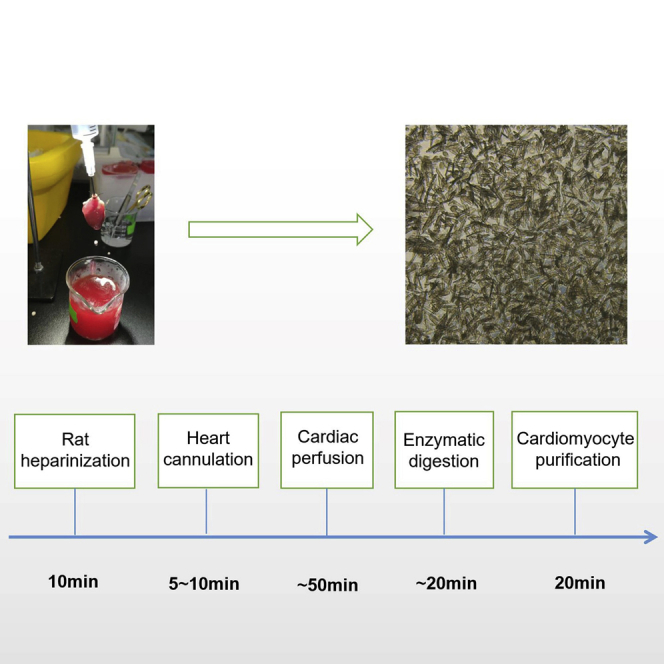
Summary
Isolation of high-quantity and high-quality ventricular cardiomyocytes from adult rats is critical to study heart physiology and pathology and for drug toxicity screening. It remains challenging to produce a high yield of viable cardiomyocytes from rats. Here, we present our modified enzymatic digestion protocol that relies on the Langendorff device to generate large numbers of viable cardiomyocytes consistently. The most critical parts of this protocol are the selection of rat age and digestion time to obtain viable cardiomyocytes.
For complete details on the use and execution of this protocol, please refer to Liu et al. (2019) and Qin et al. (2020).
Graphical Abstract
Highlights
-
•
The age of the rat is critical for the perfusion digest to obtain viable cardiomyocytes
-
•
Blebbistatin is vital for the isolation and culture of adult cardiomyocytes
-
•
Protease treatment increases the yield of cardiomyocyte
-
•
Dead cells and other cell types are separated by gravity
Isolation of high-quantity and high-quality ventricular cardiomyocytes from adult rats is critical to study heart physiology and pathology and for drug toxicity screening. It remains challenging to produce a high yield of viable cardiomyocytes from rats. Here, we present our modified enzymatic digestion protocol that relies on the Langendorff device to generate large numbers of viable cardiomyocytes consistently. The most critical parts of this protocol are the selection of rat age and digestion time to obtain viable cardiomyocytes.
Before You Begin
Prepare the solutions before you start the adult cardiomyocytes isolation. Refer to Key Resources Table and Materials and Equipment sections for a complete list of materials and equipment.
Key Resources Table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Chemicals, Peptides, and Recombinant Proteins | ||
| Heparin | Sigma-Aldrich | Cat# H3393 |
| Sodium pentobarbital | Sigma-Aldrich | Cat# P3761 |
| Type II collagenase | Biochemical Corporation | Cat# LS004176 |
| Hyaluronidase | Sigma-Aldrich | Cat# H3506 |
| Blebbistatin | TargetMol | Cat# T6038 |
| Bovine Serum Albumin | Sigma-Aldrich | Cat# V900933 |
| 0.25% trypsin-EDTA | Gibico | Cat# 25200056 |
| NaCl | Sigma-Aldrich | Cat# V900058 |
| KCl | Sigma-Aldrich | Cat# V900068 |
| KH2PO4 | Sigma-Aldrich | Cat# V900041 |
| D-glucose | Sigma-Aldrich | Cat# G8270 |
| HEPES | Sigma-Aldrich | Cat# V900477 |
| CaCl2 | Sigma-Aldrich | Cat# V900266 |
| Mg-ATP | Sigma-Aldrich | Cat# A9187 |
| NaH2PO4 | Sigma-Aldrich | Cat# V900060 |
| M199 | Sigma-Aldrich | Cat# M2520 |
| NaHCO3 | Sigma-Aldrich | Cat# V900182 |
| Glutathione | Sigma-Aldrich | Cat# G6013 |
| Creatine | Sigma-Aldrich | Cat# C3630 |
| L-carnitine | Sigma-Aldrich | Cat# C0158 |
| taurine | Sigma-Aldrich | Cat# T8691 |
| Insulin-transferrin-selenium-X | Thermo Fisher Scientific | Cat# 51500056 |
| Fetal bovine serum | Thermo Fisher Scientific | Cat# 12483020 |
| Pen/Strep(100×) | Thermo Fisher Scientific | Cat# 10378016 |
| Laminin | Thermo Fisher Scientific | Cat# 23017015 |
| Software and Algorithms | ||
| Image J | NIH | https://imagej.nih.gov/ij/download.html |
| zen | Zeiss | https://www.zeiss.com/microscopy/int/software-cameras.html |
| Experimental Models: Organisms/Strains | ||
| Rat | Shanghai SLAC | Cat# SlacSD |
| Other | ||
| 50 mL/100 mL beakers | Sinoreagent | Cat# 91110104/91110105 |
| 15 mL and 50 mL Centrifuge tube | Thermo Fisher Scientific | Cat# 339650/339651 |
| 35 mm petri dish | Thermo Fisher Scientific | Cat# 121V |
| Surgical silk | Jianhuan Medical | Cat# A221 |
| 10 mL Syringe | Thermo Fisher Scientific | Cat# S7510-10 |
| 18G flat end syringe needle cannula with graves | BD | Cat# 302032 |
| Fine-tip forceps | Jianhuan Medical | Cat# 47000500 |
| Scissors | Sangon Biotech | Cat# F519232 |
| Langendorff apparatus | Double baker warming coil from Hazvard Apparatus | Cat# 508382 |
| Peristaltic pump | Easypump | Cat# YZ1515x |
| Water bath | Shanghai Yiheng | MP-5H |
| Iron stand | Saieise | K124001 |
| Inverted microscope | Leica | DMi8 |
| Refrigerated centrifuge | Hettich | Universal 320R0 |
Resource Availability
Lead Contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Guohua Gong (guohgong@tongji.edu.cn).
Materials Availability
This study did not generate new unique reagents.
Data and Code Availability
This study did not generate any unique datasets or code.
Materials and Equipment
Solution Preparation
Note: Prepare all solutions using sterilized demineralized, ultrapure water (dd H2O). 18.2 Ω molecular biology grade H2O is highly recommended. All solutions for the isolation should be freshly prepared from the Ca2+ free Krebs-Henseleit buffer (KHB) solution. All procedures are performed with standard aseptic technique.
Note: if reagents from alternative suppliers are used, you must validate the efficiency of cardiomyocyte isolation for the first time.
-
•
Rat Cardiomyocyte Isolation Buffer (Ca2+-Free KHB, pH 7.4), 2 L
| Reagent | Final Concentration (mM) | Volume (mL) |
|---|---|---|
| NaCl (1 M) | 118 | 236 |
| KCl (0.5 M) | 4.7 | 18.8 |
| HEPES (1 M) | 10 | 20 |
| KH2PO4 (0.25 M) | 0.6 | 4.8 |
| NaH2PO4 (0.25 M) | 0.6 | 4.8 |
| MgSO4·7H2O (100 mM) | 1.2 | 24 |
| Glucose (1 M) | 11 | 22 |
| ddH2O | n/a | 1669.6 |
| Total | n/a | 2000 |
Note: The solution needs to be sterilized through the filter. It can only be stored for two weeks at 4°C.
-
•
Rat Cardiomyocyte Isolation Buffer (Ca2+ KHB): Add 10 mL CaCl2 (100 mM stock) to 1 L Ca2+-Free KHB solution.
-
•
Trypsin-ETDA solution: Dilute 0.25% trypsin-EDTA with 0.01 M PBS to a final concentration of 0.05%.
-
•
Stop Buffer: Add 1 g BSA and 5 μl 100 mM Blebbistatin to Ca2+ KHB buffer.
-
•
1000 U/mL Heparin sodium solution: Dissolve 34.72 mg heparin sodium salt (180 U/mg) in 5 mL dd H2O and store at 4°C.
-
•
80 mg/mL Pentobarbital solution: Dissolve 400 mg sodium pentobarbital in 5 mL dd H2O and store at 4°C.
-
•
Rat Collagenase Buffer (4×)
| Reagent | Final Concentration | Amount |
|---|---|---|
| collagenase type II | 472 U/mL | 9440 U |
| hyaluronidase | 0.9 mg/mL | 18 mg |
| Blebbistatin (100 mM) | 50 μM | 10 μL |
| 20 mL Ca2+-Free KHB buffer | n/a | 20 mL |
| Total | n/a | ∼20 mL |
Note: Most protocols use 2,3-butandione monoxime to inhibit myocyte contraction, but we use the inhibitor Blebbistatin to stop myocyte contraction (Plačkić and Kockskämper, 2018). It has been reported that Blebbistatin can extend the cultural life of adult cardiac myocytes (Kabaeva et al., 2008).
Note: Hyaluronidase cleaves hyaluronic acid, the main component of the extracellular matrix that links protein filaments, collagen fibers, and connective tissue cells, which can improve the osmotic ability of collagenase buffer in the cardiac tissue and thus dissociate more myocytes from cardiac tissue.
Note: The working concentration of the collagenase buffer is 1× (through adding 20 mL 4× collagenase buffer into 60 mL Ca2+-free KHB buffer in the container). Because the enzymes are mixtures, a different batch does not act identically. At first, we recommend purchasing small amount of collagenase type II. After the efficiency of digestion is verified, then purchase large quantity enzyme of the same batch. Collagenase type II from Worthington is a popular choice for cardiomyocyte isolation because it contains higher clostripain activity than other companies (Roth et al., 2014, Louch et al., 2011).
-
•
M199 Medium
| Reagent | Final Concentration | Amount |
|---|---|---|
| M199 | n/a | 1 bag (9.5 g) |
| NaHCO3 | ∼2.2 g/L | ∼2.2 g |
| Glutathione | 10 mM | 3.073 g |
| BSA | 0.2 g/L | 0.2 g |
| ddH2O | n/a | ∼1000 mL |
| Total | n/a | 1000 mL |
Note: The PH is adjusted to PH7.4 by NaHCO3 and filtered.
-
•
Culture Medium
| Reagent | Final Concentration | Amount |
|---|---|---|
| M199 medium | n/a | 93.89 mL |
| Pen/Strep(100×) | 1× | 1 mL |
| Creatine | 5 mM | 74.58 mg |
| L-carnitine | 2 mM | 32.24 mg |
| Taurine | 5 mM | 62.58 mg |
| Insulin-transferrin-selenium-X (100×) | 0.1% | 0.1 mL |
| FBS | 5% | 5 mL |
| Blebbistatin (100 mM) | 10 μM | 10 μL |
| Total | n/a | 100 mL |
Note: Warm the medium to room temperature (23–26°C) before use.
Step-by-Step Method Details
Langendorff System Preparation
Timing: 30 min
CRITICAL: Pre-warm and oxygenate the perfusion solutions.
-
1.
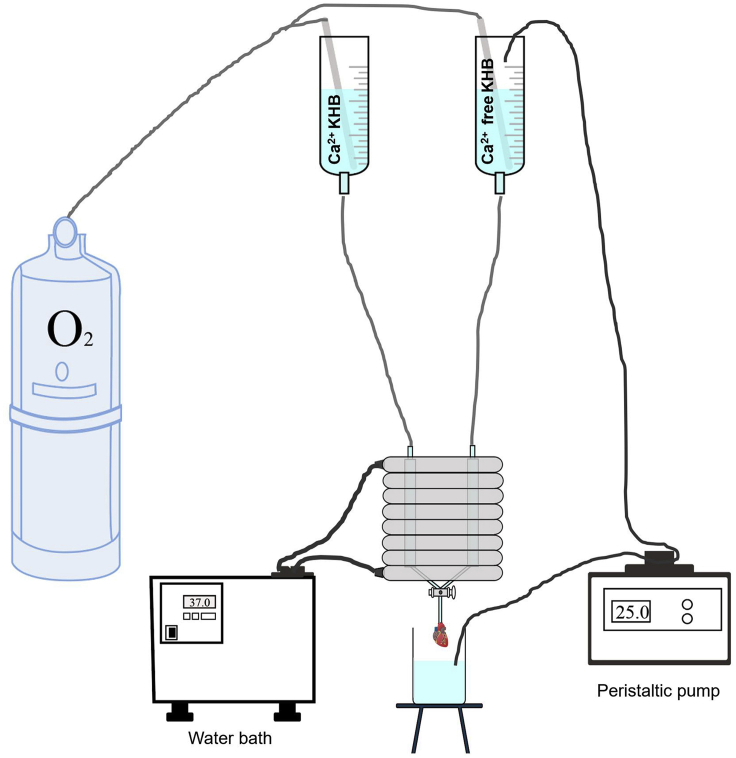
Before isolation, we need to fill up the Langendorff apparatus (Figure 1) (Liu et al., 2019), including circulating pipes, with 75% ethanol and soakfor 1–2 h.
Figure 1.
The Schematic Diagram of the Langendorff Perfusion System for Cardiomyocytes Isolation
Note: For the first use, the Langendorff device should be soaked with 25% bleach for at least 12 h to sterilize the system.
-
2.
Switch on the water bath to warm up the water bath-jacketed tubes of the Langendorff system to keep the circulating perfusion solution at 37°C into the heart.
Note: The temperature of the perfusate should also be checked regularly (Plačkić and Kockskämper, 2018).
-
3.
Rinse the system 3 times with sterilized ddH20, and empty the system after each wash.
-
4.
Fill Ca2+ KHB (∼80 mL) and Ca2+-free KHB (∼80 mL) into their containers (Figure 1).
-
5.
Turn on the oxygen to oxygenate all solutions that are going to be used for perfusion to maintain sufficient O2 supply.
-
6.
Put all the surgical tools in a beaker with 75 % ethanol, and prepare heparin sodium solution and sodium pentobarbital solution.
-
7.
Fill a 60 mm petri dish with Ca2+ KHB, and place it on ice.
-
8.
Aspirate an appropriate amount (3–4 mL) of Ca2+ KHB to a 5 mL syringe, which connects to the cannula, with care to avoid introducing air bubbles.
-
9.
Fix the 5 mL syringe on the iron stand, adjust the height so that the end of the cannula is inside the 60 mm petri dish, and then tie a surgical silk to the connecting site of the syringe for tying aorta.
-
10.
Hold 50 mL of Ca2+ KHB with a small beaker on ice.
Pause Point: The system is ready for use and is fine for a break up to 4 h.
Surgery
Timing: 30 min
CRITICAL: Use ice-cold solutions, cannulate the heart on ice to reduce heart beating. Appropriately cannulate the aorta.
-
11.
Heparinize rat (200–350 g) with sodium heparin intraperitoneally injected at 1000 U/kg to prevent the blood coagulation and the heart coronary embolism.
Note: The weight of rat (correlated with age, generally, a 200–350 g rat is 6–8 weeks old) is crucial for the quality of the cardiomyocyte preparations. Less than 4 months old rat can be used for isolation.
Note: Heparin as an anticoagulant can be used in vivo or in vitro. In some protocols, heparin has been added to the cannulation solution for use (Plačkić and Kockskämper, 2018). However, instead of using heparin in the cannulation solution, we injected heparin before harvesting the heart, which can let the anticoagulant blood flow into the coronary to prevent the formation of embolism.
-
12.
After 10 min, anesthetize the rat with sodium pentobarbital intraperitoneally injected at 80 mg/kg.
-
13.
When the rat is in deep sleep (∼5 min after injection and no response to toe pinch), spray chest and upper abdomen with 75 % ethanol, make a large incision on chest well, cut through ribs on both sides to fully expose heart and lung.
-
14.
Quickly remove the heart with the thymus and intact aortic arch. Removing the heart and some lung tissues together is better for saving time.
-
15.
Put the heart into the small beaker with ice-cold Ca2+ KHB, gently press the heart against beaker wall 2–3 times to squeeze blood out, transfer the heart to the 60 mm petri dish with ice-cold Ca2+ KHB.
-
16.
Carefully remove other tissues and clean up the aortic root (leave 3–5 mm long of the aorta) with forceps and scissors.
-
17.
Cannulate the heart with the cannula, and ligate twice with the prepared surgical silk to ensure no solution leaks from the ligating site (Liu et al., 2019).
CRITICAL: Cannulating the heart quickly is crucial for the quality of the cardiomyocyte isolation. During the isolation procedure, the cannula should not be inserted too deeply into the aorta, as this could lead to mechanical damage of the aortic valve leaflets and cause inefficient perfusion and poor digestion of the heart. The total time from the heart is removed to mounted and perfused on the Langendorff system should be as short as possible to avoid cell damage caused by ischemia and hypoxia (Plačkić and Kockskämper, 2018). We recommend a time of less than 10 min and usually perform these steps within 5 min.
-
18.
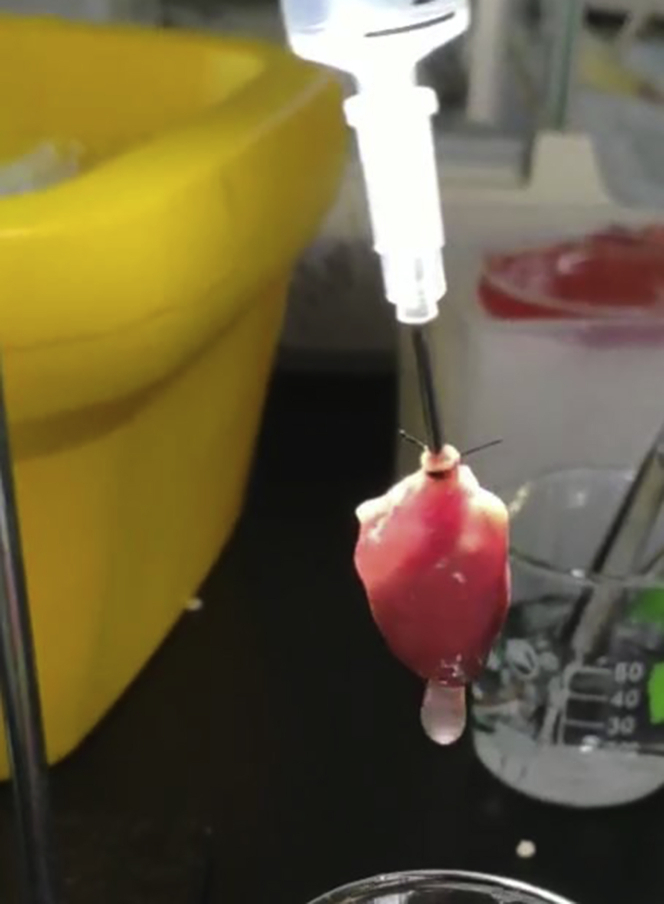
Slowly inject Ca2+ KHB into the aorta to exclude blood from the coronary artery and the heart cavity (Figure 2), and quickly hang the heart on the Langendorff apparatus.
Figure 2.
Cannulation of the Heart
Note: The cannula with the heart must be carefully connected to the Langendorff system to avoid introducing air bubbles.
Heart Perfusion
Timing: 50 min
CRITICAL: Ensure sufficient perfusion to dissociate cells from the heart tissue.
-
19.
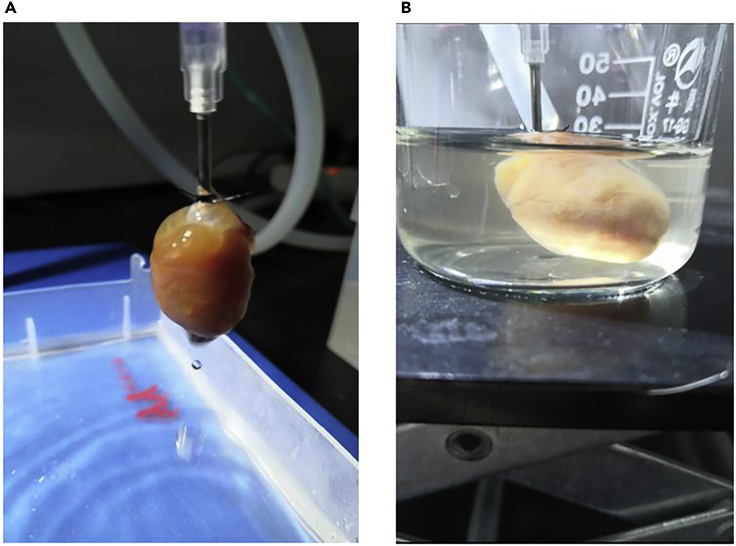
Perfuse Ca2+ KHB for 5 min at high flow rate (1–2 drops/s) (Figure 3A).
Figure 3.
Perfused Digestion of the Heart
(A) The rat heart is perfused with the KHB solution.
(B) Collagenase solution perfused heart.
Note: Enough Ca2+ KHB perfusion time leads to better myocardial cell protection.
-
20.
Switch to perfuse Ca2+-free KHB for 4–5 min at the high flow rate. The heart stops beating in 1 min.
-
21.
When there is around 60 mL Ca2+-free KHB left in the container, add 20 mL 4× Rat Collagenase Buffer to the Ca2+ -Free KHB container to mix with the Ca2+ -Free KHB to get a total 80 mL 1× collagenase buffer in the whole perfusion system. Set a 40 min timer.
Note: Fill 60 mL solution into the Ca2+ -Free KHB container and mark the solution level on the container when you use it the first time.
-
22.
Put the heart in a small beaker (50 mL), set up a tube to recirculate Collagenase buffer (set up the peristaltic pump at a speed of ∼180 mL/min) (Figure 3B).
-
23.
After 5 min, add 80 μl CaCl2 (100 mM stock) into the beaker twice at 0.5–1 min interval (the final concentration of Ca2+ is 0.2 mM in 80 mL 1× collagenase buffer).
Note: We found that a higher level of calcium ions stimulated the heart to continuously beating during digestion, which would cause the quality of isolated cardiomyocytes to decline.
-
24.
Monitor heart digestion: use the forceps to check the heart after the heart becomes swelling and white. Stop perfusion if the collagenase solution drops become quicker, and frequency reaches to 180–200 drops/min. In our experience, this process usually takes 35 min.
Note: The duration of the collagenase digestion varies and could last any time between 25 and 50 min. It depends on several factors, including weight, age, and strain of rat or disease model (cardiac remodeling)
Enzymatic Digestion (0.05% Trypsin-EDTA Solution)
Timing: 20 min
-
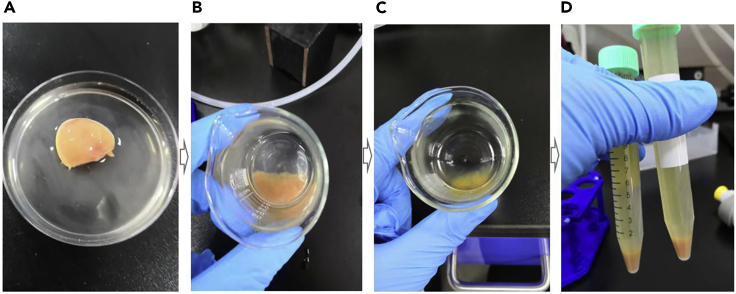
25.
Take off perfused heart, and remove atrium and aorta, put the ventricular tissues in a small beaker with 10–15 mL enzyme solution (0.05% Trypsin-EDTA), and tear the ventricular tissue into small pieces with two fine-tip forceps (Figure 4A).
Figure 4.
Enzyme Further Digests Cardiac Tissue
(A) The digested heart was cut off the atrium and aorta.
(B and C) Myocardial tissue has been digested one time with enzyme (B); myocardial tissue has been digested three times with enzyme (C).
(D) Collected cardiomyocytes in tubes.
-
26.
Pipette the tissue gently with 1 mL pipette (cut tip), and put in an incubator at 37°C for 5 min.
-
27.
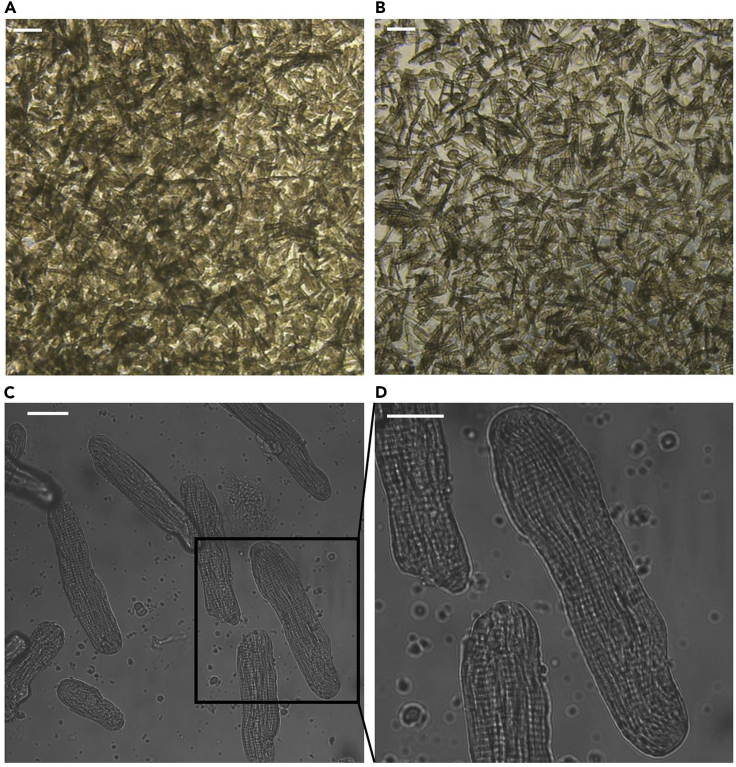
After 5 min, pipette the tissue again (Figure 4B), take one drop tissue solution to check the digest efficiency and cell viability under a microscope, stop the digestion if >80% rod shape cells are present individually (Figure 5A).
Figure 5.
Purification and Culture of Cardiomyocytes
(A) A representative image of cardiomyocytes collected from preliminary collagenase digestion of the cardiac. Scale bar, 100 μm.
(B) A representative image of cardiomyocytes obtained from trypsin digestion. Scale bar, 100 μm.
(C) A representative image of 1-day cultured cardiomyocytes. Scale bar, 30 μm.
(D) Expanded image of cardiomyocytes. Scale bar, 20 μm.
-
28.
Allow the tissues to settle by gravity for 1 min, collect the supernatant from the beaker, and transfer to two or three 15 mL conical tubes so that each tube contains 5 mL of supernatant.
-
29.
Add 5 mL Stop Buffer to the tube containing the 5 mL of supernatant, and pipette up and down 3 times to mix.
-
30.
Add 10–15 mL 0.05% Trypsin-EDTA solution to the left precipitated cardiac tissue in the beaker; repeat steps 26 to 29 (Figure 4C).
-
31.
Collect the sediment of cardiomyocytes through gravity (pipette up and down to mix and wait for 3–4 min) (Figure 4D). This will partially remove other small cells such as fibroblasts and endothelial cells.
Note: Repeat digestion with trypsin 1–2 times to get more myocytes if necessary.
Cardiomyocyte Purification
Timing: 20 min
-
32.
Sequentially add 25 μl CaCl2 (100 mM stock) to cells in the conical tubes (each containing 10 mL solution) 3 times at 5 min intervals, mix well, and the fourth time add 50 μl CaCl2, and mix well.
-
33.
Collect the precipitated live cardiomyocytes through 30 g, 30 s centrifugation, and discard the supernatant. The left fibroblasts and endothelial cells are removed from myocytes’ sedimentation by centrifuge.
-
34.
Resuspend cells with 2 mL culture medium and merge all cells into one tube, then replenish the culture medium to 10 mL and mix well. Now, these cells are ready for experiments or culture (Figure 5B).
Pause Point: The cardiomyocytes in the medium can be incubated at 37°C, 5% CO2 for 2–3 h.
Cardiomyocytes Culture
Timing: 40 min
-
35.
Pre-coat the 6 well plates or dishes with 40 μg/mL laminin (1 mL per well) for 30 min.
Note: This step should be done before cardiomyocyte purification for saving time.
-
36.
Count the cell number and plate cells with 500 μl culture medium per well at a density of 5 × 104 cells/mL, then incubate at 37°C, 5% CO2.
-
37.
Two hours later, after cardiomyocytes attached to the plate, gently change the medium with 2 mL fresh culture medium for continued culture (Figures 5C and 5D).
Expected Outcomes
In our protocol, we use collagenase and hyaluronidase to perfuse heart. Hyaluronidase cleaves hyaluronic acid, the main component of the extracellular matrix that links protein filaments, collagen fibers, and connective tissue cells, which can improve the osmotic ability of collagenase buffer in the cardiac tissue and thus dissociate more myocytes from cardiac tissue. A stronger proteinase, trypsin, is used to furtherly digest nonsingle cells (cell mass) and tissue after the perfused digestion, which can increase the yield of cardiomyocytes. At the same time, we use the cell contraction inhibitor Blebbstatin, which not only inhibits myocyte beating but also extends the cultural life of adult cardiac myocytes. Rapid and high yield viable cardiomyocytes were isolated from one adult rat heart through our protocol. It has been reported that approximately 3 million cardiomyocytes could be obtained from an adult rat heart (Campora et al., 2018). In our protocol, about 85% cells were rod shape in the digested cells, and 2.5–4.0 × 106 live cardiomyocytes could be derived from one heart. These cells are enough for seeding ten 6-well plates at a density of 3 × 104 cells/well. These cardiomyocytes can be cultured to 6–7 days with a medium change every 2 days (Liu et al., 2019).
Limitations
As stated above, the time required for cardiomyocyte isolation depends on weight, age, sex, and strain of rat or disease model; thus, it cannot be precisely defined here.
The lot-to-lot variability of protease activities from type 2 collagenase preparations is very high (Plačkić and Kockskämper, 2018). Thus, the concentration of collagenase and digestion time needs to be modified if a new batch of collagenase is used.
The yield of rod-shaped cardiomyocytes from one adult rat heart may vary (2.5–4.0 × 106 cells) with body weight, strain, and collagenase activities. (Li et al., 2014, Plačkić and Kockskämper, 2018)
Troubleshooting
Problem 1
Low yield (less than 5 × 105 cells from one heart if the cardiac tissue has not been sufficiently digested) of Rod-shaped Cardiomyocytes
Potential Solutions
Some modifications can be taken, including increasing heparin to 1250 U/kg, reducing the time of heart cannulation to 3 min, increasing oxygen supply, using more excitation-contraction coupling inhibitor (up to 15 μM Blebbistatin) (Farman et al., 2007, Kabaeva et al., 2008). The sufficient perfusion time (up to 50 min) may be needed.
Problem 2
Isolated Cardiomyocytes with Low viability (the none rod shape cells are the majority)
Potential Solution
Slightly decrease one-fourth of the concentrations of collagenase and trypsin.
Acknowledgments
This work was supported partly by the National Key Research and Development Program of China (no. 2018YFA0107102 to G.-G.H.), the National Natural Science Foundation of China (no. 31901044, 31771524 to G.-G.H. and no 81970333 to Q.-Y.), the Program for Professor of Special Appointment (Eastern Scholar) at Shanghai Institutions of Higher Learning (no. TP2017036 to G.-G.H.), and Shanghai Pujiang Program (no. 17PJ1409600 to G.-G.H.).
Author Contributions
G.-G.H. conceived, designed, and supervised the project. T.-X.G., G.-M., and L.-A.Q. conducted most of the experiments. L.-B.L. and J.-W.T. performed partial data analysis. Q.-Y. provided valuable suggestions. G.G. and T.-X.G. wrote the manuscript.
Declaration of Interests
The authors declare no competing interests.
Contributor Information
Xiangang Tian, Email: xgangtian@outlook.com.
Guohua Gong, Email: guohgong@tongji.edu.cn.
References
- Liu B.L., Li A.Q., Qin Y., Tian X.G., Gao M., Jiang W.T., Gong G.H. Comparative study on isolation and mitochondrial function of adult mouse and rat cardiomyocytes. J. Mol. Cell. Cardiol. 2019;136:64–71. doi: 10.1016/j.yjmcc.2019.09.006. [DOI] [PubMed] [Google Scholar]
- Qin Y., Li A.Q., Liu B.L., Jiang W.T., Gao M., Tian X.G., Gong G.H. Mitochondrial fusion mediated by fusion promotion and fission inhibition directs adult mouse heart function toward a different direction. FASEB. J. 2020;34:663–675. doi: 10.1096/fj.201901671R. [DOI] [PubMed] [Google Scholar]
- Roth G.M., Bader D.M., Pfaltzgraff E.R. Isolation and physiological analysis of mouse cardiomyocytes. J. Vis. Exp. 2014;91:e51109. doi: 10.3791/51109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campora S., Alberte P.S., Bruno C., Ghersi G. Isolation of adult rat cardiomyocytes using recombinant collagenases. Chem. Eng. Trans. 2018;64:25–30. [Google Scholar]
- Louch W.E., Sheehan K.A., Wolska B.M. Methods in cardiomyocyte isolation, culture, and gene transfer. J. Mol. Cell. Cardiol. 2011;51:288–298. doi: 10.1016/j.yjmcc.2011.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plačkić J., Kockskämper J. Isolation of atrial and ventricular cardiomyocytes for in vitro studies. In: Ishikawa K., editor. Experimental Models of Cardiovascular Diseases. Springer; 2018. pp. 39–54. [DOI] [PubMed] [Google Scholar]
- Li D.X., Wu J., Bai Y., Zhao X.C., Liu L.J. Isolation and culture of adult mouse cardiomyocytes for cell signaling and in vitro cardiac hypertrophy. J. Vis. Exp. 2014;87:e51357. doi: 10.3791/51357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farman G.P., Tachampa K., Mateja R., Cazorla O. Blebbistatin: use as an inhibitor of muscle contraction. Pflug. Arch. Eur. J. Phy. 2007;455:995–1005. doi: 10.1007/s00424-007-0375-3. [DOI] [PubMed] [Google Scholar]
- Kabaeva Z., Zhao Mei., Michele D.E. Blebbistatin extends the culture life of adult mouse cardiac myocytes and allows efficient and stable transgene expression. Am. J. Physiol. Heart Circ. Physiol. 2008;294:H1667–H1674. doi: 10.1152/ajpheart.01144.2007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This study did not generate any unique datasets or code.