Abstract
Aurora kinases (AURK) are key regulators of the mitotic spindle formation. AURK is frequently overexpressed in ovarian cancer and this overexpression has been frequently associated with prognosis in these tumours. Interestingly, AURK have been shown to interact with DNA repair mechanisms and other cell cycle regulators. These functions have brought light to Aurora family as a potential target for anticancer therapy. In the last years, two clinical trials with different AURK inhibitors have shown activity in epithelial and clear-cell ovarian cancer. Although there is a lack of predictive factors of AURK inhibition activity, recent trials have identified some candidates. This review will focus in the functions of the AURK family, its role as prognostic factor in epithelial ovarian cancer and potential clinical implications.
Keywords: aurora kinase, aurora inhibitors, ovarian cancer
Introduction
Aurora kinases (AURK) are a family of serin–threonin kinases which principal function is the regulation of mitotic spindle formation. The family of AURK includes Aurora kinase A (AURKA, STK15), Aurora kinase B (AURKB, STK12) and Aurora kinase C (AURKC, STK13).1,2 Recent studies have identified that AURK family plays a role not only in the mitotic process but also in cell-cycle regulation such as chromosome segregation failure, causing genetic instability, polyploidy and a significantly increased tumour incidence.3 AURK are highly conserved and hold homologous structure, constituting of a N-terminal domain, a protein kinase domain and a highly preserved C-terminal domain.
AURK family overexpression or amplification is a common alteration in cancer. In breast cancer, AURKA overexpression is related with ki67, proliferation and basal like phenotype, and AURKB with ki67 and histological grade among others. Gliomas, prostate cancer, cervical cancer and lung cancer are other tumour types in which AURKA and AURKB overexpression or amplification have been found and related to adverse clinical factors. AURKC overexpression was found to be associated to tumour grade in colorectal cancer.4
In epithelial ovarian cancer (EOC), AURK is frequently overexpressed and its expression has shown to have a prognostic impact in several published series. AURK family is implicated in mitosis, cell cycle regulation and DNA repair system. The balance AURKA–BRCA2 (has been suggested as a regulator of tumourigenesis. Preclinical data also suggest that AURK family might play a role in chemoresistance mechanisms. Moreover, AURK family has evolved as a potential target for precision medicine in cancer. In the last decade, several AURK inhibitors have been developed and tested in several cancer types. In EOC, AURK inhibitors have also been tested with different results.
Two recent clinical trials with different AURK inhibitors have shown activity in EOC and clear-cell ovarian cancer (OC). In this subtype, ARID1a mutations have been identified as a potential predictive biomarker for AURK inhibition.
This review will focus in the functions of the AURK family, its role in chemoresistance and as prognostic factor in EOC. Also, the potential clinical implications and the results of the recent trials with AURK inhibitors will be analysed.
Methods
According to the objectives of this review, a search using PubMed with the terms “Aurora kinase” and “Ovarian Cancer” has been performed.
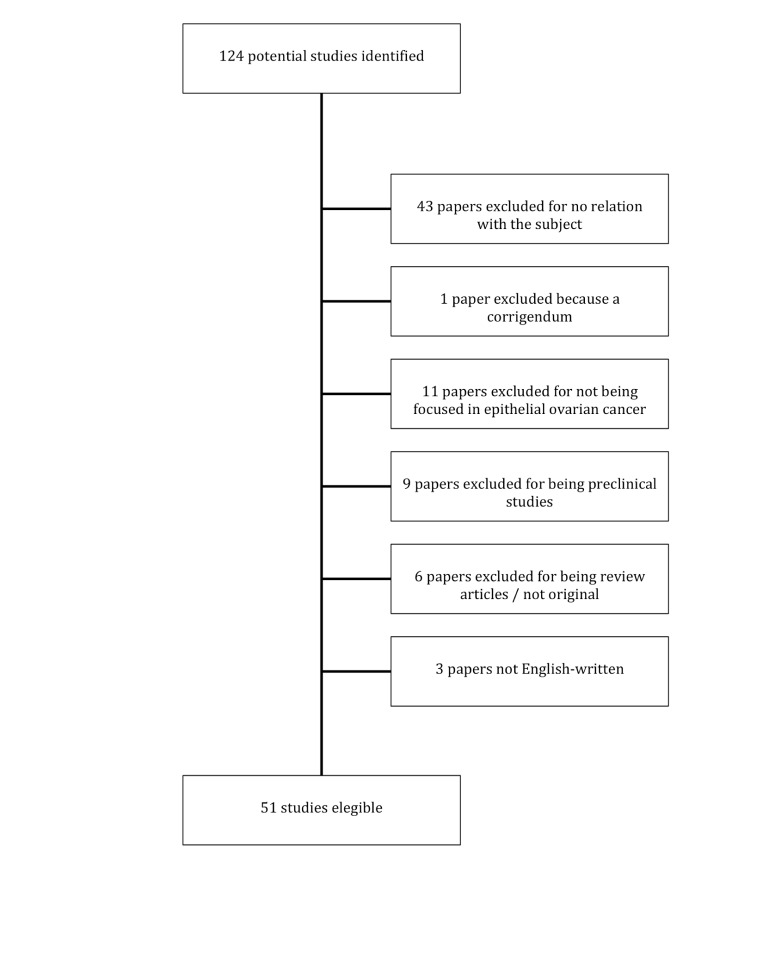
The search was limited to English language papers published in the last 15 years (between 2004 and 2019). Publications were selected by two different authors separately. References of selected publications were also checked for cross-references. Of the 118 potential entrances returned after the search, 71 were discarded (36 for not being related to the subject, 11 for being focused in other tumour types, 8 dealt with preclinical data of different compounds and 6 were review articles). Finally, 51 publications were selected for this review (see figure 1).
Figure 1.
Flow chart.
For the AURKA expression point, apart to the search review, an in silico analysis of the public database cbioportal.org was performed. This analysis was focused in the frequency of amplifications of AURKA, AURKB and AURKC using the TCGA (The Cancer Genome Atlaso) (Nature 2011) database.5
Mitotic functions of AURK
AURK family is crucial for mitosis spindle formation and progression of mitosis. Every member of the family plays a different role as a regulator of the cell division.
Aurora-A (AURKA)
AURKA plays an important role in microtubule formation in OC cell lines and its inhibition has shown an antiangiogenic effect as well as an increased cell proliferation, migration and invasion in preclinical studies.6
AURKA is involved in various process related to the spindle formation:
Centrosome maduration: AURKA is such an essential enzyme in the maduration of centrosomes that absence of AURKA leads to inhibition of centrosome maduration.7
Centrosome separation.
Mitotic entry: the active form of AURKA is first detected in the late G2 phase at the centrosome. Activation of AURKA is required for the recruitment of CDK1 to the centrosome and further progression into mitosis.
Bipolar-spindle assembling.
Chromosomal alignment on metaphase.
Cytokinesis.
At the end of the mitosis, AURKA will be degraded. AURKA is turned over through the anaphase promoting complex/cyclosome–ubiquitin–proteasome pathway. In vivo degradation study showed that this process is dependent on cadherin-1.8
Aurora-B (AURKB)
AURKB is part of the chromosome passenger complex which is formed by AURKB, INCEP (Inner Centromere Protein), survivin and borealin.9 This complex will mediate chromosome condensation and is also related to the spindle assembly checkpoint.10 Thus, AURKB plays an essential role in chromosome condensation, alignment and segregation.11
A recent publication in different cell lines, including OC lines, suggested that chromosome instability cells have a defect that limits accessibility of AURKB to the kinetochores that are important for error correction.12
It has also been demonstrated that activated AURKB mediates phosphorylation of histone H2AX at Ser121, which promotes the autophosphorylation of AURKB. This results to further accelerating AURKB activation.13
Aurora-C (AURKC)
AURKC has been identified in the centrosome during the mitosis from anaphase to cytokineses suggesting a role in the centrosome regulation in late mitosis. However, its function is no well known. Like AURKA and AURKB, AURKC is also allocated in the spindle poles but only in the late phases of the mitosis.14 Inhibition of AURKB and AURKC leads to a multinucleated cell; nevertheless, AUKRC is able to rescue a multinucleated phenotype produced by a silencing of AURKB. These findings suggest that AURKC complements and overlaps AURKB function in mitosis.15 Furthermore, AURKC may interact with transforming acidic coiled-coil 1 and colocalise to the mid-body of Hela cells during cytokinesis.16
AURK and cell cycle regulation: implications in cancer
Cell cycle control and DNA repair are two processes that are closely related. Cell-cycle arrest facilitates DNA repair system to supervise and repair any damage. High proliferative cells, such as tumour cells, acquire more genetic instability as DNA repair becomes more difficult.17
P53 is a tumorous suppressor protein that is responsible for the arrest of the cell-cycle in G2.
Several tumours such as triple negative breast cancer (TNBC) or high-grade serous OC have shown a high frequency of phenotypes with a gain of function of AURK and a loss of function of p53. The evidence of both alterations in several cases suggest the hypothesis that AURK and p53 are involved in similar molecular changes.18
These data have been confirmed by multiple evidences underlying a crosstalk between AURK and p53. P53 wild-type inhibits AURKA by a direct effect in a transactivation-dependent method. In this context, tumours associated with an inactivating p53 mutation might upregulate AURKA.
Moreover, AURK family has shown to regulate p53 by a post-transcriptional phosphorylation process.19 AURKA throughout the phosphorylation of the serine 315 of p53 facilitates the ubiquitination of p53 mediated by MDM2 and therefore the p53 degradation. Throughout the phosphorylation of serine 215 of p53 AURKA inhibits the capacity of p53 to join the DNA chain.20
Finally, AURKA regulates p53 in an indirect way by phosphorylation of other molecules such as hnRNPK or MDM2 that are positive and negative regulators of p53.
AURKA interactions also with proteins involved in the apoptosis, in particular with p73, a protein of the family of p53, implicated in the regulation of cell cycle and apoptosis. A preclinical study showed that the inhibition of AURKA in a p53-deficient cell line leads to overexpression of genes related with apoptosis mediated by p73. Other studies suggested that p73 function was inhibited by AURKA avoiding apoptosis and leading the cells to be resistant to drug-dependent cell death.21
The checkpoint kinase 1 (CHEK1) is a member of the regulation of DNA repair mediating cell-cycle arrest in response to DNA damage.22
Inhibition of AURKA has shown to be synthetic lethal in combination with CHEK1 inhibitors in the OC cell lines OVCAR3, OVCAR8, IGROV1 and SKOV3.23 The combination of alisertib and LY2603618, a CHEK1 inhibitor, triggered apoptosis and reduced the stem cell population. Moreover, this combination showed an increase of the effect of taxanes and platinum compounds.
AURKB also interactions with p53 phosphorylating multiples areas in the DNA-binding domains. This interaction leads to an inhibition of the transactivator function or the degradation of p53.24 25 AURKB decreases the expression of the cell cycle inhibitor p21WAF1/CIP1 via suppressing p53 activity, resulting in aberrant activation of CDK1. This leads to cell cycle progression and therefore promotes cell survival.26
DNA repair system and AURK
Apart from the regulatory mitotic function, it has been seen that AURK family plays other relevant non-mitotic roles. In fact preclinical studies suggested that AURK interactions with repair system mechanisms, mainly with BRCA in OC cells. By consecutive silencing of AURKA/B and BRCA1/2 in BRCA defective pancreatic and ovarian cell lines, it was seen that AURKA/B and BRCA 1/2 inhibited each other through proteasome-mediated proteolysis. This negative balance AURK–BRCA regulated cell cycle progression through p53 and cyclin A.27 In 2017, an American group published the results of a preclinical study dealing with the issue of AURKA and DNA repair mechanisms interactions in OC.28 AURKA was found to modulate the expression and activity of polyadenosine ribose phosphatase (PARP). The specific in vitro inhibition of AURKA with the selective inhibitor alisertib decreased the expression of PARP and BRCA1/2 and stimulated the non-homologous end joining (NHEJ) repair pathway by elevating DNA-dependent protein kinase catalytic subunit (DNA-PKcs) activity, a catalytic subunit required for the double strand breaking. Furthermore, alisertib stimulated error-prone NHEJ repair of DNA double strand breaks with incompatible ends. Consistent with these findings, in vivo experiments confirmed that AURKA inhibition increased phosphorylated DNA-PKcs and decreased PARP levels.
AURKA and BRCA2 balance plays a role as a key regulator of tumourigenesis and metastasis in OC. While AURKA provokes, BRCA restrains primary tumourigenesis. The metastatic promoting markers SLUG, FBN1 and MMP2 are either stimulated or suppressed by AURKA or BRCA. However, the metastatic suppressors e-cadherin and p53 are either inhibited or promoted by AURKA or BRCA, respectively. In this context, AURKA stimulates malignancy while BRCA avoid tumour development.29
In other study, BRCA2 mutations and overexpression of AURKA hyperactivated CDK1 through phosphorylation of cell division cycle phosphatase 25B (CDC25B) lead to tumourigenesis.30
Other evidences suggest that the RAS (Rat Sarcoma)-induced genomic instability and ovarian tumourigenesis induced by RAS pathway lead through the regulation of the imbalanced expression of AURKA and BRCA2.31
These findings suggest that AURKA plays a non-mitotic function by regulation of PARP and BRCA, which could represent a new potential target for OC.
AURK amplification and overexpression as biomarker in OC
One of the most important limitations of AURKA as biomarker is the identification of its expression in solid tumours. Assessment of AURK overexpression or gene amplification varies alongside the literature. Depending on the different techniques and thresholds for the definition of overexpression, the proportion of this alteration may vary accordingly. See table 1.
Table 1.
Studies assessing the expression of Aurora kinase A (AURKA) and AURKB in ovarian cancer
| N | Determination | Sample | Techn | Methods | Antibody used in IHC | Overexpression | Observations | |
| AURKA | ||||||||
| Lassmann et al32 | 107 | Protein expression | Tumour tissue | IHC | Score 0 100% − Score1 <10% + cit Score 2 10%–30%+cit Score 3 >30% + cit Scores 2–3 → overexpressed |
Clone JLM28 Novocastra | 63.5% | EOC |
| Mendiola et al 33 | 68 | Protein expression amplificatio | Tumour tissue | IHC FISH |
Score <5% − Score ≥5% + Amplified if in >5% of cells of more than 10 gene signals or three more signals as centromere |
Clone JLM28 Novocastra | 58.8% 27.6% |
EOC |
| Yang et al44 | 223 | Protein expression | Tumour Tissue | IHC | Score 0<5% +cit/nu Score 1 5%–20%+cit/nu Score 2 20%–50%+cit/nu Score 3 >50% + cit/nu Scores 1–3 → overexpressed |
GTX13824 monoclonal Ab Genetax | 40.3% | Serous OC |
| Yang et al 34 | 51 | Protein expression | Tumour Tissue | IHC | Score 0<5% +cit/nu Score 1 5%–20%+cit/nu Score 2 20%–50%+cit/nu Score 3 >50% + cit/nu Scores 1–3 → overexpressed |
GTX13824 monoclonal Ab Genetax | 48% | Primary endometrioid OC |
| Lassus et al 36 | 592 | Protein expression Amplificatio |
Tumour Tissue | IHC CISH |
AURKA Weak/negative versus Overexpression PhosphoAURKA − versus + |
Polyclonal Ab Cell Signalling Technology Polyclonal Ab Cell Signalling Technology |
27% AURKA (11% cit 17%nu) 13% phospho AURKA 9% |
Serous OC |
| Juan et al 39 | 33 | Amplification | ctDNA | NGS | Illumina | 33.3% | Platinum-resistant EOC | |
| 33 | Amplification | Archived Tumour tissue |
FISH | Gene AURKA 20q13/20q11 Amplified >2.0 copies Borderline >1.5–2 copies Not amplif <1.5 copies |
3% | Platinum-resistant EOC | ||
| AURKB | ||||||||
| Chen et al 35 | 156 | Protein expression | Tumour tissue | IHC | Score of % cells stained (0) no staining, (1) 1%–10%, (2) 11%–50% (3) 51%–80% (4) 81%–100% stained Intensity: 0) negative, (1) weak, (2) moderate, (3) strong IHS=score % × intensity if 5–12 is considered overexpressed |
Polyclonal Ab Abcam Cambridge | 34% | Overexpress more likely in poorly dif and lymph nodes |
| Mendiola et al 33 | 68 | Protein expression | Tumour tissue | IHC | Score <5% − Score ≥5% + |
Polyclonal Ab Abcam Cambridge | 83.5% | EOC |
| Beussel et al 37 | 80 | Protein expression | Tumour tissue | IHC | Score 0 100% − Score 1 <10% + cit Score 2 10%–30%+cit Score 3 >30% + cit Scores 2–3 → overexpressed |
Polyclonal Ab Novus Biologicals Cambridge | 99% (score 1–3) 19% strong expression (score 3) |
Primary EOC FIGO III |
AB, antibody; EOC, epithelial ovarian cancer; FISH, fluorescence in sity hybridation; IHC, immunohistochemistry; NGS, next-generation sequencing; Tech, technique.
AURKA overexpression by immunohistochemistry (IHC) has been assessed in many studies in different solid tumours.
One of the first studies assessing AURKA overexpression was performed by Lassmann et al.32 This group analysed AURKA mRNA and protein expression by IHC. AURKA expression was measured with a semiquantitative score and overexpression was defined as a score 2–3. Overexpression of AURKA protein was detected in 68 of 107 samples (63.5%). However, none of the later studies found such a high rate of overexpression.
The amplification of AURKA by fluorescence in sity hybridisation was assessed in only 68 EOC samples. AURKA was amplified in 27.6% of cases in this series.33
Other studies analysed AURKA expression in specific subtypes. In a series of 51 endometrioid OC samples, AURKA was found to be expressed in 48% of the samples.34
In serous OC,35 identified a 40.3% overexpression of AURKA in 223 samples. In a Finnish study36 AURKA protein expression was assessed by IHC in 592 serous OC samples, copy number by CISH (Chromogenic in situ hybridization) in 169 samples, AURKA mRNA by real-time PCR in 158 and DNA ploidy by flow cytometry in other 440 samples of serous OC. Overexpression by IHC was found in 27% of the tumours with cytoplasmic overexpression in 11% and nuclear overexpression in 17%.
AURKB expression was analysed by a semiquantitative IHC test in 80 OC tissues FIGO stage III.37 AURKB was frequently elevated (99%) in ovarian carcinomas with significant differences versus non-malignant ovarian lesions. The expression of AURKB in ovarian tumours was mild (score 1) in 51%, moderate (score 2) in 29% and strong (score 3) in 19% of the cases. The expression of AURKA and AURKB was significantly correlated (p=0.002).
AURKB levels by IHC, and reverse transcriptase PCR were analysed in 156 Taiwanese patients with EOC.35 AURKB was overexpressed in 53 samples (34%) which was significantly superior to the expression in normal ovarian tissue samples (0%), p=0.006. Overexpression of AURKB was more likely in patients with poorly and moderately differentiated versus well-differenciated carcinomas (53.6%, 28.2% and 10.0% respectively). Moreover, AURKB expression was higher in patients with lymph node metastasis (p=0.001) and a positive ascites cytology (p=0.008).
Regarding gene amplifications of AURK family in OC, an analysis in silico by a Spanish group from the cBioportal database showed that amplification of AURKA and B is an uncommon issue. In fact, AURKA amplifications were 9.6% and that AURKB was amplified in 0.6% of the samples and deleted in other 0.6%.38 cBioportal included information from 311 serous OC.
A study presented in AACR in 2016 evaluated the amplification of AURKA in serum and archived tumour samples in patients with OC in the context of a phase I trial with the pan-inhibitor AMG900. Patients included in this trial were resistant to platinum and carboplatin. The amplification determination was performed in archived tissue (mainly obtained at diagnosis of the primary tumour) and in ctDNA before treatment with AMG900. This study showed that AURKA amplification is a late event, as the amplification frequency was very low in archived tumour tissue (1 out of 33 patients) but the determination in ctDNA (extracted at study entry when the patients were resistant to conventional therapies) was higher (11 out of 33 patients).39 These data suggest that AURKA amplification could be a late and acquired alteration, probably as a clonal selection of AURKA amplified tumour cells.
Data about AURKC are scarce in the literature. In order to include some data of the frequency of AURKC alterations and mRNA expression of the three members of AURK, we performed a similar in silico analysis of the cBioportal database.40 Based on the TCGA database published in Nature in 2011, the number of copy-number alterations observed for AURKC was only 2.2% (73.6% amplifications and 36.4% deletions).
In terms of mRNA expression, there were an altered mRNA expression in 7.2% of samples for AURKA (28 samples with high and 5 with low expression), 3.1% for AURKB (5 high and 10 low) and 3.1% for AURKC (14 high and 1 low).
AURK and prognosis in OC
The role of AURK as a prognostic factor has been extensively explored in OC; nevertheless, its role is still controversial and need to be further clarified. The main concern is that the heterogeneity of AURK expression and its evaluation depending on technique and score are not completely standardised and thus this might affect to the prognostic value of this factor. See table 2.
Table 2.
Prognostic role of Aurora kinases (AURK) expression in ovarian cancer
| Reference | Type | Biomarker | Prognosis of high expression | Endpoint | HR/p value | Observations |
| Kulkarni et al41 | EOC | AURKA | Adverse | DFS | HR 1.29 (95% CI 1.06 to 1.58) |
Early stages: HR 1.72 for DFS and 1.81 for OS |
| Landen et al42 | EOC (91% serous OC) |
AURKA IHC expression (score 3) | Adverse | OS | 1.44 versus 2.81 years p=0.001 | AURKA and suboptimal cytoreduction adverse prognostic factors in multivariate analysis |
| Chen et al35 | EOC | AURKB expression | Adverse | PFS OS |
p=0.001 p=0.023 |
|
| Das et al43 | EOC | AURKA nuclear staining | Adverse | OS | 29.6 versus 106.7 months p<0.0005 | |
| Yang et al34 | Endometrioid ovarian cancer | AURKA IHC expression | Adverse | OS DFS |
p=0.001 p=0.002 |
BRCA2 and AURKA inversively regulated |
| Alcaraz Sanabria et al23 2017 | EOC FIGO I/II | AURKA expression AURKB expression |
Adverse Adverse |
PFS PFS OS |
HR 1.85 (95%CI 1.01 to 3.38) HR 1.91 (95% CI 1.05 to 3.48) HR 3.29 (95%CI 1.37 to 7.91) |
Analysis in silico |
| Yang et al44 | Serous ovarian cancer | AURKA | Adverse | OS DFS |
p=0.026 p=0.037 |
|
| Chiba et al45 | Clear-cell ovarian cancer | AURK | Adverse Not ptognostic |
OS (Stages IC3-IV) OS (all stages) |
p=0.02 p=0.18 |
|
| Lassus et al 36 | Serous ovarian cancer | AURKA IHC expression |
Adverse | OS | p<0.0001 (AURKA) p=0.0116 (citoplas AURKA) p=0.0014 (nuclear AURKA) |
AURKA IHC expression and AURKA DNA ploidy adverse factors for DFS in multivariate analysis |
| He et al48 | EOC | Adverse | DFS (seven studies) OS (five studies) |
HR 1.14 (95%CI 0.50 to 1.78) p<0.01 HR 1.40 (95%CI 0.82 to 1.98) p<0.01 |
MEtanalsis of seven studies (Kulkani 2007, Landen 2007, Mendiola 2008, Das 2010, Lassus 2010, Yang 2011 and Yang 2011) | |
| Kulbe et al50 | EOC | AURKA gene expression | Not prognostic Adverse |
PFS OS |
p=0.08 NS p=0.0125 |
Analysis in silico |
| Lassmann et al32 | Primary EOC optimally debulked | AURKA expression | Adverse if non-taxane based adjvant CT Protective if taxane-based adjuvant CT |
OS OS |
p=0.003 p=0.018 |
|
| Mendiola et al33 | EOC | AURKA (+vs −) AURKB (+ vs −) |
Protective Protective Not prognostic |
OS PFS OS PFS |
HR 0.51 (95% CI 0.27 to 0.95) HR 0.52 (95% CI 0.29 to 0.92) HR 0.3 (95% CI 0.10 to 0.87) HR 0.43 (95% CI 0.17 to 1.09) |
In multivariate analysis only AURKA remains a protective prognostic factor in both PFS and OS |
| Alcaraz Sanabria et al 2017 | EOC FIGO III/IV | AURKA Expression AURKB expression |
Protective Not prognostic |
PFS PFS |
HR 0.86 (95% CI 0.74 to 0.99) HR 0.87 (95% CI 075 to 1.01) |
Analysis in silico |
| Heilmann et al46 | EOC | AURKA AURKB |
Not prognostic Nor prognosis |
OS OS |
p=0.18 p=0.495 |
Study included breast and ovarian cancer. Results shown only for ovarian cohort. |
| Lee et al 47 | Primary EOC | AURKA IHC expression | Not prognostic | RFS | p=0.63 |
DFS, disease-free survival; EOC, epithelial ovarian cancer; OS, overall survival; PFS, progression-free survival; RFS, recurrence-free survival.
AURKA and AURKB were identified as prognostic factors of tumour progression in a cohort of 143 patients with EOC.41 AURKA and tumour ploidy states were associated with impaired disease-free survival (DFS) (HR for AURKA 1.29; 95% CI 1.06 to 1.58; p=0.001). Moreover in this cohort AURKA expression in early stages was of particular prognostic importance (DFS for early stages HR 1.72; 95% CI 1.19 to 2.48; p=0.004 and overall survival (OS) for early stages HR 1.81; 95% CI 1.14 to 2.81; p=0.01).
A study of the University of Texas42 showed that patients with strong AURKA expression (score 3) were associated with impaired survival (median survival 1.44 vs 2.81 years; p=0.001) when compared with mild or moderate expression. In fact AURKA, with suboptimal cytoreduction, were independent predictors of survival in the multivariate analysis.
In 2010, the analysis of 45 OC samples43 showed that AURKA nuclear staining was associated with decreased survival with 29.5 versus 106.7 months in AURKA non-expresssed patients (p<0.0005).
The deleterous impact of AURK expression has been observed as well in studies designed specifically in different ovarian subtypes, such as endometrioid, serous or clear-cell OC. In endometrioid ovarian carcinoma, IHC expression of AURKA was found to have an adverse prognosis.35 AURKA expression was more frequent in non-familial endometrioid OC and was negatively correlated with the expression of BRCA2 score (p=0.019) suggesting a double negative regulation. In the log-rank test, AURKA expression was related with shorter overall (OS) (p=0.001) and DFS (p=0.002). Other study asssessing the role of AURKA and BRCA2 in high-grade serous ovarian carcinoma (HGSOC) confirmed that AURKA expression predicted poor OS and DFS, but the ratio AURKA/BRCA2 was identified as a negative prognostic factor as well.44
In serous OC, cytoplasmic or nuclear IHC AURKA overexpression was found to have an adverse prognosis in terms of survival and to be associated with other adverse factors such as high grade, high proliferation index and aberrant p53.28 AURKA IHC overexpression and AURKA DNA ploidy were identified as adverse for DFS in multivariate analysis.
AURK overexpression was associated with poorer survival in ovarian clear-cell carcinoma (OCCC) while AURKA inhibition has shown to enhance the cytotoxic effect of cisplatin in this cancer subtype in a preclinical study.45
The prognosis role of AURKA and AURKB was assessed from an in silico analysis of cBioportal.18
In early stages (FIGO I/II), high expression of AURKA was associated with poorer progression-free survival (PFS; HR 1.85; 95% CI 1.01 to 3.38). AURKB showed similar results for PFS (HR 1.91; 95% CI 1.05 to 3.48) and for OS (HR 3.29; 95% CI 1.37 to 7.91). However, when the analysis was conducted for advanced stages (FIGO III/IV), it was found that the AURKA expression was related to an improvement of clinical outcomes (HR 0.86; 95% CI 0.74 to 0.99) or not significant for AURKB (HR 0.87; 95% CI 0.75 to 1.01). In line with these results, AURKA overexpression was also found as a negative prognostic factor for survival depending on the type of adjuvant chemotherapy administered in a German series of 115 EOC treated with adjuvant CT. In this study, AURKA overexpression was associated with improved OS in optimal debulked patients receiving adjuvant taxol and carboplatin (p=0.018) but was an adverse prognostic factor in patients receiving non-taxane therapy (p=0.03).25
Nevertheless, in other studies, no prognostic impact of AURKA expression was detected. A German study performed in 93 ovarian benign and malignant tumour samples, showed hat the expression of AURKA, and AURKB among other cell cycle markers, were not predictive and were not associated with prognostic factors.46
These results are in line with a second study47 in 160 patients with primary EOC in which any AURKA immunostaining (score 1–3) was not predictive of impaired survival vs absence of expression (p=0.63).
Moreover, other series found that AURKA amplification could be protective. A series in 68 EOC in Spain showed that AURKA overexpression was an independent prognostic factor of improved OS and RFS.26
In 2015, a metanalysis of the impact of IHC AURKA expression in seven studies with OS as endpoint and five other studies with DFS as endpoint was performed. AURKA levels in tumour tissue by IHC were correlated with an impaired prognosis in OS by univariate analysis in seven articles (pooled HR 1.40;95% CI 0.82 to 1.98). However the impact of AURKA on OS by multivariate analysis in three studies was not confirmed.48 In the studies with DFS as an endpoint, AURKA had a deleterous prognostic impact.
Despite the controversial results, AURKA gene has been identified as a candidate gene in different prognostic genomic platforms. A Chinese group constructed a database-based generated gene support vector machine classifier to predict recurrence and survival in OC.49 Thirty-nine genes were selected according to their prognosis relevance from three databases (GSE17260, GSE44104 and GSE51088). Among these, AURKA and AURKA-interacting protein 1 were identified as one of the relevant genes. The prediction accuracies of this SVM (Support Vector Machine) classifier for the three aforementioned databases were 92.7%, 93.3% and 90.4%, respectively. However, a very recent study of discovery and validation of new genes in OC identified AURKA as a candidate gene to discern between benign and malign pelvic mass. Candidate genes were extracted from an in silico analysis of three GSE databases and further validated in blood from patients with either benign masses or ovarian carcinoma. AURKA was identified as a potential biomarker of malignancy among four other genes. Moreover, in this study elevated levels of AURKA and T-cell differentiation protein myelin and lymphocyte were associated with poor prognosis in the OC samples.50
AURKB has been also identified as a potential marker for deleterous survival. In a study in more than 150 patients with EOC, high AURKB expression group showed a significant shorter PFS (p=0.001) and OS (p=0.023) versus the low-expression group.30
AURK and chemoresistance in OC
Preclinical studies have identified that AURKA might be a marker of chemoresistance. Several mechanisms have been suggested to explain the AURKA associated acquired resistance such as the upregulation of NF-kappaB51 pathway or the activation of AKT through a p53-dependent manner.52
A bioinformatic analyses evaluated the impact of gene panels in OC prediction to carboplatin resistance by analysing the microarrays datasets GDS1381 and GDS3592. AURKA was identified among other four genes as a potential key marker involved in carboplatin response.53
A Italian group found that AURKA overexpression assessed by a semiquantitative IHC score was significantly associated with platinum-resistance in 41 patients with HGSOC.54
Nevertheless, other studies failed to identify AURKA as a chemoresistance factor in HGSOC. Li et al performed a study evaluating the expression of different genes in 96 patients with OCCC and 113 patients with HGSOC. Four biomarkers were differently expressed in HGSOC versus OCCC. HER2 and PDL1 overexpression was common in OCCC while loss of BRCA1 and BRCA2 was frequent in HGSOC. Of note, AURKA and PDL1 were correlated with platinum-resistance only in the OCCC group (p=0.043) while no relation with platinum resistance and AURKA was seen in HGSOC.55
AURKB has been identified as well, as a potential target for chemoresistance reversion. In a preclinical study with cisplatin-resistant OVCAR-8 cells, sequential combination of AURKB inhibitors followed by cisplatin showed a synergistic effect with an enhanced apoptotic response. This effect was dependent on c-Myc expression.56
In breast cancer, AURKB overexpression has been also related with resistance to the endocrine agent tamoxifen. AURKB overexpression was also related with impaired prognosis in these tumours.57
Dacomitinib, a pan inhibitor of ErbB receptors, have been studied in chemoresistant ovarian cell lines. Dacomitinib impaired growth and increased apoptosis of resistant-ovarian cells by inhibiting PLK1-FOXM1 signalling pathway and its downstream targets including AURKB.58 This effect suggest that downregulation of AURKB with dacomitinib could have an impact in chemoresistance reversion. These results suggest that AURKB inhibition could be a potential target in chemoresistant EOC.
On contrary, a clinical analysis in 88 pleural effusions from advanced-stage OC showed that low AURKB expression in prechemotherapy effusion was related with primary chemotherapy resistance (p=0.006) and poor treatment response (p=0.013). Compared with their primary tumour, primary effusions showed a significantly higher levels of AURKA expression.59
There is scarce information about AURKC and its role in chemoresistance. Nevertheless, an AURKC interacting molecules has been identified as a potential resistant biomarker to paclitaxel in OC. The disulphide isomerase ERp57, that intearacts with AURKC and beta-actin among others, was asssociated to paclitaxel resistance.60
AURK as a target in OC: AURK inhibition as a therapeutic approach
In the recent years, several AURK inhibitors have been tested in OC. In this context, compounds with both selective Aurora or pan-Aurora activity have been developed.
In a retrospective analysis, the outcomes of more than 240 patients with high-grade EOC included in 94 phase I trials in the University of Texas MD Anderson Cancer Center were analysed.61 Patients were stratified according with the study drug administered. Among the targeted agents included in these trials, there were bevacizumab, anti-VEGFR (Vascular Endothelial Growth Factor Receptor) inhibitors and other compounds targeting PI3K-AKT-mTOR, MAPK, Src, Wee1 and AURKA signalling pathways. Those patients treated with chemotherapy plus bevacizumab or AURKA inhibitors showed a PFS longer than 6 months, suggesting the potential benefit deriving from AURKA inhibitors.
Hereby, we present the most relevant AURK inhibitors evaluated in OC in clinical trials.
Alisertib in OC
Alisertib (previously known as MLN8237) is an oral small inhibitor selective for AURKA. The selectivity in the inhibition of AURKA may result in a better toxicity profile and therapeutic index compared with the pan-Aurora inhibitors.
A preclinical study in OC cell lines showed that alisertib blocked cell cycle and induced apoptosis through p53 upregulation but also inhibited epithelial mesenchymal transition via PI3K/AKT/mTor and sirtuin-1 mediated pathways.62
In a phase 1 trial63 including different solid tumours alisertib was administered to 59 patients, of whom 3 (5%) were patients with OC. Neutropenia and stomatitis were the most commons dose limitants toxicities (DLT). More common grade 3 toxicities were neutropenia (34%), leucopenia (22%) and thrombocytopenia (12%). Signs of antitumour activity was also observed, with prolonged stable disease for more than 6 months in six patients. The recommended phase II dose for alisertib was 50 mg two times a day on a 7-day schedule.
Thirty-one patients with platinum-resistant or platinum-refractory epithelial ovarian (n=25), fallopian tube (n=5) or primary peritoneal tumours (n=1) were treated with alisertib at the aforementioned doses of the phase I study. Response rate was 10% and the duration of responses were prolonged for this adverse prognosis population (6.9–11.1 months). Moreover, 52% achieved stable disease with six patients (19%) lasting ≥3 months.64 This results suggest a modest but durable activity of alisertib as monotherapy in OC.
When tested in combination with chemotherapy, in OC cell lines and in orthotopic xenograft models of OC, alisertib in monotherapy or combined with paclitaxel showed inhibition of tumour growth and metastatisation. The combination was more effective than either drug alone.65
A recent phase Ib/II trial66 evaluated the activity of alisertib in combination with weekly paclitaxel in patients with breast (phase 1) and OC (phase 1 and phase 2). The primary endpoint for the phase 2 trial was PFS. In this trial, patients were randomised to receive alisertib 40 mg 3 days on and 4 days off for 3 weeks plus paclitaxel 60 mg/m2 intravenously days 1, 8 and 15 versus weekly paclitaxel 80 mg/m2 in 28-day cycles.
A total of 191 patients with advanced breast cancer or recurent OC were enrolled, including 142 patients with OC randomised to alisertib +paclitaxel (n=73) versus paclitaxel monotherapy (n=69). Median PFS was 6.7 months for the combination arm versus 4.7 months for paclitaxel alone (HR 0.75; 80% CI 0.58 to 0.96, p=0.14). The prespecified two sided p value cut-off to be considered for further investigations was 0.20; thus, the study was considered positive. Grade 3 or higher toxic events reported were 63 (86%) versus 14 (20%) for the patients in the alisertib versus paclitaxel alone arms, including 77% versus 10% neutropenia and 25% versus 0% stomatitis.
Other AURK inhibitors in OC
ENMD-2076
ENMD-2076 is an orally active multitarget kinase inhibitor that has selective activity against Aurora A and a potent antiangiogenic activity by VEGFR and FGFR (Fibroblast Growth Factor Receptor) inhibition.67
The phase I trial68 in solid tumours showed that ENMD-2076 was well tolerated with an maximal tolerated doses (MTD) of 160 mg/m(2). Neutropenia grade 3 was the DLT of this compound. Expanded cohorts at MTD for ovarian, colorectal and refractory tumours showed promising activity in ovarian tumours with two partial responses in refractory/resistant disease.
Recently, a phase II trial of ENMD-2076 in OCCC was published by the Princess Margaret Consortium.69 The rational for this trial was that apart to a strong expression of VEGF in OCCC, the overexpression of AURKA had been associated with chemoresistance in this subtype.55
Loss of AT-rich interactive domain 1A (ARID1A) was analysed as a potential predictive biomarker for response to ENMD-2076. Patients included had been diagnosed with an OCCC previously treated with platinum-based chemotherapy. Forty patients were finally enrolled. ENMD-2076 was well tolerated with main related grade 3 toxicities being hypertension (28%), proteinuria (10%) and diarrhoea (10%). In terms of activity, ENMD-2076 did no meet the preset bar for efficacy. The best response was partial response for three patients and stable disease for 26. Of note, the overall 6-month PFS was superior in those patients with loss of ARID1A expression (33% vs 12% p=0.023) suggesting a potential predictive role of ARID1A expression.
AMG-900
AMG-900 is an oral selective pan-AURK inhibitor. A phase 1 trial evaluated the safety, tolerability and dose-expansion phases in tree tumour types: taxane-resistant and platinum-resistant OC, taxane-resistant TNBC) and castration-resistant and taxane-resistant or cisplatin/etoposid-resistant prostate cancer (CRPC). The MTD for AMG-900 was 25 mg/day increasing to 40 mg/day with G-CSF in a 4 days on/10 days off schedule. During dose expansion, 3/29 (10.3%) evaluable patients with OC experienced a partial response by RECIST V.1.1 criteria. Moreover, seven patients with OC (24.1%) had a partial response according to GCIG criteria. No response was seen among patients with TNBC or CRPC.70
Danusertib (PHA-739358)
Danusertib hydrochloride, also known as PHA-739358, is an intravenous pan-Aurora inhibitor. In the phase I trial, one patient with refractory OC had a partial response suggesting a potential activity in this setting.71
In a further phase III trial, 223 patients with different tumour types including OC (n=34) were included. Primary endpoint was the progression-free rate (PFR) at 4 months assessed by RECIST V.1.1. Danusertib was administered at 500 mg/m2 given as a 24 hours intravenous infusion every 14 days. This compound did not met the prespecified protocol criteria for clinically relevant activity in any of the treated cancer. PFR at 4 months in OC was 12.1%. The most frequent adverse events were fatigue/astenia, nausea, diarrhoea and haematological toxicity.72
Tozasertib (VX-680, VE-465, MK-0457)
Tozasertib, a pan-Aurora inhibitor,73 has shown to enhanced carboplatin activity by MTT proliferative assay in both platinum-sensitive and platinum-resistant ovarian cell lines of varying p53 status.74 At low doses, this compound synergises paclitaxel induced apoptosis and is active in paclitaxel resistant cells.75 Moreover, the combination of tozasertib with the histone deacetylase valproic acid showed a synergistic effect on gynaecological cancer cells including three OC cell lines.76 A phase I trial with tozarsertib as a 24 hours continuous intravenous infusion in 27 patients identified the MTD as 64 mg/m(2)/h.77 Neutropenia grade 4 and herpes grade 3 were the DLTs. Other common adverse events were nausea, vomiting, diarrhoea and fatigue. Of note, almost half patient achieved stable disease and that was noteworthy in one patients with OC a prolonge stabilisation of 11 months.
Conclusions
AURK family plays an important role in tumourigenesis in OC. AURKA overexpression or amplification is a common alteration in EOC with prognosis implications. But in the last years, AURK have evolved as a potential target with some promising results of AURK inhibitors alisertib and ENMD-2076. Main concern for further development of some of this agents is the toxicity, that can be relevant when combined with chemotherapy.
Nevertheless, there is a strong need to continue exploring the therapeutic potential of the AURK pathway in this setting.
Footnotes
Contributors: JAP-F and AC: conception and design. All authors: writing and final approval.
Funding: JAP-F and BP are awarded with a Mutua Madrileña Medical Research Grant and a Spanish Society of Medical Oncology (SEOM) Grant for Emerging Groups Grant.
Competing interests: None declared.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Carmena M, Earnshaw WC. The cellular geography of aurora kinases. Nat Rev Mol Cell Biol 2003;4:842–54. 10.1038/nrm1245 [DOI] [PubMed] [Google Scholar]
- 2.Pérez Fidalgo JA, Roda D, Roselló S, et al. Aurora kinase inhibitors: a new class of drugs targeting the regulatory mitotic system. Clin Transl Oncol 2009;11:787–98. 10.1007/s12094-009-0447-2 [DOI] [PubMed] [Google Scholar]
- 3.Willems E, Dedobbeleer M, Digregorio M, et al. The functional diversity of Aurora kinases: a comprehensive review. Cell Div 2018;13:7. 10.1186/s13008-018-0040-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yan M, Wang C, He B, et al. Aurora-A kinase: a potent oncogene and target for cancer therapy. Med Res Rev 2016;36:1036–79. 10.1002/med.21399 [DOI] [PubMed] [Google Scholar]
- 5.Cancer Genome Atlas Research Network Integrated genomic analyses of ovarian carcinoma. Nature 2011;474:609–15. 10.1038/nature10166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang C, Yan Q, Hu M, et al. Effect of AURKA gene expression knockdown on angiogenesis and tumorigenesis of human ovarian cancer cell lines. Target Oncol 2016;11:771–81. 10.1007/s11523-016-0436-7 [DOI] [PubMed] [Google Scholar]
- 7.Hirota T, Kunitoku N, Sasayama T, et al. Aurora-A and an interacting activator, the LIM protein Ajuba, are required for mitotic commitment in human cells. Cell 2003;114:585–98. 10.1016/S0092-8674(03)00642-1 [DOI] [PubMed] [Google Scholar]
- 8.Taguchi Sichi, Honda K, Sugiura K, et al. Degradation of human Aurora-A protein kinase is mediated by hCdh1. FEBS Lett 2002;519:59–65. 10.1016/S0014-5793(02)02711-4 [DOI] [PubMed] [Google Scholar]
- 9.Bolton MA, Lan W, Powers SE, et al. Aurora B kinase exists in a complex with survivin and INCENP and its kinase activity is stimulated by survivin binding and phosphorylation. Mol Biol Cell 2002;13:3064–77. 10.1091/mbc.e02-02-0092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jelluma N, Brenkman AB, van den Broek NJF, et al. Mps1 phosphorylates borealin to control Aurora B activity and chromosome alignment. Cell 2008;132:233–46. 10.1016/j.cell.2007.11.046 [DOI] [PubMed] [Google Scholar]
- 11.Tang A, Gao K, Chu L, et al. Aurora kinases: novel therapy targets in cancers. Oncotarget 2017;8:23937–54. 10.18632/oncotarget.14893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang H, Lampson M, Efimov A, et al. Chromosome instability in tumor cells due to defects in Aurora B mediated error correction at kinetochores. Cell Cycle 2018;17:2622–36. 10.1080/15384101.2018.1553340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shimada M, Goshima T, Matsuo H, et al. Essential role of autoactivation circuitry on Aurora B-mediated H2AX-pS121 in mitosis. Nat Commun 2016;7:12059. 10.1038/ncomms12059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kimura M, Matsuda Y, Yoshioka T, et al. Cell cycle-dependent expression and centrosome localization of a third human aurora/Ipl1-related protein kinase, AIK3. J Biol Chem 1999;274:7334–40. 10.1074/jbc.274.11.7334 [DOI] [PubMed] [Google Scholar]
- 15.Sasai K, Katayama H, Stenoien DL, et al. Aurora-C kinase is a novel chromosomal passenger protein that can complement Aurora-B kinase function in mitotic cells. Cell Motil Cytoskeleton 2004;59:249–63. 10.1002/cm.20039 [DOI] [PubMed] [Google Scholar]
- 16.Gabillard J-C, Ulisse S, Baldini E, et al. Aurora-C interacts with and phosphorylates the transforming acidic coiled-coil 1 protein. Biochem Biophys Res Commun 2011;408:647–53. 10.1016/j.bbrc.2011.04.078 [DOI] [PubMed] [Google Scholar]
- 17.Bast RC, Hennessy B, Mills GB. The biology of ovarian cancer: new opportunities for translation. Nat Rev Cancer 2009;9:415–28. 10.1038/nrc2644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sasai K, Treekitkarnmongkol W, Kai K, et al. Functional significance of Aurora Kinases-p53 protein family interactions in cancer. Front Oncol 2016;6:247. 10.3389/fonc.2016.00247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hsueh K-W, Fu S-L, Chang C-B, et al. A novel Aurora-A-mediated phosphorylation of p53 inhibits its interaction with MDM2. Biochim Biophys Acta 2013;1834:508–15. 10.1016/j.bbapap.2012.11.005 [DOI] [PubMed] [Google Scholar]
- 20.Katayama H, Sasai K, Kawai H, et al. Phosphorylation by Aurora kinase A induces MDM2-mediated destabilization and inhibition of p53. Nat Genet 2004;36:55–62. 10.1038/ng1279 [DOI] [PubMed] [Google Scholar]
- 21.Katayama H, Wang J, Treekitkarnmongkol W, et al. Aurora kinase-A inactivates DNA damage-induced apoptosis and spindle assembly checkpoint response functions of p73. Cancer Cell 2012;21:196–211. 10.1016/j.ccr.2011.12.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhao H, Watkins JL, Piwnica-Worms H. Disruption of the checkpoint kinase 1/cell division cycle 25A pathway abrogates ionizing radiation-induced S and G2 checkpoints. Proc Natl Acad Sci U S A 2002;99:M99:14795–800. 10.1073/pnas.182557299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alcaraz-Sanabria A, Nieto-Jiménez C, Corrales-Sánchez V, et al. Synthetic lethality interaction between Aurora kinases and CHEK1 inhibitors in ovarian cancer.. Mol Cancer Ther 2017;16:2552–62. 10.1158/1535-7163.MCT-17-0223 [DOI] [PubMed] [Google Scholar]
- 24.Wu L, Ma CA, Zhao Y, et al. Aurora B interacts with NIR-p53, leading to p53 phosphorylation in its DNA-binding domain and subsequent functional suppression. J Biol Chem 2011;286:2236–44. 10.1074/jbc.M110.174755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gully CP, Velazquez-Torres G, Shin J-H, et al. Aurora B kinase phosphorylates and instigates degradation of p53. Proc Natl Acad Sci U S A 2012;109:E1513–22. 10.1073/pnas.1110287109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.González-Loyola A, Fernández-Miranda G, Trakala M, et al. Aurora B overexpression causes aneuploidy and p21Cip1 repression during tumor development. Mol Cell Biol 2015;35:3566–78. 10.1128/MCB.01286-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang Y, Wang Z, Qi Z, et al. The negative interplay between Aurora a/b and BRCA1/2 controls cancer cell growth and tumorigenesis via distinct regulation of cell cycle progression, cytokinesis, and tetraploidy. Mol Cancer 2014;13:94. 10.1186/1476-4598-13-94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Do T-V, Hirst J, Hyter S, et al. Aurora A kinase regulates non-homologous end-joining and poly(ADP-ribose) polymerase function in ovarian carcinoma cells. Oncotarget 2017;8:50376–92. 10.18632/oncotarget.18970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang Z, Liu Y, Lu L, et al. Fibrillin-1, induced by Aurora-A but inhibited by BRCA2, promotes ovarian cancer metastasis. Oncotarget 2015;6:6670–83. 10.18632/oncotarget.3118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aradottir M, Reynisdottir ST, Stefansson OA, et al. Aurora A is a prognostic marker for breast cancer arising in BRCA2 mutation carriers. J Pathol Clin Res 2015;1:33–40. 10.1002/cjp2.6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang G, Mercado-Uribe I, Multani AS, et al. RAS promotes tumorigenesis through genomic instability induced by imbalanced expression of Aurora-A and BRCA2 in midbody during cytokinesis. Int J Cancer 2013;133:275–85. 10.1002/ijc.28032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lassmann S, Shen Y, Jütting U, et al. Predictive value of Aurora-A/STK15 expression for late stage epithelial ovarian cancer patients treated by adjuvant chemotherapy. Clin Cancer Res 2007;13:4083–91. 10.1158/1078-0432.CCR-06-2775 [DOI] [PubMed] [Google Scholar]
- 33.Mendiola M, Barriuso J, Mariño-Enríquez A, et al. Aurora kinases as prognostic biomarkers in ovarian carcinoma. Hum Pathol 2009;40:631–8. 10.1016/j.humpath.2008.10.011 [DOI] [PubMed] [Google Scholar]
- 34.Yang F, Guo X, Yang G, et al. AURKA and BRCA2 expression highly correlate with prognosis of endometrioid ovarian carcinoma. Mod Pathol 2011;24:836–45. 10.1038/modpathol.2011.44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen Y-J, Chen C-M, Twu N-F, et al. Overexpression of Aurora B is associated with poor prognosis in epithelial ovarian cancer patients. Virchows Arch 2009;455:431–40. 10.1007/s00428-009-0838-3 [DOI] [PubMed] [Google Scholar]
- 36.Lassus H, Staff S, Leminen A, Leminen Isola J, et al. Aurora-A overexpression and aneuploidy predict poor outcome in serous ovarian carcinoma. Gynecol Oncol 2011;120:11–17. 10.1016/j.ygyno.2010.09.003 [DOI] [PubMed] [Google Scholar]
- 37.Beussel S, Hasenburg A, Bogatyreva L, et al. Aurora-B protein expression is linked to initial response to taxane-based first-line chemotherapy in stage III ovarian carcinoma. J Clin Pathol 2012;65:29–35. 10.1136/jclinpath-2011-200212 [DOI] [PubMed] [Google Scholar]
- 38.Ocaña A, Pérez-Peña J, Alcaraz-Sanabria A, et al. In silico analyses identify gene-sets, associated with clinical outcome in ovarian cancer: role of mitotic kinases. Oncotarget 2016;7:22865–72. 10.18632/oncotarget.8118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Juan G, Paweletz K, Anderson A, et al. Detection of aurora kinase A (AURKA) focal amplification in plasma samples of patients with recurrent ovarian cancer. Proccedings of AACR 2016. Cancer Res 2016;76. [Google Scholar]
- 40.cBioportal cBioportal for cancer genomics database. Available: cbioportal.org
- 41.Kulkarni AA, Loddo M, Leo E, et al. DNA replication licensing factors and aurora kinases are linked to aneuploidy and clinical outcome in epithelial ovarian carcinoma. Clin Cancer Res 2007;13:6153–61. 10.1158/1078-0432.CCR-07-0671 [DOI] [PubMed] [Google Scholar]
- 42.Landen CN, Lin YG, Immaneni A, et al. Overexpression of the centrosomal protein aurora-A kinase is associated with poor prognosis in epithelial ovarian cancer patients. Clin Cancer Res 2007;13:4098–104. 10.1158/1078-0432.CCR-07-0431 [DOI] [PubMed] [Google Scholar]
- 43.Das K, Lorena PDN, Kuan Ng L, Ng LK, et al. Aurora-A expression, hormone receptor status and clinical outcome in hormone related cancers. Pathology 2010;42:540–6. 10.3109/00313025.2010.508789 [DOI] [PubMed] [Google Scholar]
- 44.Yang G, Chang B, Yang F, et al. Aurora kinase A promotes ovarian tumorigenesis through dysregulation of the cell cycle and suppression of BRCA2. Clin Cancer Res 2010;16:3171–81. 10.1158/1078-0432.CCR-09-3171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chiba Y, Sato S, Itamochi H, et al. Inhibition of Aurora kinase A synergistically enhances cytotoxicity in ovarian clear cell carcinoma cell lines induced by cisplatin: a potential treatment strategy. Int J Gynecol Cancer 2017;27:1666–74. 10.1097/IGC.0000000000001081 [DOI] [PubMed] [Google Scholar]
- 46.Heilmann T, Dittmann L, van Mackelenbergh M, et al. Head-to-head comparison of the impact of Aurora A, Aurora B, repp86, Cdk1, CDK2 and Ki67 expression in two of the most relevant gynaecological tumor entities. Arch Gynecol Obstet 2016;294:813–23. 10.1007/s00404-016-4104-z [DOI] [PubMed] [Google Scholar]
- 47.Lee Y-K, Choi E, Kim MA, et al. Bubr1 as a prognostic marker for recurrence-free survival rates in epithelial ovarian cancers. Br J Cancer 2009;101:504–10. 10.1038/sj.bjc.6605161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.He Y, Jiang W, Qian X, et al. Role of Aurora-A in ovarian cancer: a meta-analysis. Oncol Res Treat 2015;38:M38(9):442–7. 10.1159/000439194 [DOI] [PubMed] [Google Scholar]
- 49.Zhou J, Li L, Wang L, et al. Establishment of a SVM classifier to predict recurrence of ovarian cancer. Mol Med Rep 2018;18:3589–98. 10.3892/mmr.2018.9362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kulbe H, Otto R, Darb-Esfahani S, et al. Discovery and validation of novel biomarkers for detection of epithelial ovarian cancer. Cells 2019;8:pii:E713:713. 10.3390/cells8070713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sun C, Chan F, Briassouli P, et al. Aurora kinase inhibition downregulates NF-kappaB and sensitises tumour cells to chemotherapeutic agents. Biochem Biophys Res Commun 2007;352:220–5. 10.1016/j.bbrc.2006.11.004 [DOI] [PubMed] [Google Scholar]
- 52.Yang H, He L, Kruk P, et al. Aurora-A induces cell survival and chemoresistance by activation of Akt through a p53-dependent manner in ovarian cancer cells. Int J Cancer 2006;119:2304–12. 10.1002/ijc.22154 [DOI] [PubMed] [Google Scholar]
- 53.Zhan S-J, Liu B, Linghu H. Identifying genes as potential prognostic indicators in patients with serous ovarian cancer resistant to carboplatin using integrated bioinformatics analysis. Oncol Rep 2018;39:2653–63. 10.3892/or.2018.6383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mignogna C, Staropoli N, Botta C, et al. Aurora kinase A expression predicts platinum-resistance and adverse outcome in high-grade serous ovarian carcinoma patients. J Ovarian Res 2016;9:31. 10.1186/s13048-016-0238-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li M, Li H, Liu F, et al. Characterization of ovarian clear cell carcinoma using target drug-based molecular biomarkers: implications for personalized cancer therapy. J Ovarian Res 2017;10:9. 10.1186/s13048-017-0304-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ma Y, Cao H, Lou S, et al. Sequential treatment with aurora B inhibitors enhances cisplatin-mediated apoptosis via c-myc. J Mol Med 2015;93:427–38. 10.1007/s00109-014-1228-0 [DOI] [PubMed] [Google Scholar]
- 57.Larsen SL, Yde CW, Laenkholm A-V, et al. Aurora kinase B is important for antiestrogen resistant cell growth and a potential biomarker for tamoxifen resistant breast cancer. BMC Cancer 2015;15:239. 10.1186/s12885-015-1210-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Momeny M, Zarrinrad G, Moghaddaskho F, et al. Dacomitinib, a pan-inhibitor of ErbB receptors, suppresses growth and invasive capacity of chemoresistant ovarian carcinoma cells. Sci Rep 2017;7:4204. 10.1038/s41598-017-04147-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hetland TE, Nymoen DA, Holth A, et al. Aurora B expression in metastatic effusions from advanced-stage ovarian serous carcinoma is predictive of intrinsic chemotherapy resistance. Hum Pathol 2013;44:777–85. 10.1016/j.humpath.2012.08.002 [DOI] [PubMed] [Google Scholar]
- 60.Cicchillitti L, Della Corte A, Di Michele M, et al. Characterisation of a multimeric protein complex associated with ERp57 within the nucleus in paclitaxel-sensitive and -resistant epithelial ovarian cancer cells: the involvement of specific conformational states of beta-actin. Int J Oncol 2010;37:445–54. 10.3892/ijo_00000693 [DOI] [PubMed] [Google Scholar]
- 61.Hou M-M, Wang Z, Janku F, et al. Continuous anti-angiogenic therapy after tumor progression in patients with recurrent high-grade epithelial ovarian cancer: phase I trial experience. Oncotarget 2016;7:35132–43. 10.18632/oncotarget.9048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ding Y-H, Zhou Z-W, Ha C-F, et al. Alisertib, an aurora kinase A inhibitor, induces apoptosis and autophagy but inhibits epithelial to mesenchymal transition in human epithelial ovarian cancer cells. Drug Des Devel Ther 2015;9:425–64. 10.2147/DDDT.S74062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cervantes A, Elez E, Roda D, et al. Phase I pharmacokinetic/pharmacodynamic study of MLN8237, an investigational, oral, selective Aurora a kinase inhibitor, in patients with advanced solid tumors. Clin Cancer Res 2012;18:4764–74. 10.1158/1078-0432.CCR-12-0571 [DOI] [PubMed] [Google Scholar]
- 64.Matulonis UA, Sharma S, Ghamande S, et al. Phase II study of MLN8237 (alisertib), an investigational Aurora a kinase inhibitor, in patients with platinum-resistant or -refractory epithelial ovarian, fallopian tube, or primary peritoneal carcinoma. Gynecol Oncol 2012;127:63–9. 10.1016/j.ygyno.2012.06.040 [DOI] [PubMed] [Google Scholar]
- 65.Do T-V, Xiao F, Bickel LE, Klein-Szanto AJ, et al. Aurora kinase A mediates epithelial ovarian cancer cell migration and adhesion. Oncogene 2014;33:539–49. 10.1038/onc.2012.632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Falchook G, Coleman RL, Roszak A, et al. Alisertib in combination with weekly paclitaxel in patients with advanced breast cancer or recurrent ovarian cancer: a randomized clinical trial. JAMA Oncol 2019;5:e183773. 10.1001/jamaoncol.2018.3773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fletcher GC, Brokx RD, Denny TA, et al. ENMD-2076 is an orally active kinase inhibitor with antiangiogenic and antiproliferative mechanisms of action. Mol Cancer Ther 2011;10:126–37. 10.1158/1535-7163.MCT-10-0574 [DOI] [PubMed] [Google Scholar]
- 68.Diamond JR, Bastos BR, Hansen RJ, et al. Phase I safety, pharmacokinetic, and pharmacodynamic study of ENMD-2076, a novel angiogenic and Aurora kinase inhibitor, in patients with advanced solid tumors. Clin Cancer Res 2011;17:849–60. 10.1158/1078-0432.CCR-10-2144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lheureux S, Tinker A, Clarke B, et al. A clinical and molecular phase II trial of oral ENMD-2076 in ovarian clear cell carcinoma (OCCC): a study of the Princess Margaret phase II Consortium. Clin Cancer Res 2018;24:6168–74. 10.1158/1078-0432.CCR-18-1244 [DOI] [PubMed] [Google Scholar]
- 70.Carducci M, Shaheen M, Markman B, et al. A phase 1, first-in-human study of AMG 900, an orally administered pan-Aurora kinase inhibitor, in adult patients with advanced solid tumors. Invest New Drugs 2018;36:1060–71. 10.1007/s10637-018-0625-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cohen RB, Jones SF, Aggarwal C, et al. A phase I dose-escalation study of danusertib (PHA-739358) administered as a 24-hour infusion with and without granulocyte colony-stimulating factor in a 14-day cycle in patients with advanced solid tumors. Clin Cancer Res 2009;15:6694–701. 10.1158/1078-0432.CCR-09-1445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Schöffski P, Besse B, Gauler T, et al. Efficacy and safety of biweekly i.v. administrations of the Aurora kinase inhibitor danusertib hydrochloride in independent cohorts of patients with advanced or metastatic breast, ovarian, colorectal, pancreatic, small-cell and non-small-cell lung cancer: a multi-tumour, multi-institutional phase II study. Ann Oncol 2015;26:598–607. 10.1093/annonc/mdu566 [DOI] [PubMed] [Google Scholar]
- 73.Lin YG, Immaneni A, Merritt WM, et al. Targeting aurora kinase with MK-0457 inhibits ovarian cancer growth. Clin Cancer Res 2008;14:5437–46. 10.1158/1078-0432.CCR-07-4922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fu S, Li Y, Huang J, et al. Aurora kinase inhibitor ve 465 synergistically enhances cytotoxicity of carboplatin in ovarian cancer cells through induction of apoptosis and downregulation of histone 3. Cancer Biol Ther 2012;13:1034–41. 10.4161/cbt.21045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Scharer CD, Laycock N, Osunkoya AO, et al. Aurora kinase inhibitors synergize with paclitaxel to induce apoptosis in ovarian cancer cells. J Transl Med 2008;6:79. 10.1186/1479-5876-6-79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Li Y, Liu T, Ivan C, et al. Enhanced cytotoxic effects of combined valproic acid and the Aurora kinase inhibitor VE465 on gynecologic cancer cells. Front Oncol 2013;3:58. 10.3389/fonc.2013.00058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Traynor AM, Hewitt M, Liu G, et al. Phase I dose escalation study of MK-0457, a novel aurora kinase inhibitor, in adult patients with advanced solid tumors. Cancer Chemother Pharmacol 2011;67:305–14. 10.1007/s00280-010-1318-9 [DOI] [PMC free article] [PubMed] [Google Scholar]