Abstract
Background
Antibiotic therapies targeting multiple regenerative mechanisms have the potential for neuroprotective effects, but the diversity of experimental strategies and analyses of non-standardised therapeutic trials are challenging. In this respect, there are no cases of successful clinical application of such candidate molecules when it comes to human patients.
Methods
After 24 hours of culturing, three different minocycline (Sigma-Aldrich, M9511, Germany) concentrations (1 µM, 10 µM and 100 µM) were added to the primary cortical neurons 15 minutes before laser axotomy procedure in order to observe protective effect of minocycline in these dosages.
Results
Here, we have shown that minocycline exerted a significant neuroprotective effect at 1 and 100μM doses. Beyond confirming the neuroprotective effect of minocycline in a more standardised and advanced in-vitro trauma model, our findings could have important implications for future studies that concentrate on the translational block between animal and human studies.
Conclusion
Such sophisticated approaches might also help to conquer the influence of human-made variabilities in critical experimental injury models. To the best of our knowledge, this is the first study showing that minocycline increases in-vitro neuronal cell survival after laser-axotomy.
Keywords: Minocycline, laser-axotomy, in-vitro cortical cell culture, translational neuroscience, trauma, propidium
1. INTRODUCTION
Axotomy often initiates alterations in the cytology of nerve cells, changes in specific gene expression, transport of some neurotrophins and, degeneration of neurons. This process is induced retrogradely by depriving these cells of target-derived trophic support that finally lead to neuronal cell death. It has been already shown that the evolution of abnormalities in neurons depends on several factors. These include the type of axotomized neurons, location of cell body lesion, and the intensity of injury [1]. Some studies have even recognized significant post-lesional differences after axotomy in specific neuronal cell types indicating that there is a variability in implementation of the severity of trauma that depends on the different type, severity and location of the lesion [1]. Accordingly, studies have already indicated that differences in the Central Nervous System (CNS) injury (such as dissection and acceleration) might cause a wide range of clinical complexity [2]. Several types of in-vitro damage methods in nervous tissue elements have been shown in controlled culture conditions. In this context, there have been many tool and techniques (i.e., different mechanical impactors and stretchers) to induce the in-vitro neuronal injury. However, these techniques did not offer enough standardization for the applied trauma and failed to mimic direct mechanical damage on neuronal axons [2]. These findings together suggested the importance of conducting well-designed and highly controlled experimental studies to evaluate the pathophysiological changes of damaged neuronal cells. These novel experimental approaches would also provide real-time monitoring of the ongoing biomechanical parameters. In the light of these findings, automatized controlled dissection of the culture of cortical cells might serve as a suitable trauma model to determine the mechanical damage of neurons with high accuracy and low-variability [3]. Considering all the evidences, it can be hypothesized that enhanced trauma models combined with feasible candidate neuroprotective agents could be beneficial to overcome sampling variation and human-made errors. Laser beam axotomy is a feasible standardized trauma method for neuroscientists to evaluate the neuroprotective outcomes [2]. This method serves as a precise control of laser parameters for modulating the intensity of axonal damage (i.e., total or localised axotomy damage), through very small scales (i.e., micro or nanoseconds). Making such standardized in-vitro brain trauma models readily available might open up a new window for understanding the pathophysiological role of individual axons in the CNS injury [4]. Minocycline is a potential candidate neuroprotective agent with its long half-life and well documented gastrointestinal tract absorption. Studies have already shown that minocycline also posseses a high lipophilic ability which is responsible for its high blood-brain barrier penetration [5]. The underlying mechanisms of the neuroprotective role of minocycline include the inhibition of microglial activity and the induction of anti-apoptotic cascades [5].
TBI and spinal cord injury have both been defined with a prominent diffuse axonal injury that is related to either acceleration/deceleration trauma or whiplash injury [6]. Glaucoma, is another progressive disease that involves secondary degeneration of RGCs that is due to the damage of optic nerve axons [7].
In our present study, we aimed to evaluate the neuroprotective role of minocycline (1μM, 10μM and 100μM) in an in-vitro model of cortical culture (Figs. 1-3) after laser-induced axonal damage. We assessed the neuronal cell survival /vitality rate via propidium iodide staining which is a critical marker for neuroregenerative response. Here, we also aimed to test if laser-induced axonal damage is an accurate, feasible and easy model to imitate the mechanical damage of neurons with cultured Cortical Neurons (CNs).
2. MATERIALs AND METHODS
In this study 20 neonatal (P0-P1) Balb-C male mice were used and the study has been approved by the Ethical Committee of Experimental Animals of Istanbul Medipol University (38828770-604.0101-E.35856).
Inclusion criteria: The predilection toward using only male mice is often based on that male mice are larger that can offer an easier target for manipulating (such as surgery for preparing for cell culture). It should also be noted that they do not have estrous cycles that can lead to significant variability in the pharmacology. Furthermore, there is a larger body of literature and data sets for male mice on which a reliable comparative data can be built.
2.1. Primary Cortical Neuron Culturing
Neonatal mice were decapitated under 4% isofluorane anesthesia, cortical layer was taken carefully and meninges were removed. The dissection procedure was performed in ice-cold Leibovitz’s Medium (L15) (Sigma-Aldrich, L5520, Germany) containing 2 mM glutamax (Gibco, 35050061, USA), 100 U/100 µg penicillin/streptomycin, 250 ng amphotericine (Sigma-Aldrich, A5955, Germany), 2% (v/v) B-27 (Gibco, 1750444, USA). Briefly, dissected cortex was minced into small pieces and then collected in Leibovitz’s Medium (L15) (Sigma-Aldrich, L5520, Germany) containing 2 mM glutamax (Gibco, 35050061, USA), 100 U/100 µg penicillin/streptomycin, 250 ng amphotericine (Sigma-Aldrich, A5955, Germany), 2% (v/v) B-27 (Gibco, 1750444, USA). 6 U/ml papain (Sigma-Aldrich, P4762, Germany) and 50 μg/ml DNaseI (Sigma-Aldrich, D4513, Germany) were also added and it was incubated at +4oC for 45 minutes for digestion. After incubation the tissue was gently triturated by pasteur pipette and the sample was centrifuged at 800 rpm for 3 minutes at +4ºC. After discarding the supernatant, the pellet was resuspended in Leibovitz’s Medium (L15) (Sigma-Aldrich, L5520, Germany) containing 10% (v/v) fetal bovine serum (FBS) (Thermo Fisher Scientific, 10500064, USA), 2 mM glutamax (Gibco, 35050061, USA), 100 U/100 µg penicillin/streptomycin, 250 ng amphotericine (Sigma-Aldrich, A5955, Germany), 2% (v/v) B-27 (Gibco, 1750444, USA) and was incubated at room temperature for 10 minutes. After incubation, it was re-centrifuged at 800 rpm for 3 minutes at +4ºC and supernatant was discarded. Pelleted cells were resuspended in Neurobasal A medium (Gibco, 10888022, USA) containing 2 mM glutamax (Gibco, 35050061, USA), 100 U/100 µg penicillin/streptomycin, 250 ng amphotericine (Sigma-Aldrich, A5955, Germany), 2% (v/v) B-27 (Gibco, 1750444, USA).
Neurons were cultured on poly-L-lysine (Sigma-Aldrich, P6282, Germany) coated glass-bottomed 35 mm petri dishes and incubated in a cell culture incubator with 5% CO2 at 37ºC. After 24 hours incubation, imaging was performed under Primo Vert invert microscop (Zeiss Technologies, Germany) with 20X objective. Cells were seeded into 35 mm petri dishes at a density of 25x103 cells for cell viability experiments. Each experiment was repeated at least three times.
2.2. Minocycline Application
After 24 hours of culturing, three different minocycline (Sigma-Aldrich, M9511, Germany) concentrations (1 µM, 10 µM and 100 µM) were added to the primary cortical neurons 15 minutes before laser axotomy procedure in order to observe protective effect of minocycline in these dosages.
2.3. Laser Axotomy
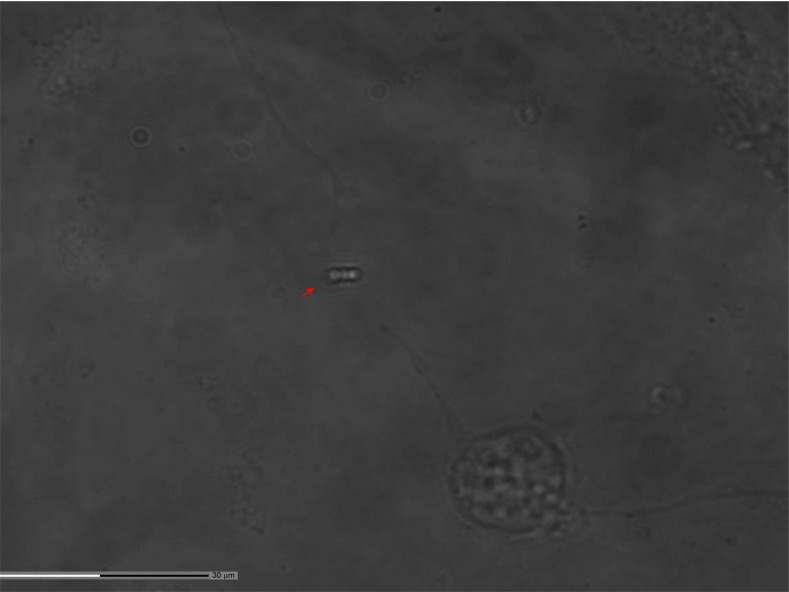
In order to cut the axons of cultured primary cortical neurons, PALM Combisystem Microdissection microscobe (Zeiss Technologies, Germany) was used. For axotomy a laser pulse at 337 nm with a 300 lJ energy was applied and the application point was chosen in such a way that the distance of the point from the cell body should be at least two times greater than the diameter of the cell soma (Fig. 3). For each experimental group, ten different areas from each petri dish were chosen randomly and thirty neurons were axotomized.
Fig. (3).
For axotomy a laser pulse energy was applied in a significant distance of the point from the cell body. Please see the red arrowhead.
After laser axotomy, cortical neurons were placed into cell culture incubator with 5% CO2 at 37ºC for 24 hours.
2.4. Cell Viability Assay
After 24 hours of incubation of axotomized cells, Propidium Iodide (PI) (Sigma-Aldrich, P4170, Germany), a nuclear dye labeling only dead cells with red fluorescence, was used to distinguish the axotomized dead cells from axotomized viable cells. Cells were treated with PI at 7.5 µM concentration and the number of dead and live cells were counted using PALM Combisystem Microdissection microscobe (Zeiss Technologies, Germany). Numbers of PI-positive dead cells were counted from chosen areas at each petri dish as mentioned above.
2.5. Statistical Analysis
The cell viability data were statistically evaluated by using one-way ANOVA test in SPSS (version 18, IBM, USA) and p values less than 0.05 are considered significant. All values are given as mean ± SD.
3. RESULTS
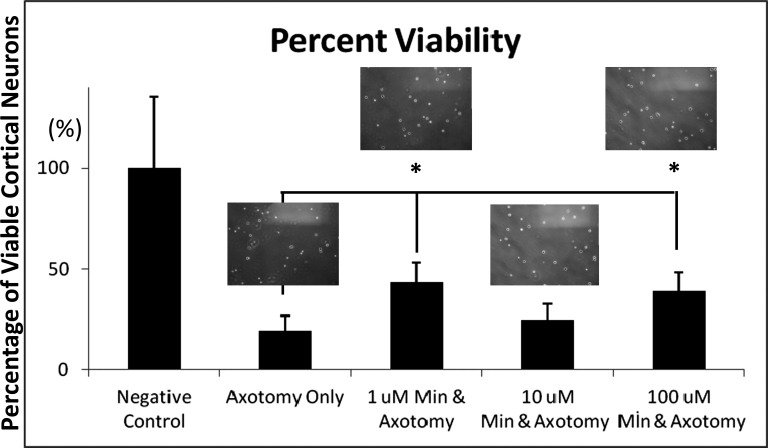
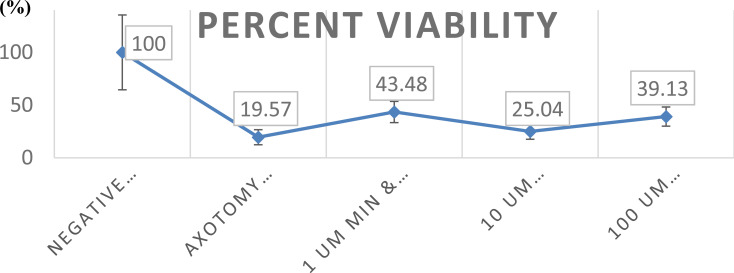
Treatment of cortical cultures with minocycline affected the survival of cortical neurons in a statistically significant manner as compared to the non-minocycline-treated group (Figs. 1 and 2). Most importantly, no toxic effect of minocycline was noted up to a final concentration of 100 μM (Figs. 1 and 2). Laser axotomy administration to cortical cultures resulted in a reduction of surviving cortical neurons to approximately 80.43% of control values.
Minocycline at concentrations of 1 and 100μM significantly increased the number of surviving axotomized cortical neurons as compared to the axotomized-only group (122.2% and 99.5% of axotomized values, respectively) (Figs. 1 and 2).
4. DISCUSSION
The primary aim of the study was to evaluate whether laser-induced axotomy is a reliable and standardized in-vitro injury model to assess the neuroprotective effect of minocycline. To clarify this, we have identified injured neurons by defining the cytostructural changes after 24 hours. Our present model allowed us to assess the direct effect of the minocycline through excluding the influence of other experimental parameters on neurons (i.e. inflammation and hypoxia). In the present study, we have assessed only the cell vitality which can be considered as a weakness of our study. However, our main aim was to conduct a preliminary study and collect the most reliable data regarding the role of the laser-axotomy model in evaluating the neuroprotective effect of candidate neuroprotective antibiotics. To the best of our knowledge, this is the first study showing that minocycline increases in-vitro neuronal cell survival after laser-axotomy. After considering previous evidence showing that secondary neuronal cell death occurs within the 24 h after axotomy injury [3], it is not unreasonable to assume that minocycline (1 and 100 μM) reversed laser-axotomy induced cell damage and increased the survival of neurons within this critical time period. We have observed that 10 μM minocycline increased the survival of axotomized cortical neurons although this difference was not statistically significant. It is difficult to estimate what has caused this difference, however, one possible explanation would be that nearly every intra- or inter-cellular mechanisms show a nonlinear character due to the presence of complex and multi-component relationships seen in every level of a cell or group of cells that might affect the linear cellular response to a drug or a molecule. The neuroprotective role of minocycline has been confirmed in various animal models. In agreement with this, there are increasing data suggesting that minocycline exerts strong neuroprotective effects in translational experimental models which are relevant to human brain and spinal trauma [8-10]. For instance, a recent study indicated that minocycline blocked the neuronal cell death which was induced by optic nerve axotomy. Taken together, these studies suggested that the neuroprotective effect of minocycline involved the activation of specific intracellular anti-apoptotic pathways [9, 10].
Although there are promising results in preclinical studies, the translation of these study results into human studies are weak. In line with this, there are a small number of translational experimental studies which failed to confirm the neuroprotective effect of minocycline.
For instance, a recent study showed that the combination of simvastatin and minocycline failed to alter the histopathological outcomes in a clinically relevant model of cervical spinal cord injury [11]. Furthermore, minocycline exerted differential effects on the immature brain after trauma, which suggested that minocycline may not be an effective therapeutic strategy for TBI in the immature brain [12]. Accordingly, randomised ALS trials showed that minocycline deteriorated the clinical outcomes [13] and minocycline was not found sufficiently effective to improve the motor recovery after spinal cord injury in humans [14]. This was in line with a recent human phase II trial showing that minocycline exerted no efficacy on the CSF markers in acute spinal cord injury patients [15]. Also, a very recent study by Scott et al. have conferred that minocycline increased neurodegeneration in brain trauma patients despite its significant inhibitory effect on microglial activation [16].
CONCLUSION
In addition to suggesting that the minocycline exerts a neuroprotective role in a mechanical CNS injury model, our results also indicate that laser-axotomy is an attractive neuronal mechanical injury model to study the in-vitro neuroprotective effect of critical candidate neuroprotective drugs in cortical cells. After considering all these controversial data, laser-axotomy might be considered as a valuable tool for evaluating the drug-induced neuroregenerative pathways with a greater accuracy. Such an approach could also help us to overcome the translational challenges in animal and human studies.
Fig. (1).
Minocycline at concentrations of 1 and 100μM significantly increased the number of surviving axotomized cortical neurons as compared to the axotomized-only group. *P≤0.05. Data are given as mean ± SD.
Fig. (2).
Laser axotomy administration to cortical cultures resulted in a reduction of surviving cortical neurons to approximately 80.43% of control values. Minocycline at concentrations of 1 and 100μM significantly increased the number of surviving axotomized cortical neurons as compared to the axotomized-only group (122.2% and 99.5% of axotomized values, respectively). *P≤0.05. Data are given as mean ± SD.
Acknowledgements
Declared none.
Ethics Approval and Consent to Participate
The study has been approved by the Ethical Committee of Experimental Animals of Istanbul Medipol University with registration number: (38828770-604.0101-E.35856).
Human and Animal Rights
No humans were used in this research. All animal research procedures followed were in accordance with the standards set forth in the eighth edition of Guide for the Care and Use of Laboratory Animals published by the National Academy of Sciences, The National Academies Press, Washington, D.C.
Consent for Publication
Not applicable.
Availability of Data and Materials
Not applicable.
Funding
None.
Conflict of Interest
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Koliatsos V.E., Price D.L. Axotomy as an experimental model of neuronal injury and cell death. Brain Pathol. 1996;6(4):447–465. doi: 10.1111/j.1750-3639.1996.tb00875.x. [DOI] [PubMed] [Google Scholar]
- 2.Cengiz N., Oztürk G., Erdoğan E., Him A., Oğuz E.K. Consequences of neurite transection in vitro. J. Neurotrauma. 2012;29(15):2465–2474. doi: 10.1089/neu.2009.0947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ma Y.H., Zeng X., Zhang K., Zeng Y.S. A new in vitro injury model of mouse neurons induced by mechanical scratching. Neurosci. Lett. 2012;510(1):14–19. doi: 10.1016/j.neulet.2011.12.061. [DOI] [PubMed] [Google Scholar]
- 4.Wu T., Mohanty S., Gomez-Godinez V., et al. Neuronal growth cones respond to laser-induced axonal damage. J. R. Soc. Interface. 2012;9(68):535–547. doi: 10.1098/rsif.2011.0351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Levkovitch-Verbin H., Waserzoog Y., Vander S., Makarovsky D., Ilia P. Minocycline mechanism of neuroprotection involves the Bcl-2 gene family in optic nerve transection. Int. J. Neurosci. 2014;124(10):755–761. doi: 10.3109/00207454.2013.878340. [DOI] [PubMed] [Google Scholar]
- 6.Czeiter E., Pal J., Kovesdi E., et al. Traumatic axonal injury in the spinal cord evoked by traumatic brain injury. J. Neurotrauma. 2008;25(3):205–213. doi: 10.1089/neu.2007.0331. [DOI] [PubMed] [Google Scholar]
- 7.Howell G.R., Libby R.T., Jakobs T.C., et al. Axons of retinal ganglion cells are insulted in the optic nerve early in DBA/2J glaucoma. J. Cell Biol. 2007;179(7):1523–1537. doi: 10.1083/jcb.200706181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kelestemur T., Yulug B., Caglayan A.B., et al. Targeting different pathophysiological events after traumatic brain injury in mice: Role of melatonin and memantine. Neurosci. Lett. 2016;612:92–97. doi: 10.1016/j.neulet.2015.11.043. [DOI] [PubMed] [Google Scholar]
- 9.Yulug B., Kilic E., Altunay S., et al. Cinnamon polyphenol extract exerts neuroprotective activity in traumatic brain injury through modulation of Nfr2 and cytokine expression. CNS Neurol. Disord. Drug Targets. 2018;17(6):439–447. doi: 10.2174/1871527317666180501110918. [DOI] [PubMed] [Google Scholar]
- 10.Sanchez Mejia R.O., Ona V.O., Li M., Friedlander R.M. Minocycline reduces traumatic brain injury-mediated caspase-1 activation, tissue damage, and neurological dysfunction. Neurosurgery. 2001;48(6):1393–1399. doi: 10.1227/00006123-200106000-00051. [DOI] [PubMed] [Google Scholar]
- 11.Lee J.H., Tigchelaar S., Liu J., et al. Lack of neuroprotective effects of simvastatin and minocycline in a model of cervical spinal cord injury. Exp. Neurol. 2010;225(1):219–230. doi: 10.1016/j.expneurol.2010.06.018. [DOI] [PubMed] [Google Scholar]
- 12.Hanlon L.A., Raghupathi R., Huh J.W. Differential effects of minocycline on microglial activation and neurodegeneration following closed head injury in the neonate rat. Exp. Neurol. 2017;290:1–14. doi: 10.1016/j.expneurol.2016.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gordon P.H., Moore D.H., Miller R.G., et al. Western ALS Study Group Efficacy of minocycline in patients with amyotrophic lateral sclerosis: A phase III randomised trial. Lancet Neurol. 2007;6(12):1045–1053. doi: 10.1016/S1474-4422(07)70270-3. [DOI] [PubMed] [Google Scholar]
- 14.Casha S., Zygun D., McGowan M.D., Bains I., Yong V.W., Hurlbert R.J. Results of a phase II placebo-controlled randomized trial of minocycline in acute spinal cord injury. Brain. 2012;135(Pt 4):1224–1236. doi: 10.1093/brain/aws072. [DOI] [PubMed] [Google Scholar]
- 15.Casha S., Rice T., Stirling D.P., et al. Cerebrospinal fluid biomarkers in human spinal cord injury from a phase II minocycline trial. J. Neurotrauma. 2018;35(16):1918–1928. doi: 10.1089/neu.2018.5899. [DOI] [PubMed] [Google Scholar]
- 16.Scott G., Zetterberg H., Jolly A., et al. Minocycline reduces chronic microglial activation after brain trauma but increases neurodegeneration. Brain. 2018;141(2):459–471. doi: 10.1093/brain/awx339. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.