Summary
The multidimensional cargo of extracellular vesicles (EV) released in urine is a reflection of the pathophysiological processes occurring within their cells and tissues of origin in the urogenital system. Here, we describe a step-by-step protocol for density-based separation of urinary EV with high specificity and repeatability. The implementation of integrative omics allows the study of the molecular complexity of highly purified urinary EV, supporting the identification of EV-specific functions and biomarkers.
For complete details on the use and execution of this protocol, please refer to Dhondt et al. (2020).
Graphical Abstract
Highlights
-
•
Separation of EV from urine with high specificity and repeatability
-
•
Density gradient centrifugation separates EV from other extracellular particles
-
•
Size-exclusion chromatography recovers EV from density gradient fractions
-
•
Generation of reliable multi-omics data for clinical and research applications
The multidimensional cargo of extracellular vesicles (EV) released in urine is a reflection of the pathophysiological processes occurring within their cells and tissues of origin in the urogenital system. Here, we describe a step-by-step protocol for density-based separation of urinary EV with high specificity and repeatability. The implementation of integrative omics allows the study of the molecular complexity of highly purified urinary EV, supporting the identification of EV-specific functions and biomarkers.
Before You Begin
Preparation of Size-Exclusion Chromatography (SEC) Columns
Timing: 6–8 h
Prepare size-exclusion chromatography (SEC) columns at least 1 d before use, because washing the Sepharose CL-2B resin by gravity sedimentation takes several hours.
-
1.
Transfer the Sepharose CL-2B resin to a sterile glass laboratory bottle. Wait at least 2 h to obtain complete gravity sedimentation of the resin (Figure 1A). Decant the supernatant and replace with 1 volume of PBS per 3 volumes of resin. Mix thoroughly. Repeat this washing step two more times. Do not decant the liquid after the final washing step.
Figure 1.
Preparation of SEC Columns
(A) Sepharose CL-2B resin after gravity sedimentation.
(B) A nylon net (20 μm pore size) is placed on the bottom of a disposable 10 mL syringe.
(C) Washed resin in transferred into the syringe.
(D) A SEC column filled with exactly 10 mL of stacked resin.
-
2.
Place a 1 cm2 piece of nylon-net filter with a 20-μm pore size on the bottom of a 10 mL disposable syringe (Figure 1B).
-
3.
Clamp the syringe to a laboratory stand and pipet the washed Sepharose CL-2B resin into the syringe (Figure 1C). Let the resin settle and add more until the syringe is filled with exactly 10 mL of stacked resin (Figure 1D). Make sure the column never dries out.
-
4.
Close the lower opening of the syringe with a closing cone and the upper opening with Parafilm and store upright in a rack at 4°C until use.
SEC columns can be stored at 4°C for up to 1 week.
CRITICAL: The recommended syringe for in-house preparation of SEC columns has a concentric Luer slip nozzle and an internal diameter of 14.8 mm. Since the diameter of the SEC column and the location of the syringe nozzle impact the recovery efficiency of EV, we recommend the use of a syringe with identical dimensions if researchers are unable to use this particular syringe.
Alternatives: Commercial SEC columns can be used as an alternative to in-house made SEC columns. The qEVoriginal-70 nm (IZON Science - Cat#SP1) is an off-the-shelf SEC column with identical characteristics as the in-house made column presented in this protocol.
Preparation of the Ultracentrifuge
Timing: 5 min
Prior to Urinary EV Separation, place the SW 32.1 Ti rotor and buckets in a cold room at 4°C. Turn on the ultracentrifuge and cool down to 4°C.
Key Resources Table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Alix | Cell Signaling Technology | Cat#2171S |
| TSG-101 | Santa Cruz Biotechnology | Cat#sc-7964 |
| Flotillin-1 | BD Biosciences | Cat#610820 |
| Syntenin-1 | Abcam | Cat#ab133267 |
| CD9 | Cell Signaling Technology | Cat#D3H4P |
| PODXL | Abcam | Cat#ab150358 |
| AQP2 | ThermoFisher Scientific | Cat#PA5-38004 |
| UPK1B | Sigma-Aldrich | Cat#HPA031800 |
| PSA/KLK3 | Cell Signaling Technology | Cat#D11E1 |
| GM130 | BD Biosciences | Cat#610822 |
| THP | Santa Cruz Biotechnology | Cat#sc-20631 |
| PMP70 | Sigma-Aldrich | Cat#P0497 |
| Sheep anti-mouse HRP-linked Ab | GE Healthcare life sciences | Cat#NA931V |
| Donkey anti-rabbit HRP-linked Ab | GE Healthcare life sciences | Cat#NA934V |
| Biological Samples | ||
| Urine | Human | Ethical Committee of Ghent University Hospital approval EC/2015/0260 |
| Chemicals, Peptides, and Recombinant Proteins | ||
| OptiPrep (60% w/v) iodixanol | Axis-Shield | Cat#AXS-1114542 |
| Sucrose | Sigma-Aldrich | Cat#S0389 |
| EDTA (Titriplex III) | Merck Millipore | Cat#1084180100 |
| Tris base | Sigma-Aldrich | Cat#T6066 |
| HCl (standardized solution 5.0 N) | VWR | Cat#35638.K2 |
| Ultrapure water | Invitrogen | Cat#AM9932 |
| PBS (pH 7.2) | ThermoFisher Scientific | Cat#20012019 |
| Sepharose CL-2B | GE Healthcare | Cat#17014001 |
| Critical Commercial Assays | ||
| Multistix 10SG Urinalysis Reagent Test Strips (Siemens Healthineers) | VWR | Cat#10789-338 |
| Deposited Data | ||
| EV-TRACK | (Van Deun et al., 2017) | EV-TRACK ID: EV190064 |
| ProteomeXchange | (Vizcaíno et al., 2014) | Dataset identifier: PXD015289 |
| GEO | (Edgar, 2002) | Dataset identifier: GSE131689 |
| Other | ||
| 100 mL urine collection container with screw cap (sterile) | Sarstedt | Cat#75.562.105 |
| 1.5 mL Eppendorf Safe-Lock microcentrifuge tubes (sterile) | Sigma-Aldrich | Cat#EP022363212 |
| 5 mL Eppendorf tubes (sterile) | Sigma-Aldrich | Cat#EP0030119460 |
| 17 mL Thinwall polypropylene tube | Beckman Coulter | Cat#337986 |
| Conical 50 mL polypropylene tubes (sterile) | Greiner Bio One | Cat#210261 |
| Centricon Plus-70 centrifugal filter units (10 kDa MWCO) | Merck Millipore | Cat#UFC701008 |
| Amicon Ultra-2 centrifugal filter units (10 kDa MWCO) | Merck Millipore | Cat#UFC201024 |
| 10 mL Disposable syringe (sterile) | Romed | Cat#3SYR-10ML |
| Combi-Stopper closing cones | B Braun | Cat#4495101 |
| Parafilm M laboratory film | Bemis Company | Cat#PM-996 |
| Nylon-net filters (20.0 μm pore size) | Merck Millipore | Cat#NY2002500 |
| Bottle-top vacuum filters (0.22 μm pore size) | Sigma-Aldrich | Cat#CLS430624 |
| Optima XPN-80 Ultracentrifuge | Beckman Coulter | Cat#A99839 |
| SW 32.1 Ti Swinging-Bucket Rotor and SW 32 Ti Rotor Bucket Set | Beckman Coulter | Cat#369651 |
| Biomek 4000 Laboratory Automation Workstation | Beckman Coulter | Cat#A99749 |
| Magnetic stirrer | N/A | N/A |
| P100 - P5000 Manual pipettes | N/A | N/A |
| P100 - P5000 Pipette tips (sterile) | N/A | N/A |
| Electronic pipettor | BrandTech Scientific | Cat#26330 |
| 5 mL and 25 mL Serological pipettes | nerbe plus | Cat#12-441-9105 Cat#12-481-9102 |
| Cooled benchtop centrifuge with swinging-bucket rotor | Eppendorf | Cat#5811000320 |
| 100 mL and 1 L Glass laboratory bottles (sterile) | Duran | Cat#218012417 Cat#218015414 |
| Vacuum pump | N/A | N/A |
| Laboratory stand with three-prong extension clamps | VWR | Cat#470135-122 |
| Vortex mixer | N/A | N/A |
| Multi-well microplate reader | Biotek | Cat#SynergyHTX |
| Nunc MicroWell 96-Well Microplate | VWR | Cat#734-2097 |
Materials and Equipment
We recommend that all materials are prepared in a class II laminar flow cabinet to avoid contamination. Sterile, omics-grade resources are used for all steps.
Density Gradient Buffers
Density Gradient Buffer 1
| Reagent | Final Concentration | Add to 1L |
|---|---|---|
| Sucrose | 0.25 M | 85.58 g |
| EDTA | 6 mM | 1.75 g |
| Tris base | 60 mM | 7.27 g |
| HCl 5 M | n/a | ~6.20 mL |
| Ultrapure water | n/a | Up to 1 L |
-
•
To prepare 1 L of density gradient buffer 1, dissolve 85.58 g sucrose, 1.75 g EDTA and 7.27 g Tris in 800 mL of ultrapure water at 20°C–25°C (use magnetic stirrer). Adjust the pH to 7.4 with HCl (5 M). Dilute the buffer to 1 L with ultrapure water.
-
•
Filter the buffer by passing it through a vacuum-connected 0.22-μm bottle-top filter on top of a sterile bottle.
Density Gradient Buffer 2
| Reagent | Final Concentration | Add to 1L |
|---|---|---|
| Sucrose | 0.25 M | 85.58 g |
| EDTA | 1 mM | 0.29 g |
| Tris base | 10 mM | 1.21 g |
| HCl 5 M | n/a | ~1.05 mL |
| Ultrapure water | n/a | Up to 1 L |
-
•
To prepare 1 L of density gradient buffer 2, dissolve 85.58 g sucrose, 0.29 g EDTA and 1.21 g Tris in 800 mL of ultrapure water at 20°C–25°C (use magnetic stirrer). Adjust the pH to 7.4 with HCl (5 M). Dilute the buffer to 1 L with ultrapure water.
-
•
Filter the buffer by passing it through a vacuum-connected 0.22-μm bottle-top filter on top of a sterile bottle.
Density gradient buffers can be stored at 4°C for up to 6 months.
CRITICAL: HCl is highly corrosive. Avoid contact with eyes and skin. Use protective goggles, gloves and clothing.
CRITICAL: To analyze the molecular complexity of urinary EV preparations by integrative omics approaches (e.g. mass spectrometry based proteomics, RNA sequencing), the use of ultrapure water (ASTM type 1) is a prerequisite to avoid interference with organic, biological and elemental contaminants. Avoid the use of deionized water (diH2O), which only controls for ionic contaminants.
General requirements for Type 1 ultrapure water (ASTM D1193-06, 2011) are:
| Parameter | Type 1 Reagent Water |
|---|---|
| Conductivity, max. μS/cm (25°C) | 0.056 |
| Resistivity, min. MΩ-cm (25°C) | 18 |
| pH, units (25°C) | n/a |
| Total Organic Carbon (TOC), max. (μg/L) | 50 |
| Sodium, max. (μg/L) | 1 |
| Chloride, max. (μg/L) | 1 |
| Total Silica, max. (μg/L) | 3 |
| Filter, min. μm | 0.2 |
Step-By-Step Method Details
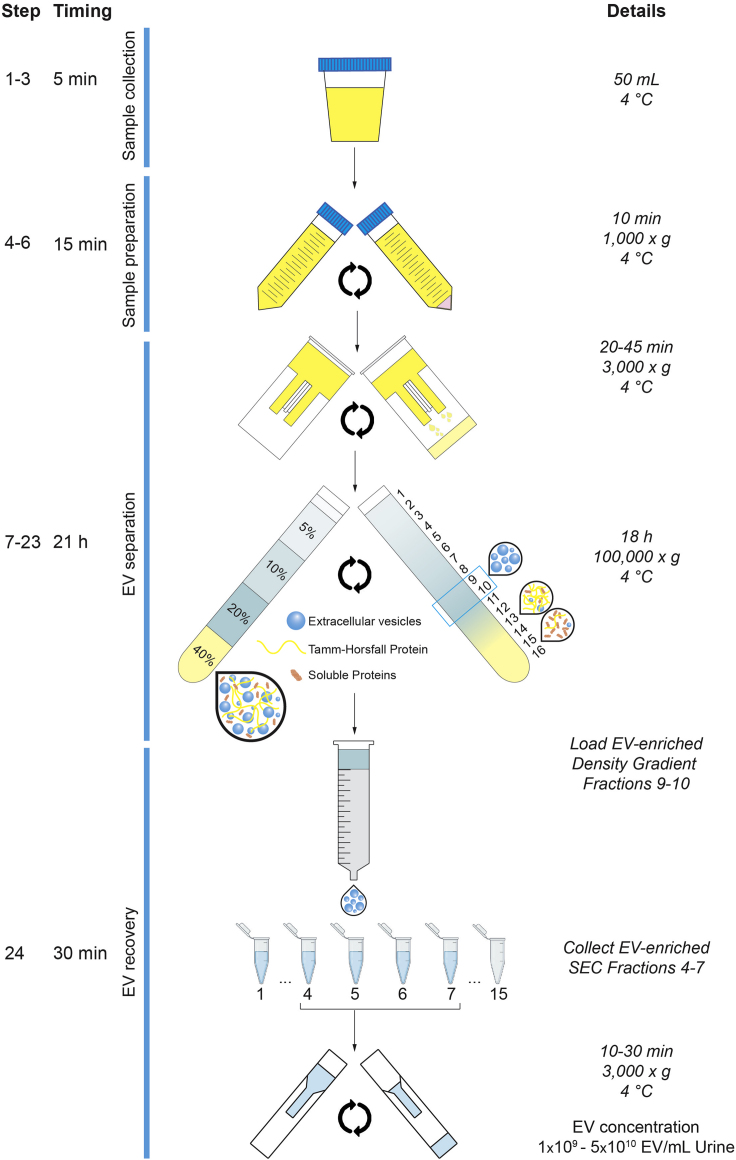
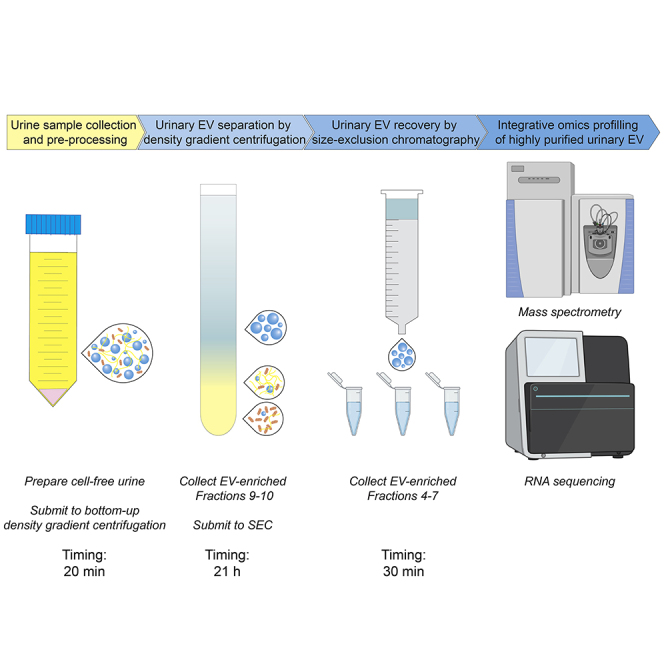
This protocol provides step-by-step instructions on performing density-based fractionation of urine to prepare highly purified, multi-omics grade, urinary EV. Figure 2 highlights the 4 major steps of the protocol. Urine samples are collected (1), pre-processed (2) and stored using validated standard operating procedures. Bottom-up density gradient centrifugation of pre-processed urine separates urinary EV from other extracellular particles (3). Finally, urinary EV are recovered from density gradient fractions by SEC and concentrated to a volume suitable for downstream integrative omics profiling (4).
Figure 2.
Illustrative Overview of the Urinary EV Separation Protocol
After collection and preparation of the urine sample, pre-enrichment of urinary EV is performed by ultrafiltration. Urinary EV are separated and recovered from this ultrafiltrate by the orthogonal implementation of a bottom-up density gradient and size-exclusion chromatography. The EV sample is concentrated to a volume suitable for downstream EV characterization and integrative omics analysis.
Urine Sample Collection
Timing: 5 min
Urine samples are collected using a validated standard operating procedure.
-
1.
Collect urine in a sterile container with a screw cap. Collect a mid-stream sample of random-catch or second morning urine (∼50 mL).
Note: The use of second morning urine is recommended to minimize variability between donors based on diurnal patterns and to omit the use of first morning urine collected from large bladder residues.
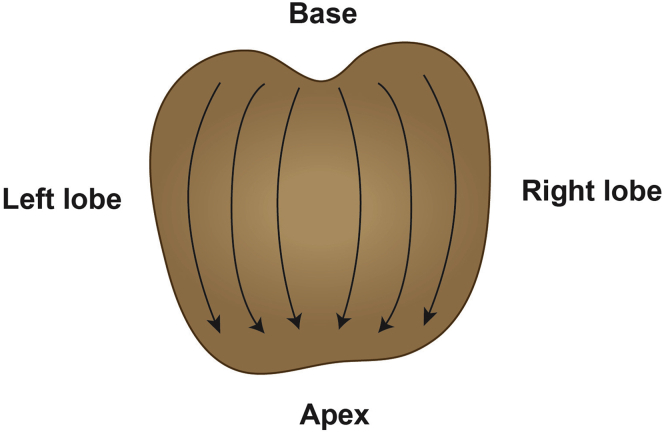
Alternatives: If urine enriched in prostate-derived secretions is required, collect a first-catch urine sample (∼50 mL) immediately following digital rectal examination (DRE). DRE is performed by applying pressure on the prostate, depressing the surface ∼1 cm, from the base to the apex and from the lateral to the median line for each prostate lobe. Perform 3 finger strokes per lobe (Figure 3) (Groskopf et al., 2006).
Figure 3.
Standard Operating Procedure to Collect Urine Enriched in Prostatic Secretions
A first-catch urine sample is collected immediately following standardized digital rectal examination.
CRITICAL: Experiments using human biological specimens must conform to local and national regulations, and in accordance to the principles of the Helsinki Declaration. Written informed consent must be obtained from urine sample donors. Strict compliance with Biosafety Level 2 practices, containment equipment, and facilities are recommended when handling human body fluids (U.S. Department of Health and Human Services; Centers for Disease Control; National Institutes of Health, 2009).
-
2.
Perform test strip urinalysis to determine pathological changes in the collected urine sample.
Note: Bacterial contamination, urinary tract infection, hematuria and proteinuria may affect downstream analysis of urinary EV.
Alternatives: Urinalysis can also be performed using an automated urine chemistry analyzer.
-
3.
If immediate sample preparation after collection cannot be achieved, refrigerate the urine sample at 4°C.
CRITICAL: Correct preservation of urine samples before sample preparation is essential to prevent microbial overgrowth, which can interfere with downstream urinary EV separation and characterization.
CRITICAL: Do not freeze urine samples prior to sample preparation. This leads to cell lysis and release of cell organelles into the sample.
Note: There is no consensus regarding short-term storage stability of urinary EV. Therefore, we recommend Urine Sample Preparation within the same day, no later than 4 h after sample collection (Yamamoto, 2010). The addition of protease inhibitors is not recommended, since urinary EV are largely resistant to the endogenous proteolytic activity of urine (Mitchell et al., 2009).
Urine Sample Preparation
Timing: 15 min
Urine samples are pre-processed and stored using validated standard operating procedures.
-
4.
Transfer the urine sample to a sterile 50 mL conical centrifugation tube.
-
5.
Place the centrifugation tube into a pre-cooled benchtop centrifuge with swinging bucket rotor and centrifuge at 1,000 × g for 10 min at 4°C. Counterbalance when needed.
-
6.
Collect the supernatant, using an electronic pipettor and serological pipette, and transfer it to a new sterile 50 mL conical centrifugation tube. Discard the pellet.
CRITICAL: Avoid transferring or disturbing the pellet, which contains cells and cell debris, bacteria and urinary crystal precipitates.
Pause Point: Cell-free urine samples can be used immediately or stored at −80°C for up to 1 year.
Note: When collecting and storing urine samples in a biorepository, report all pre-analytical and patient-related details according to the Biospecimen Reporting for Improved Study Quality (BRISQ) recommendations to better evaluate, interpret, compare, and reproduce experimental results obtained with these samples (Moore et al., 2011).
Urinary EV Separation
Timing: 21 h
Urinary EV are separated from other extracellular particles using bottom-up density gradient centrifugation.
When working with frozen urine samples that were stored in a biorepository, continue with step 7. When working with fresh urine samples, jump to step 8.
Optional: To calculate recovery efficiencies and allow the normalization of quantitative results between protocol runs, spike a control urine sample with a known amount of GFP-positive recombinant EV (rEV). These rEV can be tracked during separation from urine using fluorescence-, protein- or RNA based methods. For detailed instructions on the generation and use of rEV, we refer to (Geeurickx et al., 2019).
-
7.
Thaw the 50 mL urine sample at 20°C–25°C. After thawing, vortex the sample for 1 min.
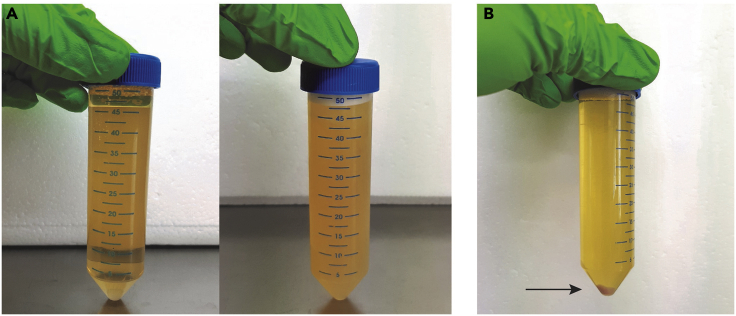
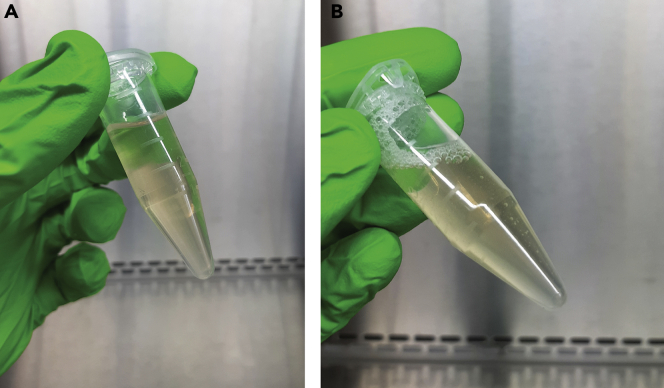
CRITICAL: Urinary crystalline salts, such as calcium oxalate and amorphous phosphate or urate crystals, precipitate at low storage temperatures. Crystal precipitation is observed as an increased turbidity of the sample (Figure 4A). These precipitates interfere with downstream EV separation. Therefore, urine samples containing crystal precipitates after thawing require additional centrifugation at 1,000 × g for 10 min at 4°C, as described in steps 5–6 of the protocol. The pellet will be pink in appearance (Figure 4B). Alternatively, precipitates can be resolubilized by a 5 min temperature equilibration in a 37°C water bath or by correction of the urinary pH to 8.0 by addition of a 1 M Tris buffer (Yamamoto, 2010).
Figure 4.
Removal of Urinary Crystalline Salts
(A) Fresh urine sample (left) versus thawed urine sample containing crystal precipitates (right).
(B) Crystal precipitates are removed by centrifugation. The pellet (arrow) is pink in appearance.
Note: Limit the amount of freeze-thaw cycles to one. Avoid repeated freeze-thaw cycles, since this might negatively impact EV recovery from urine (Yuana et al., 2015).
-
8.
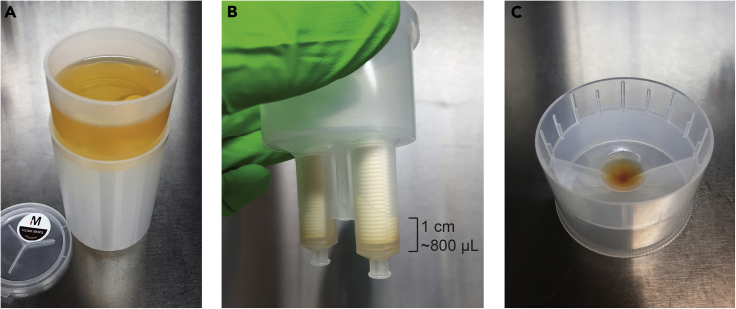
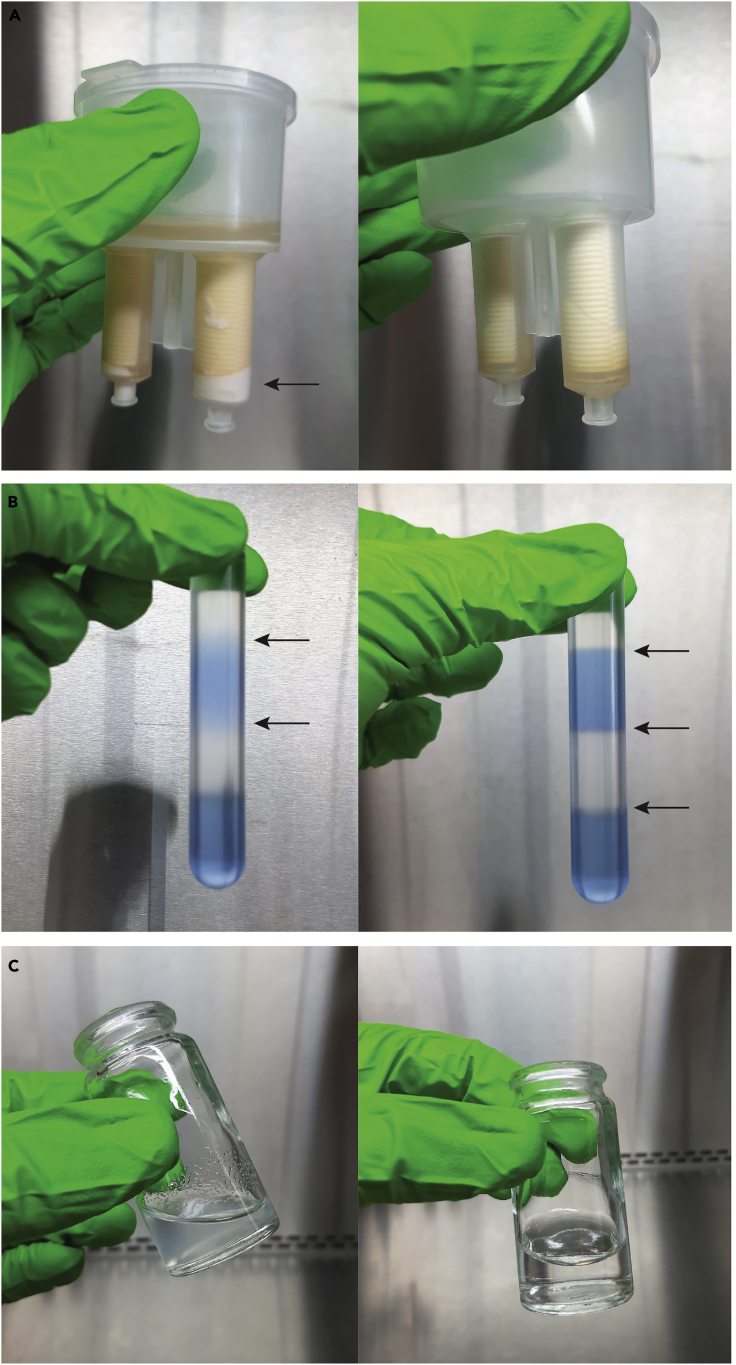
Transfer the urine sample, using an electronic pipettor and serological pipette, into the sample filter cup of an assembled 10-kDa Millipore Centricon Plus-70 centrifugal filter unit (Figure 5A).
Figure 5.
Preparation of a Concentrated Urine Sample
(A) The cell-free urine sample is transferred into the sample filter cup of a centrifugal filter device (step 8).
(B) The sample is concentrated to a target volume of 800 μL, corresponding to ~1 cm of remaining sample in the filter core (step 9).
(C) The concentrated urine sample is recovered in the concentrate collection cup of the centrifugal filter device (steps 10–11).
Note: The choice of centrifugal filter device for ultrafiltration is crucial and impacts the EV recovery efficiency of the protocol. Ultrafiltration devices equipped with a regenerated cellulose membrane and a 10 kDa pore size recover EV the most efficiently (Vergauwen et al., 2017).
-
9.
Place the filter unit into a pre-cooled benchtop centrifuge with swinging bucket rotor and centrifuge at 3,000 × g for minimally 20 min at 4°C until the sample is concentrated to at least 800 μL. Counterbalance with a similar device when needed.
CRITICAL: Differences in concentration of urinary solutes due to donor fluid intake and renal function, cause variability in centrifugation times between samples to obtain the required sample volume. Overconcentration of the urine sample will negatively impact EV recovery. Therefore, regular visual assessment of the concentrate at several time points (e.g. 20, 25, 30 min, etc.) is critical. The target volume of 800 μL corresponds to approximately 1 cm of remaining sample in the filter core (Figure 5B). See Problem 1 in the Troubleshooting section.
-
10.
Connect a concentrate collection cup to the sample filter cup, invert the device and place it in the benchtop centrifuge. Counterbalance with a similar device when needed. Spin the concentrate down at 1,000 × g for 2 min at 4°C (Figure 5C).
-
11.
Remove the concentrate cup containing the concentrated urine sample from the sample filter cup. Collect the sample with a pipette and, if necessary, correct the volume to 800 μL with pre-cooled (4°C) Density Gradient Buffer 2. Transfer to a 5 mL Eppendorf tube and place on ice.
CRITICAL: The concentrated urine sample will form the basis for the preparation of a 40% (w/v) iodixanol sample suspension in step 14 of the protocol. It is critical that the volume of this sample is exactly 800 μL in order to prepare the suspension correctly.
-
12.
Prepare the iodixanol working solution (50% w/v) by mixing 1 volume of Density Gradient Buffer 1 with 5 volumes of OptiPrep in a 50 mL conical tube according to the following table. Keep the working solution cooled on ice.
| Gradients | OptiPrep (mL) | Buffer 1 (mL) |
|---|---|---|
| 1 | 7 | 1.4 |
| 2 | 14 | 2.8 |
| 3 | 21 | 4.2 |
| 4 | 28 | 5.6 |
| 5 | 35 | 7.0 |
| 6 | 42 | 8.4 |
Note: Fresh working solution should be prepared for each experiment. Protect Optiprep from prolonged exposure to direct sunlight, since this leads to release of iodine from the iodixanol molecule.
-
13.
Prepare 5%, 10% and 20% (w/v) iodixanol solutions by mixing appropriate volumes of working solution (50% w/v) and Density Gradient Buffer 2 in 50 mL conical tubes according to the following table. Turn the tubes gently up and down until homogeneous solutions have been obtained. Keep the solutions cooled on ice.
| Gradients | Iodixanol Solutions |
|||||
|---|---|---|---|---|---|---|
| 5 (% w/v) |
10 (% w/v) |
20 (% w/v) |
||||
| Working Solution (mL) | Buffer 2 (mL) | Working Solution (mL) | Buffer 2 (mL) | Working Solution (mL) | Buffer 2 (mL) | |
| 1 | 0.5 | 4.5 | 1.0 | 4.0 | 2.0 | 3.0 |
| 2 | 1.0 | 9.0 | 2.0 | 8.0 | 4.0 | 6.0 |
| 3 | 1.5 | 13.5 | 3.0 | 12.0 | 6.0 | 9.0 |
| 4 | 2.0 | 18.0 | 4.0 | 16.0 | 8.0 | 12.0 |
| 5 | 2.5 | 22.5 | 5.0 | 20.0 | 10.0 | 15.0 |
| 6 | 3.0 | 27.0 | 6.0 | 24.0 | 12.0 | 18.0 |
Note: Fresh solutions should be prepared for each experiment.
-
14.
Prepare a 40% (w/v) iodixanol sample suspension by adding 3.2 mL of iodixanol working solution (50% w/v) to the concentrated urine sample (800 μL), prepared in step 11 of the protocol. Homogenize the mixture by gently pipetting up and down.
CRITICAL: Gentle pipetting to homogenize the 40% (w/v) iodixanol sample suspension is a prerequisite to avoid the introduction of air bubbles, which may disturb the density layers of the gradient (Figure 6).
Figure 6.
Preparation of a 40% Iodixanol Sample Suspension
(A) The suspension is homogenized by gently mixing the concentrated urine sample and the appropriate volume of iodixanol working solution (step 14).
(B) Imprudent homogenization of the suspension generates air bubbles and should be avoided.
-
15.
Prepare a discontinuous density gradient in a clean 17 mL Thinwall polypropylene tube by layering iodixanol solutions with successively lower densities on top of each other.
Timing: 10–20 min per density gradient
-
a.
Hold the tube upright and gently dispense the 4 mL of 40% (w/v) iodixanol sample suspension, prepared in step 15 of the protocol, to the bottom of the tube.
-
b.
Carefully tilt the tube to 70°. Gently dispense 4 mL of the 20% (w/v) iodixanol solution to the surface of the liquid close to the opening of the tube. Keep the pipette tip close to the wall of the tube.
Note: transferring the solution dropwise using a P1000 pipette (4 × 1,000 μL) provides a slow and smooth flow of liquid.
-
c.
Gently dispense 4 mL of the 10% (w/v) iodixanol solution to the surface of the liquid close to the opening of the tube. Keep the pipette tip close to the wall of the tube.
Note: transferring the solution dropwise using a P1000 pipette (4 × 1,000 μL) provides a slow and smooth flow of liquid.
-
d.
Gently dispense 3.5 mL of the 5% (w/v) iodixanol solution to the surface of the liquid close to the opening of the tube. Keep the pipette tip close to the wall of the tube.
Note: transferring the solution dropwise using a P1000 pipette (4 × 875 μL) provides a slow and smooth flow of liquid.
-
e.
Gently dispense 1.0 mL of PBS to the surface of the liquid close to the opening of the tube. Keep the pipette tip close to the wall of the tube.
-
f.
Place the density gradient upright in a tube rack on ice or in a pre-cooled (−20°C) aluminum cooling block.
CRITICAL: Keep the work area free from vibrations and disturbances. Dispense the iodixanol solutions carefully to avoid mixing and ensure the formation of distinct layers with a sharp interface (Figure 7). Do not repeatedly shift between holding the tube tilted and upright, since this negatively affects gradient layering (Methods Video S1). See Problem 2 in the Troubleshooting section.
Figure 7.
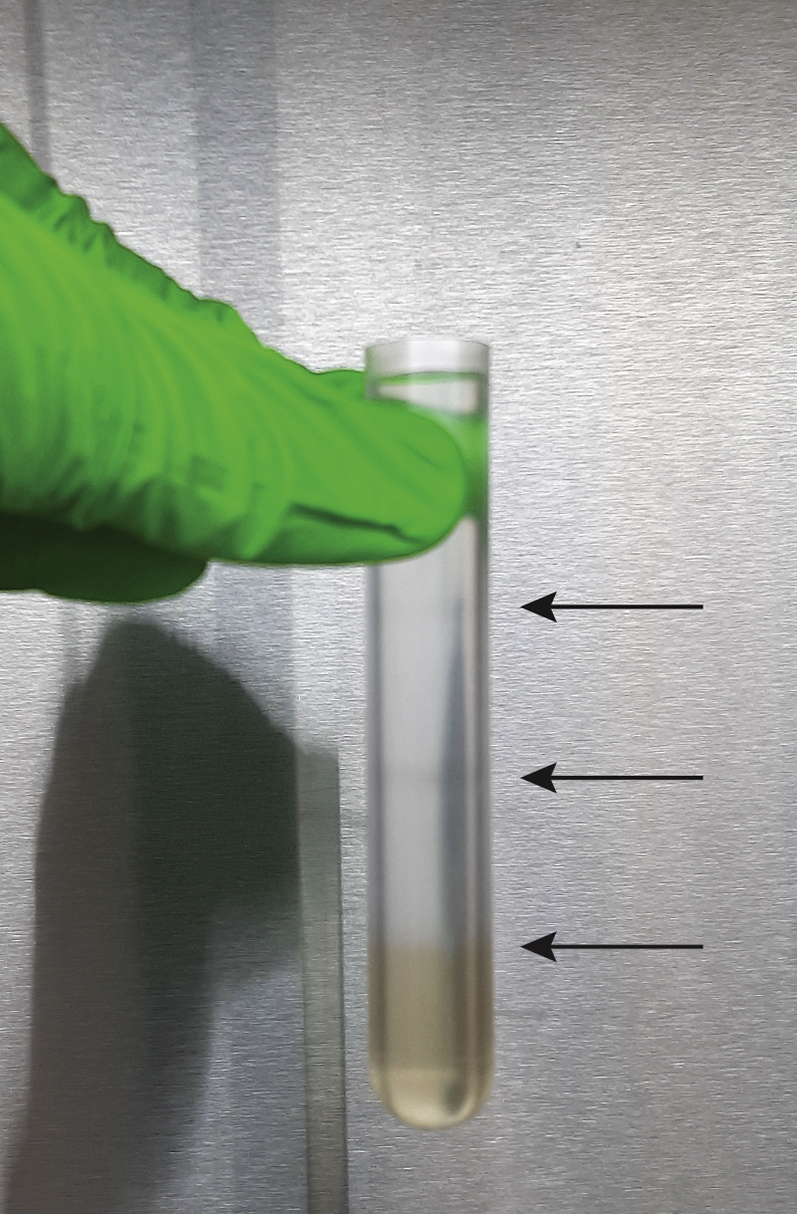
Successful Preparation of a Discontinuous Bottom-Up Density Gradient
The iodixanol solutions form distinct layers (arrows) with a sharp interface (step 15).
Alternatives: Robot-assisted layering of discontinuous density gradients eliminates user variability, is less time-consuming, and contributes to consistent and reproducible density-based EV separation from urine. In case an automated liquid handler with liquid level sensing (e.g. Biomek 4000 Workstation) is available to the researcher, we recommend robot-assisted preparation of density gradients. For detailed instructions on automated gradient layering, we refer to (Tulkens et al., 2019).
-
16.
Place the SW 32.1 Ti rotor into the ultracentrifuge.
-
17.
Gently place the density gradient(s), prepared in step 16, into the rotor bucket.
-
18.
Gently place the rotor buckets into the rotor. Counterbalance when needed.
-
19.
Program the ultracentrifuge run using the following settings:
-
a.
Rotor type: SW 32.1 Ti Rotor
-
b.
Type of centrifugation tube: 17 mL Thinwall
-
c.
Speed: 100,000 × g
-
d.
Time: 18 h
-
e.
Temperature: 4°C
-
f.
Acceleration: 0 (MAX)
-
g.
Deceleration: 9
-
20.
Start the ultracentrifuge.
Pause Point: Plan the ultracentrifuge run as an overnight run and continue the protocol the next day.
-
21.
After completion of the ultracentrifuge run, carefully transfer the rotor buckets into a rack.
-
22.
Take the fractionated density gradient out of the bucket and collect the fractions from top to bottom.
Timing: 10–15 min per density gradient
-
a.
Hold the tube upright. Gently place the pipette tip on the center of the fluid meniscus of the density gradient and carefully collect individual 1 mL fractions from the top of the gradient.
CRITICAL: Collection of density gradient fractions is technically challenging and prone to error. Hold the centrifugation tube between your thumb and index finger to ensure the tube stays upright during the entire process. Use a P1000 pipette with smooth and precise aspiration control (Methods Video S2).
In step 15, a discontinuous density gradient is prepared in a 17 mL Thinwall polypropylene tube by layering iodixanol solutions with successively lower densities on top of each other. The iodixanol solutions are dispensed carefully to avoid mixing and ensure the formation of distinct layers with a sharp interface.
-
b.
Transfer the collected density gradient fractions to sterile 1.5 mL Eppendorf tubes and keep them cooled on ice.
Alternatives: As outlined in step 15, robot-assisted collection of density gradient fractions has several advantages over manual collection, contributing to standardization and reproducibility of the protocol by eliminating user variability. In case an automated liquid handler with liquid level sensing (e.g. Biomek 4000 Workstation) is available to the researcher, we highly recommend robot-assisted collection of density gradient fractions. For detailed instructions on automated gradient fractionation, we refer to (Tulkens et al., 2019).
Optional: Determine the final density of the fractions collected from a blank control gradient using a multi-well plate reader.
-
(i)
Transfer 200 μL of density gradient buffer 2 in the well of a 96-well microplate to use as a blank
-
(ii)
Transfer 100 μL of 5%, 10%, 20% and 40% iodixanol solutions into 100 μL of diH2O in the wells of a 96-well microplate to make 1:1 aqueous dilutions
-
(iii)
Transfer 100 μL of each of the fractions into 100 μL of diH2O in the wells of a 96-well plate microplate to make 1:1 aqueous dilutions
-
(iv)
Place the 96-well plate in a multi-well plate reader and measure the absorbance values at 340 nm of the solutions in each well
-
(v)
Calculate the density of the gradient fractions using a standard curve of the absorbance values of the aqueous dilutions of 5, 10, 20 and 40% iodixanol solutions. Corresponding densities of the iodixanol solutions (in 0.25 M sucrose) are provided in the table:
| Iodixanol Solution (% w/v) | Density (g/mL) |
|---|---|
| 5 | 1.054 |
| 10 | 1.079 |
| 20 | 1.127 |
| 40 | 1.223 |
Note: For iodixanol concentrations above 35% (w/v), it may be necessary to make a second 1:1 aqueous dilution to avoid absorbance values above 1.2.
-
23.
Pool density gradient fractions 9–10, enriched in urinary EV, and continue with urinary EV recovery.
Optional: Other density gradient fractions can be collected and processed in function of the intended research objective. Soluble urinary proteins are enriched in high-density fractions 14–16 (Dhondt et al., 2020).
In step 22a, density gradient fractions are collected from the top of the gradient. The tube is held upright. The pipette tip is gently placed on the center of the fluid meniscus and individual 1 mL fractions are carefully collected.
Urinary EV Recovery
Timing: 30 min per sample
EV are recovered from density gradient fractions using size-exclusion chromatography.
Note: Several methods can be used for urinary EV recovery from density gradient fractions (e.g. SEC and ultracentrifugation). However, complete separation of the iodixanol matrix from EV can only be guaranteed using size-exclusion chromatography (SEC) (Vergauwen et al., 2017). Since iodixanol remnants in urinary EV preparations interfere with downstream mass spectrometry-based proteomics and RNA sequencing, complete removal is desirable. In addition, the efficiency of EV recovery from density gradient fractions by SEC is higher compared to that of EV recovery by ultracentrifugation. Therefore, if the final goal of urinary EV separation is performing integrative omics profiling, SEC should be used in order to obtain maximal EV purity and yield, qualitative tandem mass spectra for mass spectrometry-based proteomics, and optimal sequencing quality and depth for RNA sequencing.
-
24.
Size-exclusion Chromatography
-
a.
Clamp the SEC column to a laboratory stand (See Materials and Equipment for instructions on how to prepare SEC columns). Check if the column did not dry out and remove the closing cone (Figure 8).
Figure 8.
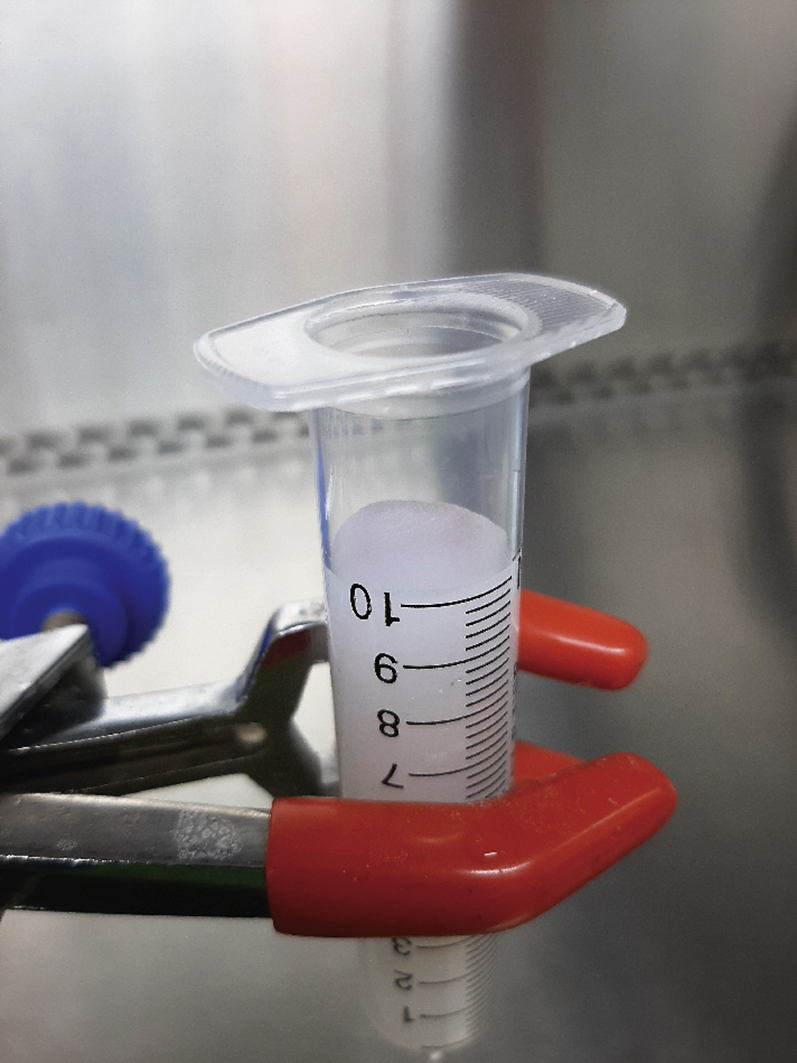
Size-Exclusion Chromatography Quality Assurance
Prior to or during SEC, the column should be prevented from drying out (step 24).
-
b.
Rinse the column with 2mL of pre-cooled PBS (4°C). Collect the eluate in a translucent vial and confirm the column does not leak Sepharose resin. See Problem 3 in the Troubleshooting section.
-
c.
Load pooled density fractions 9–10 (2 mL), collected in steps 22–23 of the protocol, onto the column as soon as the PBS buffer has completely disappeared into the resin.
CRITICAL: When loading PBS or sample onto the column, ensure not to disturb the Sepharose resin in the column. Load dropwise and close to the surface of the resin. Ensure a slow and smooth flow of liquid. Make sure the SEC column does not dry out (Methods Video S3).
-
d.
Immediately following sample loading onto the SEC column, start collecting individual fractions of 1 mL. SEC fractions 1–3 are void volume, while EV elute in SEC fractions 4–7. When the loaded sample has completely disappeared into the resin, load PBS buffer onto to column as long as it takes to collect the SEC fractions of interest and to prevent the column from drying out.
Note: Collect the first 3 mL of eluate (SEC fractions 1–3) in a 5 mL Eppendorf tube and collect the next 4 mL of eluate (SEC fractions 4–7 containing EV) in a separate 5 mL Eppendorf tube.
-
e.
Transfer SEC fractions 4–7 into a 10-kDa centrifugal filter unit.
Note: The choice of centrifugal filter device for ultrafiltration is crucial and impacts the EV recovery efficiency of the protocol. Ultrafiltration devices equipped with a regenerated cellulose membrane and a 10 kDa pore size recover EV the most efficiently (Vergauwen et al., 2017).
-
f.
Place the filter unit into a pre-cooled benchtop centrifuge with swinging bucket rotor and centrifuge at 3,000 × g at 4° C until the sample is concentrated to 100 μL (10–30 min). Counterbalance with a similar device when needed.
-
g.
Collect the concentrated urinary EV sample by a reverse spin at 1,000 × g for 2 min at 4°C. Transfer to a 1.5 mL Eppendorf tube and place on ice.
Pause Point: Urinary EV can be characterized immediately or stored at −80°C for several months.
In step 24c, EV-enriched density gradient fractions are loaded on top of a SEC column. Sample or PBS are loaded dropwise and close to the surface of the resin. Immediately following sample loading onto the SEC column, individual fractions of 1 mL are collected.
Urinary EV Preparation for Integrative Omics Profiling
Urinary EV samples are prepared for integrative omics profiling.
Note: we provide different procedures for urinary EV preparation for integrative omics profiling. However, a detailed step-by-step description of these methods is outside the scope of this protocol. The approach to downstream sample processing is at the discretion of the researcher.
-
25.
Prepare urinary EV samples for mass spectrometry-based proteomic analysis (a) and/or RNA sequencing (b). A 100 μL urinary EV suspension is sufficient for both downstream applications.
-
a.
Perform protein extraction and tryptic digestion of the urinary EV sample for mass spectrometry-based proteomics by filter-aided sample preparation (FASP) (Dhondt et al., 2020; Wiśniewski et al., 2009). Desalt the peptides and proceed to liquid chromatography–mass spectrometry (LC-MS/MS).
-
b.
Perform RNA extraction of the urinary EV sample for RNA sequencing by spin column-based purification of total RNA (eg. miRNeasy Serum/Plasma Kit – Qiagen – Cat# 217184). We recommend the use of Sequin (Hardwick et al., 2016) and ERCC (ThermoFisher – Cat# 4456740) RNA spikes to control for variation in RNA isolation and RNA expression data, respectively. Proceed to library preparation and RNA sequencing.
Expected Outcomes
Consistent characterization of urinary EV preparations by multiple, complementary methods is essential to analyze the performance of the protocol, as well as its specificity and repeatability (De Wever and Hendrix, 2019). We advise to characterize urinary EV in compliance with EV-TRACK (Van Deun et al., 2017) and MISEV2018 guidelines (Théry et al., 2018) prior to downstream integrative omics profiling (e.g. mass-spectrometry based proteomics and RNA sequencing). We recommend a combination of complementary particle- and protein-based measurement methods to obtain a quantitative and qualitative assessment of urinary EV. Western blot (or ELISA) confirms the enrichment of EV-associated proteins and depletion of non-EV-associated proteins such as urinary high-molecular weight proteins. Total particle number and size distribution are measured by light scattering methods such as nanoparticle tracking analysis (NTA). EV ultrastructure, integrity and purity are evaluated by electron microscopy (EM). For detailed instructions on how to perform EV characterization, we refer to (Tulkens et al., 2019).
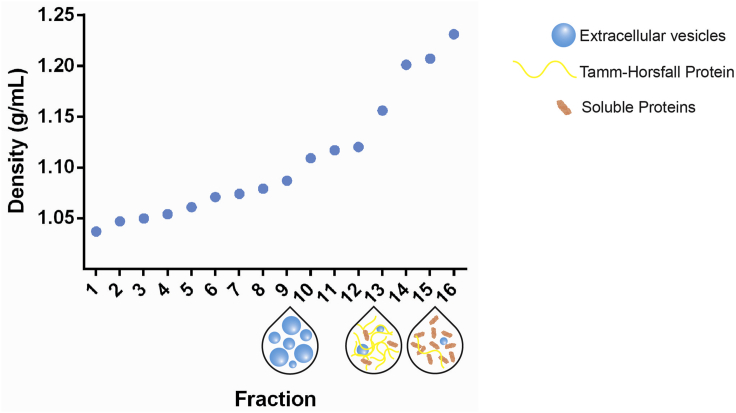
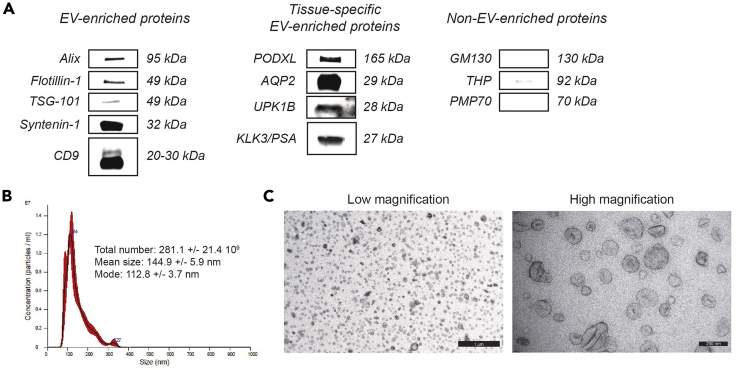
EV-enriched density gradient fractions are defined by distinct physical and biochemical characteristics (Figures 9 and 10). Urinary EV (1.087–1.109 g/mL) differ in buoyant density from Tamm-Horsfall protein (THP) polymers (1.156–1.201 g/mL) and protein aggregates (1.207–1.231 g/mL) (Figure 9). Western blot analysis reveals the presence of EV-enriched proteins (e.g. ALIX, TSG-101, Flotillin-1, Syntenin-1, CD9) and depletion of non-EV-enriched proteins (e.g. GM130, THP, PMP70). In addition, urinary EV are enriched in proteins associated with the specific genitourinary organs they are derived from, such as PODXL and AQP2 (kidney), UPK1B (urinary bladder) and KLK3/PSA (prostate) (Figure 10A). The total urinary EV yield and size distribution profile is obtained by NTA (NTA-specific size range: 90–250 nm) (Figure 10B). An overall high urinary EV yield can be expected, but is variable depending on sample volume, time of collection, hydration status and renal function (Dhondt et al., 2018). In our experience, 1.0 × 109–5.0 × 1010 EV per mL urine are obtained, corresponding to an EV-derived protein recovery of 0.1–4.0 μg per mL urine. The combination of bottom-up density-gradient centrifugation and SEC achieves an average urinary EV recovery efficiency of 30% (Dhondt et al., 2020; Geeurickx et al., 2019). In general, urinary EV preparations obtained by this protocol contain 1.0 × 1010 particles per μg of protein, corresponding to high-purity urinary EV preparations (Webber and Clayton, 2013). Transmission electron microscopy (TEM) uncovers urinary EV ultrastructure and identifies extensive numbers of vesicles (TEM-specific size range: 30–250 nm) surrounded by a lipid bilayer, in the absence of polymeric THP networks and protein complexes (Figure 10C).
Figure 9.
Density Gradient Centrifugation to Separate Urinary EV from Tamm-Horsfall Protein Polymers and Soluble Proteins
Density of the collected fractions as determined by 340 nm absorbance. EV (1.087–1.109 g/mL) differ in buoyant density from Tamm-Horsfall protein polymers (1.156–1.201 g/mL) and protein aggregates (1.207–1.231 g/mL).
Figure 10.
Characterization of Urinary EV Preparations by Protein- and Particle-Based Analysis Methods
(A) The presence of (tissue-specific) EV-enriched or depletion of non-EV-enriched proteins is assessed by western blot analysis.
(B) EV yield and size-distribution profile are obtained by nanoparticle tracking analysis. The NTA calculated size distribution is depicted as mean (black line) with standard error (red area) and total particle number, mean particle size and mode are shown.
(C) Low (scale bar: 1000 nm) and high magnification transmission electron microscopy images (scale bar: 200 nm) of urinary EV.
Integrative omics profiling enables researchers to unravel the molecular complexity of urinary EV. Mass spectrometry-based proteomics reveals the enrichment of a variety of endosomal, cytosolic and membrane-derived proteins (Dhondt et al., 2020), while RNA sequencing identifies distinct classes of RNA molecules, including mRNA, miRNA, lncRNA and circRNA (Everaert et al., 2019).
We demonstrated the ability of this protocol to separate urinary EV with high specificity and repeatability, which is a prerequisite to obtain reliable multi-omics data for clinical and research applications. For full details, we refer to (Dhondt et al., 2020).
Limitations
While this protocol was developed to recover urinary EV with high specificity and repeatability, a prerequisite to accurately map their biochemical composition, the long turn-around time and low-throughput aspect of a density gradient approach, as well as the need for relatively high sample volumes, limits its use beyond a scientific research setting. The implementation of density-based urinary EV separation also requires technical expertise and might be prone to replication bias in inexperienced hands. Urine is a diluted solution with a relatively low absolute concentration of EV (Webber and Clayton, 2013). Therefore, pre-enrichment of EV by ultrafiltration, starting from a sufficiently high volume of urine (30–50 mL) is necessary in order to generate a useful quantity of EV for downstream integrative omics analysis and should be considered when creating biorepositories for the study of urinary EV.
EV can be separated from urine using techniques different from the one described in this protocol. According to the EV-TRACK knowledgebase (Van Deun et al., 2017), serial ultracentrifugation (Pisitkun et al., 2004), polymer-based precipitation (Alvarez et al., 2012) and size-exclusion chromatography (Lozano-Ramos et al., 2015) are the most frequently used. In addition, ultrafiltration (Vergauwen et al., 2017), microfluidics- (Liang et al., 2017) and immunoaffinity-based methods (Islam et al., 2019; Mussack et al., 2019) and flow cytometric sorting (Campos-Silva et al., 2019) have been applied for urinary EV separation. The separation method of choice is guided by a tradeoff between efficiency and the degree of EV-specificity required to answer the experimental question (Théry et al., 2018). Despite the incompatibility between density-gradient centrifugation and high-throughput sample processing, the implementation of this step is critical to separate urinary EV with high specificity from other components such as Tamm-Horsfall protein polymers and urinary protein aggregates. A similar level of specificity cannot be realized with alternative separation methods available to date (Van Deun et al., 2014; Dhondt et al., 2020). However, certain limitations of a density gradient approach can be overcome by automation of gradient layering and fraction collection, rendering the process reproducible, robust, operator-independent and less time consuming (Tulkens et al., 2019). The use of biological reference materials such as trackable GFP-positive rEV assists in mitigating technical variation introduced during sample preparation and analysis (Geeurickx et al., 2019).
Although the potential impact of various pre-analytical factors during clinical sample processing on EV studies is increasingly recognized, this issue has not been fully addressed in this protocol. For collection, handling and storage of urine samples, we recommend following the tentative standard operating procedure provided by the Human Kidney and Urine Proteome Project (HKUPP) from the Human Proteome Organization (HUPO) (Yamamoto, 2010). Finally, normalized analysis of EV is important to allow patient-to-patient comparison of samples. To which extent the intrinsic inter- and intrasubject variability of urine is reflected in urinary EV is not clear. Various methods of normalization have been described, including urinary flow rate and volume, urinary creatinine and protein concentration, particle numbers and EV-enriched protein (CD9, ALIX, PSA) signal (Dhondt et al., 2018). However, comparative studies searching for optimal normalization strategies of EV in urine are largely missing.
Troubleshooting
Problem 1
Insufficient removal of crystal precipitates from the urine sample may cause obstruction of the filter pores. This will result in a significantly slower or ineffective clearance of the urine sample trough the filter. Pink precipitates can be observed on the filter membrane (Figure 11A).
Figure 11.
Troubleshooting
(A) Obstructed centrifugal filter due to the presence of residual urinary crystals on the filter membrane (left) versus normal situation (right).
(B) Mixed iodixanol solutions and the absence of a clear interface (arrows) between layers (left) versus correct gradient layering and the presence of a clear interface (arrows) between layers (right).
(C) Presence of Sepharose resin in the SEC eluate (left) versus normal appearance of the eluate (right).
Potential Solution
Collect the remaining urine sample from the sample filter cup. Remove the crystal precipitates by centrifugation, as described in step 8 of the protocol and transfer the supernatant to a new centrifugal filter device.
Problem 2
Manual gradient layering is technically challenging. Improper handling of the layering technique may result in mixing of the iodixanol solutions and reduce the resolving power of the gradient. If this is the case, no distinct interface can be observed between the gradient layers (Figure 11B).
Potential Solution
Prepare a new density gradient. Researchers with limited experience in making density gradients are recommended to familiarize themselves with the procedure by preparing colored test gradients. Add 50 μL of 0.4% (w/v) trypan blue solution to the 10% and 40% (w/v) iodixanol solutions. A sharp interface should be observed between the different layers of the gradient.
Problem 3
When a SEC column has been incorrectly prepared, Sepharose resin may leak out of the syringe into the eluate. This may interfere with the separation of the sample components and downstream analyses (Figure 11C).
Potential Solution
Prepare a new SEC column. A Sepharose leak is usually caused by incorrect placement of the nylon-net filter on the bottom of the syringe. Ensure that the filter sufficiently covers the opening of the syringe nozzle. Wetting the filter with PBS might allow more accurate manipulation. Carefully add the Sepharose resin to the syringe.
Resource Availability
Lead Contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Prof. Dr. An Hendrix (an.hendrix@ugent.be).
Materials Availability
This study did not generate any unique materials or reagents.
Data and Code Availability
The mass spectrometry proteomics data have been deposited to the ProteomeXchange Consortium via the PRIDE partner repository with the dataset identifier PXD015289. Total RNA sequencing data were deposited in GEO with the dataset identifier GSE131689. All relevant data of our experiments were submitted to the EV-TRACK knowledgebase with the dataset identifier EV190064.
Acknowledgments
This work was supported by the Fund for Scientific Spearheads of Ghent University Hospital, Concerted Research Actions from Ghent University, “Stichting tegen Kanker,” the Fund for Scientific Research-Flanders, and “Kom op tegen Kanker (Stand up to Cancer), the Flemisch cancer society”.
Author Contributions
Conceptualization: B.D., N.L., O.D.W., and A.H.; Investigation: B.D.; Methodology: B.D., O.D.W., and A.H.; Resources: B.D., N.L., O.D.W., and A.H.; Supervision: N.L., O.D.W., and A.H.; Validation: B.D., O.D.W., and A.H.; Visualization: B.D.; Writing: B.D., N.L., O.D.W., and A.H.
Declaration of Interests
The authors declare no competing interests.
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.xpro.2020.100073.
Contributor Information
Bert Dhondt, Email: bertdhon.dhondt@ugent.be.
An Hendrix, Email: an.hendrix@ugent.be.
References
- Alvarez M.L., Khosroheidari M., Kanchi Ravi R., Distefano J.K. Comparison of protein, microRNA, and mRNA yields using different methods of urinary exosome isolation for the discovery of kidney disease biomarkers. Kidney Int. 2012;82:1024–1032. doi: 10.1038/ki.2012.256. [DOI] [PubMed] [Google Scholar]
- ASTM D1193-06 . ASTM International; W. Conshohocken, PA: 2011. Standard Specification for Reagent Water. [Google Scholar]
- Campos-Silva C., Suárez H., Jara-Acevedo R., Linares-Espinós E., Martinez-Piñeiro L., Yáñez-Mó M., Valés-Gómez M. High sensitivity detection of extracellular vesicles immune-captured from urine by conventional flow cytometry. Sci. Rep. 2019;9:1–12. doi: 10.1038/s41598-019-38516-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Deun J., Mestdagh P., Sormunen R., Cocquyt V., Vermaelen K., Vandesompele J., Bracke M., De Wever O., Hendrix A. The impact of disparate isolation methods for extracellular vesicles on downstream RNA profiling. J. Extracell. Vesicles. 2014;3 doi: 10.3402/jev.v3.24858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services. Centers for Disease Control. National Institutes of Health . Fifth Edition. U.S. Government Printing Office; 2009. Biosafety in Microbiological and Biomedical Laboratories. [Google Scholar]
- Van Deun J., Mestdagh P., Agostinis P., Akay Ö., Anand S., Anckaert J., Martinez Z.A., Baetens T., Beghein E., Bertier L. EV-TRACK: Transparent reporting and centralizing knowledge in extracellular vesicle research. Nat. Methods. 2017;14:228–232. doi: 10.1038/nmeth.4185. [DOI] [PubMed] [Google Scholar]
- Dhondt B., Van Deun J., Vermaerke S., de Marco A., Lumen N., De Wever O., Hendrix A. Urinary extracellular vesicle biomarkers in urological cancers: from discovery towards clinical implementation. Int. J. Biochem. Cell Biol. 2018;99:236–256. doi: 10.1016/j.biocel.2018.04.009. [DOI] [PubMed] [Google Scholar]
- Dhondt B., Geeurickx E., Tulkens J., Van Deun J., Vergauwen G., Lippens L., Miinalainen I., Rappu P., Heino J., Ost P. Unravelling the proteomic landscape of extracellular vesicles in prostate cancer by density-based fractionation of urine. J. Extracell. Vesicles. 2020;9:1736935. doi: 10.1080/20013078.2020.1736935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar R. Gene Expression Omnibus: NCBI gene expression and hybridization array data repository. Nucleic Acids Res. 2002;30:207–210. doi: 10.1093/nar/30.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everaert C., Helsmoortel H., Decock A., Hulstaert E., Van Paemel R., Verniers K., Nuytens J., Anckaert J., Nijs N., Tulkens J. Performance assessment of total RNA sequencing of human biofluids and extracellular vesicles. Sci. Rep. 2019;9:1–16. doi: 10.1038/s41598-019-53892-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geeurickx E., Tulkens J., Dhondt B., Van Deun J., Lippens L., Vergauwen G., Heyrman E., De Sutter D., Gevaert K., Impens F. The generation and use of recombinant extracellular vesicles as biological reference material. Nat. Commun. 2019;10:3288. doi: 10.1038/s41467-019-11182-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groskopf J., Aubin S.M.J., Deras I.L., Blase A., Bodrug S., Clark C., Brentano S., Mathis J., Pham J., Meyer T. APTIMA PCA3 molecular urine test: Development of a method to aid in the diagnosis of prostate cancer. Clin. Chem. 2006;52:1089–1095. doi: 10.1373/clinchem.2005.063289. [DOI] [PubMed] [Google Scholar]
- Hardwick S.A., Chen W.Y., Wong T., Deveson I.W., Blackburn J., Andersen S.B., Nielsen L.K., Mattick J.S., Mercer T.R. Spliced synthetic genes as internal controls in RNA sequencing experiments. Nat. Methods. 2016;13:792–798. doi: 10.1038/nmeth.3958. [DOI] [PubMed] [Google Scholar]
- Islam M.K., Syed P., Lehtinen L., Leivo J., Gidwani K., Wittfooth S., Pettersson K., Lamminmäki U. A nanoparticle-based approach for the detection of extracellular vesicles. Sci. Rep. 2019;9:10038. doi: 10.1038/s41598-019-46395-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang L.G., Kong M.Q., Zhou S., Sheng Y.F., Wang P., Yu T., Inci F., Kuo W.P., Li L.J., Demirci U. An integrated double-filtration microfluidic device for isolation, enrichment and quantification of urinary extracellular vesicles for detection of bladder cancer. Sci. Rep. 2017;7:46224. doi: 10.1038/srep46224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozano-Ramos I., Bancu I., Oliveira-Tercero A., Armengol M.P., Menezes-Neto A., Del Portillo H.A., Lauzurica-Valdemoros R., Borràs F.E. Size-exclusion chromatography-based enrichment of extracellular vesicles from urine samples. J. Extracell. Vesicles. 2015;4:1–11. doi: 10.3402/jev.v4.27369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell P.J., Welton J., Staffurth J., Court J., Mason M.D., Tabi Z., Clayton A. Can urinary exosomes act as treatment response markers in prostate cancer? J. Transl. Med. 2009;7:4. doi: 10.1186/1479-5876-7-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore H.M., Kelly A.B., Jewell S.D., McShane L.M., Clark D.P., Greenspan R., Hayes D.F., Hainaut P., Kim P., Mansfield E. Biospecimen reporting for improved study quality (BRISQ) J. Proteome Res. 2011;10:3429–3438. doi: 10.1021/pr200021n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mussack V., Wittmann G., Pfaffl M.W. Comparing small urinary extracellular vesicle purification methods with a view to RNA sequencing—Enabling robust and non-invasive biomarker research. Biomol. Detect. Quantif. 2019;17:100089. doi: 10.1016/j.bdq.2019.100089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisitkun T., Shen R.-F., Knepper M.A. Identification and proteomic profiling of exosomes in human urine. Proc. Natl. Acad. Sci. U S A. 2004;101:13368–13373. doi: 10.1073/pnas.0403453101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Théry C., Witwer K.W., Aikawa E., Alcaraz M.J., Anderson J.D., Andriantsitohaina R., Antoniou A., Arab T., Archer F., Atkin-Smith G.K. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles. 2018;7:1535750. doi: 10.1080/20013078.2018.1535750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulkens J., De Wever O., Hendrix A. Analyzing bacterial extracellular vesicles in human body fluids by orthogonal biophysical separation and biochemical characterization. Nat. Protoc. 2019 doi: 10.1038/s41596-019-0236-5. [DOI] [PubMed] [Google Scholar]
- Vergauwen G., Dhondt B., Van Deun J., De Smedt E., Berx G., Timmerman E., Gevaert K., Miinalainen I., Cocquyt V., Braems G. Confounding factors of ultrafiltration and protein analysis in extracellular vesicle research. Sci. Rep. 2017;7:2704. doi: 10.1038/s41598-017-02599-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vizcaíno J.A., Deutsch E.W., Wang R., Csordas A., Reisinger F., Ríos D., Dianes J.A., Sun Z., Farrah T., Bandeira N. ProteomeXchange provides globally coordinated proteomics data submission and dissemination. Nat. Biotechnol. 2014;32:223–226. doi: 10.1038/nbt.2839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webber J., Clayton A. How pure are your vesicles? J. Extracell. Vesicles. 2013;2 doi: 10.3402/jev.v2i0.19861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Wever O., Hendrix A. A supporting ecosystem to mature extracellular vesicles into clinical application. EMBO J. 2019;38:e101412. doi: 10.15252/embj.2018101412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiśniewski J.R., Zougman A., Nagaraj N., Mann M. Universal sample preparation method for proteome analysis. Nat. Methods. 2009;6:359–362. doi: 10.1038/nmeth.1322. [DOI] [PubMed] [Google Scholar]
- Yamamoto T. The 4th Human Kidney and Urine Proteome Project (HKUPP) Workshop 26 September 2009, Toronto, Canada. Proteomics. 2010;10:2069–2070. doi: 10.1002/pmic.201090041. [DOI] [PubMed] [Google Scholar]
- Yuana Y., Böing A.N., Grootemaat A.E., van der Pol E., Hau C.M., Cizmar P., Buhr E., Sturk A., Nieuwland R. Handling and storage of human body fluids for analysis of extracellular vesicles. J. Extracell. Vesicles. 2015;4:29260. doi: 10.3402/jev.v4.29260. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
In step 15, a discontinuous density gradient is prepared in a 17 mL Thinwall polypropylene tube by layering iodixanol solutions with successively lower densities on top of each other. The iodixanol solutions are dispensed carefully to avoid mixing and ensure the formation of distinct layers with a sharp interface.
In step 22a, density gradient fractions are collected from the top of the gradient. The tube is held upright. The pipette tip is gently placed on the center of the fluid meniscus and individual 1 mL fractions are carefully collected.
In step 24c, EV-enriched density gradient fractions are loaded on top of a SEC column. Sample or PBS are loaded dropwise and close to the surface of the resin. Immediately following sample loading onto the SEC column, individual fractions of 1 mL are collected.
Data Availability Statement
The mass spectrometry proteomics data have been deposited to the ProteomeXchange Consortium via the PRIDE partner repository with the dataset identifier PXD015289. Total RNA sequencing data were deposited in GEO with the dataset identifier GSE131689. All relevant data of our experiments were submitted to the EV-TRACK knowledgebase with the dataset identifier EV190064.