JGP study describes a novel quantitative assay combining fluorescence microscopy and electrophysiology, which reveals that transport of small molecules through CALHM1 and connexin channels is saturable
Abstract
JGP study describes a novel quantitative assay combining fluorescence microscopy and electrophysiology, which reveals that transport of small molecules through CALHM1 and connexin channels is saturable.
Large-pore channels, including those formed by connexin, LRRC8, and CALHM proteins, are permeable to both atomic ions and small molecules such as ATP. Despite their physiological importance, little is known about how these channels transport molecules. In this issue of JGP, Gaete et al. describe a new assay to analyze the kinetic properties and permselectivity of this diverse family of channels (1).
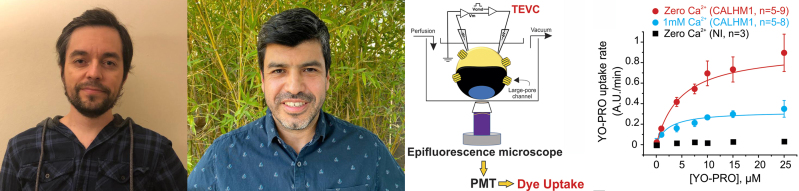
Pablo Gaete (left), Jorge Contreras (center), and colleagues develop a novel voltage-clamp/dye uptake assay (second from right) that allows researchers to simultaneously monitor the permeation of small molecules and atomic ions through large-pore channels. Among other new details, the researchers reveal that molecular transport through CALHM1 channels is saturable and Ca2+-dependent (far right).
Ion transport through large-pore channels can be measured using standard electrophysiological techniques, which are often easiest to perform in large cells such as Xenopus oocytes. Small molecule transport, in contrast, is normally assessed using fluorescent dye uptake assays. “But those assays are hard to do in oocytes because they’re not transparent and have a lot of autofluorescence,” explains Jorge Contreras from Rutgers New Jersey Medical School.
This means that ion and small molecule transport through large-pore channels cannot be measured simultaneously, and the voltage-dependence of molecular transport cannot be examined by varying membrane potential. To address these problems, Contreras and colleagues, including first author Pablo Gaete, devised a technique to perform dye uptake assays in voltage-clamped Xenopus oocytes (1).
First, the oocytes are centrifuged to clear yolk and pigment granules from the animal pole, creating a translucent window into the cell. Next, the oocytes are injected with salmon DNA to provide an almost infinite number of binding sites for fluorescent, DNA-intercalating dyes such as ethidium or YO-PRO, which can be taken up into cells by exogenously expressed large-pore channels. Dye uptake can then be measured while the oocytes are voltage-clamped, allowing researchers to control the membrane potential and also quantify the number of functional channels in each cell, in turn enabling them to obtain accurate kinetic parameters, including Vmax and Km, for molecular transport.
Using this approach, Gaete et al. analyzed the uptake of YO-PRO into oocytes expressing CALHM1, a protein that forms voltage-gated and Ca2+-sensitive channels and is thought to control neuronal excitability (2). Surprisingly, given that large-pore channels are often thought to allow the free diffusion of small molecules and ions, the researchers found that YO-PRO uptake through CALHM1 became saturated at low micromolar concentrations of the dye. “That indicates that these channels share properties with molecular transporters,” Contreras says.
The researchers found that YO-PRO transport was voltage- and Ca2+-dependent, but still occurred at membrane potentials/extracellular Ca2+ concentrations at which ion permeation through CALHM1 is negligible.
Gaete et al. then analyzed the uptake of ethidium into oocytes expressing connexins, components of gap junctions that can also form large-pore “hemichannels” mediating transport between the cytosol and the extracellular milieu (3). Ethidium uptake through Cx30 channels was also saturable, the researchers discovered, but the dye isn’t transported at all by Cx26 channels, even though the two connexins share >90% sequence similarity and, according to MD simulations, form channels with similar pore sizes.
The two connexins mostly differ in a short N-terminal helix that projects into the pore. Gaete et al. found that disease-causing mutations in this region can alter the channels’ selectivity for molecular transport. For example, Cx30G11R, which causes hidrotic ectodermal dysplasia, abolished ethidium transport even though it increases ionic currents through the channel.
“Again, this suggests that small molecules and ions permeate via different mechanisms,” Contreras says. “We believe this might be associated with distinct channel conformations, which is something we want to look at next.”
References
- 1.Gaete, P.S., et al. 2020. J. Gen. Physiol. 10.1085/jgp.202012607 [DOI] [Google Scholar]
- 2.Ma, Z., et al. 2012. Proc. Natl. Acad. Sci. USA. 10.1073/pnas.1204023109 [DOI] [Google Scholar]
- 3.Saez, J.C., et al. 2003. Physiol. Rev. 10.1152/physrev.00007.2003 [DOI] [Google Scholar]