Abstract
Background
Neonatal Respiratory Distress Syndrome (NRDS) is one of the most frequent causes of neonatal mortality especially in premature infants. Although it has been well established that maternal antenatal corticosteroid therapy has a positive effect on NRDS reduction, yet the effectiveness of this treatment in multifetal pregnancies is dubious.
Objective
We aimed to investigate the effect of betamethasone therapy on the incidence of NRDS in multifetal pregnancies through a randomized controlled trial.
Methods
140 women with a multifetal pregnancy at less than 28 weeks’ gestational age were randomly allocated into intervention and control groups. Women at the intervention group received intramuscularly betamethasone (12 mg/kg/BW twice). Neonatal outcomes were evaluated between two groups of intervention and control, and two subgroups of preterm and term births. This study is registered with the Iranian Clinical Trials Registry, number IRCT20180227038879N1.
Results
The incidence of NRDS was significantly lower in infants of betamethasone group than the ones in the control group (4.9% vs 18.1%, P=0.034) while it did not show a significant reduction in preterm infants compared to mature ones. Also, the intervention group presented a significant lower neonatal ventilation than the control group (47.2% vs 63.2%, P=0.041). Other neonatal outcomes, including age at birth, birth weight, Apgar score, NICU admission, and the number of mortalities were not significantly different between study groups.
Conclusion
Betamethasone therapy during 28-32 weeks of gestation in multifetal pregnancies was associated with better neonatal outcomes through significant reductions in NRDS incidence and requiring ventilator treatment. However, betamethasone administration did not reduce the chance of NRDS in premature infants.
Keywords: Neonatal respiratory distress syndrome, multifetal pregnancies, betamethasone, antenatal corticosteroid, clinical trial, gestation
1. INTRODUCTION
Neonatal Respiratory Distress Syndrome (NRDS) is a consequence of the lung’s incomplete evolution, especially in premature infants. Unfortunately, this syndrome results in early neonatal mortality in most cases and the survivors remain at a high risk of short or long-term disabilities [1]. Along with several factors, such as low birth weight, asphyxia, caesarean, and diabetic mothers that increase the risk of NRDS in infants, preterm birth has been considered as one of the most important risk factors of it [2-4]; Up to 50% of preterm births at 26-28 weeks, and 30% of them at 30-31 weeks end up in babies with NRDS [5]. Multifetal pregnancies indirectly increase the risk of NRDS through their high possibility of preterm birth [6]. The first randomized controlled trial of human betamethasone therapy for NRDS prevention which was held in 1972, showed the effectiveness of betamethasone in NRDS reduction especially in babies of under 32 weeks’ gestation [7]. After that, several trials examined Antenatal Corticosteroid (ACS) treatment in terms of neonatal outcomes and a systematic review of those studies (eighteen trials with data on about 3700 infants) indicated that antenatal administration of ACS including betamethasone to mothers in risk of preterm birth is correlated with a significant reduction in neonatal mortality, NRDS and maternal Intraventricular Hemorrhage (IVH) [8].
Although the effects of ACS treatment are well studied in singleton pregnancies, there are few studies on multifetal pregnancies which also present inconsistent findings [9-13], as a recent Cochrane systematic review claimed that evidence for the effectiveness of ACS in multiple pregnancies is insufficient especially in low and middle-income countries [14]. Since the positive effects of ACS therapy in multiple pregnancies is yet doubtful and the previous trials compared the outcomes between singleton and multifetal pregnancies, we aimed to explore the effect of betamethasone therapy on incidence of NRDS in women with multifetal pregnancies through a randomized controlled trial.
2. MATERIALS AND METHODs
We retrospectively assessed the betamethasone therapy outcomes in women with multifetal pregnancies who were referred to Alzahra teaching hospital (the tertiary referral hospital in northwestern Iran), from January 2016 to December 2018.
2.1. Participants
Eligible participants were the women with a multifetal pregnancy at less than 28 weeks’ gestational age whose responsible physician declared their risk of preterm birth and stated no contraindication to further betamethasone therapy on them. In the current study, being pregnant with more than one fetuses was considered as the potential risk of preterm birth before 32 weeks of gestation. Women who were in any potential risk of preterm birth other that multifetal pregnancy such as anatomical uterine disorders, heart or renal diseases, etc.; or if their physician considered ACS therapy to be essential or to be limited for them, were excluded from the study. We started samples recruitment in May 2016 and completed it in August 2018.
2.2. Procedures
Initial samples were selected through hospital records using the random numbers table technique. Afterwards, selected women who signed the informed consent were evaluated regarding inclusion criteria. Eligible women were randomly assigned to the betamethasone therapy group or the control group. For random assigning the participants into the study groups, Random Allocation Software version 2.0 was used.
In the exposure group, 26-28 weeks’ pregnant women were given two doses of betamethasone 12 mg IM 24 hours apart by intramuscular injection in the presence of their responsible physician; they also were monitored for any side-effects of the injections. The control group was considered as a non-placebo group. Women and their infants in both groups were followed up until their hospital discharge. The maternal data (including age, history of previous pregnancies considering number of fetuses and preterm deliveries, IVH occurrence during current pregnancy, gestational age at trial entry), and neonatal outcomes (including gestational age at birth, birth weight, Apgar score, NICU admission, ventilator treatment, mortality, and incidence of NRDS) were collected.
NRDS was diagnosed based on respiratory grunting and retracting, an increased oxygen requirement (FiO2 > 0.4) and chest radiographies. And, IVH was detected in case of intraventricular bleeding with or without ventricular dilatation, or with parenchymal involvement [15].
2.3. Statistical Analysis
The baseline characteristic was analyzed using descriptive statistics. The outcomes were compared between two study groups using the chi-squared test or Fisher’s exact test. The paired t-test and McNemar’s test were run to compare the outcomes between the subgroups (according to the delivery date). The results were considered statistically significant in case of P-value < 0.05. All data analysis was done using SPSS software, version 24.
3. RESULTS
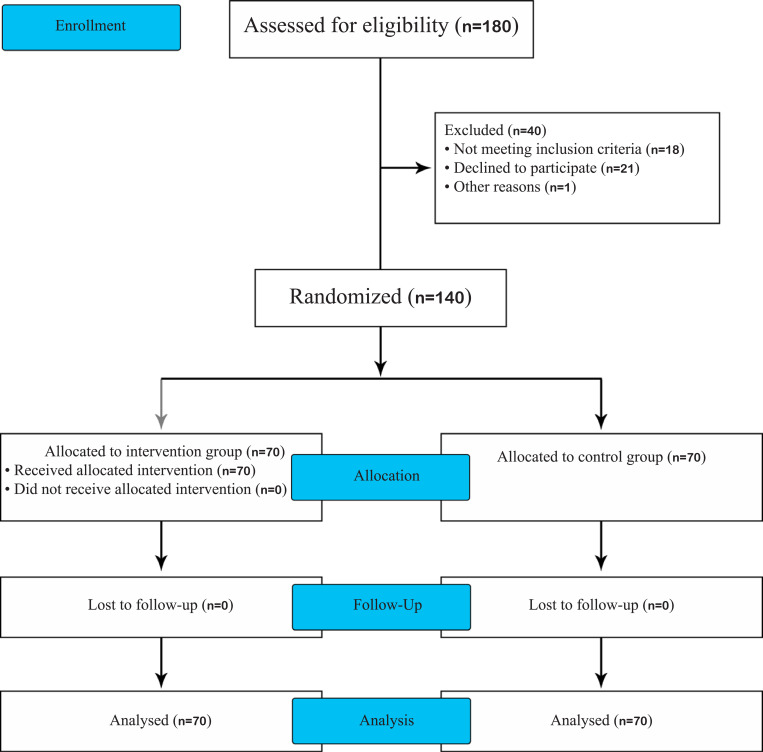
140 women with multifetal pregnancies who met the inclusion criteria were included in the study, of which, 70 cases were allocated to each betamethasone therapy and control groups (Fig. 1 shows the trial profile). As it is presented in Table 1, baseline variables among two groups were similar with respect to maternal characteristics (age, history of previous pregnancies and preterm deliveries) and current pregnancy status (number of fetuses and gestational age at trial entry).
In our study, 134 and 6 women were pregnant with twins and triplets, respectively; No evidence of IVH was seen in women of two groups during their pregnancy. The study mothers gave birth to overall 286 infants, 142 infants in betamethasone group including 144 ones in the control group, of which, 5 and 8 infants died in intervention and control groups, respectively. Our results showed that the proportion of preterm birth does not differ between the two groups (64.1% vs 70.8%, P>0.005, chi-squared test) (Table 2).
In general, neonatal outcomes, including age at birth, gender, birth weight, Apgar score <7, NICU admission, and mortalities were not significantly different between betamethasone and control groups, except for the incidence of NRDS and ventilator treatment. Following the data in Table 2, NRDS incidence was significantly lower in betamethasone group’s infants than control group’s (4.9% vs 18.1%, P=0.034, Fisher’s exact test) as well as the ventilator treatment (47.2% vs 63.2%, P=0.041, chi-squared test).
In addition, subgroups analysis in terms of preterm birth within each study group revealed that birth weight, Apgar score <7 at 1 minute and 5 minutes, and ventilator treatment were significantly different in premature infants compared to mature ones within both groups. Also, the premature infants in the intervention group had higher incidence of NRDS
(6.6% vs 2.0%, P=0.032, McNemar’s test) and ventilator treatment (57.1% vs 29.4%, P=0.021, McNemar’s test) than mature ones; the same correlations were observed between the premature and mature infants of control group (ventilator treatment: 70.6% vs 45.2%, P=0.041, McNemar’s test & NRDS incidence: 21.6% vs 9.5%, P=0.004, McNemar’s test). Data from subgroups analysis did not differ for NIUCU admission and neonatal mortalities within two groups (Table 3).
4. DISCUSSION
While it was well documented in the literature that incidence of NRDS among multiple-birth infants is higher than singleton infants [4, 13] and the prenatal ACS treatment in women at risk of preterm delivery helps fetal lung development and prevents the incidence of NRDS [14, 16], our study on efficacy of prenatal maternal administration of two doses of betamethasone 12 mg in multifetal pregnancies which are at high risk of preterm delivery, showed a significant reduction in occurrence of NRDS.
Furthermore, two Cochrane studies indicated that prenatal corticosteroid treatment of mothers at risk of preterm birth results in significant reductions in neonatal NRDS plus shorter ventilator treatment and less need for respiratory support [6, 14]; In parallel to their findings, our infants in the betamethasone group did not only show an NRDS reduction, but they also presented a significant reduction in ventilator treatment than the ones in the control group.
Concerning the high incidence of preterm birth in both betamethasone (64%) and control groups (71%) in our study, all premature infants had lower birth weight and Apgar score compared to babies born at term, as expected. About the respiratory situation, NRDS and ventilator treatment were more common in premature newborns in both groups, suggesting that betamethasone could not prevent the incidence of NRDS in premature infants. A South Korean trial also claimed that none of the single or multiple courses of ACS administration prevent the NRDS in preterm twins [13].
Also, the current study tried to make an insight into the efficacy of betamethasone on NRDS reduction in multifetal pregnancies, but our small sample size resulted in a majority of twin pregnancies so that we could not assess the outcomes across twin-pregnancies and pregnancies with more than two fetuses. Moreover, we did not examine the betamethasone therapy at different gestational age ranges, so that the most beneficial administration of betamethasone is yet subject to debate.
CONCLUSION
In conclusion, this trial study showed a positive effect of betamethasone therapy on the reduction of NRDS and ventilator treatment in infants of multifetal pregnancies; However, it did not reduce the risk of NRDS in premature babies. Further randomized controlled trials are required to study the effectiveness of betamethasone therapy on the incidence of NRDS between twin-pregnancies and pregnancies with more than two fetuses and realize the best performance of betamethasone therapy in multifetal pregnancies through its administration at different gestational age ranges.
Fig. (1).
Trial profile based on CONSORT flow diagram.
Table 1.
Baseline characteristics of women in betamethasone and control groups.
| - |
Betamethasone
(n=70) |
Control
(n=70) |
P-value |
|---|---|---|---|
| Maternal age (years)¥ | 30.1 (4.4) | 29.8 (5.2) | 0.548 |
| Number of Previous Pregnancies≠ | |||
| 0 | 47 (67.1%) | 43 (61.4%) | 0.347 |
| 1-2 | 19 (27.1%) | 24 (34.3%) | 0.213 |
| ≥3 | 4 (5.7%) | 3 (4.3%) | 0.678 |
| Previous Preterm deliveries (<32 weeks)≠ | 3 (4.3%) | 4 (5.7%) | 0.642 |
| Number of Fetuses at Trial Entry≠ | - | - | - |
| 2 | 64 (91.4%) | 62 (88.6%) | 0.452 |
| 3 | 6 (8.6%) | 8 (11.4%) | 0.221 |
| ≥4 | 0 | 0 | - |
| Gestational Age at Trial Entry (weeks)¥ | 26.0 (2.5) | 26.7 (2.7) | 0.682 |
¥ Mean (SD) was presented. ≠ N (%) was presented.
Table 2.
Neonatal outcomes in betamethasone and control groups.
| - |
Betamethasone
(n=142) |
Control
(n=144) |
P-value |
|---|---|---|---|
| Gestational Age at Birth (week)¥ | 30.2 (2.6) | 28.8 (2.3) | 0.341 |
| Number of preterm births (<32 weeks)≠ | 91 (64.1%) | 102 (70.8%) | 0.171 |
| Sex≠ | |||
| Male | 66 (46.5%) | 70 (48.6%) | 0.544 |
| Female | 76 (53.5%) | 74 (51.4%) | 0.512 |
| Birthweight (kg)¥ | 1.45 (0.5) | 1.42 (0.5) | 0.742 |
| Apgar score <7≠ | - | - | - |
| At 1 minute | 29 (20.4%) | 40 (27.8%) | 0.252 |
| At 5 minutes | 13 (9.1%) | 21 (14.6%) | 0.128 |
| NICU Admission≠ | 136 (95.8%) | 140 (97.2%) | 0.871 |
| Ventilator Treatment≠ | 67 (47.2%) | 91 (63.2%) | 0.041 |
| Neonatal Mortality≠ | 5 (3.5%) | 8 (5.5%) | 0.269 |
| Respiratory Distress Syndrome≠ | 7 (4.9%) | 26 (18.1%) | 0.034 |
¥ Mean (SD) was presented. ≠ N (%) was presented.
Table 3.
Comparison of neonatal outcome among subgroups in terms of preterm birth.
| - | Betamethasone | Control | ||||
|---|---|---|---|---|---|---|
|
Preterm Birth
(<32 weeks) (n=91) |
Term Birth
(≥32 weeks) (n=51) |
P-value |
Preterm Birth
(<32 weeks) (n=102) |
Term Birth
(≥32 weeks) (n=42) |
P-value | |
| Birthweight (kg)¥ | 1.29 (0.6) | 1.51 (0.7) | 0.046 | 1.22 (0.6) | 1.47 (0.6) | 0.038 |
| Apgar score <7≠ | - | - | - | - | - | - |
| At 1 minute | 20 (22.0%) | 9 (17.6%) | 0.028 | 35 (34.3%) | 5 (11.9%) | 0.003 |
| At 5 minutes | 11 (12.1%) | 2 (3.9%) | 0.001 | 19 (18.6%) | 2 (4.8%) | 0.000 |
| NICU Admission≠ | 91 (100%) | 45 (88.2%) | 0.274 | 102 (100%) | 38 (90.5%) | 0.366 |
| Ventilator Treatment≠ | 52 (57.1%) | 15 (29.4%) | 0.021 | 72 (70.6%) | 19 (45.2%) | 0.041 |
| Neonatal Mortality≠ | 4 (4.4%) | 1 (2.0%) | 0.251 | 6 (5.9%) | 2 (4.8%) | 0.426 |
| Respiratory Distress Syndrome≠ | 6 (6.6%) | 1 (2.0%) | 0.032 | 22 (21.6%) | 4 (9.5%) | 0.004 |
¥ Mean (SD) was presented. ≠ N (%) was presented.
ACKNOWLEDGEMENTS
We also thank the staff of Alzahra Teaching Hospital and members of Women’s Reproductive Health Research Center of Tabriz University of Medical Sciences for their great support in study development.
LIST OF ABBREVIATIONS
- ACS
Antenatal Corticosteroid
- IVH
Intraventricular Hemorrhage
- NICU
Neonatal Intensive Care Unit
- NRDS
Neonatal Respiratory Distress Syndrome
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The study protocol was approved by the ethical committee of Tabriz University of Medical Sciences, number IR.TBZMED.REC.1396.1000. This clinical trial is registered with the Iranian Clinical Trials Registry (http://www.irct.ir/), number IRCT20180227038879N1.
HUMAN AND ANIMAL RIGHTS
No Animal subjects were used in the study. All research procedures followed were in accordance with the ethical standards of the committee responsible for human experimentation (institutional and national), and with the Helsinki Declaration of 1975, as revised in 2008 (http://www.wma.net/en/20activities/10ethics/10helsinki/).
CONSENT FOR PUBLICATION
Written informed consent was acquired from all potential mothers before including them in the study.
AVAILABILITY OF DATA AND MATERIALS
The data supporting the findings of the article is available in the research deputy of Tabriz University of Medical Sciences at [https://researchvice-en.tbzmed.ac.ir/], reference number [58323].
Funding
We would like to thank Tabriz University of Medical Sciences [https://portal-en.tbzmed.ac.ir/] for funding this study under the grant number of 58323.
CONFLICT OF INTEREST
The study authors declare no conflict of interest in terms of scientific collaboration and financial benefits.
REFERENCES
- 1.Laws P.J., Sullivan D.E., Grayson N. Australia’s mothers and babies. Sydney: AIHW National Perinatal Statistics Unit; 2004. [Google Scholar]
- 2.Kliegman R.M., Stanton B.M., Geme J.S., Schor N.F. Nelson Text-book of Pediatrics E-Book. Maryland: Elsevier Health Scienc-es; 2015. [Google Scholar]
- 3.Gerten K.A., Coonrod D.V., Bay R.C., Chambliss L.R. Cesarean delivery and respiratory distress syndrome: Does labor make a difference? Am. J. Obstet. Gynecol. 2005;193(3 Pt 2):1061–1064. doi: 10.1016/j.ajog.2005.05.038. [DOI] [PubMed] [Google Scholar]
- 4.Qiu X., Lee S.K., Tan K., Piedboeuf B., Canning R. Canadian Neonatal Network. Comparison of singleton and multiple-birth outcomes of infants born at or before 32 weeks of gesta-tion. Obstet. Gynecol. 2008;111(2 Pt 1):365–371. doi: 10.1097/AOG.0b013e318162688f. [DOI] [PubMed] [Google Scholar]
- 5.Hintz S.R., Van Meurs K.P., Perritt R., et al. NICHD Neonatal Research Network. Neurodevelopmental outcomes of premature infants with severe respiratory failure enrolled in a randomized controlled trial of inhaled nitric oxide. J. Pediatr. 2007;151(1):16–22. doi: 10.1016/j.jpeds.2007.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Crowther C.A., Haslam R.R., Hiller J.E., Doyle L.W., Robinson J.S., Australasian Collaborative Trial of Repeat Doses of Steroids (ACTORDS) Study Group Neonatal respiratory distress syndrome after repeat exposure to antenatal corticosteroids: A randomised controlled trial. Lancet. 2006;367(9526):1913–1919. doi: 10.1016/S0140-6736(06)68846-6. [DOI] [PubMed] [Google Scholar]
- 7.Liggins G.C., Howie R.N. A controlled trial of antepartum glucocorticoid treatment for prevention of the respiratory distress syndrome in premature infants. Pediatrics. 1972;50(4):515–525. [PubMed] [Google Scholar]
- 8.Crowley P. Prophylactic corticosteroids for preterm birth. Cochrane Database Syst. Rev. 1996:1. doi: 10.1002/14651858.CD000065. [DOI] [PubMed] [Google Scholar]
- 9.Turrentine M.A., Wilson P.D., Wilkins I.A. A retrospective analysis of the effect of antenatal steroid administration on the incidence of respiratory distress syndrome in preterm twin pregnancies. Am. J. Perinatol. 1996;13(6):351–354. doi: 10.1055/s-2007-994355. [DOI] [PubMed] [Google Scholar]
- 10.Quist-Therson E.C., Myhr T.L., Ohlsson A. Antenatal steroids to prevent respiratory distress syndrome: Multiple gestation as an effect modifier. Acta Obstet. Gynecol. Scand. 1999;78(5):388–392. doi: 10.1080/j.1600-0412.1999.780508.x. [DOI] [PubMed] [Google Scholar]
- 11.Al-Yatama M.K., Al Essa M., Omu A.E., Al-Shamali I., Egbase P., Rashwan N. Effect of repeated doses of dexamethasone on the incidence and severity of respiratory distress syndrome in multifetal gestation between 24 and 34 weeks. Gynecol. Obstet. Invest. 2001;52(1):26–33. doi: 10.1159/000052936. [DOI] [PubMed] [Google Scholar]
- 12.Blickstein I., Shinwell E.S., Lusky A., Reichman B., Network I.N. Israel Neonatal Network. Plurality-dependent risk of respiratory distress syndrome among very-low-birth-weight infants and antepartum corticosteroid treatment. Am. J. Obstet. Gynecol. 2005;192(2):360–364. doi: 10.1016/j.ajog.2004.10.604. [DOI] [PubMed] [Google Scholar]
- 13.Choi S.J., Song S.E., Seo E.S., Oh S.Y., Kim J.H., Roh C.R. The effect of single or multiple courses of antenatal corticosteroid therapy on neonatal respiratory distress syndrome in singleton versus twin pregnancies. Aust. N. Z. J. Obstet. Gynaecol. 2009;49(2):173–179. doi: 10.1111/j.1479-828X.2009.00970.x. [DOI] [PubMed] [Google Scholar]
- 14.Roberts D, Brown J, Medley N, Dalziel SR. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth Cochrane Database Syst Rev. 2017. [DOI] [PMC free article] [PubMed]
- 15.American Academy of Pediatrics An international classification of retinopathy of prematurity. Pediatrics. 1984;74(1):127–133. [PubMed] [Google Scholar]
- 16.Gilstrap L.C., Christensen R., Clewell W.H., et al. NIH consensus development panel on the effect of corticosteroids for fetal maturation on perinatal outcomes. JAMA. 1995;273(5):413–418. doi: 10.1001/jama.1995.03520290065031. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data supporting the findings of the article is available in the research deputy of Tabriz University of Medical Sciences at [https://researchvice-en.tbzmed.ac.ir/], reference number [58323].