosomal LINC00461 has been reported to enhance cell proliferation via binding to miR‐15a/16 and promoting the expression of BCL‐2. 75 Similarly, in oesophageal squamous cell carcinoma (ESCC) cells, exosomal lncRNA PCAT1 promotes cell proliferation through binding to miR‐326. 76 Exosomal ZFAS1 also promotes the cell proliferation of ESCC cells by regulating the miR‐124/STAT3 axis. 77
4.2. Metastasis
Metastasis is one of the major malignant characteristics of cancer, and patients with metastatic cancer have a poor prognosis. Lymphangiogenesis and epithelial‐mesenchymal transition (EMT) facilitate the process of cancer metastasis.
LncRNA SOX2OT is reported to take part in the progress of several cancers, including lung cancer, breast cancer, ESCC and pancreatic ductal adenocarcinoma (PDAC). In PDAC cells, SOX2OT promotes SOX2 expression by binding to miR‐200 and facilitates EMT and stem cell like properties. The exosomal SOX2OT from PDAC cells enhances the metastasis of acquired PDAC cells. 78
MALAT1 is overexpressed in several cancer cells and regulates tumour development. In a previous study, exosomal MALAT1 derived from metastatic colorectal cancer (CRC) cells was found to promote the expression of FUT4 in the primary CRC cells through binding to miR‐26a and miR‐26b. 79
In CRC, lncRNA RPPH1 interacts with TUBB3 to inhibit its ubiquitination and promotes EMT of CRC cells. 80 In addition to regulating metastasis, the exosomal RPPH1 from CRC cells also regulates the behaviour of macrophages. Exosomal RPPH1 mediates macrophage M2 polarization to induce metastasis in CRC.
Lymph node metastasis is associated with poor survival in patients with bladder cancer. It has been found that in bladder cancer, lncRNA LNMAT2 is incorporated into exosomes through binding with hnRNPA2B1. 35 A previous report demonstrated that the exosomes derived from bladder cancer cells were transmitted to human lymphatic endothelial cells (HLECs). Subsequently, LNMAT2 increased the expression of PROX1 via epigenetic modulation of H3K4me3 levels in PROX1 promoter sequences. Thus, it was suggested that exosomal LNMAT2 promoted lymphangiogenesis and lymph node metastasis in bladder cancers.
Thyroid cancer is one of the most lethal human cancers. Thyroid cancer stem‐like cells (CSCs) are significantly associated with tumorigenesis. LINC‐ROR plays multiple roles in several kinds of cancers, such as regulating the proliferation and drug sensitivity of hepatocellular carcinoma, promoting metastasis of pancreatic cancer and enhancing the EMT in ovarian cancer cells. In thyroid cancer, CSCs‐derived exosomes containing LINC‐ROR have been found to promote the EMT when transferred to normal cells. 81
Not only the exosomes from cancer cells but also exosomes from other kinds of cells take part in cancer metastasis. Exosomes from cancer‐associated fibroblasts contain up‐regulated lncRNA TIRY, which regulates the Wnt/β‐catenin signalling pathway by binding to miR‐14 and enhances metastasis in oral squamous cell carcinoma. 82
In summary, exosomal lncRNAs regulate the metastasis of cancer cells through the following ways: cancer cell‐derived exosomes are transferred to cancer cells and exosomal lncRNAs regulate the metastatic property of cancer cells; cancer cell‐derived exosomes transferred to non‐cancer cells (normal cells/HLECs/macrophage) enhances metastatic potential via exosomal lncRNAs; non‐cancer cell (cancer‐associated fibroblast)‐derived exosomes transferred to cancer cells regulate their metastasis via lncRNAs.
4.3. Chemoresistance
Drug resistance is a big challenge in cancer treatment and understanding the underlying mechanism of drug resistance will provide therapeutic strategy for cancer patients with drug resistance. Exosomal lncRNAs were reported to take part in drug resistance (Figure 3).
Figure 3.
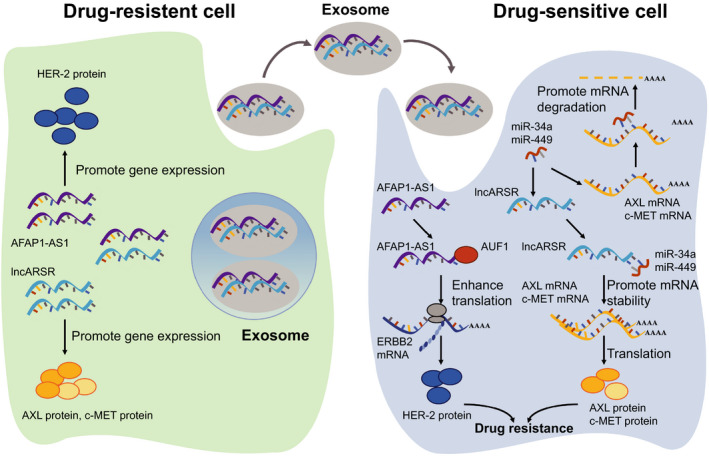
Exosomal lncRNAs transfer chemoresistance in cancer. Exosomal LncRNAs derived from drug‐resistant cells are received by drug‐sensitive cells and promote drug resistance by regulating the expression of drug resistance related genes in sensitive cells. In renal cancer cells, exosomal lncARSR derived from sunitinib resistant cells are transferred to sensitive cells. Exosomal lncARSR competitively bind to miR‐34 and miR‐449 and enhance AXL and c‐MET expression. In breast cancer, exosomal AFAP1‐AS1 transfers the trastuzumab resistance from resistant cells to sensitive cells. Exosomal AFAP1‐AS1 interacts with AUF1 and the interaction promote ERBB2 translation
Sunitinib is an oral drug used for treating advanced renal cell carcinoma (RCC). Most of the patients receiving sunitinib therapy develop drug resistance after 6‐15 months of treatment. It is reported that exosomal lncRNAs are involved in the modulation of drug resistance. LncARSR regulates the expression of AXL and c‐MET by binding to miR‐34/miR‐449 and thereby promotes sunitinib resistance in RCC cells. 34 Exosomal lncARSR from sunitinib‐resistant RCC cells was found to induce drug resistance in previously sensitive cells.
Gefitinib is a type of tyrosine kinase inhibitor that blocks epidermal growth factor receptor tyrosine kinase (EGFR‐TK). In NSCLC, overexpressed lncRNA UCA1 was reported to be responsible for gefitinib resistance. 83 Moreover, FOSL2 was found to be up‐regulated in gefitinib‐resistant NSCLC cells and UCA1 protected FOSL2 by sponging miR‐143 in NSCLC cells. Some previous studies showed that gefitinib‐resistant NSCLC cells transferred exosomal UCA1 to drug‐sensitive cells which then acquired gefitinib resistance. Along with UCA1, H19 present in the exosomes of NSCLC also modulates gefitinib resistance. 84 In ESCC, up‐regulated lncRNA PART1 promotes gefitinib resistance by modulating BCL‐2 expression. Exosomes containing PART1 disseminate drug resistance to other sensitive ESCC cells. 85
Breast cancer is the most common cancer and one of the leading causes of cancer death in females worldwide. Trastuzumab is a humanized monoclonal antibody used in the treatment of HER2‐positive breast cancer patients. Over half of the patients develop drug resistance after trastuzumab therapy. Exosomal AGAP1‐AS1, AFAP1‐AS1 and SNHG14 are reported to participate in the regulation of trastuzumab resistance in HER‐positive breast cancer. 86 , 87 , 88 It is suggested that AFAP1‐AS1 enhances the translation of ERBB2 by associating with AUF1, whereas SNHG14 regulates the BCL‐2/BAX signalling pathway in breast cancer cells.
Cisplatin is an extensively used chemotherapeutic drug for many cancers, such as ovarian cancer, gastric cancer and cervical cancer. In ovarian cancer, exosomal UCA1 promotes cisplatin resistance by modulating the expression of FOSL2. 89 Exosomal HOTTIP regulates the expression of HMGA1 through binding to miR‐218, thereby promoting cisplatin resistance in gastric cancer. 90 Additional, HNF1A‐AS1 binds to miR‐34b to up‐regulate TUFT1 expression and subsequently promotes cisplatin resistance in cervical cancer. 91
In general, lncRNAs serve as ceRNAs to interact with miRNAs and then protect the target genes of miRNAs, that are considered vital in drug resistance. As a result, these lncRNAs are incorporated into the exosomes which then induce drug resistance in previously sensitive cells.
4.4. Angiogenesis
Exosomal RAMP‐AS1 is up‐regulated in chondrosarcoma patients and high levels of RAMP‐AS1 are often associated with poor survival. RAMP‐AS1 acts as a ceRNA to bind to miR‐2355‐5p and enhances the expression of VEGFR2, an important factor in angiogenesis. 92
Cervical cancer cells secrete exosomes containing lncRNA TUG1. Researchers observed that when exosomal TUG1 was transferred to the human umbilical vein endothelial cells (HUVECs), it promoted HUVECs proliferation via inhibiting caspase‐3 activity and regulating apoptosis‐related proteins. Thus, it was suggested that exosomal TUG1 plays a role in angiogenesis. 93
GAS5 is down‐regulated in lung cancer tissues. The exosomal GAS5 binds to miR‐29‐3p and increases the expression of PTEN. Additionally, GAS5 decreases the phosphorylation of AKT and PI3K. To summarize, exosomal GAS5 represses HUVECs proliferation and enhances their apoptosis, thus, inhibiting angiogenesis in lung cancer. 94
In glioma, exosomal lncRNA CCAT2 transferred to HUVECs is shown to activate VEGFA and TGFβ. CCAT2 increased BCL‐2 expression while suppressed the expression of BAx and caspase‐3, which inhibited apoptosis. Overall, exosomal CCAT2 promotes angiogenesis. 95 Another report found that gliomas can induce angiogenesis via secreting exosomes enriched in LINC‐POU3F3. 96
5. EXOSOMAL LNCRNAS IN CANCER—CLINICAL APPLICATIONS
The variations in the composition and expression of exosomal lncRNAs typically reflect the changes in the clinical status of the cell. A wide variety of exosomal lncRNAs take part in tumorigenesis and tumour development, making them potential biomarkers and therapeutic targets for diagnosis, prognosis and therapy of cancer. 97 , 98
5.1. Diagnostic and prognostic biomarkers
Exosomes from serum, urine and other body fluids usually reflect the clinical condition of patients with cancer. The alteration of exosomal lncRNAs may draw a clue to tumour stage. Detailed investigations in exosomal lncRNAs provide more choices for cancer diagnosis and prognosis (Figure 4).
Figure 4.
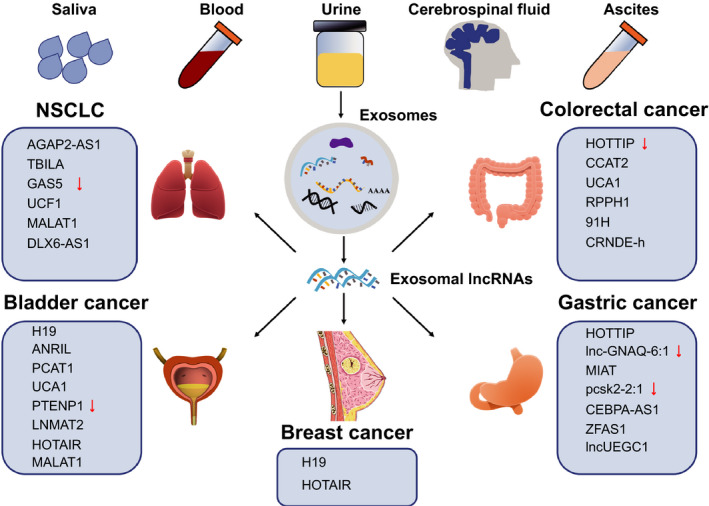
The clinical application of exosomal lncRNAs in cancer. Exosomal lncRNAs from human fluids will serve as biomarkers for diagnosis and prognosis of cancer
Exosomal H19 is overexpressed in patients with breast cancer compared to healthy individuals and those with benign breast disease. Statistical analysis found that the high expression levels of exosomal H19 are associated with lymph node metastasis, advanced TNM stages and distant metastasis. It has been observed that exosomal H19 decreases in patients with breast cancer post‐surgery. Thus, exosomal H19 can be a potentially useful biomarker for the diagnosis of breast cancer. 99 Similar observations have been reported for bladder cancer. Higher levels of exosomal H19 have been detected in preoperative samples compared with those in the postoperative samples. Furthermore, levels of exosomal H19 are lower in healthy individuals and patients with benign disease compared with those with bladder cancer. Bladder cancer patients with high level of exosomal H19 generally have poor survival. 100 In brief, serum exosomal H19 can serve as a non‐invasive diagnostic and prognostic biomarker for patients with breast cancer or bladder cancer.
Exosomal HOTAIR is up‐regulated in patients with laryngeal squamous cell carcinoma (LSCC). Serum exosomal HOTAIR correlates with clinical parameters including lymph node metastasis, clinical stages, and T classification. Exosomal HOTAIR may be a prognostic biomarker for LSCC patients. 101 In patients with breast cancer, high levels of serum exosomal HOTAIR have been detected as compared to those in healthy individuals. In addition, exosomal HOTAIR is down‐regulated in patients with breast cancer post‐surgery. A high level of exosomal HOTAIR is correlated with poor disease‐free survival and overall survival. Patients with high level of exosomal HOTAIR usually demonstrate a poor response to neoadjuvant chemotherapy and tamoxifen hormonal therapy. Exosomal HOTAIR is further correlated with the expression level of ErbB2. These correlations indicate that exosomal HOTAIR might be a promising liquid biopsy biomarker for breast cancer. 102
The expression levels of exosomal HOTTIP in gastric cancer patients are higher than those in healthy individuals. Patients with high exosomal HOTTIP have poor overall survival. Considering this, COX analysis found that up‐regulated exosomal HOTTIP can be an independent prognostic factor in patients with gastric cancer. 103 Contrastingly in CRC, exosomal HOTTIP plays different roles. 104 Exosomes from CRC patients demonstrate lower expression level of HOTTIP than those from healthy individuals. As opposed to gastric cancer, patients with low level of exosomal HOTTIP have poor overall survival.
The exosomal lncRNAs reflect clinical status in cancer patients. In different types of cancer, specific exosomal lncRNAs reflect different pathophysiological states. Multiple studies are needed to determine the indicative roles of exosomal lncRNA in cancer.
5.2. Therapeutic target
Exosomal lncRNAs play important roles in cancer progression. A detailed investigation of the functional roles of exosomal lncRNAs will facilitate their development as promising therapeutic targets for cancer treatment.
LncARSR is an oncogene in RCC and the exosomal lncARSR transfers drug resistance to the drug‐sensitive cells. In vivo study, nude mice were orthotopically xenografted with sunitinib‐resistant RCC cells or subcutaneously xenografted with sunitinib‐resistant patient‐derived xenograft, and the treatment was performed by intravenous injection or intratumoural injection of lncARSR locked nucleic acids (LNAs), respectively. Studies have shown that LNAs targeting lncARSR reduced the expression of lncARSR and restored the sunitinib response. LncARSR served as a promising therapeutic target in cancer with drug resistance. 34
LNMAT2 is up‐regulated in lymph node‐positive bladder cancer tissues. Exosomal LNMAT2 derived from bladder cancer cells are transmitted to HLECs and promote lymphatic metastasis in bladder cancer. In vivo study, bladder cancer cells were injected to the footpads of nude mice and nude mice were treated with intratumoural injection with exosomes. Exosomes secreted by LNMAT2‐transfected cells promoted the lymph node metastasis of bladder cancer cells in nude mice. In vitro study, exosomes secreted by LNMAT2‐silenced cells inhibited HLECs tube formation and migration. It is indicated that decreased expression levels of exosomal LNMAT2 inhibit the malignant characteristics of bladder cancer cells; thus, LNMAT2 might be a therapeutic target for LN metastasis in bladder cancer. 35
With the development on the investigation of promising lncRNA targets, the effective delivery of oligonucleotides targeting lncRNAs to the tumour cells is a key step in cancer treatment. There are several oligonucleotides delivery strategies, including lipid‐based carrier, polyethylenimine‐based carrier, protamine delivery, antibody‐mediated targeted delivery. 105 , 106 , 107 Mice treated with pre‐miRNA mixed with liposomes had a higher expression of miRNA than the control ones. 108 Protamine‐condensed siRNAs reduced the expression of cyclin D1 in vitro and in vivo. 109 Effective delivery of oligonucleotides will accelerate the clinical application of the exosomal lncRNAs.
6. CONCLUSION
Exosomes are important factors involved in the progression and development of cancer. The cancer‐associated roles of exosomes mainly depend on their cargoes. The component proteins and RNAs exert different functions in pathological processes of cancer. Although lncRNAs participate in tumorigenesis through various mechanisms, the exosomal lncRNAs have same characteristics. Exosomal lncRNAs bind to miRNAs and release the genes targeted by miRNAs. Furthermore, they regulate gene expression through epigenetic modulation. Through various mechanisms of gene regulation, exosomal lncRNAs play critical roles in cancer cell proliferation, metastasis, drug resistance and angiogenesis. The significance of exosomal lncRNAs in cancer makes them eligible to serve as therapeutic targets for the treatment of cancers. As exosomes are widely existed in multiple fluids and those secreted by donor cells reflect the status of donor cells, the exosomal lncRNAs have a great value in prognosis and diagnosis of cancers.
Although there is a promising prospect in the clinical application of exosomal lncRNAs, some problems still remain unsolved. For instance, is there any difference between exosomal lncRNAs transferred to the recipient cells and those left behind in the recipient cells? As most exosomal lncRNAs exert similar functions in the recipient cells and in the donor cells, maybe these exosomal lncRNAs are labelled with some special modifications, which do not change the biological activity of lncRNAs. Secondly, what is the mechanism of exosomal fusion with recipient cells? There may be some molecules that regulate the interaction of exosomes and recipient cells. If we figure out the underlying mechanisms, we can easily manipulate the exosomes, pack wanted lncRNAs, proteins or DNA into the exosomes, and make exosomes to fuse with particular recipient cells. More detailed investigations are needed to interpret the complex process, which will enlarge the insight of biological functions of exosomes and facilitate the clinical application of exosomes.
CONFLICTS OF INTEREST
We have no conflicts of interest to declare.
AUTHOR CONTRIBUTIONS
WY conceived and drafted the manuscript. WY and ZM discussed the concepts of the manuscript. ZM drew the figures. LZ approved the version to be submitted.
ACKNOWLEDGEMENTS
We would like to apologize to those researchers whose related work we were not able to cite in this review. This work was supported by a special programme from the Chinese National Natural Science Funds (31871405 and 31571460 to FZ), Jiangsu National Science Foundation (BK20180043 and 19KJA550003 to FZ).
Wang Y, Zhang M, Zhou F. Biological functions and clinical applications of exosomal long non‐coding RNAs in cancer. J Cell Mol Med. 2020;24:11656–11666. 10.1111/jcmm.15873
Wang and Zhang have equal contribution.
REFERENCES
- 1. Théry C. Exosomes: secreted vesicles and intercellular communications. F1000 Biol Rep. 2011;3:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wilusz JE, Sunwoo H, Spector DL. Long noncoding RNAs: functional surprises from the RNA world. Genes Dev. 2009;23:1494‐1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Huarte M. The emerging role of lncRNAs in cancer. Nat Med. 2015;21:1253‐1261. [DOI] [PubMed] [Google Scholar]
- 4. Niel V. Intestinal epithelial exosomes carry MHC class II/peptides able to inform the immune system in mice. Gut. 2003;52:1690‐1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rui Z, Xue L, Liu X, et al. Heat shock protein 70 is secreted from endothelial cells by a non‐classical pathway involving exosomes. Biochem Biophys Res Comm. 2009;387:229‐233. [DOI] [PubMed] [Google Scholar]
- 6. Ventimiglia LN, Alonso MA. Biogenesis and function of T cell‐derived exosomes. Front Cell Dev Biol. 2016;4:84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pan BT, Teng K, Wu C, Adam M, Johnstone RM. Electron microscopic evidence for externalization of the transferrin receptor in vesicular form in sheep reticulocytes. J Cell Biol. 1985;101:942‐948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Johnstone RM, Adam M, Hammond JR, Orr L, Turbide C. Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes). J Biol Chem. 1987;262:9412‐9420. [PubMed] [Google Scholar]
- 9. van der Pol E, Böing AN, Harrison P, Sturk A, Nieuwland R. Classification, functions, and clinical relevance of extracellular vesicles. Pharmacol Rev. 2012;64:676‐705. [DOI] [PubMed] [Google Scholar]
- 10. Ostrowski M, Carmo NB, Krumeich S, et al. Rab27a and Rab27b control different steps of the exosome secretion pathway. Nat Cell Biol. 2010;12:19‐30. [DOI] [PubMed] [Google Scholar]
- 11. Cao S‐Q, Zheng H, Sun B‐C, et al. Long non‐coding RNA highly up‐regulated in liver cancer promotes exosome secretion. World J Gastroenterol. 2019;25:5283‐5299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Stenmark H. Rab GTPases as coordinators of vesicle traffic. Nat Rev Mol Cell Biol. 2009;10:513‐525. [DOI] [PubMed] [Google Scholar]
- 13. Yun HJ, Kim H, Ga I, et al. An early endosome regulator, Rab5b, is an LRRK2 kinase substrate. J Biochem. 2015;157:485‐495. [DOI] [PubMed] [Google Scholar]
- 14. Hsu C, Morohashi Y, Yoshimura S, et al. Regulation of exosome secretion by Rab35 and its GTPase‐activating proteins TBC1D10A‐C. J Cell Biol. 2010;189:223‐232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jahn R, Scheller RH. SNAREs — engines for membrane fusion. Nat Rev Mol Cell Biol. 2006;7:631‐643. [DOI] [PubMed] [Google Scholar]
- 16. Südhof TC, Rothman JE. Membrane fusion: grappling with SNARE and SM proteins. Science. 2009;323:474‐477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cosen‐Binker LI, Binker MG, Wang CC, Hong W, Gaisano HY. VAMP8 is the v‐SNARE that mediates basolateral exocytosis in a mouse model of alcoholic pancreatitis. J Clin Investig. 2008;118:2535‐2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pulido IR, Jahn R, Gerke V. VAMP3 is associated with endothelial weibel‐palade bodies and participates in their Ca(2+)‐dependent exocytosis. Biochem Biophys Acta. 2011;1813:1038‐1044. [DOI] [PubMed] [Google Scholar]
- 19. Guo Z, Turner C, Castle D. Relocation of the t‐SNARE SNAP‐23 from lamellipodia‐like cell surface projections regulates compound exocytosis in mast cells. Cell. 1998;94:537‐548. [DOI] [PubMed] [Google Scholar]
- 20. Wei Y, Wang D, Jin F, et al. Pyruvate kinase type M2 promotes tumour cell exosome release via phosphorylating synaptosome‐associated protein 23. Nat Commun. 2017;8:14041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zhu Q, Yamakuchi M, Lowenstein CJ. SNAP23 regulates endothelial exocytosis of von Willebrand factor. PLoS One. 2015;10:e0118737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wang FW, Cao CH, Han K, et al. APC‐activated long noncoding RNA inhibits colorectal carcinoma pathogenesis through reduction of exosome production. J Clin Investig. 2019;129:727‐743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yang L, Peng X, Li Y, et al. Long non‐coding RNA HOTAIR promotes exosome secretion by regulating RAB35 and SNAP23 in hepatocellular carcinoma. Mol Cancer. 2019;18:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pant S, Hilton H, Burczynski ME. The multifaceted exosome: biogenesis, role in normal and aberrant cellular function, and frontiers for pharmacological and biomarker opportunities. Biochem Pharmacol. 2012;83:1484‐1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Valadi H, Ekström K, Bossios A, Sjöstrand M, Lee JJ, Lötvall JO. Exosome‐mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9:654‐659. [DOI] [PubMed] [Google Scholar]
- 26. Melo SA, Sugimoto H, O'Connell JT, et al. Cancer exosomes perform cell‐independent microRNA biogenesis and promote tumorigenesis. Cancer Cell. 2014;26:707‐721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kalluri R. The biology and function of exosomes in cancer. J Clin Investig. 2016;126:1208‐1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Colombo M, Raposo G, Thery C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu Rev Cell Dev Biol. 2014;30:255‐289. [DOI] [PubMed] [Google Scholar]
- 29. Théry C, Zitvogel L, Amigorena S. Exosomes: composition, biogenesis and function. Nat Rev Immunol. 2002;2:569. [DOI] [PubMed] [Google Scholar]
- 30. Simons M, Raposo GA. Exosomes – vesicular carriers for intercellular communication. Curr Opin Cell Biol. 2009;21:575‐581. [DOI] [PubMed] [Google Scholar]
- 31. Runz S, Keller S, Rupp C, et al. Malignant ascites‐derived exosomes of ovarian carcinoma patients contain CD24 and EpCAM. Gynecol Oncol. 2007;107:563‐571. [DOI] [PubMed] [Google Scholar]
- 32. Baran J, Baj‐Krzyworzeka M, Weglarczyk K, et al. Circulating tumour‐derived microvesicles in plasma of gastric cancer patients. Cancer Immunol Immunother. 2010;59:841‐850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ciravolo V, Huber V, Ghedini GC, et al. Potential role of HER2‐overexpressing exosomes in countering trastuzumab‐based therapy. J Cell Physiol. 2012;227:658‐667. [DOI] [PubMed] [Google Scholar]
- 34. Qu L, Ding J, Chen C, et al. Exosome‐transmitted lncARSR promotes sunitinib resistance in renal cancer by acting as a competing endogenous RNA. Cancer Cell. 2016;29:653‐668. [DOI] [PubMed] [Google Scholar]
- 35. Chen C, Luo Y, He W, et al. Exosomal long noncoding RNA LNMAT2 promotes lymphatic metastasis in bladder cancer. Journal of Clinical Investigation. 2019;130:404‐421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Cao L‐Q, Yang X‐W, Chen Y‐B, Zhang D‐W, Xue P. Exosomal miR‐21 regulates the TETs/PTENp1/PTEN pathway to promote hepatocellular carcinoma growth. Mol Cancer. 2019;18:148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Yang J‐K, Yang J‐P, Tong J, et al. Exosomal miR‐221 targets DNM3 to induce tumor progression and temozolomide resistance in glioma. J Neurooncol. 2017;131(2):255‐265. [DOI] [PubMed] [Google Scholar]
- 38. Balaj L, Lessard R, Dai L, et al. Tumour microvesicles contain retrotransposon elements and amplified oncogene sequences. Nat Commun. 2011;2:180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kahlert C, Melo SA, Protopopov A, et al. Identification of double‐stranded genomic DNA spanning all chromosomes with mutated KRAS and p53 DNA in the serum exosomes of patients with pancreatic cancer. J Biol Chem. 2014;289(7):3869‐3875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Robbins PD, Morelli AE. Regulation of immune responses by extracellular vesicles. Nat Rev Immunol. 2014;14:195‐208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. van Niel G, D'Angelo G, Raposo G. Shedding light on the cell biology of extracellular vesicles. Nat Rev Mol Cell Biol. 2018;19:213‐228. [DOI] [PubMed] [Google Scholar]
- 42. Raposo G, Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol. 2013;200:373‐383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. De Toro J, Herschlik L, Waldner C, Mongini C. Emerging roles of exosomes in normal and pathological conditions: new insights for diagnosis and therapeutic applications. Front Immunol. 2015;6:203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Witwer KW, Buzas EI, Bemis LT, et al. Standardization of sample collection, isolation and analysis methods in extracellular vesicle research. J Extracell Vesicles. 2013;2:20360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Guo W, Gao Y, Li N, et al. Exosomes: new players in cancer (Review). Oncol Rep. 2017;38:665‐675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Vanni I, Alama A, Grossi F, Dal Bello MG, Coco S. Exosomes: a new horizon in lung cancer. Drug Discovery Today. 2017;22:927‐936. [DOI] [PubMed] [Google Scholar]
- 47. Zhang X, Yuan X, Shi H, Wu L, Qian H, Xu W. Exosomes in cancer: small particle, big player. J Hematol Oncol. 2015;8:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Becker A, Thakur BK, Weiss JM, Kim HS, Peinado H, Lyden D. Extracellular vesicles in cancer: cell‐to‐cell mediators of metastasis. Cancer Cell. 2016;30:836‐848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Li D, Liu X, Zhou J, et al. Long noncoding RNA HULC modulates the phosphorylation of YB‐1 through serving as a scaffold of extracellular signal–regulated kinase and YB‐1 to enhance hepatocarcinogenesis. Hepatology. 2017;65:1612‐1627. [DOI] [PubMed] [Google Scholar]
- 50. Liu X, Li D, Zhang W, Guo M, Zhan Q. Long non‐coding RNAgadd7 interacts with TDP‐43 and regulates Cdk6mRNA decay. EMBO J. 2012;31:4415‐4427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Gupta RA, Shah N, Wang KC, et al. Long non‐coding RNA HOTAIR reprograms chromatin state to promote cancer metastasis. Nature. 2010;464:1071‐1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Yuan JH, Yang F, Wang F, et al. A long noncoding RNA activated by TGF‐beta promotes the invasion‐metastasis cascade in hepatocellular carcinoma. Cancer Cell. 2014;25:666‐681. [DOI] [PubMed] [Google Scholar]
- 53. Zhang E, Han L, Yin D, et al. H3K27 acetylation activated‐long non‐coding RNA CCAT1 affects cell proliferation and migration by regulating SPRY4 and HOXB13 expression in esophageal squamous cell carcinoma. Nucleic Acids Res. 2017;45:3086‐3101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Liu B, Sun L, Liu Q, et al. A cytoplasmic NF‐κB interacting long noncoding RNA blocks IκB phosphorylation and suppresses breast cancer metastasis. Cancer Cell. 2015;27:370‐381. [DOI] [PubMed] [Google Scholar]
- 55. Cui J, Placzek WJ. PTBP1 modulation of MCL1 expression regulates cellular apoptosis induced by antitubulin chemotherapeutics. Cell Death Differ. 2016;23:1681‐1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Damas ND, Marcatti M, Come C, et al. SNHG5 promotes colorectal cancer cell survival by counteracting STAU1‐mediated mRNA destabilization. Nat Commun. 2016;7:13875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Wang Z, Yang B, Zhang M, et al. lncRNA epigenetic landscape analysis identifies EPIC1 as an oncogenic lncRNA that interacts with MYC and promotes cell‐cycle progression in cancer. Cancer Cell. 2018;33:706‐720.e709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Xie JJ, Jiang YY, Jiang Y, et al. Super‐enhancer‐driven long non‐coding RNA LINC01503, regulated by TP63, is over‐expressed and oncogenic in squamous cell carcinoma. Gastroenterology. 2018;154:2137‐2151. [DOI] [PubMed] [Google Scholar]
- 59. Olivero CE, Martinez‐Terroba E, Zimmer J, et al. p53 activates the long noncoding RNA Pvt1b to inhibit Myc and suppress tumorigenesis. Mol Cell. 2020;77:761‐774.e768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Schmitt AM, Chang HY. Long noncoding RNAs in cancer pathways. Cancer Cell. 2016;29:452‐463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Sahu A, Singhal U, Chinnaiyan AM. Long noncoding RNAs in cancer: from function to translation. Trends Cancer. 2015;1:93‐109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Mandriota SJ, Jussila L, Jeltsch M, et al. Vascular endothelial growth factor‐C‐mediated lymphangiogenesis promotes tumour metastasis. EMBO J. 2001;20:672‐682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Skobe M, Hawighorst T, Jackson DG, et al. Induction of tumor lymphangiogenesis by VEGF‐C promotes breast cancer metastasis. Nat Med. 2001;7:192‐198. [DOI] [PubMed] [Google Scholar]
- 64. He W, Zhong G, Jiang N, et al. Long noncoding RNA BLACAT2 promotes bladder cancer‐associated lymphangiogenesis and lymphatic metastasis. J Clin Investig. 2018;128:861‐875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Chen C, He W, Huang J, et al. LNMAT1 promotes lymphatic metastasis of bladder cancer via CCL2 dependent macrophage recruitment. Nat Commun. 2018;9:3826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Zhuo W, Liu Y, Li S, et al. Long noncoding RNA GMAN, up‐regulated in gastric cancer tissues, is associated with metastasis in patients and promotes translation of ephrin A1 by competitively binding GMAN‐AS. Gastroenterology. 2019;156:676‐691.e611. [DOI] [PubMed] [Google Scholar]
- 67. Zhao W, Liu Y, Zhang C, Duan C. Multiple roles of exosomal long noncoding RNAs in cancers. Biomed Res Int. 2019;2019:1460572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Li Y, Yin Z, Fan J, Zhang S, Yang W. The roles of exosomal miRNAs and lncRNAs in lung diseases. Signal Transduct Target Ther. 2019;4:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Sun Z, Yang S, Zhou Q, et al. Emerging role of exosome‐derived long non‐coding RNAs in tumor microenvironment. Mol Cancer. 2018;17:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Cheng J, Meng J, Zhu L, Peng Y. Exosomal noncoding RNAs in Glioma: biological functions and potential clinical applications. Mol Cancer. 2020;19:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Zhou R, Chen KK, Zhang J, et al. The decade of exosomal long RNA species: an emerging cancer antagonist. Mol Cancer. 2018;17:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Zang X, Gu J, Zhang J, Shi H, Zhang X. Exosome‐transmitted lncRNA UFC1 promotes non‐small‐cell lung cancer progression by EZH2‐mediated epigenetic silencing of PTEN expression. Cell Death Dis. 2020;11:215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Zhang P, Zhou H, Lu K, Lu Y, Wang Y, Feng T. Exosome‐mediated delivery of MALAT1 induces cell proliferation in breast cancer. OncoTargets and Therapy. 2018;11:291‐299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Zhang R, Xia Y, Wang Z, et al. Serum long non coding RNA MALAT‐1 protected by exosomes is up‐regulated and promotes cell proliferation and migration in non‐small cell lung cancer. Biochem Biophys Res Comm. 2017;490:406‐414. [DOI] [PubMed] [Google Scholar]
- 75. Deng M, Yuan H, Liu S, Hu Z, Xiao H. Exosome‐transmitted LINC00461 promotes multiple myeloma cell proliferation and suppresses apoptosis by modulating microRNA/BCL‐2 expression. Cytotherapy. 2019;21:96‐106. [DOI] [PubMed] [Google Scholar]
- 76. Huang L, Wang Y, Chen J, et al. Long noncoding RNA PCAT1, a novel serum‐based biomarker, enhances cell growth by sponging miR‐326 in oesophageal squamous cell carcinoma. Cell Death Dis. 2019;10:513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Li Z, Qin X, Bian W, et al. Exosomal lncRNA ZFAS1 regulates esophageal squamous cell carcinoma cell proliferation, invasion, migration and apoptosis via microRNA‐124/STAT3 axis. J Exp Clin Cancer Res. 2019;38:477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Li Z, Jiang P, Li J, et al. Tumor‐derived exosomal lnc‐Sox2ot promotes EMT and stemness by acting as a ceRNA in pancreatic ductal adenocarcinoma. Oncogene. 2018;37:3822‐3838. [DOI] [PubMed] [Google Scholar]
- 79. Xu J, Xiao Y, Liu B, et al. Exosomal MALAT1 sponges miR‐26a/26b to promote the invasion and metastasis of colorectal cancer via FUT4 enhanced fucosylation and PI3K/Akt pathway. J Exp Clin Cancer Res. 2020;39:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Liang Z‐X, Liu H‐S, Wang F‐W, et al. LncRNA RPPH1 promotes colorectal cancer metastasis by interacting with TUBB3 and by promoting exosomes‐mediated macrophage M2 polarization. Cell Death Dis. 2019;10:829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Hardin H, Helein H, Meyer K, et al. Thyroid cancer stem‐like cell exosomes: regulation of EMT via transfer of lncRNAs. Lab Invest. 2018;98:1133‐1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Jin N, Jin N, Bu W, et al. Long non‐coding RNA TIRY promotes tumor metastasis by enhancing epithelial‐to‐mesenchymal transition in oral cancer. Exp Biol Med. 2020;245:585‐596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Chen X, Wang Z, Tong F, Dong X, Wu G, Zhang R. lncRNA UCA1 promotes gefitinib resistance as a ceRNA to target FOSL2 by sponging miR‐143 in non‐small cell lung cancer. Mol Ther Nucleic Acids. 2020;19:643‐653. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 84. Lei Y, Guo W, Chen B, Chen L, Gong J, Li W. Tumor‐released lncRNA H19 promotes gefitinib resistance via packaging into exosomes in non‐small cell lung cancer. Oncol Rep. 2018;40(6):3438‐3446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Kang M, Ren M, Li Y, Fu Y, Deng M, Li C. Exosome‐mediated transfer of lncRNA PART1 induces gefitinib resistance in esophageal squamous cell carcinoma via functioning as a competing endogenous RNA. J Exp Clin Cancer Res. 2018;37:171. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 86. Zheng Z, Chen M, Xing P, Yan X, Xie B. Increased expression of exosomal AGAP2‐AS1 (AGAP2 antisense RNA 1) in breast cancer cells inhibits trastuzumab‐induced cell cytotoxicity. Med Sci Monit. 2019;25:2211‐2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Han M, Gu Y, Lu P, et al. Exosome‐mediated lncRNA AFAP1‐AS1 promotes trastuzumab resistance through binding with AUF1 and activating ERBB2 translation. Mol Cancer. 2020;19:26. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 88. Dong H, Wang W, Chen R, et al. Exosome‐mediated transfer of lncRNA‐SNHG14 promotes trastuzumab chemoresistance in breast cancer. Int J Oncol. 2018;53(3):1013‐1026. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 89. Li Z, Niu H, Qin Q, et al. lncRNA UCA1 mediates resistance to cisplatin by regulating the miR‐143/FOSL2‐signaling pathway in ovarian cancer. Mol Ther Nucleic Acids. 2019;17:92‐101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Wang J, Lv B, Su Y, Wang X, Bu J, Yao L. Exosome‐mediated transfer of lncRNA HOTTIP promotes cisplatin resistance in gastric cancer cells by regulating HMGA1/miR‐218 axis. OncoTargets Ther. 2019;12:11325‐11338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Luo X, Wei J, Yang F‐L, et al. Exosomal lncRNA HNF1A‐AS1 affects cisplatin resistance in cervical cancer cells through regulating microRNA‐34b/TUFT1 axis. Cancer Cell Int. 2019;19:323. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 92. Cheng C, Zhang Z, Cheng F, Shao Z. Exosomal lncRNA RAMP2‐AS1 derived from chondrosarcoma cells promotes angiogenesis through miR‐2355‐5p/VEGFR2 axis. OncoTargets Ther. 2020;13:3291‐3301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Lei L, Mou Q. Exosomal taurine up‐regulated 1 promotes angiogenesis and endothelial cell proliferation in cervical cancer. Cancer Biol Ther. 2020;21:717‐725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Cheng Y, Dai X, Yang T, Zhang N, Liu Z, Jiang Y. Low long noncoding RNA growth arrest‐specific transcript 5 expression in the exosomes of lung cancer cells promotes tumor angiogenesis. J Oncol. 2019;2019:1‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Lang H‐L, Hu G‐W, Zhang B, et al. Glioma cells enhance angiogenesis and inhibit endothelial cell apoptosis through the release of exosomes that contain long non‐coding RNA CCAT2. Oncol Rep. 2017;38:785‐798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Lang HL, Hu GW, Chen Y, Liu Y, Xu GH. Glioma cells promote angiogenesis through the release of exosomes containing long non‐coding RNA POU3F3. Eur Rev Med Pharmacol Sci. 2017;21:959. [PubMed] [Google Scholar]
- 97. Baassiri A, Nassar F, Mukherji D, Shamseddine A, Nasr R, Temraz S. Exosomal non coding RNA in LIQUID biopsies as a promising biomarker for colorectal cancer. Int J Mol Sci. 2020;21:1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Jiang N, Pan J, Fang S, et al. Liquid biopsy: circulating exosomal long noncoding RNAs in cancer. Clin Chim Acta. 2019;495:331‐337. [DOI] [PubMed] [Google Scholar]
- 99. Zhong G, Wang K, Li J, Xiao S, Wei W, Liu J. Determination of serum exosomal H19 as a noninvasive biomarker for breast cancer diagnosis. OncoTargets Ther. 2020;13:2563‐2571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Wang J, Yang K, Yuan W, Gao Z. Determination of serum exosomal H19 as a noninvasive biomarker for bladder cancer diagnosis and prognosis. Med Sci Monit. 2018;24:9307‐9316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Wang J, Zhou Y, Lu J, et al. Combined detection of serum exosomal miR‐21 and HOTAIR as diagnostic and prognostic biomarkers for laryngeal squamous cell carcinoma. Med Oncol. 2014;31:148. [DOI] [PubMed] [Google Scholar]
- 102. Wang Y‐L, Liu L‐C, Hung Y, et al. Long non‐coding RNA HOTAIR in circulatory exosomes is correlated with ErbB2/HER2 positivity in breast cancer. Breast. 2019;46:64‐69. [DOI] [PubMed] [Google Scholar]
- 103. Zhao R, Zhang Y, Zhang X, et al. Exosomal long noncoding RNA HOTTIP as potential novel diagnostic and prognostic biomarker test for gastric cancer. Mol Cancer. 2018;17:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Oehme F, Krahl S, Gyorffy B, et al. Low level of exosomal long non‐coding RNA HOTTIP is a prognostic biomarker in colorectal cancer. RNA Biol. 2019;16:1339‐1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Zhang Y, Wang Z, Gemeinhart RA. Progress in microRNA delivery. J Controlled Release. 2013;172:962‐974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Jepsen JS, Sørensen MD, Wengel J. Locked nucleic acid: a potent nucleic acid analog in therapeutics and biotechnology. Oligonucleotides. 2004;14:130‐146. [DOI] [PubMed] [Google Scholar]
- 107. Johannes L, Lucchino M. Current challenges in delivery and cytosolic translocation of therapeutic RNAs. Nucleic Acid Therapeutics. 2018;28:178‐193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Wu Y, Crawford M, Yu B, Mao Y, Nana‐Sinkam SP, Lee LJ. MicroRNA delivery by cationic lipoplexes for lung cancer therapy. Mol Pharm. 2011;8:1381‐1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Peer D, Park EJ, Morishita Y, Carman CV, Shimaoka M. Systemic leukocyte‐directed siRNA delivery revealing cyclin D1 as an anti‐inflammatory target. Science. 2008;319:627‐630. [DOI] [PMC free article] [PubMed] [Google Scholar]


