Key Points
Question
Does robotic ventral hernia repair with intraperitoneal mesh offer a clinical benefit compared with the traditional laparoscopic approach?
Findings
In this single-blinded, randomized clinical trial of 75 patients, no significant difference in pain, complications, quality of life, and hospital length of stay was found. The 52-minute increase in median operative time of the robotic approach incurring additional cost is not countered by a measurable benefit.
Meaning
There is no apparent clinical benefit to the robotic approach when compared with the traditional laparoscopic ventral hernia repair with intraperitoneal mesh.
Abstract
Importance
Despite rapid adoption of the robotic platform for ventral hernia repair with intraperitoneal mesh in the United States, there is no level I evidence comparing it with the traditional laparoscopic approach. This randomized clinical trial sought to demonstrate a clinical benefit to the robotic approach.
Objective
To determine whether robotic approach to ventral hernia repair with intraperitoneal mesh would result in less postoperative pain.
Design, Setting, and Participants
A registry-based, single-blinded, prospective randomized clinical trial at the Cleveland Clinic Center for Abdominal Core Health, Cleveland, Ohio, completed between September 2017 and January 2020, with a minimum follow-up duration of 30 days. Two surgeons at 1 academic tertiary care hospital. Patients with primary or incisional midline ventral hernias of an anticipated width of 7 cm or less presenting in the elective setting and able to tolerate a minimally invasive repair.
Interventions
Patients were randomized to a standardized laparoscopic or robotic ventral hernia repair with fascial closure and intraperitoneal mesh.
Main Outcomes and Measures
The trial was powered to detect a 30% difference in the Numerical Rating Scale (NRS-11) on the first postoperative day. Secondary end points included the Patient-Reported Outcomes Measurement Information System Pain Intensity short form (3a), hernia-specific quality of life, operative time, wound morbidity, recurrence, length of stay, and cost.
Results
Seventy-five patients completed their minimally invasive hernia repair: 36 laparoscopic and 39 robotic. Baseline demographics and hernia characteristics were comparable. Robotic operations had a longer median operative time (146 vs 94 minutes; P < .001). There were 2 visceral injuries in each cohort but no full-thickness enterotomies or unplanned reoperations. There were no significant differences in NRS-11 scores preoperatively or on postoperative days 0, 1, 7, or 30. Specifically, median NRS-11 scores on the first postoperative day were the same (5 vs 5; P = .61). Likewise, postoperative Patient-Reported Outcomes Measurement Information System 3a and hernia-specific quality-of-life scores, as well as length of stay and complication rates, were similar. The robotic platform adds cost (total cost ratio, 1.13 vs 0.97; P = .03), driven by the cost of additional operating room time (1.25 vs 0.85; P < .001).
Conclusions and Relevance
Laparoscopic and robotic ventral hernia repair with intraperitoneal mesh have comparable outcomes. The increased operative time and proportional cost of the robotic approach are not offset by a measurable clinical benefit.
Trial Registration
ClinicalTrials.gov Identifier: NCT03283982
This randomized clinical trial evaluates whether robotic approach to ventral hernia repair with intraperitoneal mesh would result in less postoperative pain.
Introduction
The robotic platform has been rapidly adopted in the realm of general surgery, with little high-level evidence to support its use.1,2,3 Ventral hernia repair in particular is a popular application for robotic implementation, with several large retrospective series demonstrating appealing clinical benefits.4,5,6 While prospective trials are lacking, retrospective data on robotic hernia repair with intraperitoneal mesh have demonstrated a reduction in length of stay (LOS) and postoperative morbidity compared with the traditional laparoscopic approach originally described by LeBlanc and Booth.7,8,9 When compared with open mesh repair, randomized controlled data have shown the laparoscopic alternative does provide decreased wound morbidity and shorter hospital LOS.10,11,12,13,14,15 However, laparoscopic intraperitoneal mesh placement is notoriously painful in the early postoperative period, and rates of chronic pain, bulging, and patient dissatisfaction as high as 25% are significant.16,17,18 Introduction of the robotic platform allows for several potential advantages vs the traditional laparoscopic approach when repairing a ventral hernia with intraperitoneal mesh19:
Intracorporeal suturing allows for closure of the fascial defect with a running self-locking suture, a practice associated with improved quality of life (QoL) and reduced recurrence for laparoscopic closures with the shoelacing technique.20,21
Peritoneal mesh fixation with a running stitch as opposed to tacks and transfascial sutures often implicated as a source of postoperative pain following laparoscopic repairs.17,22
Here, we hypothesized that the robotic approach to ventral hernia repair with intraperitoneal mesh would provide a measurable clinical benefit in regards to early postoperative pain compared with the traditional laparoscopic approach. To our knowledge, this is the first prospective randomized clinical trial that sought to answer this question.
Methods
Design, Eligibility, and Randomization
This registry-based, prospective, single-blinded RCT enrolled patients between September 2017 and January 2020 at the Cleveland Clinic Center for Abdominal Core Health in Cleveland, Ohio. The Cleveland Clinic’s institutional review board approved the trial protocol, and all participants provided written consent. Eligible participants were aged 18 years or older, presenting in the elective setting with primary or incisional midline ventral hernias of an anticipated width of 7 cm or less who were candidates for minimally invasive hernia repair. The formal trial protocols can be found in Supplement 1.
Recruitment was performed by surgeons (A.P. and C.P.) who screened for eligibility. Both surgeons completed fellowships that included training in advanced laparoscopy and complex abdominal wall reconstruction. Both surgeons also had robotic training and credentialing that was in line with requirements defined by Intuitive Surgical and our department of General Surgery. A concealed randomization scheme was performed by using a random number of blocks with a 1:1 ratio of assigning patients to each arm. Data managers randomized patients to the robotic or laparoscopic approach before scheduling their operation to confirm the availability of the robotic platform if needed. Patients were blinded to the operative approach throughout the study. Patient demographics, hernia characteristics, and operative details were recorded in the Americas Hernia Society Quality Collaborative (AHSQC) Database. Additional patient-reported outcomes were stored in a separate database (Research Electronic Data Capture; Vanderbilt University).
Operative Details
All operations began by achieving laparoscopic access using a technique according to each surgeon’s discretion. If used, a cutdown incision onto the hernia could not be greater than what would be necessary for a traditional Hassan technique. After achieving safe intraperitoneal access, additional 5-mm/12-mm laparoscopic or 8-mm robotic ports would be placed to sufficiently allow for safe reduction of the hernia contents, 5 cm of circumferential adhesiolysis around the defect, and mesh placement. At the discretion of the surgeon, those randomized to the robotic technique could have their adhesiolysis performed with the robot or laparoscopically. After intracorporeal measurement of the defect, patients undergoing the robotic technique (DaVinci Si or Xi; Intuitive Inc) underwent defect closure with a running 0 permanent monofilament self-locking suture (V-loc; Medtronic). Barrier-coated monofilament polypropylene (Parietene DS; Medtronic or Ventralight ST; Bard) was then secured circumferentially with 3-0 monofilament absorbable self-locking suture (V-loc; Medtronic or Stratafix; Ethicon), with 5 cm of overlap from the initial measurement prior to defect closure. For those randomized to the laparoscopic approach, the fascial defects were closed with serial figure of 8’s using 0 monofilament permanent suture (Prolene; Ethicon) passed by a Carter-Thomason (ie, shoelacing technique) in 1-cm increments.23 The mesh was secured circumferentially with 4 permanent transfascial sutures at each apex followed by fixation with a permanent tacking device (ProTack; Medtronic) using the double crown technique.24 Port sites were injected with bupivacaine, 0.25%, for both approaches.
Outcome Measurements
The primary outcome was pain on the first postoperative day as measured by the 0 to 10 Numerical Rating Scale (NRS-11), which was collected preoperatively and in the postanesthesia care unit (PACU) as well as 1, 7, 30, and 365 days after surgery.25 Secondary outcomes measured preoperatively, at a mean (SD) of 30 (15) days and a mean (SD) of 12 (3) months, included pain as measured by the Patient-Reported Outcomes Measurement Information System (PROMIS) Pain Intensity short form 3a and abdominal-wall–specific QoL using the hernia-specific quality of life (HerQLes) survey.26,27,28 Additional secondary outcomes included operating room time, PACU opioid consumption measured in morphine equivalents, rates of same-day discharge, hospital LOS, as well as surgical site infection, surgical site occurrence, surgical site occurrence requiring a procedural intervention, ventral hernia recurrence, and cost.29
Because our institution does not permit reporting of cost in dollars, values for cost are reported as ratios. Total cost includes operating room cost (as calculated by cost per minute of operating room time required for the case) and disposable/reusable cost, which was calculated to include disposable materials as well as reusable materials including the robotic instruments. Robotic and laparoscopic capital equipment costs were not amortized for the purpose of this analysis.
Statistical Analysis
In the absence of data regarding postoperative pain, QoL, wound morbidity, and recurrence for robotic IPOM available at the time of the trial design, the investigators determined that a 30% reduction in NRS-11 on the first postoperative day would be a minimal clinically important difference.30 A mean (SD) reported postoperative day 1 NRS-11 data for laparoscopic ventral hernia repair of 4.76 (1.98) is fortunately available.31 Assuming a 2-sided α of .05 and β of 0.20, a total sample size of 62 patients (31 per arm) was initially calculated. Considering a 20% dropout rate to occur in each arm, 74 patients (37 patients per arm) were defined as the sample size necessary to detect a difference in the primary end point. Patients converted to an open procedure would be removed from analysis. As surgeons were permitted to change minimally invasive platforms if necessary, patients would be analyzed in intent to treat fashion based on their initial randomization.
Bivariate analysis was first conducted to compare all characteristics and short-term end points among groups. Unpaired, 2-tailed t test; Mann-Whitney test; χ2 test; and Fisher exact test were used when appropriate.
Results
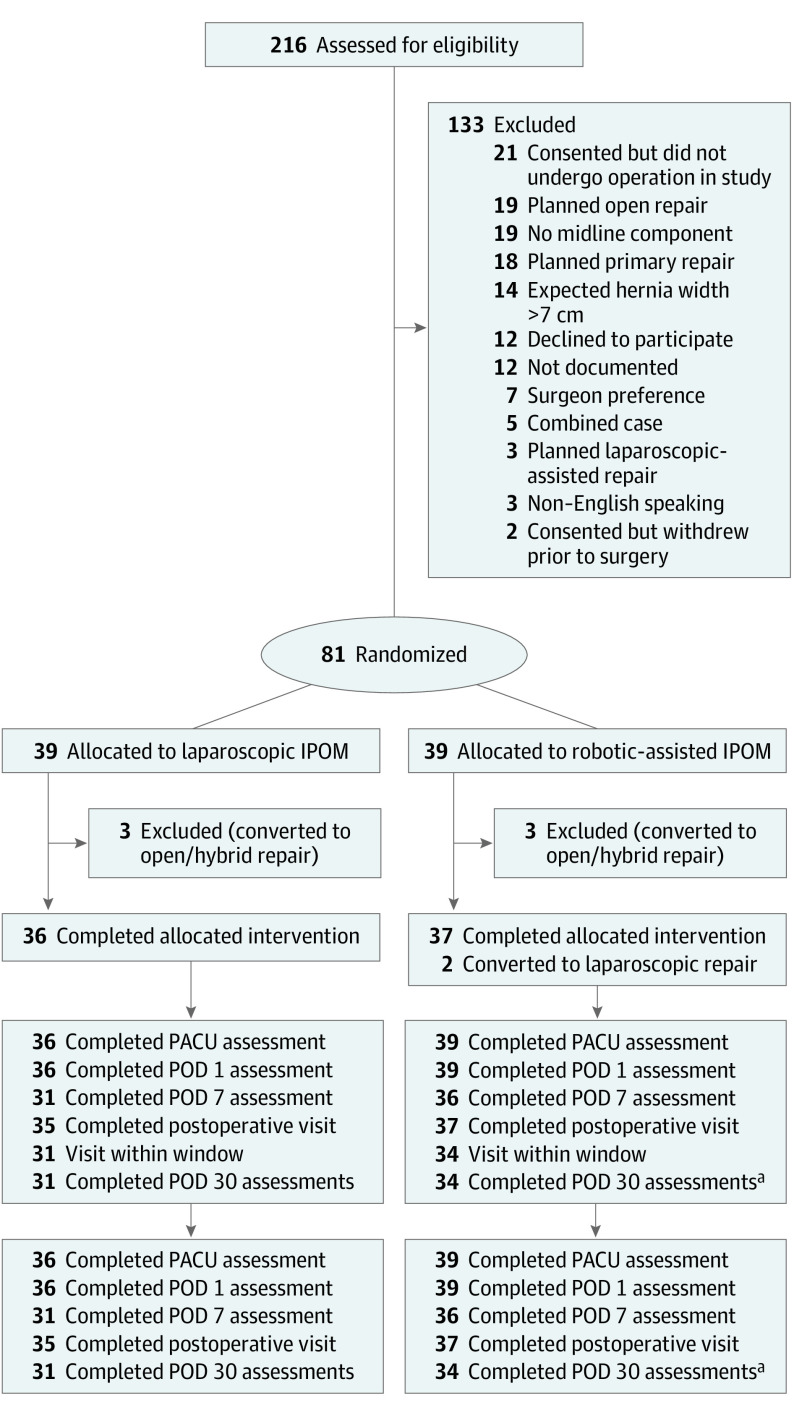
During the enrollment period, 105 of 216 eligible patients were consented for the trial (Figure 1). Of those enrolled, 2 subsequently withdrew, and 21 were awaiting their operation when the study closed. Ultimately, 81 patients were operated on, and 6 of these cases were converted to an open approach, 3 from the laparoscopic group and 3 from the robotic group, owing to the surgeon’s judgment of an inability to perform safe adhesiolysis or failure to progress. Of the 75 patients completing their minimally invasive operative intervention, baseline patient demographics, medical comorbidities, and hernia characteristics summarized in Table 1 were similar, with the exception that laparoscopic patients had a lower median body mass index (BMI; calculated as weight in kilograms divided by height in meters squared; 31 vs 35; P = .02) (Table 1). All patients achieved fascial closure and mesh placement with adequate overlap adhering to the study protocol. Two patients randomized to the robotic platform were converted to a laparoscopic technique, one owing to an operating room bed malfunction that made docking the robot unsafe and one owing to a lack of intraperitoneal space to allow for intracorporeal suturing. These patients were analyzed in intention-to-treat fashion (Table 2).
Figure 1. CONSORT Flow Diagram.
aThirty-five patients had hernia-specific quality of life (HerQLes) and Patient-Reported Outcomes Measurement Information System (PROMIS) scores within window. However, only 34 patients had Numerical Rating Scale (NRS) scores. IPOM indicates intraperitoneal onlay mesh; PACU, postanesthesia care unit; POD, postoperative day.
Table 1. Patient Demographics and Hernia Characteristics.
| Patient demographic | No. (%) | P value | |
|---|---|---|---|
| Laparoscopic (n = 36) | Robotic (n = 39) | ||
| Age at the assessment date, median (IQR), y | 55 (49-60) | 56 (50-70) | .18 |
| Female, % | 58 | 41 | .21 |
| Race/ethnicity, % | |||
| Black | 17 | 19 | .87 |
| White | 83 | 81 | |
| BMI, median (IQR) | 31 (27-36) | 35 (31-39) | .02 |
| Hypertension requiring medication | 17 (47) | 17 (44) | .93 |
| COPD | 1 (3) | 4 (10) | .36 |
| Diabetes mellitus | 3 (8) | 9 (23) | .15 |
| Current smoker (active within 1 mo of surgery) | 4 (11) | 2 (5) | .42 |
| Chronic immunosuppression | 3 (8) | 2 (5) | .66 |
| History of abdominal wall SSI | 0 | 2 (5) | .49 |
| ASA | |||
| 1 | 1 (3) | 1 (3) | >.99 |
| 2 | 2 (19) | 7 (18) | |
| 3 | 27 (75) | 29 (74) | |
| 4 | 1 (3) | 2 (6) | |
| Hernia characteristics | |||
| Primary | 9 (25) | 8 (20.5) | .85 |
| Incisional | 27 (75) | 31 (79.5) | |
| Recurrent incisional hernia | 8 (22) | 5 (13) | .44 |
| Modified ventral hernia working group stage | |||
| 1 | 10 (28) | 5 (13) | .18 |
| 2 | 26 (72) | 34 (87) | |
| Hernia, median (IQR) | |||
| Width, cm | 4 (2-5) | 3(2.5-5) | .88 |
| Length | 5 (2-8) | 5(3-8) | .36 |
Abbreviations: ASA, American Society of Anesthesiologists; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); COPD, chronic obstructive pulmonary disease; IQR, interquartile range; SSI, surgical site infection.
Table 2. Operative Details.
| Variable | No. (%) | P value | |
|---|---|---|---|
| Laparoscopic (n = 36) | Robotic (n = 39) | ||
| Antibiotics given according to SCIP protocol | 36 (100) | 39 (100) | >.99 |
| Type of robot used | |||
| Si | NA | 14 (36) | NA |
| Xi | NA | 25 (64) | NA |
| Fascial closure | 36 (100) | 39 (100) | >.99 |
| Sublay intraperitoneal permanent mesh fixation | |||
| Transfascial suture and permanent tack fixation | 36 (100) | 2 (6) | <.001 |
| Peritoneal suture fixation | 0 | 37 (94) | |
| Conversion to laparoscopy | NA | 2 (6) | NA |
| Conversion to robotic repair | 0 | NA | NA |
| Intraoperative complications | 2 (6) | 2 (6) | |
| Bowel serosal injury | 2 (6) | 1 (3) | >.99 |
| Liver injury | 0 | 1 (3) | |
| Operative time, median (IQR), min | 94 (69-116) | 146 (123-192) | |
| Surgeon A | 94 (57-128) | 142 (124.5-194) | <.001 |
| Surgeon B | 89 (61.5-123) | 147 (121.5-185.5) | |
Abbreviations: IQR, interquartile range; NA, not applicable; SCIP, Surgical Care Improvement Project.
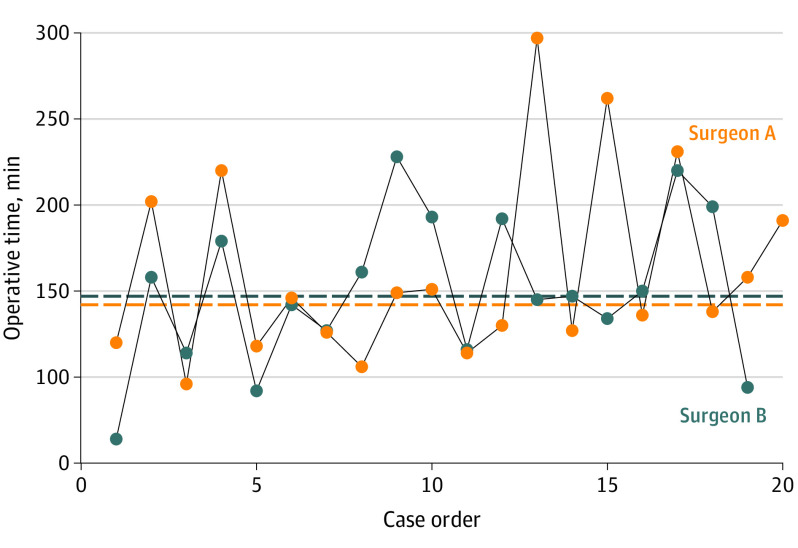
There were 4 intraoperative complications that did not warrant conversion to an open procedure. Two serosal injures in the laparoscopic group were repaired laparoscopically with Lembert sutures. In the robotic group, 1 serosal injury was repaired robotically with Lembert sutures, and a liver injury that occurred during optical entry was cauterized for hemostasis. Laparoscopic operations had a significantly shorter median operative time than robotic counterparts (94 vs 146 minutes; P < .001). Both enrolling surgeons had comparable median operative times for laparoscopic (89 and 94 minutes; P = .98) and robotic procedures (142 and 147 minutes; P = .51) (Figure 2).
Figure 2. Sequential Operative Times for Robotic Cases.
Median operative time in minutes (surgeon A, 142; surgeon B, 147; P = .51).
Median NRS-11 scores on the first postoperative day, the primary end point of the study, were the same (5 vs 5; P = .61) (Table 3). There was no significant difference in hospital LOS (10 vs 25 hours; P = .17), same-day discharge (56% vs 44%; P = .42), opioid consumption in PACU morphine equivalents (45 vs 46; P = .88), or overall complication rates (8% vs 6%; P > .99) for laparoscopic patients and patients undergoing the robotic procedure, respectively. Laparoscopic patients developed 1 seroma that did not require intervention, 1 readmission for an ileus, and 1 reoperation for a thin patient who could feel a transfascial suture at her waistline and requested its excision 3 months later. One patient undergoing the robotic procedure developed deep vein thrombosis and had a readmission for pain control. There were no other unplanned reoperations or procedural interventions. Additional NRS-11 scores at baseline, in PACU, and on postoperative days 7 and 30 were similar. Likewise, PROMIS 3a scores preoperatively and at 30 days were similar, although patients in the laparoscopic group demonstrated statistically significant improvement (−3 vs 0; P = .03). Baseline and postoperative HerQLes scores demonstrated no difference in baseline or postoperative hernia-specific QoL. Finally, to address the statistically significant difference in baseline BMI, we performed a covariate-adjusted analysis accounting for BMI and randomization. The effect of robotic/laparoscopic randomization remained insignificant (effect of difference, −0.46; 95% CI −1.59 to 0.67; P = .42) in regards to the primary outcome (NRS-11 on the first postoperative day) after adjusting for BMI.
Table 3. Outcomes.
| Outcome | Data captured, No. | Median (IQR) | P value | |
|---|---|---|---|---|
| Laparoscopic (n = 36) | Robotic (n = 39) | |||
| Length of hospital stay, h | 75 | 10 (8 to 31) | 25 (10 to 30) | .17 |
| Discharged home, No. (%) | 75 | 20 (56) | 17 (44) | .42 |
| PACU, morphine equivalents | 75 | 45 (29 to 71) | 46 (28 to 68) | .88 |
| Postoperative complications, No. (%) | 75 | 3 (8) | 2 (6) | >.99 |
| Pulmonary embolism | NA | 0 | 1 (3) | >.99 |
| SSO | NA | 1 (3) | 0 | >.99 |
| Readmission | NA | 1 (3) | 1 (3) | >.99 |
| Reoperation | NA | 1 (3) | 0 | >.99 |
| NRS-11 | ||||
| Preoperative | 75 | 1.5 (0 to 4) | 1 (0 to 3) | .86 |
| PACU | 75 | 6 (4 to 8) | 6 (5 to 8) | .97 |
| Postoperative day | ||||
| 1st | 73 | 5 (3 to 7) | 5 (3 to 6) | .61 |
| 7th | 68 | 3 (2 to 5) | 4 (2 to 5) | .58 |
| 30th | 65 | 2 (0 to 2) | 1 (0 to 2) | .71 |
| PROMIS 3a | ||||
| Preoperative | 75 | 49 (40 to 49) | 44 (31 to 51) | .29 |
| Postoperative day 30 | 65 | 44 (38 to 48) | 46 (42 to 51) | .28 |
| Delta | 66 | −3 (−9.4 to .41) | 0 (−2.9 to 9.5) | .01 |
| HerQLes | ||||
| Preoperative | 75 | 51 (37 to 73) | 55 (35 to 73) | .91 |
| Postoperative day 30 | 66 | 75 (41 to 81) | 67 (45 to 79) | .66 |
| Cost | ||||
| Disposable/reusable median cost ratio | NA | 1.00 (0.87 to 1.19) | 0.97 (0.85 to 1.51) | .60 |
| Operating room time-cost ratio | NA | 0.85 (0.67 to 1.00) | 1.25 (0.98.1.49) | <.001 |
| Total cost ratio | NA | 0.97 (0.85 to 1.16) | 1.13 (0.90 to 1.52) | .03 |
Abbreviations: HerQLes, hernia-specific quality of life; IQR, interquartile range; NRS-11, Numerical Rating Scale; PACU, postanesthesia care unit; PROMIS, Patient-Reported Outcomes Measurement Information System; SSO, surgical site occurrence.
Cost ratios are summarized in Table 3 as well. Total cost was significantly less for the laparoscopic cohort (0.97 vs 1.13; P = .03). The discrepancy between total cost was driven by a difference in operating room time cost (0.85 vs 1.25; P < .001), while the cost of disposables/reusables was comparable (1.00 vs 0.97; P = .60).
Discussion
To our knowledge, this is the first prospective, randomized, single-blinded trial comparing laparoscopic and robotic ventral hernia repair with intraperitoneal mesh that reveals no difference in postoperative pain. Complication rates, QoL, and hospital LOS likewise demonstrated no discernable difference. Both techniques appear to have similar rates of maintaining and completing the intended minimally invasive approach. However, the 52-minute increase in median operative time incurring additional cost does not appear to be offset by a measurable clinical benefit.
First, the decision to pursue a reduction in early postoperative pain as the primary end point should be addressed. A previous analysis of data from the AHSQC database compared propensity-matched groups of laparoscopic (n = 454) and robotic (n = 177) hernia repairs with intraperitoneal mesh and fascial closure. There, the longer operative time for robotic repairs (46% vs 30% > 2 hours; P < .001) was countered by a shorter median LOS (0 vs 1 day; interquartile range, 3.00; P < .001), lower rate of seroma (4% vs 9%; P = .02), and fewer overall complications (8% vs 19%; P < .001).7 Several analyses acknowledge the confounded nature of LOS, often affected by a multitude of social factors and the distance a patient has traveled for care, and therefore, we decided this was not a reliable primary end point.32,33 Presumably, the touted benefit of reduced LOS by the robotic approach was the consequence of less early postoperative pain. Therefore, this was chosen as the more salient variable off of which to power the analysis, and a 30% reduction has been consistently defined elsewhere as a minimal clinically important difference.34,35 The resultant homogeneity of outcomes among our cohorts in regards to pain, complications, QoL, and hospital LOS are likely a consequence of the randomized design, neutralizing the selection bias inherent to any retrospective analysis. Because the higher BMI in the robotic group was potentially clinically significant (35 vs 31; P = .02) in regards to increased pain on the first postoperative day, we did feel it was important to confirm that after adjusting for BMI, randomization did not have an independent effect on the primary outcome.
Next, some of the secondary outcomes and exclusions should be contextualized. The difference in the change (Δ) of the PROMIS pain intensity 3a scores suggesting more pain improvement for laparoscopic repairs (−3 vs 0; P = .03), while statistically significant, may not be clinically relevant. While there is no validation of the PROMIS 3a for patients with a hernia, data from orthopedic literature would suggest that an SD of 5 is the minimal clinically important difference for that specific tool.36 A separate issue concerns our 6 conversions to an open procedure. The summative experience of randomized clinical trials comparing laparoscopic and open ventral hernia repair have shown less wound morbidity and shorter hospital stays for laparoscopic repairs, often at the expense of an increased rate of bowel injury, demonstrated in several meta-analyses and systematic reviews.37,38,39 While our 7% conversion rate could be considered high, the absence of any missed enterotomies or unplanned reoperations could be framed as prudent surgical judgment in a context where bowel injury is somewhat notorious. Importantly, the platform also did not appear to affect the conversion rate. A more legitimate criticism is our exclusion of these 6 patients from analysis. While they could have been included in intent to treat fashion, we ultimately excluded them to isolate the comparison of the minimally invasive techniques alone.
The discrepancy in operative times warrants a thorough discussion. The median operative time for the robotic repairs (146 minutes) is significantly longer than the laparoscopic arm (94 minutes). Retrospective data by which to compare our robotic OR time are widely variable. Indeed, the aforementioned retrospective analysis7 from the AHSQC found that robotic repairs took more than 2 hours in 46% of cases but offers no additional granularity.7 Alternatively, several large series of robotic ventral hernias repairs, including Gonzalez et al (n = 368)40 and Kudsi et al (n = 68),41 have reported median operative times of 89 and 80 minutes, respectively. To account for some of the time discrepancy, it is worth noting that in the series by Gonzalez et al,41 more than 30% of patients did not have their fascial defect closed, and 40% of patients had their mesh tacked in place despite use of the robotic platform. In the 2020 series by Kudsi et al,40 20% of patients did not have their fascial defect closed and more than 40% were primary defects. Our robotic cohort included 80% incisional and 13% recurrent incisional defects, likely requiring a more time-consuming adhesiolysis. Furthermore, all of the patients undergoing robotic surgery achieved fascial closure and a near-complete rate of intraperitoneal mesh suture fixation. In full transparency, because one of the enrolling surgeons had several years of robotic experience while the other was in their first year of clinical practice after completing an abdominal wall reconstruction fellowship, we thought it was important to demonstrate the similarity in operative times of robotic cases (Figure 2). This is critically important because our data would suggest that the cost discrepancy would be neutralized if surgeons were able to overcome the difference in operative time.
The cost analysis for this operation is unique. Previously, our trial of laparoscopic vs robotic transabdominal preperitoneal inguinal hernia repair found that the robotic platform not only added cost in regards to operative time but also added to the disposable/reusable costs (median $1784 vs $623; P < .001).42 In this study, disposable/reusable costs were similar, likely because several tacking devices were required for laparoscopic repairs, offsetting the cost of self-fixating suture and robotic disposables. Therefore, if comparable operative times were achieved, the value discrepancy could theoretically be mitigated, and other authors have reported advancements in robotic efficiency in a short time.43 That said, our operative times between an experienced and less experienced robotic surgeon were comparable, suggesting that we were not necessarily en route to overcoming the 52-minute discrepancy. While some may contend the robot offers benefits to the surgeon that are not conventionally measured, the aforementioned randomized trial on inguinal hernia repair also found no measurable clinical benefit to the robot while causing more surgeon frustration and no difference in ergonomics.42 To date, as long as the time and associated cost discrepancy exists, the onus remains on the robotic platform and its users to either become very efficient or provide evidence of an objective benefit to justify its use.
Currently, adoption of the robotic platform without high-level evidence, particularly in the realm of general surgery, has become commonplace. From 2012 to 2018, use of the robot for general surgery procedures increased from 1.8% to 15.1%.1 Commonly, robotic inguinal repair and intraperitoneal mesh placement for ventral hernias are regarded as basic procedures that the surgeons should be comfortable with before pursuing advanced techniques.44,45,46 Having completed 2 randomized clinical trials that failed to show a measurable benefit of these procedures, an alternative argument could be made that the robotic outcomes are comparable, safe, and allow surgeons to gain comfort with the robotic platform while working toward more complex approaches. Namely, these advanced operations are ventral hernia repair techniques with extraperitoneal mesh placement, including the robotic transabdominal preperitoneal repair, robotic endoscopic totally extraperitoneal approach, and robotic transversus abdominis release (TAR). In retrospective series, both Martin-Del-Campo et al5 and Bittner et al6 described a reduction in complications and LOS for robotic TAR compared with open TAR historical controls. A separate AHSQC analysis comparing all robotic retromuscular approaches with open repairs in a matched group of patients with hernia likewise found a similar benefit in LOS for robot repairs.4 Most recently, in 2020 Kudsi et al40 demonstrated fewer complications and wound morbidity favoring robotic totally extraperitoneal when compared with robotic placement of intraperitoneal mesh, suggesting that the more complex robotic approach adds even greater value. The need for randomized trials evaluating these robotic techniques now grows more important than ever. If advanced techniques can likewise not elucidate a clinical benefit, then use of basic techniques as a training platform becomes a bridge to nowhere.
Limitations
Remaining limitations include a lack of long-term follow-up to elucidate the durability of each repair technique, as well as implications regarding prolonged pain. Long-term follow-up will further establish the safety, efficacy, and unanticipated benefits of the robotic technique. While 3% to 10% of the short-term data points for pain and QoL assessments were not collected, the analysis was powered for 20% attrition, and therefore, that missing data should not affect the validity of our primary end point. Finally, granular data regarding postdischarge opioid consumption would have been a timely addition to this assessment but was not built into the initial protocol.
Conclusions
Laparoscopic and robotic ventral hernia repair with intraperitoneal mesh offer similar early postoperative outcomes in regards to pain, QoL, and complication rates. Owing to the increased operative time and associated cost, there is currently no measurable benefit to justify the robotic approach.
Trial Protocol
Data Sharing Statement.
References
- 1.Sheetz KH, Claflin J, Dimick JB. Trends in the adoption of robotic surgery for common surgical procedures. JAMA Netw Open. 2020;3(1):e1918911. doi: 10.1001/jamanetworkopen.2019.18911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jung M, Morel P, Buehler L, Buchs NC, Hagen ME. Robotic general surgery: current practice, evidence, and perspective. Langenbecks Arch Surg. 2015;400(3):283-292. doi: 10.1007/s00423-015-1278-y [DOI] [PubMed] [Google Scholar]
- 3.Ahmad A, Ahmad ZF, Carleton JD, Agarwala A. Robotic surgery: current perceptions and the clinical evidence. Surg Endosc. 2017;31(1):255-263. doi: 10.1007/s00464-016-4966-y [DOI] [PubMed] [Google Scholar]
- 4.Carbonell AM, Warren JA, Prabhu AS, et al. Reducing length of stay using a robotic-assisted approach for retromuscular ventral hernia repair: a comparative analysis from the Americas Hernia Society Quality Collaborative. Ann Surg. 2018;267(2):210-217. doi: 10.1097/SLA.0000000000002244 [DOI] [PubMed] [Google Scholar]
- 5.Martin-Del-Campo LA, Weltz AS, Belyansky I, Novitsky YW. Comparative analysis of perioperative outcomes of robotic versus open transversus abdominis release. Surg Endosc. 2018;32(2):840-845. doi: 10.1007/s00464-017-5752-1 [DOI] [PubMed] [Google Scholar]
- 6.Bittner JG IV, Alrefai S, Vy M, Mabe M, Del Prado PAR, Clingempeel NL. Comparative analysis of open and robotic transversus abdominis release for ventral hernia repair. Surg Endosc. 2018;32(2):727-734. doi: 10.1007/s00464-017-5729-0 [DOI] [PubMed] [Google Scholar]
- 7.Prabhu AS, Dickens EO, Copper CM, et al. Laparoscopic vs robotic intraperitoneal mesh repair for incisional hernia: an Americas Hernia Society Quality Collaborative Analysis. J Am Coll Surg. 2017;225(2):285-293. doi: 10.1016/j.jamcollsurg.2017.04.011 [DOI] [PubMed] [Google Scholar]
- 8.LeBlanc KA, Booth WV. Laparoscopic repair of incisional abdominal hernias using expanded polytetrafluoroethylene: preliminary findings. Surg Laparosc Endosc. 1993;3(1):39-41. [PubMed] [Google Scholar]
- 9.LeBlanc KA, Booth WV. Avoiding complications with laparoscopic herniorrhaphy. Surg Laparosc Endosc. 1993;3(5):420-424. [PubMed] [Google Scholar]
- 10.Rogmark P, Petersson U, Bringman S, et al. Short-term outcomes for open and laparoscopic midline incisional hernia repair: a randomized multicenter controlled trial: the ProLOVE (prospective randomized trial on open versus laparoscopic operation of ventral eventrations) trial. Ann Surg. 2013;258(1):37-45. doi: 10.1097/SLA.0b013e31828fe1b2 [DOI] [PubMed] [Google Scholar]
- 11.Carbajo MA, Martín del Olmo JC, Blanco JI, et al. Laparoscopic treatment vs open surgery in the solution of major incisional and abdominal wall hernias with mesh. Surg Endosc. 1999;13(3):250-252. doi: 10.1007/s004649900956 [DOI] [PubMed] [Google Scholar]
- 12.Olmi S, Scaini A, Cesana GC, Erba L, Croce E. Laparoscopic versus open incisional hernia repair: an open randomized controlled study. Surg Endosc. 2007;21(4):555-559. doi: 10.1007/s00464-007-9229-5 [DOI] [PubMed] [Google Scholar]
- 13.Navarra G, Musolino C, De Marco ML, Bartolotta M, Barbera A, Centorrino T. Retromuscular sutured incisional hernia repair: a randomized controlled trial to compare open and laparoscopic approach. Surg Laparosc Endosc Percutan Tech. 2007;17(2):86-90. doi: 10.1097/SLE.0b013e318030ca8b [DOI] [PubMed] [Google Scholar]
- 14.Misra MC, Bansal VK, Kulkarni MP, Pawar DK. Comparison of laparoscopic and open repair of incisional and primary ventral hernia: results of a prospective randomized study. Surg Endosc. 2006;20(12):1839-1845. doi: 10.1007/s00464-006-0118-0 [DOI] [PubMed] [Google Scholar]
- 15.Othman IH, Metwally YH, Bakr IS, Amer YA, Gaber MB, Elgohary SA. Comparative study between laparoscopic and open repair of paraumbilical hernia. J Egypt Soc Parasitol. 2012;42(1):175-182. doi: 10.12816/0006305 [DOI] [PubMed] [Google Scholar]
- 16.Eker HH, Hansson BM, Buunen M, et al. Laparoscopic vs. open incisional hernia repair: a randomized clinical trial. JAMA Surg. 2013;148(3):259-263. doi: 10.1001/jamasurg.2013.1466 [DOI] [PubMed] [Google Scholar]
- 17.Liang MK, Clapp M, Li LT, Berger RL, Hicks SC, Awad S. Patient Satisfaction, chronic pain, and functional status following laparoscopic ventral hernia repair. World J Surg. 2013;37(3):530-537. doi: 10.1007/s00268-012-1873-9 [DOI] [PubMed] [Google Scholar]
- 18.Carter SA, Hicks SC, Brahmbhatt R, Liang MK. Recurrence and pseudorecurrence after laparoscopic ventral hernia repair: predictors and patient-focused outcomes. Am Surg. 2014;80(2):138-148. [PubMed] [Google Scholar]
- 19.Allison N, Tieu K, Snyder B, Pigazzi A, Wilson E. Technical feasibility of robot-assisted ventral hernia repair. World J Surg. 2012;36(2):447-452. doi: 10.1007/s00268-011-1389-8 [DOI] [PubMed] [Google Scholar]
- 20.Bernardi K, Olavarria OA, Liang MK. Primary fascial closure during minimally invasive ventral hernia repair. JAMA Surg. 2019;(Dec):26. [DOI] [PubMed] [Google Scholar]
- 21.Christoffersen MW, Westen M, Rosenberg J, Helgstrand F, Bisgaard T. Closure of the fascial defect during laparoscopic umbilical hernia repair: a randomized clinical trial. Br J Surg. 2020;107(3):200-208. doi: 10.1002/bjs.11490 [DOI] [PubMed] [Google Scholar]
- 22.Heniford BT, Park A, Ramshaw BJ, Voeller G. Laparoscopic repair of ventral hernias: nine years’ experience with 850 consecutive hernias. Ann Surg. 2003;238(3):391-399. doi: 10.1097/01.sla.0000086662.49499.ab [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Orenstein SB, Dumeer JL, Monteagudo J, Poi MJ, Novitsky YW. Outcomes of laparoscopic ventral hernia repair with routine defect closure using “shoelacing” technique. Surg Endosc. 2011;25(5):1452-1457. doi: 10.1007/s00464-010-1413-3 [DOI] [PubMed] [Google Scholar]
- 24.Muysoms F, Vander Mijnsbrugge G, Pletinckx P, et al. Randomized clinical trial of mesh fixation with “double crown” versus “sutures and tackers” in laparoscopic ventral hernia repair. Hernia. 2013;17(5):603-612. doi: 10.1007/s10029-013-1084-9 [DOI] [PubMed] [Google Scholar]
- 25.Breivik EK, Björnsson GA, Skovlund E. A comparison of pain rating scales by sampling from clinical trial data. Clin J Pain. 2000;16(1):22-28. doi: 10.1097/00002508-200003000-00005 [DOI] [PubMed] [Google Scholar]
- 26.Cella D, Riley W, Stone A, et al. ; PROMIS Cooperative Group . The Patient-Reported Outcomes Measurement Information System (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005-2008. J Clin Epidemiol. 2010;63(11):1179-1194. doi: 10.1016/j.jclinepi.2010.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stone AA, Broderick JE, Junghaenel DU, Schneider S, Schwartz JE. PROMIS fatigue, pain intensity, pain interference, pain behavior, physical function, depression, anxiety, and anger scales demonstrate ecological validity. J Clin Epidemiol. 2016;74:194-206. doi: 10.1016/j.jclinepi.2015.08.029 [DOI] [PubMed] [Google Scholar]
- 28.Krpata DM, Schmotzer BJ, Flocke S, et al. Design and initial implementation of HerQLes: a hernia-related quality-of-life survey to assess abdominal wall function. J Am Coll Surg. 2012;215(5):635-642. doi: 10.1016/j.jamcollsurg.2012.06.412 [DOI] [PubMed] [Google Scholar]
- 29.Haskins IN, Horne CM, Krpata DM, et al. A call for standardization of wound events reporting following ventral hernia repair. Hernia. 2018;22(5):729-736. doi: 10.1007/s10029-018-1748-6 [DOI] [PubMed] [Google Scholar]
- 30.Morino M, Pellegrino L, Castagna E, Farinella E, Mao P. Acute nonspecific abdominal pain: a randomized, controlled trial comparing early laparoscopy versus clinical observation. Ann Surg. 2006;244(6):881-886. doi: 10.1097/01.sla.0000246886.80424.ad [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Asencio F, Aguiló J, Peiró S, et al. Open randomized clinical trial of laparoscopic versus open incisional hernia repair. Surg Endosc. 2009;23(7):1441-1448. doi: 10.1007/s00464-008-0230-4 [DOI] [PubMed] [Google Scholar]
- 32.Celio DA, Poggi R, Schmalzbauer M, Rosso R, Majno P, Christoforidis D. ERAS, length of stay and private insurance: a retrospective study. Int J Colorectal Dis. 2019;34(11):1865-1870. doi: 10.1007/s00384-019-03391-2 [DOI] [PubMed] [Google Scholar]
- 33.Fawcett W. Outcome following surgery. Anaesthesia. 2019;74(9):1204. doi: 10.1111/anae.14766 [DOI] [PubMed] [Google Scholar]
- 34.Jordan K, Dunn KM, Lewis M, Croft P. A minimal clinically important difference was derived for the Roland-Morris Disability Questionnaire for low back pain. J Clin Epidemiol. 2006;59(1):45-52. doi: 10.1016/j.jclinepi.2005.03.018 [DOI] [PubMed] [Google Scholar]
- 35.Katz NP, Paillard FC, Ekman E. Determining the clinical importance of treatment benefits for interventions for painful orthopedic conditions. J Orthop Surg Res. 2015;10:24. doi: 10.1186/s13018-014-0144-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stephan A, Mainzer J, Kümmel D, Impellizzeri FM. Measurement properties of PROMIS short forms for pain and function in orthopedic foot and ankle surgery patients. Qual Life Res. 2019;28(10):2821-2829. doi: 10.1007/s11136-019-02221-w [DOI] [PubMed] [Google Scholar]
- 37.Zhang Y, Zhou H, Chai Y, Cao C, Jin K, Hu Z. Laparoscopic versus open incisional and ventral hernia repair: a systematic review and meta-analysis. World J Surg. 2014;38(9):2233-2240. doi: 10.1007/s00268-014-2578-z [DOI] [PubMed] [Google Scholar]
- 38.Sauerland S, Walgenbach M, Habermalz B, Seiler CM, Miserez M. Laparoscopic versus open surgical techniques for ventral or incisional hernia repair. Cochrane Database Syst Rev. 2011;(3):CD007781. doi: 10.1002/14651858.CD007781.pub2 [DOI] [PubMed] [Google Scholar]
- 39.Al Chalabi H, Larkin J, Mehigan B, McCormick P. A systematic review of laparoscopic versus open abdominal incisional hernia repair, with meta-analysis of randomized controlled trials. Int J Surg. 2015;20:65-74. doi: 10.1016/j.ijsu.2015.05.050 [DOI] [PubMed] [Google Scholar]
- 40.Kudsi OY, Gokcal F, Chang K. Robotic intraperitoneal onlay versus totally extraperitoneal (TEP) retromuscular mesh ventral hernia repair: a propensity score matching analysis of short-term outcomes. Am J Surg. 2020;(Jan):8. doi: 10.1016/j.amjsurg.2020.01.003 [DOI] [PubMed] [Google Scholar]
- 41.Gonzalez A, Escobar E, Romero R, et al. Robotic-assisted ventral hernia repair: a multicenter evaluation of clinical outcomes. Surg Endosc. 2017;31(3):1342-1349. doi: 10.1007/s00464-016-5118-0 [DOI] [PubMed] [Google Scholar]
- 42.Prabhu AS, Carbonell A, Hope W, et al. Robotic inguinal vs transabdominal laparoscopic inguinal hernia repair: the RIVAL randomized clinical trial. JAMA Surg. 2020;(Mar):18. doi: 10.1001/jamasurg.2020.0034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Muysoms F, Van Cleven S, Pletinckx P, Ballecer C, Ramaswamy A. Robotic transabdominal retromuscular umbilical prosthetic hernia repair (TARUP): observational study on the operative time during the learning curve. Hernia. 2018;22(6):1101-1111. doi: 10.1007/s10029-018-1825-x [DOI] [PubMed] [Google Scholar]
- 44.Pakula AM, Skinner RA. From ipom to tar: robotic herniorraphy is in the armamentarium of the acute care surgeon: a video based guide to the technique. J Trauma Acute Care Surg. 2019;87(1):251-253. [DOI] [PubMed] [Google Scholar]
- 45.Fuenmayor P, Lujan HJ, Plasencia G, Karmaker A, Mata W, Vecin N. Robotic-assisted ventral and incisional hernia repair with hernia defect closure and intraperitoneal onlay mesh (IPOM) experience. J Robot Surg. 2020;(Jan):2. doi: 10.1007/s11701-019-01040-y [DOI] [PubMed] [Google Scholar]
- 46.Kozman MA, Tonkin D, Eteuati J, Karatassas A, McDonald CR. Robotic-assisted ventral hernia repair with surgical mesh: how I do it and case series of early experience. ANZ J Surg. 2019;89(3):248-254. doi: 10.1111/ans.15071 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
Data Sharing Statement.