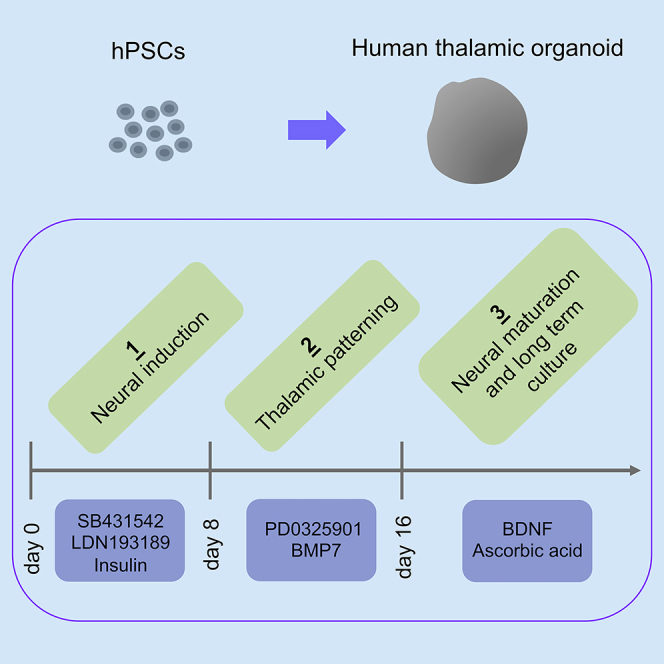
Summary
Thalamus is a critical information relay hub in the cortex; its malfunction causes multiple neurological and psychiatric disorders. However, there are no model systems to study the development and function of human thalamus. Here, we present a protocol to generate regionally specified human brain organoids that recapitulate the development of the thalamus using human pluripotent stem cells (hPSCs). Thalamic organoids can be used to study human thalamus development, to model related diseases, and to discover potential therapeutics.
For complete information on human thalamic organoids and their application, please refer to the paper by Xiang et al. (2019).
Graphical Abstract
Highlights
-
•
Feeder-free culture of hESCs or hiPSCs
-
•
Neural induction of hESC- or hiPSC-derived embryoid bodies
-
•
Production of human thalamus-like brain organoids from hESCs or hiPSCs
Thalamus is a critical information relay hub in the cortex; its malfunction causes multiple neurological and psychiatric disorders. However, there are no model systems to study the development and function of human thalamus. Here, we present a protocol to generate regionally specified human brain organoids that recapitulate the development of the thalamus using human pluripotent stem cells (hPSCs). Thalamic organoids can be used to study human thalamus development, to model related diseases, and to discover potential therapeutics.
Before You Begin
Prepare the below materials before starting the differentiation. Refer to Key Resources Table for a complete list of materials and equipment.
Note: All procedures are performed in a Class II biological hood with standard aseptic technique. Cells and brain organoids are cultured in a humidified 37 °C incubator with 5% CO2.
Alternatives: Here, we describe the generation of thalamic organoids from hESCs (HES-3 NKX2-1GFP/w cells and H-1 cells). We have also had success with peripheral blood mononuclear cell (PBMC)-derived hiPSCs.
Note: if reagents from alternative suppliers are used, you must validate the organoids generated for the first time.
Neural Induction Medium
| DMEM/F-12 | |
|---|---|
| Knockout serum replacement | 15% (v/v) |
| MEM-NEAA | 1% (v/v) |
| Glutamax supplement | 1% (v/v) |
| β-Mercaptoethanol | 100 μM |
| LDN-193189 | 100 nM |
| SB431542 | 10 μM |
| Insulin | 4 μg/mL |
Store up to 10 days at 4°C.
Thalamic Patterning Medium
| DMEM/F-12 | |
|---|---|
| Dextrose | 0.15 (w/v) |
| β-Mercaptoethanol | 100 μM |
| N2 supplement | 1% (v/v) |
| B27 supplement without vitamin A | 2% (v/v) |
| BMP7 | 30 ng/mL |
| PD325901 | 1 μM |
Store up to 8 days at 4°C.
Neural Differentiation Medium
| DMEM/F-12∗ | |
|---|---|
| Neurobasal medium∗ | |
| N2 supplement | 0.5% (v/v) |
| B27 supplement | 1% (v/v) |
| MEM-NEAA | 0.5% (v/v) |
| Glutamax supplement | 1% (v/v) |
| Penicillin/Streptomycin | 1% (v/v) |
| Insulin | 0.025% (v/v) |
| β-Mercaptoethanol | 50 μM |
| BDNF | 20 ng/mL |
| Ascorbic acid | 200 μM |
| Optional: FGF2 | 20 ng/mL (See Troubleshooting step 4) |
Store up to 2 weeks at 4°C.
DMEM/F-12 and neurobasal medium are mixed at 1:1 ratio.
Key Resources Table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| TCF7L2 | Cell Signaling | Cat# 2569; RRID:AB_2199816 |
| Chemicals, Peptides, and Recombinant Proteins | ||
| mTeSR1 | Stem Cell Technologies | Cat# 05875 |
| DMEM-F12 | Life Technologies | Cat# 11330057 |
| Neurobasal Media | Life Technologies | Cat# 2110349 |
| FBS | Life Technologies | Cat# 10437028 |
| Amino acids, non-essential | Life Technologies | Cat# 11140050 |
| Penicillin/Streptomycin | Life Technologies | Cat# 15140-122 |
| Glutamax | Life Technologies | Ca# 35050 |
| Insulin | Sigma | Ca# I9278 |
| β-Mercaptoethanol | Sigma | Ca# M7522 |
| N2 | Life Technologies | Cat# 17502-048 |
| B27 | Life Technologies | Cat# 17504-044 |
| B27 supplement without vitamin A | Life Technologies | Cat# 12587010 |
| bFGF | Millipore | Cat# GF003AF |
| KnockOut Serum Replacement | Life Technologies | Cat# 10828-028 |
| Matrigel | BD | Cat# 354230 |
| Y-27632 | Stem Cell Technologies | Cat# 72304 |
| Dispase (100 mL) | Stem Cell Technologies | Cat# 07913 |
| Accutase (100 mL) | Stem Cell Technologies | Cat# AT104 |
| LDN-193189 | Sigma | Cat# SML0559 |
| SB431542 | Abcam | Cat# ab120163 |
| XAV939 | Sigma | Cat# X3004 |
| BMP7 | GIBCO | Cat# PHC9544 |
| PD0325901 | Axon Medchem | Cat# Axon 1408 |
| BDNF | Prepotech | Cat# 450-02 |
| Ascorbic acid | Sigma | Cat# A92902 |
| Experimental Models: Cell Lines | ||
| HES-3 NKX2-1GFP/w | Elefanty lab | https://www.ncbi.nlm.nih.gov/pubmed/21425409 |
| Other | ||
| U-bottom ultra-low-attachment 96-well plate | Corning | CLS7007-24EA |
| Ultra-low-attachment 24-well plate | Corning | CLS3473 |
| Ultra-low-attachment 6-well plate | Corning | CLS3471-24EA |
| Orbital shaker | IKA | KS260 |
Step-by-Step Method Details
Maintaining Culture of hESCs or hiPSCs
TIMING: 1 week
hESCs or hiPSCs are cultured under feeder-free condition. Passaging is performed once a week when cells approach ∼80% confluency.
Note: Culturing of hPSCs is established by Yale Human Embryonic Stem Cell Core, based on the protocol from Eric Bouhassira’s laboratory to grow human H-1 and H-9 cell lines derived by the James Thomson’s lab at Wisconsin, as well as hESC lines from other labs.
-
1.Thaw 100 μl aliquoted Matrigel on ice. Dilute Matrigel with 6 mL ice-cold DMEM/F-12 medium.Note: see below for the calculation of Matrigel volume required for plate coating, as suggested by WiCell. 1 mL/X mL = given concentration/1 mg (X = mL of Matrigel aliquoted per tube; 1 mg is suggested for use in two 6-well plates).
-
2.
Add 1 mL of diluted Matrigel to each well of the 6-well tissue culture plate.
-
3.
Place the plate in the incubator for 2 h before use. We suggest that the Matrigel-coated plate is used directly 2 h after coating. If storage is needed, keep the coated plate at 4°C before use.
-
4.
Under the stereomicroscope, scrape off differentiated colonies using a 200 μl pipette tip.
-
5.
In the cell culture hood, remove the medium in the well, then add 1 mL DMEM/F-12 medium to each well. DMEM/F-12 medium is warmed up to room temperature (22 - 25°C) before use.
-
6.
Add 166 μl Dispase (5 U/mL) to each well, return the plate to the incubator, and incubate for 7 min. This time period is suitable for different cell lines. At the end of treatment, the edge of the colony should curl up while the major of the colony remain attached to the plate.
-
7.
Take out the plate, remove the Dispase-containing medium from each well, and wash each well with 2 mL DMEM/F-12 medium twice.
-
8.
Remove the wash medium, add another 1 mL DMEM/F-12 medium to each well. Scrape down the colonies vertically and horizontally with 1 mL general pipette tip.
-
9.
Gently mix the cell suspension, and transfer 100 μl colony-containing medium to a 15 mL centrifuge tube. Centrifuge for 3 min at 200 × g.
-
10.
Remove medium from the tube, and re-suspend the cells with 2 mL mTeSR1 medium. mTeSR1 medium was developed for feeder-independent culture of hPSCs (Ludwig et al., 2006a, Ludwig et al., 2006b) and has been widely used. mTeSR1 medium is warmed up to room temperature (22 - 25°C) before use.
-
11.Take out the Matrigel-coated plate (prepared in Step 2), remove Matrigel solution, and add the 2 mL cell suspension to 1 well of the 6-well tissue culture plate.Note: depending on the scale of the organoid induction (or other experiments) to be performed with these cells, the volume of cell suspension and the numbers of plated wells can be adjusted.
-
12.
Return the plate to incubator. Medium is changed daily (2 mL/well) starting from 48 h after the passaging. Unused cells from step 8 can be discarded at this point.
Neural Induction
TIMING: 8 days
When hESCs or hiPSCs approach ∼80% confluency, which typically occurs one week after passaging the cells, single cell suspension is prepared to perform neural induction.
-
1.Under the stereomicroscope, scrape off any differentiated cells.
 CRITICAL: Differentiated cells may interrupt the neural induction and the following differentiation process (Figure 1).
CRITICAL: Differentiated cells may interrupt the neural induction and the following differentiation process (Figure 1). -
2.
In the cell culture hood, remove the medium from each well. Wash the well once with 1 mL DMEM/F-12 medium.
-
3.
Remove the DMEM/F-12 medium, and add 1 mL of pre-warmed Accutase to each well of the 6-well plate.
-
4.
Return the plate to the incubator for 10 min.
-
5.Tap the plate gently to make sure colonies are dissociated (Methods Video 1).Optional: Increase incubation time if dissociation is not complete. Figure 2 shows an example of successful dissociation.Methods Video 1. Neural Induction, Step 5Tap the plate gently to make sure colonies are dissociated.Download video file (4.7MB, mp4)
-
6.
Using 1 mL pipette tip, pipette up and down the Accutase solution in the well ∼4 times to make a single-cell suspension.
-
7.
Transfer the single-cell suspension to a 15 mL centrifuge tube, which contains 5 mL of DMEM/F-12 medium.
-
8.
Spin down the cells for 3 min at 200 × g at room temperature (22 - 25°C).
-
9.
Remove the supernatant, and re-suspend the cells with 1 mL of neural induction medium. Neural induction medium is warmed up to room temperature before use.
-
10.
Mix 10 μl cell suspension with 10 μl of trypan blue solution. Load 10 μl of the mixture to the hemocytometer. Count and calculate live cell concentration.
-
11.Dilute cells in neural induction medium to make final concentration to 60,000 live cells/mL. Add Y-27632 (final concentration of 50 μM) and heat-inactivated FBS (final concentration of 5% [v/v]) to the cell suspension and mix well. Prepare 15 mL cell suspension for the use of a whole 96-well plate.
 CRITICAL: With the concentration of 60,000 live cells/mL, each embryoid body will contain 9,000 live cells (9,000 live celles/150 μl of medium). Here, HES-3 NKX2-1GFP/w cells is used for thalamic organoid generation. The cell number required for optimal thalamic organoid generation can be cell line-dependent and should be determined for the specific cells being used.
CRITICAL: With the concentration of 60,000 live cells/mL, each embryoid body will contain 9,000 live cells (9,000 live celles/150 μl of medium). Here, HES-3 NKX2-1GFP/w cells is used for thalamic organoid generation. The cell number required for optimal thalamic organoid generation can be cell line-dependent and should be determined for the specific cells being used.
-
12.
Adding FBS helps the formation of embryoid body after the single cell suspension is plated. If the cell lines used do not require FBS to efficiently form embryoid body 24 h after plating the cells, then FBS can be removed from this step.
-
13.
Add 150 μl of the single cell suspension in step 11 into each well of the ultra-low attachment 96-well plate, and place the plate to incubator. This is day 0 of the neural induction period.
-
14.
On day 1, gently take out the plate from the incubator, and observe under microscope. An embryoid body with a smooth surface should be formed in each well. Return the plate to the incubator for further culturing. (Figure 3)
-
15.
On day 2, take out the plate, and remove 75 μl of the medium from each well. Add 150 μl of neural induction medium containing 50 μM Y-27632 to each well.
-
16.
On day 4 and 8, remove 100 μl medium from each well, and add 150 μl neural induction medium (without Y-27632) to each well.
Figure 1.
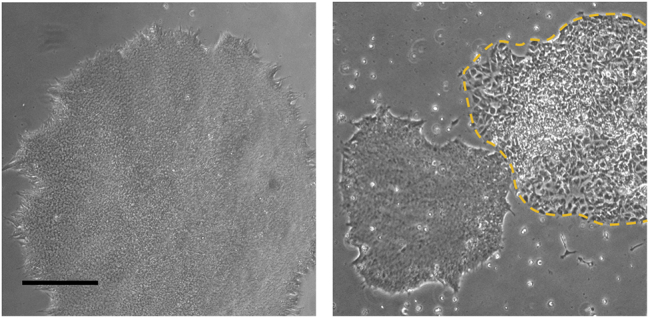
hESC Colonies under Culture
A non-differentiated colony ideal for differentiation (left side), and colonies with differentiated parts that need to be removed before single cell dissociation (labeled by the yellow dash line). The scale bar represents 200 μm.
Figure 2.
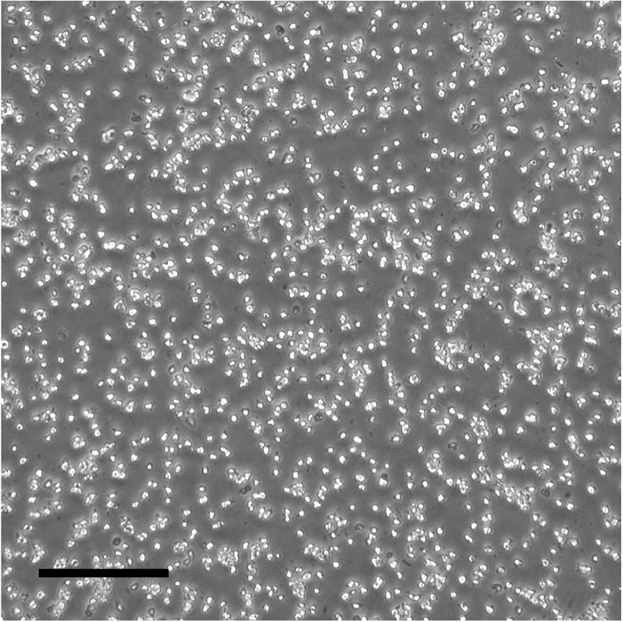
Dissociation of hESCs
A ideal single cell suspension after Accutase treatment. The scale bar represents 200 μm.
Figure 3.
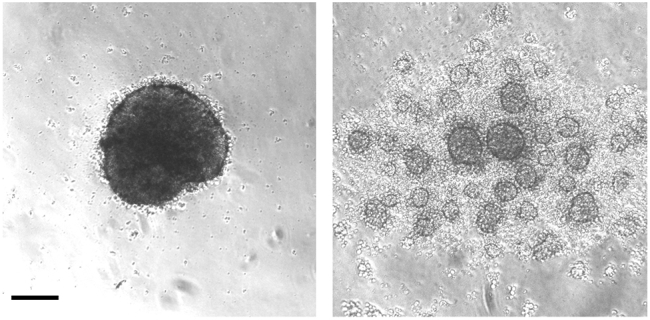
Formation of Embryoid Bodies
Sucessful (left side) and failed (right side) formation of central embryoid body 24 h after plating single cells. The scale bar represents 200 μm.
Thalamic Patterning
TIMING: 8 days
Starting from day 8, embryoid bodies will be collected from the ultra-low attachment 96-well plate and transferred to ultra-low attachment 24-well plate for spinning culture. Eight days of patterning will be performed to achieve thalamic fate.
-
1.On day 8 of differentiation, take out the ultra-low attachment 96-well plate from the incubator. Using a 5 mL pipette, transfer the embryoid bodies from each well to an ultra-low attachment 24-well plate.
 CRITICAL: Transfer the embryoid bodies cautiously, avoid damaging of the samples. Transfer 1 embryoid body to each well of the ultra-low attachment 24-well plate.
CRITICAL: Transfer the embryoid bodies cautiously, avoid damaging of the samples. Transfer 1 embryoid body to each well of the ultra-low attachment 24-well plate. CRITICAL: During transfer, tilt the ultra-low attachment 24-well plate, so that embryoid bodies will fall down in the well with the medium. This will avoid drying the embryoid bodies (Figure 4;Methods Video 2).
CRITICAL: During transfer, tilt the ultra-low attachment 24-well plate, so that embryoid bodies will fall down in the well with the medium. This will avoid drying the embryoid bodies (Figure 4;Methods Video 2).
Figure 4.

The Setting to Tilt the Plate when Transferring Embryoid Bodies
Tilt the ultra-low attachment 24-well plate, so that embryoid bodies will fall down in the well with the medium.
During transfer, tilt the ultra-low attachment 24-well plate, so that embryoid bodies will fall down in the well with the medium. This will avoid drying out the embryoid bodies.
-
2.
After the transferring is done, remove the neural induction medium in each well of the ultra-low attachment 24-well plate.
-
3.Add 1 mL of thalamic patterning medium to each well of the ultra-low attachment 24-well plate.
 CRITICAL: Avoid drying the embryoid bodies when replacing the medium, e.g., if there are 24 embryoid bodies, divide them into four groups. Perform medium replacement 6 embryoid bodies per group.
CRITICAL: Avoid drying the embryoid bodies when replacing the medium, e.g., if there are 24 embryoid bodies, divide them into four groups. Perform medium replacement 6 embryoid bodies per group. -
4.
Place the plate on an orbital shaker inside the incubator, and start spinning culture at a speed of 80 rpm.
-
5.On day 10, 12, and 14, remove the medium from each well and add 1 mL of thalamic patterning medium to each well.
 CRITICAL: Avoid drying the embryoid bodies when replenishing the medium.
CRITICAL: Avoid drying the embryoid bodies when replenishing the medium.
Neural Maturation and Long-Term Culture
TIMING: 3–12 months
-
1.Starting from day 16, change medium with neural differentiation medium, and continue spinning culture. Carefully change medium as described in step 5 in thalamic patterning.Optional: Organoids can be collected and transferred to ultra-low attachment 6-well plate for long term culture. In this case, transfer no more than 6 organoids to each well of an ultra-low attachment 6-well plate.
-
2.Medium is replenished every other day before day 25, then every four days after that.
 CRITICAL: There is no pause point after the neural induction begins. The period of neural differentiation and maturation depends on the purpose of the experiment. Typically, after three months’ culturing, electrophysiologically active neurons can be readily detected inside the organoids.
CRITICAL: There is no pause point after the neural induction begins. The period of neural differentiation and maturation depends on the purpose of the experiment. Typically, after three months’ culturing, electrophysiologically active neurons can be readily detected inside the organoids.
Expected Outcomes
High quality and non-differentiated hPSC colonies are maintained for single cell dissociation. hPSCs being used should possess normal karyotype and are free of any form of microbial contamination (e.g., mycoplasma, fungus, bacteria et al.).
Twenty-four h after plating the single cell suspension, a single central embryoid body with smooth surface should form (Figure 3), and maintain healthy during neural induction (i.e., a smooth surface, no disintegration).
Note: as long as a central embryoid body is formed (Figure 3, left side), a small amount of debris around the embryoid body should be fine. In contrast, a failure to form a central embryoid body (Figure 3, right side) will lead to failure in organoid development. During thalamic patterning, embryoid body should maintain a smooth surface (Figure 5).
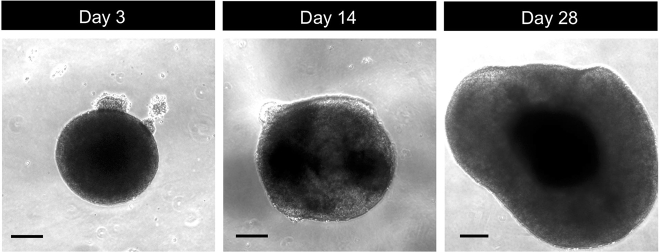
Figure 5.
Human Thalamic Organoids at Different Stages
Photomicrographs of different stages of human thalamic organoid differentiation. Scale bars represent 200 μm.
Organoids remain intact and continue growing during further differentiation and maturation (Figure 5). Size increase may stop after two months’ of culturing.
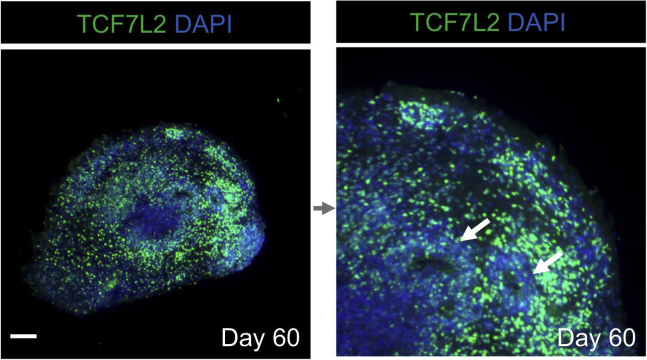
Organoids will express thalamus-specific markers, and produce a cell diversity in a way similar to human thalamus in vivo (Xiang et al., 2019). For a basic confirmation of thalamic induction, qPCR and/or immunostaining analysis for representative markers (e.g., caudal forebrain marker OTX2 and thalamic marker DBX1, GBX2, as well as TCF7L2) can be detected. In particular, TCF7L2+ cells should be widely distributed in thalamic organoids (Figure 6). Once the protocol is established for a specific cell line, it is expected that thalamic organoid will be produced in each differentiation; thus, marker analysis for thalamic organoids is only necessary when working with a new line for the first time).
Figure 6.
Immunostaining of Human Thalamic Organoids
Immunostaining of human thalamic organoid for the thalamus-specific transcriptional factor TCF7L2. Arrows indicate ventricle-like regions in the organoid. The scale bars represent 200 μm.
Limitations
As stated above, the time required for long-term culture depends on specific experimental purpose thus cannot be defined here. For fully functional maturation, long term culturing (e.g., over three months) is required.
Compared to the human brain organoid representing the cerebral cortex, human thalamic organoids display smaller ventricle-like organizations (Xiang et al., 2019). The fundamental difference requires further investigation.
Differentiation efficiency can be cell line-dependent (Lancaster et al., 2017), thus optimization may be needed for specific cell lines to be used. Once the optimization is defined, differentiation tends to remain consistent given the tight signaling control of the development. We have generated thalamic organoids from hESCs (HES-3 NKX2-1GFP/w cells and H-1 cells) and peripheral blood mononuclear cell (PBMC)-derived hiPSCs. All these cell lines efficiently produce thalamic organoids.
Troubleshooting
Problem 1
Low Efficiency of Directed Differentiation.
Potential Solutions
The quality of starting cells should be carefully controlled before differentiation. hESCs or hiPSCs should be maintained according to the standard culturing protocol. For alternative protocol of hESCs or hiPSCs culturing, refer to WiCell for details (https://www.wicell.org/home/stem-cells/support/stem-cell-protocols/-home-stem-cells-support-stem-cell-protocols-stem-cell-protocols-cmsx-.cmsx).
If cells are cultured under MEF condition, a transition to MEF-free culture condition is needed before using the current differentiation protocol.
Make sure any differentiated colonies is removed before single cell dissociation.
Make sure any form of microbial contamination is avoided before and throughout the differentiation.
Make sure all the reagents used are in the correct concentration.
Problem 2
Failure in Embryoid Body Growth.
Potential Solutions
Make sure a single embryoid body is formed 24 h after plating the single cell suspension; otherwise, successful organoid development is unlikely.
Double check the quality of the hESCs or hiPSCs used for differentiation (see problem-1).
Avoid excessive dissociation by Accutase during single cell preparation.
Make sure the neural induction medium is correctly prepared.
As described here and in previous reports, to ensure embryoid body formation, Y-27632 needs to be supplemented for the first 4 days of differentiation (Lancaster and Knoblich, 2014, Xiang et al., 2017, Xiang et al., 2018, Xiang et al., 2019). Make sure the correct concentration of Y-27632 is used.
Problem 3
Disintegration of Brain Organoids Occurs during Differentiation.
Potential Solutions
Check the presence of any contamination in the culture system (e.g., bacteria, fungus, mycoplasma, et al.).
Ensure the medium is prepared correctly, and all the chemicals and cytokines supplemented are in the correct concentration.
Make sure not to break the surface of organoids whenever transferring them.
During thalamic patterning, instead of transferring embryoid bodies to ultra-low attachment 24-well plate, they can also be transferred to ultra-low attachment 6-well plate to start the spinning culture. Researchers can choose either option according to their preference or specific scale of differentiation.
Adding dissolved Matrigel (e.g., 1% ∼2% [v/v]) may benefit organoid integrity.
Problem 4
Failure in Long-Term Development of Organoids.
Potential Solutions
Extra growth factors (e.g., FGF2 or EGF) are not included in the protocol. These factors, however, may promote the development of organoids (Birey et al., 2017, Yoon et al., 2019). For cell lines that display low proliferation rate and poor organoid development, supplementation of growth factors such as FGF2, EGF, or both, could be an alternative option.
For most of the cases, 9000 cells/embryoid body will be an ideal starting point to achieve successful organoid differentiation. The optimal cell number required can be cell line-dependent (Lancaster et al., 2017), thus desires to be tested if needed.
Acknowledgments
We thank Dr. Andrew G. Elefanty for sharing HES-3 NKX2-1GFP/w human ES cell line. I.-H.P. was partly supported by NIH (GM111667-01, R01AA025080-01, R01CA203011-2), CSCRF (14-SCC-YALE-01, 16-RMB-YALE-04), Kavli Foundation, Simons Foundation, and KRIBB/KRCF research initiative program (NAP-09-3).
Author Contributions
Y.X. and I.-H.P. conceived the study. Y.X. and B.C. performed the experiments. Y.X. wrote the manuscript.
Declaration of Interests
The authors declare no competing interests.
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.xpro.2019.100001.
Contributor Information
Yangfei Xiang, Email: yangfei.xiang@yale.edu.
In-Hyun Park, Email: inhyun.park@yale.edu.
References
- Birey F., Andersen J., Makinson C.D., Islam S., Wei W., Huber N., Fan H.C., Metzler K.R.C., Panagiotakos G., Thom N. Assembly of functionally integrated human forebrain spheroids. Nature. 2017;545:54–59. doi: 10.1038/nature22330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster M.A., Knoblich J.A. Generation of cerebral organoids from human pluripotent stem cells. Nat. Protoc. 2014;9:2329–2340. doi: 10.1038/nprot.2014.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster M.A., Corsini N.S., Wolfinger S., Gustafson E.H., Phillips A.W., Burkard T.R., Otani T., Livesey F.J., Knoblich J.A. Guided self-organization and cortical plate formation in human brain organoids. Nat. Biotechnol. 2017;35:659–666. doi: 10.1038/nbt.3906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig T.E., Bergendahl V., Levenstein M.E., Yu J., Probasco M.D., Thomson J.A. Feeder-independent culture of human embryonic stem cells. Nat. Methods. 2006;3:637–646. doi: 10.1038/nmeth902. [DOI] [PubMed] [Google Scholar]
- Ludwig T.E., Levenstein M.E., Jones J.M., Berggren W.T., Mitchen E.R., Frane J.L., Crandall L.J., Daigh C.A., Conard K.R., Piekarczyk M.S. Derivation of human embryonic stem cells in defined conditions. Nat. Biotechnol. 2006;24:185–187. doi: 10.1038/nbt1177. [DOI] [PubMed] [Google Scholar]
- Xiang Y., Tanaka Y., Patterson B., Kang Y.J., Govindaiah G., Roselaar N., Cakir B., Kim K.Y., Lombroso A.P., Hwang S.M. Fusion of Regionally Specified hPSC-Derived Organoids Models Human Brain Development and Interneuron Migration. Cell Stem Cell. 2017;21:383–398.e7. doi: 10.1016/j.stem.2017.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang Y., Yoshiaki T., Patterson B., Cakir B., Kim K.Y., Cho Y.S., Park I.H. Generation and Fusion of Human Cortical and Medial Ganglionic Eminence Brain Organoids. Curr. Protoc. Stem Cell Biol. 2018;47:e61. doi: 10.1002/cpsc.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang Y., Tanaka Y., Cakir B., Patterson B., Kim K.Y., Sun P., Kang Y.J., Zhong M., Liu X., Patra P. hESC-Derived Thalamic Organoids Form Reciprocal Projections When Fused with Cortical Organoids. Cell Stem Cell. 2019;24:487–497.e7. doi: 10.1016/j.stem.2018.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon S.J., Elahi L.S., Pașca A.M., Marton R.M., Gordon A., Revah O., Miura Y., Walczak E.M., Holdgate G.M., Fan H.C. Reliability of human cortical organoid generation. Nat. Methods. 2019;16:75–78. doi: 10.1038/s41592-018-0255-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tap the plate gently to make sure colonies are dissociated.
During transfer, tilt the ultra-low attachment 24-well plate, so that embryoid bodies will fall down in the well with the medium. This will avoid drying out the embryoid bodies.