Abstract
Background:
We have shown previously in multivariable analysis that black men had 19% lower risk of death than white men with metastatic castration resistant prostate cancer (mCRPC) treated with a docetaxel (D) and prednisone (P)-based regimen. The primary goal of this analysis was to compare progression-free survival (PFS), biochemical PFS, ≥50% decline in PSA from baseline and objective response rate (ORR) in white, black and Asian men with mCRPC treated with a DP-based regimen.
Patients and Methods:
Individual patient data from 8,820 mCRPC men randomized on nine phase III trials to DP-containing regimen were combined. Race used in the analysis was based on self-report. Endpoints were PFS, biochemical PSA, ≥50% decline in PSA from baseline and ORR. The proportional hazards and the logistic regression models were employed to assess the prognostic importance of race in predicting outcomes adjusting for established prognostic factors.
Results:
Of 8,820 patients, 7,528 (85%) were white, 500 (6%) were black, 424 were Asian (5%) and 368 (4%) had race unspecified. Median PFS were in months 8.3 (95% CI 8.1-8.5), 8.2 (95% CI 7.4-8.8), and 8.3 (95% CI 7.6-8.8) in white, black and Asian men, respectively. Median PSA PFS were 9.7 months (95% CI 9.4-10), 8.5 months (95% CI 7.6-10) and 10.0 (95% CI 9.5-11.8) in white, black and Asian men, respectively.
Conclusions:
We observed no differences in clinical outcomes by race and ethnic groups in men with mCRPC enrolled on these phase III clinical trials with DP.
Keywords: Docetaxel, disparity, progression-free survival, biochemical progression, PSA decline, objective response rate
Introduction
Racial disparities in treatment outcomes in men with localized prostate cancer has been well documented, although the causes for these differences are not fully understood [1-7]. In contrast, data on racial disparities in men with advanced prostate cancer are more limited. Results of analyses of clinical outcomes in men with metastatic castration-resistant prostate cancer (mCRPC) as a function of race is not well documented and have been inconsistent [8-12]. We have previously reported two pooled analyses of disparities in overall survival in men with mCRPC [10-12]. The first report was based on 1,188 mCRPC patients enrolled in eight phase II and phase III trials between 1991 and 2002, in which we demonstrated that the hazard ratio for death was 0.77 for black compared to white men, corresponding to a 23% lower risk of death in black men [10-12]. The second analysis was based on 8,028 men treated with docetaxel plus prednisone (DP) enrolled in nine phase III trials conducted between 1990 and 2014 [10, 11]. In the latter report, we demonstrated that although black men had similar median overall survival to white men, in an adjusted analysis black men with mCRPC had a 19% lower risk of death compared to white men treated with DP-based regimen [12].
Analyses by race in men with mCRPC based on intermediate clinical outcomes, such as progression-free survival, PSA decline and objective response rate are sparse. We undertook a pooled analysis to determine whether black men had worse clinical outcomes than white men with mCRPC who were enrolled in phase III trials. Our motivation for this analysis was to assess if there are treatment differences in black versus white men with mCRPC. If differences in clinical outcomes by race are observed, this may reflect differences in the biology of disease as the data in our analysis are based on patients who had access to clinical trials. We also explored whether Asian men and Hispanic men had worse clinical outcomes than white men. The clinical outcomes evaluated across these ethnic groups were established endpoints: progression-free survival (PFS), biochemical PFS, 50% decline in PSA from baseline and objective response (ORR).
Methods
This analysis was approved by the Duke University Medical Center Institutional Review Board. We performed a systematic search of the literature encompassing the period January 2004-July 2015 using PubMed and clinicaltrials.gov to identify trials testing docetaxel/prednisone (DP) versus DP plus an experimental agent in men with mCRPC, as described previously [12-13]. The first author had access to the individual patient data from each of the selected trials that was requested from the pharmaceutical sponsor or the National Cancer Institute National Clinical Trials Network (NCI NCTN) coordinating group [14-23]. Data on 8,820 patients were available (supplementary Figure S1A).
Endpoints
Progression-free survival was determined for every patient, based on a per-protocol definition of PFS and was provided as a composite endpoint by the sponsor. Other endpoints considered were biochemical PFS, ≥50% decline in PSA from baseline and objective response rates (ORR). Biochemical progression-free survival (bPFS), was defined per the PSA Working Group 2 as the time between date of random allocation to date of biochemical progression or death, whichever occurred first [24]. Fifty percent decline in PSA from baseline was defined as a binary endpoint following the standard definition of PSA-working group 2 [24]. Objective response rate (ORR) was defined as a binary endpoint based on whether a patient experienced a best overall tumor response of complete or partial response. The ORR was evaluated for patients with bidimensionally measurable disease. PSA data from the TAX 327 trial was not available from the sponsor. Thus, this trial (TAX327) was excluded from the analysis of the biochemical PFS and PSA-decline endpoints. Objective response data were not available for the TAX 327, ENTHUSE 33, and SYNERGY trials and the patients enrolled on these trials (2,633 of 5,502 patients with measurable disease) were excluded from the ORR analysis.
Statistical Analysis
Race and ethnicity were self-reported; and the collection of race across the trials is listed in supplementary Table S1. Ethnicity was collected only in the three NCI NCTN trials (CALGB 90401, SWOG9916 and SWOG 0421). 368 (4%) men with unspecified/unknown racial category were excluded from the analysis. We conducted several analyses to estimate the pooled hazard ratio (HR for PFS and biochemical PFS) or the pooled odds ratio (OR for the binary outcomes: PSA decline and ORR) to test if there were differences in PFS, bPFS, PSA decline, and ORR in: 1) black vs. white men and 2) in Asian vs. white men. In addition, we performed sensitivity analyses by assessing: (1) only patients enrolled on the NCI NCTN trials, and in (2) patients who were randomized to the DP arm only by excluding patients who received experimental therapy. Since race and ethnicity were only collected only in the NCI NCTN trials, we performed additional analyses and explored the clinical outcomes including overall survival (OS) in Hispanic vs. non-Hispanic white men who were enrolled in the NCTN trials.
We used a two-stage fixed mixed effect model, as previously reported for obtaining the pooled HR and the odds ratios [12-13]. In the first stage, we estimated the HRs (for PFS and biochemical PFS) or ORs (for PSA decline and ORR) for each of the racial groups within each trial. We conducted both univariate and multivariable analyses of race and ethnicity predicting clinical outcomes by employing the proportional hazards or the logistic regression models. There were no statistically significant differences in OS by treatment arms (supplementary Figure S1A), however, differences in clinical outcomes by treatment arms were observed across the nine trials in biochemical progression-free survival (supplementary Figure S1B), in PFS (CALGB 90401 & MAINSAIL), in PSA decline and ORR (CALGB 90401 & VENICE). To minimize the impact of confounding, in multivariable analyses we adjusted for treatment assignment, age, performance status, alkaline phosphatase, PSA, hemoglobin, and site of metastases. These were the common baseline factors that were collected across the trials. Thus, our primary analyses were based on the results of the multivariable analyses of the proportional hazards and logistic regression models. In the second stage, we combined the estimates from all the individual trials in order to acquire summary estimates of the HR (or OR) along with the estimated variance. We used both Cochran Q and I2 statistics to test for heterogeneity across the trials. The Q statistic tested for the homogeneity of the estimated HRs or ORs across the studies, whereas the I2 determined the fraction of the total variation in the study estimates that can be accounted for heterogeneity. All p-values were computed by the chi-square and we considered a two-sided p-value <0.05 as statistically significant.
Within each clinical trial, we analyzed the data using a modified intent-to-treat analysis. TAX327 and SWOG9916 trials included patients that were randomized to non-docetaxel arms; thus patients in these arms were excluded from the analysis. The Kaplan-Meier product-limit approach was utilized to estimate the PFS and bPFS distributions by the three racial groups. Furthermore, we summarized the individual hazard ratios and pooled hazard ratio (or ORs) estimates along with the 95% confidence intervals and presented the results in forest plots. All analyses were conducted in R 3.6.1.
Results
Baseline Characteristics
Ten trials that enrolled 8,820 mCRPC patients between 1990 and 2014 were identified (Figure 1). The number of patients, treatment arm and accrual period are listed in supplement Table S2. The baseline characteristics of patients across the three racial groups are presented in Table 1. Of 8,452 men with mCRPC, 7,528 (89%) were white, 500 (6%) were black, and 424 were Asian (5%). While roughly similar, there were several differences in baseline characteristics across the racial groups. Ninety percent of black men had a performance status of 0-1 compared with 95% of white men. The median PSA levels were 60 ng/ml for Asians, 85 ng/ml for white men, and 127 ng/ml for black men. The median hemoglobin levels were 13 g/dL for white men, 12 g/dL for black men, and 12 g/dL for Asians. In addition, the median alkaline phosphatase levels were different between white (median=138 U/L) and Asians (median=150 U/L).
Figure 1.
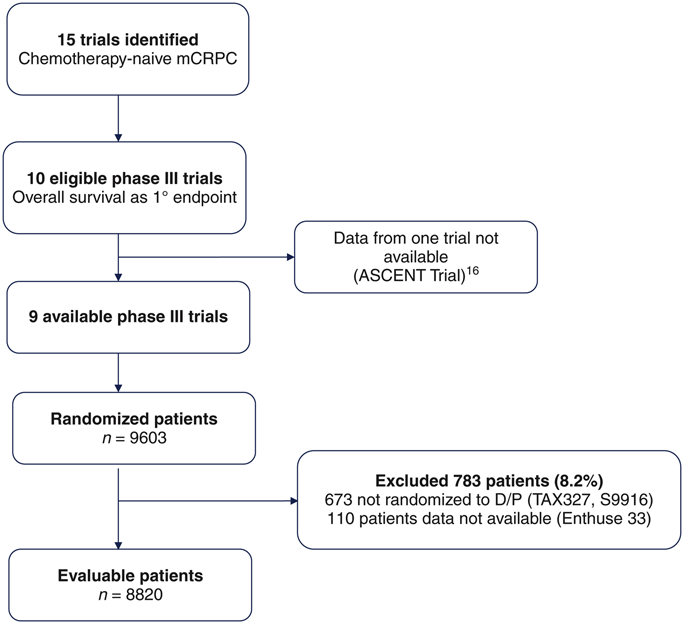
PRISMA diagram.
DP, docetaxel/prednisone; mCRPC, metastatic castration-resistant prostate cancer.
Table 1:
Baseline Characteristics Baseline Characteristics of 8,452 Men by Racial Groups
| Baseline Characteristic | Black (N=500) |
Asian (N=424) |
White (N=7,528) |
Total (N=8,452) |
|---|---|---|---|---|
| Median Age, years (25th, 75th percentile) | 67.6 (60.3, 73.0) | 69.0 (62.0, 73.0) | 69.0 (63.0, 74.0) | 69.0 (63.0, 74.0) |
| Performance status (%) | ||||
| 0 | 211 (42.2) | 214 (50.5) | 3352 (44.5) | 3777 (44.7) |
| 1 | 240 (48.0) | 190 (44.8) | 3746 (49.8) | 4176 (49.4) |
| 2 | 48 (0.6) | 20 ( 4.7) | 410 (5.4) | 478 (5.6) |
| Missing | 1 (0.2) | 20 (0.3) | 21 (0.3) | |
| Treatment Assignment | ||||
| Docetaxel (%) | 283 (56.6) | 219 (56.6) | 3889 (51.7) | 4391(51.9) |
| Experimental (%) | 217 (43.4) | 205 (43.4) | 3639 (48.3) | 4061(48.1) |
| Median testosterone ng/dL (25th, 75th percentile) | 20.0 (10.0, 31.0) | 15.0 (7.3, 25.0) | 18.0 (10.0, 26.0) | 18.0 (10.0, 26.0) |
| Median alkaline phosphatase U/L (25th, 75th percentile) | 127.0 (82.0, 263.0) | 150.0 (91.5, 343.5) | 138.0 (85.0, 284.0) | 137.0 (85.0, 287.0) |
| Median PSA ng/ml (25th, 75th percentile) | 126.8 (44.7, 350.4) | 60.1 (19.1, 170.0) | 84.9 (30.5, 246.6) | 85.1 (30.4, 249.9) |
| Median hemoglobin g/dL (25th, 75th percentile) | 11.9 (10.7, 13.0) | 12.2 (11.0, 13.4) | 13.0 (11.8, 14.2) | 12.9 (11.7, 14.1) |
| Site of metastases (%) | ||||
| Lymph Nodes (LN) | 31 (6.2) | 19 (4.5) | 507 (6.7) | 557 (6.6) |
| Bone/Bone+LN | 347 (69.4) | 313 (73.9) | 5428 (72.1) | 6088(72.1) |
| Lung | 61 (12.2) | 21 (5.0) | 680 (9.0) | 762 (9.0) |
| Liver | 32 (6.4) | 53 (12.5) | 634 (8.4) | 719 (8.5) |
| Other | 29 (5.8) | 18 ( 4.1) | 279 (3.8) | 326 (3.8) |
Among the 2,337 mCRPC men enrolled in three NCTN trials, 110 patients were Hispanic, 90 were identified as Hispanic white and 1,843 as non-Hispanic whites. In general, Hispanic white men had similar baseline characteristics to non-Hispanic white men. Hispanic white men were diagnosed at an earlier age (median 66.5 years) compared to non-Hispanic white (69.8 years, supplementary Table S3). Hispanic white men also had higher alkaline phosphatase (150 U/L vs. 122 U/L) and lower hemoglobin levels (12.1 g/dL vs. 12.8 g/dL) than non-Hispanic white patients.
Clinical Outcomes
Progression-Free Survival (PFS)
There were no differences in the PFS distributions of white, black and Asian men with median PFS 8.3 months (95% CI=8.2-8.5), 8.2 months (95% CI=7.4-8.8); and 8.3 months (95% CI=7.6-8.8), respectively (Figure 2A). Table 2 summarizes the pooled HRs and pooled ORs for the clinical outcomes by race and ethnic groups adjusting for important prognostic variables. In addition, we present the results of the pooled HRs and ORs of clinical outcomes by race/ethnicity based on univariate analyses (Table 2). The pooled HRs from the univariate analysis for black men vs. white men were 1.08 (95% CI=0.96-1.17, supplementary Figure S2A) and the pooled HR for PFS for Asian men vs. white men was 1.10 (95% CI=0.98-1.23, supplementary Figure S2B). From the multivariable analysis, the pooled HR for progression or death for black men vs. white men was 0.96 (1.06 (95%CI=0.86-1.07; Figure 2B). Furthermore, the pooled HR for Asian men vs. white men was 1.00 (95% CI=0.89-1.12, Figure 2C).
Figure 2.
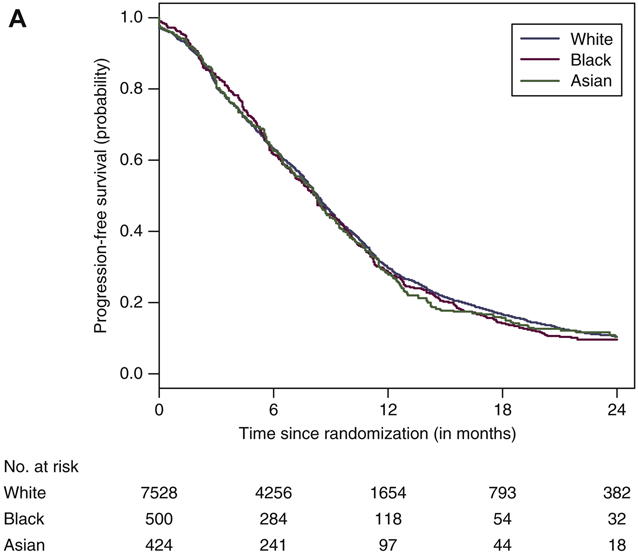
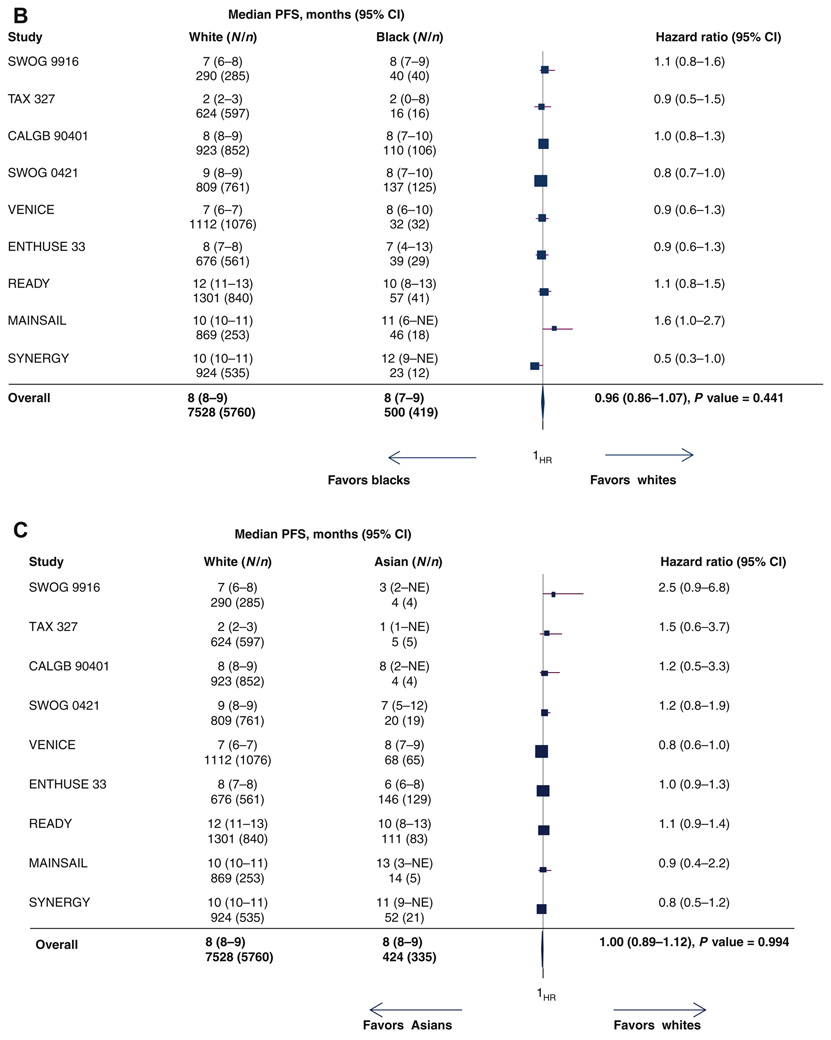
(A) Progression-free survival curves by race. (B) Forest plot with hazard ratios (HR) for progression-free survival (PFS) for black versus white men (reference group [ white men, Q [ 11.565, df [ 8, P [ 0.172, I2 [ 0.308). (C) Forest plot with HR for PFS for Asian versus white men (reference group [ white men, Q [ 9.844, df [ 8, P [ 0.276, I2 [ 0.187).
CI, confidence interval; N, number of patients; n, number of progression or death events; NE, not estimated.
Table 2.
Summary of Clinical Outcomes by Race and Ethnic Groups
| All Patients | Randomized to DP Arm Only | Enrolled on the NCTN Trials | |||||
|---|---|---|---|---|---|---|---|
| Black vs. White | Asian vs. White | Black vs. White | Asian vs. White | Black vs. White | Asian vs. White | Hispanic White vs. non- Hispanic White+ |
|
| OS: Univariate HR (95% CI) | 1.00(0.89-1.12) | 1.13(1.00-1.29) | 0.99 (0.85-1.15) | 1.16(0.98-1.39) | 0.94 (0.82-1.09) | 1.18(0.78-1.81) | 1.06 (0.83-1.35) |
| Adjusted HR (95% CI)† | 0.81(0.72-0.91)* | 0.95 (0.84-1.09)** | 0.79(0.67- 0.93)* | 1.04(0.86-1.24)** | 0.76 (0.66-0.88)* | 1.24(0.81-1.89)** | 0.98 (0.77-1.25) |
| PFS: Univariate HR (95% CI) | 1.06 (0.96-1.17) | 1.10 (0.98-1.23) | 1.08(0.95-1.24) | 1.14(0.97-1.33) | 1.08(0.95-1.23) | 1.36(0.93-1.99) | 1.11(0.89-1.38) |
| Adjusted HR (95% CI)† | 0.96(0.86- 1.07) | 1.00 (0.89-1.12) | 0.96(0.86-1.07) | 1.02(0.87-1.20) | 0.96(0.84-1.10) | 1.35(0.92-1.99) | 1.02 (0.82-1.28) |
| bPFS: Unadjusted HR (95% CI) | 1.12 (0.98-1.28) | 1.09 (0.93-1.27) | 1.09 (0.92-1.30) | 1.11(0.90-1.37) | 1.10 (0.94-1.30) | 1.10(0.62-1.95) | 1.11 (0.84-1.45) |
| Adjusted HR (95% CI)† | 1.05 (0.93-1.21) | 0.99 (0.84-1.12) | 1.07(0.93-1.22) | 0.99( 0.85-1.16) | 1.04 (0.88-1.24) | 1.17 (0.66-2.08) | 1.08 (0.81-1.44) |
| PSA decline: Univariate Odds Ratio (95% CI) | 0.86 (0.70-1.04) | 0.94(0.75-1.17) | 0.86 (0.70-1.04) | 0.94 (0.75-1.17) | 0.79(0.61-1.03) | 1.23 (0.55-2.75) | 0.54 (0.33-0.87) |
| Adjusted Odds Ratio (95% CI)† | 1.05 (0.85-1.30) | 1.13 (0.90-1.42) | 0.91(0.69-1.22) | 1.06 (0.77-1.46) | 0.99 (0.75-1.31) | 1.38 (0.61-3.14) | 0.56 (0.33-0.93) |
| Objective Response Rate: Univariate Odds Ratio (95% CI) | 0.93 (0.66-1.31) | 0.80 (0.52-1.22) | 0.84(0.51-1.38) | Small numbers | 0.87(0.56-1.36) | 1.86(0.49-6.94) | 0.85 (0.41-1.74) |
| Adjusted Odds Ratio (95% CI)† | 1.07 (0.74-1.55) | 0.95 (0.61-1.49) | 1.03(0.60-1.77) | 0.98 (0.63-1.60) | 2.57 (0.64-10.3) | 1.04 (0.50-2.30) | |
Collected only in the NCI National Clinical Trials Network (NCTN)
Published in Halabi et al. JCO, 2019 [12]
In press Halabi et al. JNCI Cancer Spectrum
Adjusted for treatment assignment, age, performance status, alkaline phosphatase, PSA, hemoglobin, and site of metastases
In sensitivity analyses, we estimated the median PFS in white, black, and Asian men enrolled in NCTN trials. The median PFS in black and white men were the same (8 months), whereas the median PFS was shorter in Asian men (6 months). In men who were enrolled in the NCI NCTN trials, the pooled HRs for PFS from univariate analyses for black men and for Asian men vs. white men were 1.08 (95% CI=0.95-1.23) and 1.36 (95% CI=0.93-1.99, Table 2), respectively. From the multivariable analysis, the pooled HRs for PFS for black men vs. white men and for Asian men vs. white men were 0.96 (95% CI=0.84-1.10) and 1.35 (95% CI=0.92-1.99; Table 2), respectively. It is noteworthy that the number of events in the Asian group is particularly small; thus, caution should be exercised in interpreting the results from the NCTN trials
In a separate sensitivity analysis, we estimated the hazard ratio for PFS by the three racial groups in men who were randomized to DP alone (Table 2). The univariate pooled HRs for PFS for black men and for Asian men vs. white men were 1.08 (95% CI=0.95-1.24) and 1.14 (95% CI=0.97-1.33). From the multivariable analysis, the pooled HRs for PFS for black men and for Asian men vs. white men were 0.96 (95% CI=0.87-1.07) and 1.02 (95% CI=0.87-1.20), respectively.
Biochemical PFS
Biochemical progression events occurred in 49% of white men, 56% of Black men, and 44% of Asian men. The median bPFS for white, black and Asian men were 9.9 months (95% CI=9.4-10.5), 8.5 months (95% CI= 8.0-10.3) and 11.1 months (95% CI= 9.9-12.5; Figure 3A), respectively. From the univariate analyses, the HRs for biochemical PFS for black and Asian men were 1.12 (95% CI= 0.98-1.28; supplementary Figure S3A) and 1.09 (95% CI=0.93-1.27; supplementary Figure S3B), respectively. In multivariable analysis, the pooled HRs for biochemical PFS for black men vs. white men and for Asian men vs. white men were 1.05 (95% CI=0.97-1.26; Figure 3B) and 0.99 (95% CI=0.84-1.12; Figure 3C).
Figure 3.

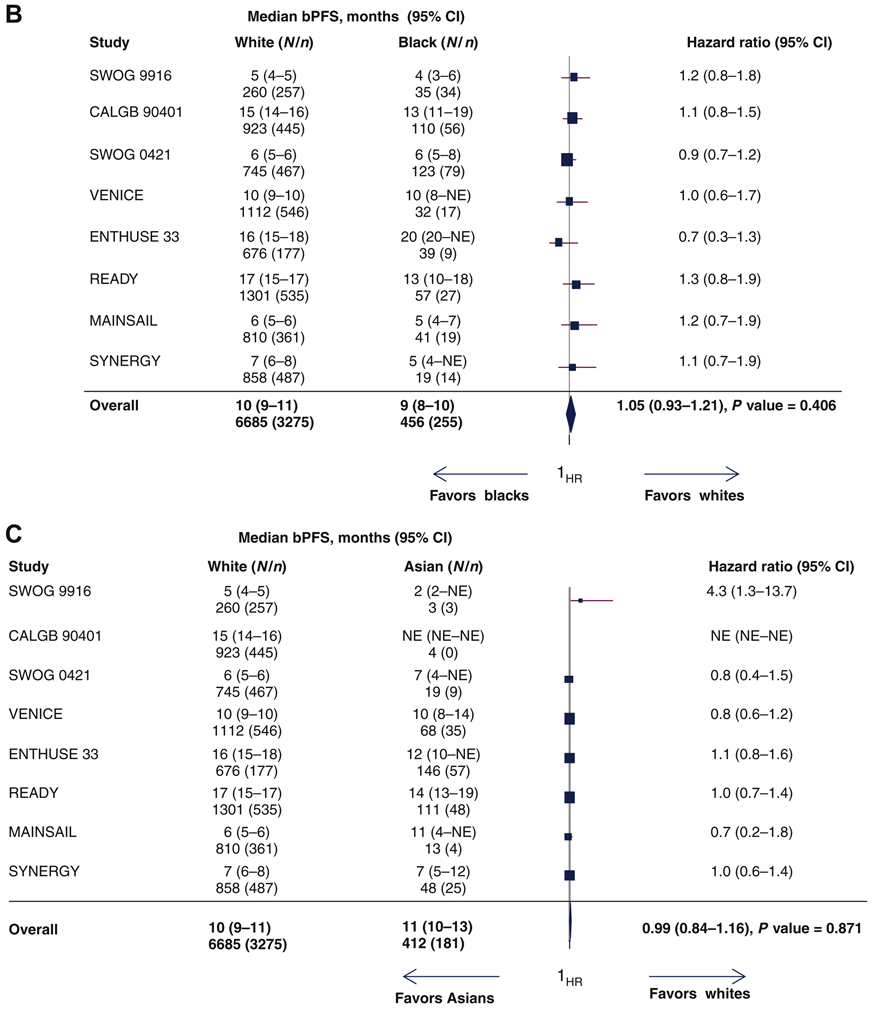
(A) PSA progression-free survival curves by race. (B) Forest plot with hazard ratios (HR) for biochemical progression-free survival (bPFS) for black versus white men (reference group [ white men, Q [ 3.965, df [ 7, P [ 0.784, I2 [ 0.000). (C) Forest plot with HR for bPFS for Asian versus white patients (reference group [ white men, Q [ 8.949, df [ 7, P [ 0.256, I2 [ 0.218).
CI, confidence interval; N, number of patients; n, number of biochemical progression or death events; NE, not estimated; PSA, prostate-specific antigen.
In the NCTN trials, the median bPFS was 9 months (95% CI= 8.8-10) in white, 8 months (95% CI= 7-9) in black, and 7 months (95% CI=4-NE) in Asian men. From the univariate analyses, the pooled HRs for bPFS for black men and Asian men were 1.10 (95% CI=0.94-1.30) and 1.10 (95% CI=0.62-1.95; Table 2). In addition, the pooled HRs for bPFS for black and Asian men vs. white men were 1.04(95% CI=0.88-1.24) and 1.17 (95% CI=0.66-2.08) from the multivariable analyses. In the subgroup of men randomized to DP alone, the pooled HR of black vs. white patients was 1.07 (95% CI=0.93-1.22), while the pooled HR for bPFS for Asian vs. white men was 0.99 (95% CI=0.85-1.16; Table 2).
≥50% Decline in PSA from Baseline
Of the 8,820 patients, PSA data were available on 7,687 men (no PSA outcome data were available from the TAX327 trial and 464 patients enrolled on other trials did not have baseline (on treatment) PSA values available (supplementary Figure S4A). The overall proportion of white, black and Asian men who experienced ≥50% decline in PSA from baseline were 64%, 58% and 62%, respectively. From the univariate analyses, the pooled ORs for PSA response for black and Asian vs. white men were 0.86 (95% CI=0.7-1.04; supplementary Figure S4B) and 0.94 (95% CI=0.75-1.17; supplementary Figure S4C). From the multivariable analyses, the pooled OR for PSA response for black vs. white men were 1.05 (95% CI=0.85-1.30; Figure 4A) whereas the pooled OR for Asian vs. white men was 1.13 (95% CI=0.90 −1.42; Figure 4B). For the subgroup of patients treated with DP alone (Table 2), the pooled ORs for PSA response for black and for Asian men vs. white men were 0.91 (95% CI=0.69-1.22) and 1.06 (95% CI=0.77-1.46), respectively. The number of patients enrolled on the NCTN were small, precluding performing any analyses on the PSA decline and ORR endpoints.
Figure 4.
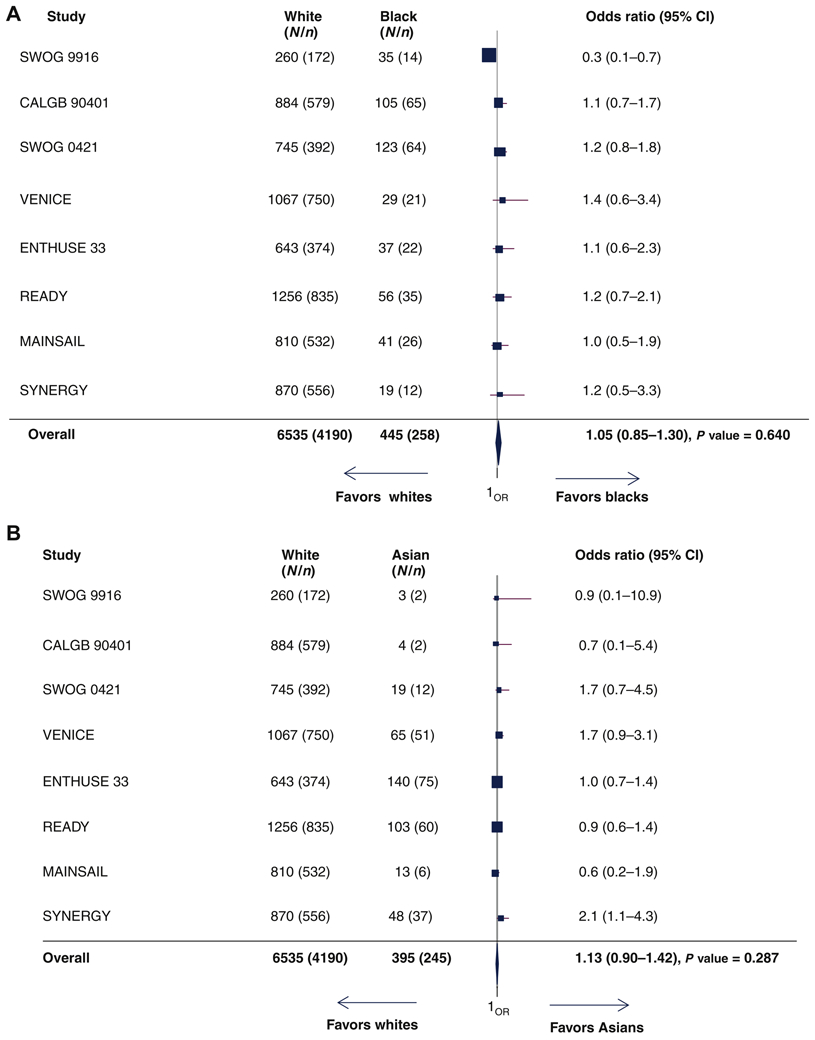
(A) Forest plot with odds ratios (OR) for ‡50% decline in PSA from baseline for black versus white patients (reference group [ white men, Q [ 8.995, df [ 7, P [ 0.256, I2 [ 0.218). (B) Forest plot with OR for ‡50% decline in PSA from baseline for Asian versus white patients (reference group [ white men, Q [ 8.052, df [ 7, P [ 0.328, I2 [ 0.131).
CI, confidence interval; N, number of patients; n, number of patients who experienced ≥50% decline in PSA from baseline; PSA, prostate-specific antigen.
Objective Response Rate
A total of 2,760 men with measurable disease had response data available (supplementary Figure S5A). Eighty-nine percent were white, 7% were black and 4% were Asian. The proportion of white, black and Asian men with a complete or partial response were 39%, 30%, and 34%, respectively. From the univariate analysis, the pooled OR for objective response rate for black and Asian men were 0.93 (95% CI= 0.66-1.31; supplementary Figure 5B) and 0.80 (95% CI=0.52-1.22; supplementary Figure 5C). While the pooled ORs for objective response rates from the multivariable analyses were 1.07 (95% CI=0.74-1.55; supplementary Figure 5D) and 0.95 (95% CI=0.61-1.49; supplementary Figure 5E).
For the subgroup of patients who were randomized to DP alone, the HR for the pooled OR for objective response rate for black men vs. white men was 1.03 (95% CI= 0.61-1.77; Table 2). The number of Asian men with measurable disease that were treated with DP alone or those enrolled in the NCI NCTN trials were too small to conduct these analyses.
Comparing Outcomes of Hispanic White vs. Non-Hispanic White
The median overall survival for Hispanic white and non-Hispanic white was 20 months (supplementary Figure S6A). From univariate analysis, the pooled HR for death for Hispanic white men versus non-Hispanic white men was 1.06 (95% CI=0.83-1.35, Table 2). In multivariable analysis, the pooled HR for death for Hispanic white men vs. non-Hispanic white men was 0.98 (95% CI=0.77-1.25); supplementary Figure S6B). The median PFS for Hispanic and non-Hispanic white was 7 months (95% CI=5-9) and 9 months (95% CI=8-9; supplementary Figure S7A), respectively. From the univariate analysis, the pooled HR for PFS for Hispanic white men vs. non-Hispanic white men was 1.11 (95% CI=0.89-1.38; Table 2). From the multivariable analysis, the pooled HR for PFS for Hispanic white men vs. non-Hispanic White men was 1.02 (95% CI=0.82-1.28; supplementary Figure S7B). The median bPFS for Hispanic and non-Hispanic white was 7 months (95% CI=5-9) and 10 months (95% CI=9-10; supplementary Figure S8A), respectively. The pooled HR for bPFS for Hispanic white men vs. non-Hispanic white men was 1.08 (95% CI=0.81-1.44; supplementary Figure S8B).
The proportion of Hispanic white and non-Hispanic white who experienced a ≥50% decline in PSA from baseline were 47.5% and 61.1%, respectively. From the univariate analysis, the pooled OR for PSA response for Hispanic white men vs. non-Hispanic white men was 0.54 (95% CI=0.33-0.8; Table 2). The OR for PSA response for Hispanic white vs. non-Hispanic white was 0.56 (95% CI=0.33-0.93; supplementary Figure S9) from the multivariable analysis. A total of 51 and 847 Hispanic white and non-Hispanic white men had measurable disease. The proportion of Hispanic and non-Hispanic white men who experienced complete or partial response was 24% and 29%, respectively. The pooled OR for objective response rate for Hispanic white vs. non-Hispanic white men was 1.04 (95% CI=0.50-2.30; supplementary Figure S10).
Discussion
In this meta-analysis, 7,528 were white men, 500 were black, 424 were Asian and 90 were Hispanic white men with mCRPC who were enrolled on randomized phase III trials. We found no differences in PFS and biochemical progression across three racial and ethnic groups: black, white and Asian men. These data suggest that they share a similar hazard of disease progression or death. These data were consistent when evaluated in patients enrolled on the NCTN trials, although there were only 28 Asian men who were enrolled on such trials.
Although a lower proportion of black men experienced a PSA decline than white men, or had an objective response rate, these differences were not statistically significant either in univariate or multivariable analyses. Asian men had a similar proportion of PSA decline and objective response rates to white men. Moreover, we found no differences in overall survival, progression-free survival and biochemical progression in Hispanic white compared to non-Hispanic whites. However, a lower proportion of Hispanic white men had PSA or objective response rates compared to the non-Hispanic men with mCRPC. Our sample size of Hispanic white men enrolled in the NCTN trials was limited and caution must be taken in interpreting the data.
Our results contribute to the ongoing discussion regarding health disparities in prostate cancer outcomes and are in agreement with several analyses based from large clinical trials that have shown that there are no differences in clinical outcomes by racial group in men with prostate cancer [10-12, 25]. In a comprehensive analysis from three large cohorts of men with non-metastatic prostate cancer from: the NRG trials, the Veteran Administration (VA) health care system, and the Surveillance, Epidemiology, and End Results (SEER) registry, no differences in prostate cancer-specific mortality nor in other cause mortality by race were observed in the NRG trials and the VA system [25].
Nevertheless, it is a well-known fact that healthy disparity exits based on the incidence and mortality data from the SEER and other population-based registries [26-30]. Using the SEER database, Dess et al. noted differences in other causes of mortality between black and white men and a higher hazard of death in black vs. white men [25]. In another report, Mahal et al indicated that black men were at a higher risk of prostate cancer-specific death compared to non-black men and described significant interactions between race and PSA-screening eligibility [29]. Aizer et al. reported a higher hazard ratio of prostate cancer mortality in black men than other racial groups [30].
Discrepancies in outcomes in patients enrolled on clinical trials vs. outcomes at the population-level may be due to a selection bias for patients enrolled on clinical trials vs. broader population-based analyses of prostate cancer patients. It has been reported that the prevalence of comorbid factors is adversely related to participation in clinical trials [31]. Men with mCRPC that are eligible for, and choose to participate in, a clinical study are unlikely to be representative of the broader prostate cancer population, and having the social support, access to health care and other intangible elements that allow them to be included in a clinical trial might help to reduce disparities in outcomes.
It has been debated whether the racial disparities seen in cancer outcomes are due to cultural, socioeconomic factors, diet, access to health care, and preventive health factors. Also debated is how much is due to innate biologic differences in prostate cancer as manifested in different ethnic groups [32-40]. There is a body of literature suggesting biological differences that may contribute to the health disparity in prostate cancer outcomes, although these differences have not been well delineated [32-37]; furthermore, the trials included in this analysis did not collect any biologic or genomic data.
The NCI NCTN trials included in this analysis were successful in enrolling a higher proportion of black men (12%) than industry trials (4%), but enrolled only 28 Asian men. This may reflect the fact that many industry trials had large accrual contributions from areas of the world (e.g. Europe and Asia) where the proportion of patients of African heritage is lower. Despite inclusion of 500 black, 424 Asian and 90 Hispanic white men in this analysis, the proportion of black, Hispanic, and Asian men is much lower than the estimated US black, Hispanic and Asian male population of about 14%, 18% and 6%, respectively. Health disparity research is critical to guide treatment for all populations and underrepresented patients with mCRPC. Concerted efforts should be directed to engaging and enrolling more underrepresented patients on clinical trials with mCRPC.
Our results must be considered within the limitations of a retrospective pooled analysis. The study population was highly selected. Thus, these results cannot be generalized to mCRPC men who are treated with non-docetaxel therapies or to men without mCRPC. Further, this analysis does not account for the fact that the population of Asian and Hispanic men with prostate cancer is heterogeneous as race was not further categorized in the clinical trials. Nevertheless the large size of this analysis provides reasonable confidence in concluding that in mCRPC men enrolled on clinical trials, and certainly among men enrolled on trials in which they were treated with DP, there is no compelling evidence to suggest that black men, Asian men, or Hispanic men treated with DP on clinical trials had worse clinical outcomes than white men.
In summary, our analysis shows that patients that were eligible for clinical trials and received the same treatment had no differences in clinical outcomes. Thus, we need to better focus on other variables (such as health care access, biology) which can lead to poor outcomes for black men and other underrepresented groups.
Supplementary Material
Highlights.
Health disparity research is critical to guide treatment for underrepresented patients with mCRPC.
The proportion of black, Asian and Hispanic patients enrolled on the phase III trials is low.
No differences in PFS and biochemical progression across the three racial and ethnic groups were observed.
Concerted efforts should be directed at engaging and enrolling more underrepresented patients on clinical trials with mCRPC.
Acknowledgement
This research was supported by the United States Army Medical Research W81XWH-15-1-0467. The authors thank Alliance, SWOG, AstraZeneca, BMS, Celgene, Oncogenex, Regeneron and Sanofi for sharing their data with them and making this analysis possible. The data from the READY trial was obtained via Supporting Open Access for Research (SOAR) data sharing program which is a collaboration initiative between Duke Clinical Research Institute and Bristol Myers Squibb.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Presented in part at the 2019 GU ASCO and ASCO Annual Meeting (Journal of Clinical Oncology, 37 abstract 5021).
Disclosures
Conflict of Interest: SH reports other from Bayer, Eisai and Ferring; outside the submitted work.
DPP Consultant fees: Ada Cap (Advanced Accelerator Applications), Amgen, Astellas, AstraZeneca, Bayer, Bicycle Therapeutics (added 1/2020), Boehringer Ingelheim, Bristol Myer Squibb, Clovis, Eli Lilly, Exelixis, Incyte, Janssen, Pfizer, Pharmacyclics, Roche Laboratories, Seattle Genetics, Urogen. Grant Support: Ada Cap (Advanced Accelerator Applications), Astellas, AstraZeneca, Bayer, Bristol Myer Squibb, Clovis, Eli Lilly, Endocyte, Genentech, Innocrin, MedImmune, Merck, Novartis, Pfizer, Progenics, Roche Laboratories, Sanofi Aventis, Seattle Genetics ; Ownership interest/investment: Bellicum, Tyme (sold 10/2019).
KC reports grants and personal fees from Janssen, Astellas, Sanofi, Bayer, Roche, and AstraZeneca, outside the submitted work.
JDB has served on advisory boards and received fees from many companies including Astra Zeneca, Astellas, Bayer, Boehringer Ingelheim, Cellcentric, Daiichi, Genentech/Roche, Genmab, GSK, Janssen, Merck Serono, Merck Sharp & Dohme, Menarini/Silicon Biosystems, Orion, Pfizer, Qiagen, Sanofi Aventis, Sierra Oncology, Taiho, Vertex Pharmaceuticals. He is an employee of The ICR, which have received funding or other support for his research work from AZ, Astellas, Bayer, Cellcentric, Daiichi, Genentech, Genmab, GSK, Janssen, Merck Serono, MSD, Menarini/Silicon Biosystems, Orion, Sanofi Aventis, Sierra Oncology, Taiho, Pfizer, Vertex, and which has a commercial interest in abiraterone, PARP inhibition in DNA repair defective cancers and PI3K/AKT pathway inhibitors (no personal income). He was named as an inventor, with no financial interest, for patent 8,822,438. He has been the CI/PI of many industry sponsored clinical trials. JDB is a National Institute for Health Research (NIHR) Senior Investigator. The views expressed in this article are those of the author(s) and not necessarily those of the NHS, the NIHR, or the Department of Health.
CL reports honoraria and consultant fees from Sanofi, Bayer, Janssen, Astellas Pharma. In addition, he reports research grants from Sanofi, Bayer, Janssen, Astellas Pharma and Pfizer.
DIQ reports Compensated consultant: Astellas, Bayer, BMS, Advanced Accelerator Applications, Roche/Genentech, Janssen, Merck, Novartis, Pfizer. Travel: Astellas, Bayer, BMS, Genentech, Merck, Pfizer. Research to institution: Bayer, BMS, Roche/Genentech, Merck, Pfizer
KF : Participation to advisory boards/honorarium for: Astellas, Bayer, Curevac, Janssen, MSD, Orion, Sanofi
MJM reports consultant fees from Bayer, Endocyte, Advanced Accelerator Applications, Blue Earth Diagnostics, Tokai Pharmaceuticals, Tolmar Pharmaceuticals, ORIC Pharmaceutical. Travel: Bayer, Endocyte. In addition he reports research funding to the institution from Bayer, Sanofi, Endocyte, Progenics, Corcept Therapeutics, Roche/Genentech
CSH reports other from Aptevo, Aragon Pharma, Astellas, AstraZeneca, Bayer, Clovis, Dendreon, eFFECTOR Therapeutics, Emergent, Ferring, Genentech, Hoffman-Laroche, Medivation, and Pfizer. She reports personal fees from Aptevo, Asana, Astellas, Bayer, Blue Earth Diagnostics, Pharma, fees from Clovis, Dendreon, Endocyte, Ferring, Hinova, Janssen, Merck, Myriad, Orion, Pfizer, Tolmar, Carrick Therapeutics,Novartis, outside the submitted work.
IFT reports other from Sanofi, during the conduct of the study; other from Janssen, Bayer, Roche-Genentech, outside the submitted work.
EJS reports consultant fees and honoraria from Fortis, Janssen Oncology, Beigene, Tolero Pharmaceuticals. Travel from Janssen. In addition, he reports research grants to institution from Janssen, Merck.
These authors have no COI (Drs. Dutta, Tangen, Rosenthal, Thompson Jr, Araujo, Eisenberger, and Kelly).
References
- 1.Freedland SJ, Isaacs WB. Explaining racial differences in prostate cancer in the United States: sociology or biology? The Prostate 2005; 62:243–252. [DOI] [PubMed] [Google Scholar]
- 2.Eastham JA, Carver B, Katz J, et al. : Clinical stage T1c prostate cancer: pathologic outcomes following radical prostatectomy in black and white men. The Prostate 2002;50:236–240. [DOI] [PubMed] [Google Scholar]
- 3.Freeman VL, Durazo-Arvizu R, Keys LC, et al. Racial differences in survival among men with prostate cancer and comorbidity at time of diagnosis. American J Public Health 2004; 94: 803–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chornokur G, Dalton K, Borysova ME, et al. Disparities at presentation, diagnosis, treatment, and survival in African American men, affected by prostate cancer. The Prostate 2001;71: 985–997, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hoffman RM, Gilliland FD, Eley JW, et al. Racial and ethnic differences in advanced-stage prostate cancer: the Prostate Cancer Outcomes Study. Journal of the National Cancer Institute 2001;93: 388–395. [DOI] [PubMed] [Google Scholar]
- 6.Brawley OW, Knopf K, Thompson I. The epidemiology of prostate cancer part II: the risk factors. Seminars Urol Oncol 1998;16:193–201. [PubMed] [Google Scholar]
- 7.Cooperberg MR. Re-examining racial disparities in prostate cancer outcomes. J Clin Oncol 2013;20:2979–2980. [DOI] [PubMed] [Google Scholar]
- 8.Thompson IM, Tangen CM, Tolcher A, et al. : Association of African-American ethnic background with survival in men with metastatic prostate cancer. JNCI 2001;93:219–225. [DOI] [PubMed] [Google Scholar]
- 9.Tangen CM, Hussain MHA, Higano CS, et al. : Improved overall survival trends of men with newly diagnosed m1 prostate cancer: A SWOG phase iii trial experience (S8494, S8894 and S9346). J Urol 2012;188:1164–1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Halabi S, Small EJ, Vogelzang NJ, et al. : Impact of race on survival in men with metastatic hormone refractory prostate cancer. Urol 2004;64:212–217. [DOI] [PubMed] [Google Scholar]
- 11.Halabi S, Vogelzang NJ, Ou SS, et al. : Clinical outcomes by age in men with hormone refractory prostate cancer: A pooled analysis of eight Cancer and Leukemia Group B (CALGB) studies. J Urol 2006;176:81–86. [DOI] [PubMed] [Google Scholar]
- 12.Halabi S, Dutta S, Tangen C, et al. : Overall survival of black and white men with metastatic castration-resistant prostate cancer treated with docetaxel. J Clin Oncol 2019;37:403–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Halabi S, Kelly WK, Ma H, et al. : A meta-analysis evaluating the impact of site of metastasis on overall survival in men with castration-resistant prostate cancer. J Clin Oncol 2016; 34:1652–1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tannock IF, DeWit R, Berry W, et al. : Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med 2004;351:1502–1512. [DOI] [PubMed] [Google Scholar]
- 15.Petrylak DP, Tangen CM, Hussain MHA, et al. : Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med 2004;351:1513–1520. [DOI] [PubMed] [Google Scholar]
- 16.Scher HI, Jia X, Chi K et al. : Randomized, open-label phase III Trial of docetaxel plus high-dose calcitriol versus docetaxel plus prednisone for patients with castration-resistant prostate cancer. J Clin Oncol 2011;29:2191–2198. [DOI] [PubMed] [Google Scholar]
- 17.Kelly WM, Halabi S, Carducci M, et al. : Randomized, double-blind, placebo-controlled phase III trial comparing docetaxel and prednisone with or without bevacizumab in men with metastatic castration-resistant prostate cancer: CALGB 90401. J Clin Oncol 2012;30:1534–1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fizazi K, Higano C, Nelson J et al. : Phase III, randomized, placebo-controlled study of docetaxel in combination with zibotentan (ZD4054) in patients with metastatic castration-resistant prostate cancer. J Clin Oncol 2013;31:1740–1747. [DOI] [PubMed] [Google Scholar]
- 19.Quinn DI, Tangen CM, Hussain M, et al. : Docetaxel and atrasentan versus docetaxel and placebo for men with advanced castration-resistant prostate cancer (SWOG S0421): A randomised phase 3 trial. Lancet Oncol 2013;14:893–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tannock IF, Fizazi K, Ivanov S, et al. : Aflibercept versus placebo in combination with docetaxel and prednisone for treatment of men with metastatic castration-resistant prostate cancer (VENICE): a phase 3, double-blind randomised trial. Lancet Oncol 2013;14:760–768. [DOI] [PubMed] [Google Scholar]
- 21.Araujo JC, Trudel GC, Saad F, et al. : Docetaxel and dasatinib or placebo in men with metastatic castration-resistant prostate cancer (READY): a randomised, double-blind phase 3 trial. Lancet Oncol 2013;14:1307–1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Petrylak DP, Vogelzang NJ, Budnik N, et al. : Docetaxel and prednisone with or without lenalidomide in chemotherapy-naive patients with metastatic castration-resistant prostate cancer (MAINSAIL): A randomised, double-blind, placebo-controlled phase 3 trial. Lancet Oncol 2015;16:417–425. [DOI] [PubMed] [Google Scholar]
- 23.Chi KN, Higano CS, Blumenstein BA, et al. : Custirsen in combination with docetaxel and prednisone for patients with metastatic castration-resistant prostate cancer (SYNERGY trial): a phase 3, multicentre, open-label, randomised trial. Lancet Oncol 2017;18:473–485. [DOI] [PubMed] [Google Scholar]
- 24.Scher HI, Halabi S, Tannock I, et al. : Design and endpoints of clinical trials for Patients with progressive prostate cancer and castrate levels of testosterone: Recommendations of the Prostate Cancer Clinical Trials Working Group (PCWG2). J Clin Oncol 2008; 26:1148–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dess RT, Hartman HE, Mahal BA, et al. : Association of black race with prostate cancer-specific and other mortality. JAMA Oncol 2019. doi: 10.1001/jamaoncol.2019.0826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chinea FM, Patel VN, Kwon D, et al. : Ethnic heterogeneity and prostate cancer mortality in Hispanic/ Latino men: a population-based study. Oncotarget, 2017;8:69709–69721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pinheiro PS, Sherman RL, Trapido EJ, et al. : Cancer incidence in first generation U.S. Hispanics: Cubans, Mexicans, Puerto Ricans, and new Latinos. Cancer Epidemiology, Biomarkers & Prevention. 2009;18:2162–2169. [DOI] [PubMed] [Google Scholar]
- 28.Miller KD, Goding Sauer A, Ortiz AP, et al. : Cancer statistics for Hispanics/Latinos, CA Cancer J Clin 2018;68:425–445. [DOI] [PubMed] [Google Scholar]
- 29.Mahal BA, Chen Y-W, Muralidhar V et al. : Racial disparities in prostate cancer outcome among prostate-specific antigen screening eligible populations in the United States. Ann Oncol 2017;28:1098–1104. [DOI] [PubMed] [Google Scholar]
- 30.Aizer AA, Wilhite TJ, Chen MH, et al. Lack of reduction in racial disparities in cancer-specific mortality over a 20-year period. Cancer 2014;120:1532–1539. [DOI] [PubMed] [Google Scholar]
- 31.Unger JM, Cook E, Tai E, et al. Role of clinical trial participation in cancer research: barriers, evidence, and strategies. Am Soc Clin Oncol Educ Book 2016;35:185–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rebbeck TR. Prostate cancer disparities by race and ethnicity: from nucleotide to neighborhood. Cold Spring Harb Perspect Med 2018;8:a030387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hait WN, Yang JM: The individualization of cancer therapy: the unexpected role of p53. Transactions of the American clinical and climatological association 2016;117:85–101. [PMC free article] [PubMed] [Google Scholar]
- 34.Komura K, Jeong SH, Hinohara K, et al. : Resistance to docetaxel in prostate cancer is associated with androgen receptor activation and loss of KDM5D expression. Proceedings of the National Academy of Sciences 2016; 113: 6259–6264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.de Morrée ES, Böttcher R, van Soest RJ, et al. Loss of SLCO1B3 drives taxane resistance in prostate cancer. British J Cancer 2016; 115: 674–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marín-Aguilera M, Codony-Servat J, Kalko SG, et al. : Identification of docetaxel resistance genes in castration-resistant prostate cancer. Molecular Cancer therapeutics 2012;11:329–339. [DOI] [PubMed] [Google Scholar]
- 37.Ledet EM, Sartor O, Rayford W, et al. Suggestive evidence of linkage identified at chromosomes 12q24 and 2p16 in African American prostate cancer families from Louisiana. Prostate 2012;72:938–947. [DOI] [PubMed] [Google Scholar]
- 38.Moses KA, Orom H, Brasel A, et al. : Racial/ethnic disparity in treatment for prostate cancer: Does cancer severity matter? Urol 2017;99:76–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Watson M, Grande D, Radhakrishnan A, et al. : Racial differences in prostate cancer treatment: the role of socioeconomic status. Ethn Dis 27:201–208; 2017. doi: 10.18865/ed.27.3.201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Warnecke RB, Oh A, Breen N, et al. Approaching health disparities from a population perspective: the National Institutes of Health Centers for Population Health and Health Disparities. Am J Public Health 2008; 98(9):1608–1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


