SUMMARY
WNT signaling is crucial for embryonic development, adult tissue homeostasis, and injury repair. The poor biophysical characteristics of WNTs and their lack of receptor selectivity have hindered their use as research tools or potential therapeutics. We have developed a platform to generate potent, soluble, selective WNT mimetics with drug-like properties for both research and therapeutic applications. To help researchers adapt and expand on this platform, we describe these protocols and key considerations in generating and studying WNT mimetics.
For complete details on the use and execution of this protocol, please refer to Chen et al., 2020.
Graphical Abstract

Highlights
-
•
Described the approach to generate potent and selective WNT mimetics
-
•
Multivalent binding to FZD and LRP is a requirement for maximal activation
-
•
Activity matching protein peak on SEC is necessary to eliminate artifacts
-
•
Important to identify the necessary controls to avoid artifacts
WNT signaling is crucial for embryonic development, adult tissue homeostasis, and injury repair. The poor biophysical characteristics of WNTs and their lack of receptor selectivity have hindered their use as research tools or potential therapeutics. We have developed a platform to generate potent, soluble, selective WNT mimetics with drug-like properties for both research and therapeutic applications. To help researchers adapt and expand on this platform, we describe these protocols and key considerations in generating and studying WNT mimetics.
Before you Begin
WNT signaling plays essential roles in the development, homeostasis and regeneration of many organs and tissues (Nusse and Clevers, 2017). There are 19 mammalian WNTs that can induce WNT/β-catenin signaling through binding to receptors Frizzled (FZD) and low-density lipoprotein receptor-related protein (LRP) on the cell surface. WNTs are lipoglycoproteins. As a result of palmitoleoylation on a conserved serine residue, WNTs are highly hydrophobic, making them difficult to express, solubilize, and purify. Due to the conserved interaction sites between WNTs and FZDs, WNTs are also highly promiscuous in their interactions with the ten FZDs (FZD1–10) and the two LRPs (LRP5 and LRP6), with each WNT able to activate multiple FZD and LRP pairs (Janda et al., 2012, Dijksterhuis et al., 2015). Therefore, while WNT signaling plays a central role in tissue regeneration and holds promise for the treatment of degenerative diseases, the difficulty in manufacturing the ligands, the complexity in signaling, and lack of specificity have hindered the uses of endogenous ligands as research tools or potential therapeutics.
We have developed a platform to generate potent, soluble, selective WNT mimetics with drug-like properties. Unlike antagonist molecules, generation of agonist is more prone to artifacts. Some common practices in the field to purify and assess activities of agonists could potentially result in inaccurate conclusions. Therefore, in this protocol, not only we describe the methodologies to generate active molecules, but more importantly, we highlight methods to avoid artifacts in generating and studying WNT mimetics.
Identification and Selection of Binders
Binders against WNT receptors, FZDs and LRPs, can be obtained with various techniques, including utilization of either full-length or fragments of wild-type or mutant proteins or ligands that bind these receptors, various phage or yeast display of antibody or other scaffolds, or in vivo immunization campaigns. While only antibody or antibody fragments will be discussed here, any of the binders from various sources can be assembled in the manner described in this report for the purpose of WNT mimetic generation. The following detailed protocols start after the selection of the desired binders based on, but not limited to, affinity, epitope, and specificity.
Maintenance of Cell Cultures
-
1.
Maintain the Expi293F cells in Expi293 Expression Medium at 37°C with 8% CO2 and humidity set at 85% on an orbital shaking incubator. Make sure the cell viability is higher than 97%.
Note: FreeStyle 293-F cells in FreeStyle 293 Expression System could be used instead, although the protein expression levels tend to be lower than Expi293 Expression System.
-
2.
Maintain HEK293 STF cells in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum and GlutaMAX at 37°C in a 5% CO2 environment.
Preparation of Intestinal Organoid Media
Timing: 1 h
-
3.
Matrigel: thaw a bottle of Matrigel on ice. Meanwhile, prechill sterile 1.5 mL Eppendorf tubes on ice. After thawing, aliquot Matrigel into prechilled Eppendorf tubes on ice (we usually make 200 μL aliquots). Freeze aliquots at −20°C until use.
-
4.
Organoid growth media additives: reconstitute growth factors and growth media additives with tissue culture grade water, aliquot and store at −20°C until use.
-
5.
Stock concentration of growth factors and additives: hEGF 500 μg/mL, hNoggin 100 μg/mL, hRSPO-1 100 μg/mL, B27 supplement 50X, N2 supplement 100X, N-acetylcysteine 500 mM.
-
6.
IntestiCult™ Organoid Growth Medium: reconstitute IntestiCult™ Organoid Growth Medium following manufacture’s instruction and aliquot (we make 100 mL aliquots). The IntestiCult™ Organoid Growth Medium is stable at 4°C for 1 month. Freeze aliquots for longer-term use.
-
7.
Intestine organoid Basal Medium: Mix DMEM and F12K at 1:1 ratio. Thaw stock aliquots of growth factors and additives. Dilute growth factors and additives to the indicated final concentration in DMEM: F12K (10 mM HEPES, 1x penicillin/streptomycin, 1x GlutaMAX, 1x N2 supplement, 1x B27 supplement, 1.25 mM N-acetylcysteine, 50 ng/mL hEGF, 50 ng/mL hNoggin and 500 ng/mL hRSPO-1).
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| anti-Flag M2 affinity gel | Sigma-Aldrich | A2220 |
| Chemicals, Peptides, and Recombinant Proteins | ||
| IWP2 | Tocris Biosciences | 3533/10 |
| Recombinant human WNT3A | R&D systems | 5036-WN |
| Recombinant human EGF | Peprotech | AF-100-15 |
| Recombinant human Noggin | Peprotech | 120-10C |
| Recombinant human RSPO-1 | R&D Systems | 4645-RS-025 |
| Opti-MEM I Reduced Serum Medium | Thermo Fisher Scientific | 31985070 |
| 3X FLAG Peptide | Sigma-Aldrich | F4799-25MG |
| Imidazole | Sigma-Aldrich | 56749-250G |
| DMEM | Thermo Fisher Scientific | MT10013CV |
| Ham's F-12K (Kaighn's) Medium | Thermo Fisher Scientific | 21127022 |
| HEPES | Thermo Fisher Scientific | 15630 |
| Penicillin/streptomycin 100X | Thermo Fisher Scientific | 15140122 |
| GlutaMAX 100X | Thermo Fisher Scientific | 35050061 |
| IntestiCult™ Organoid Growth Medium | STEMCELL technologies | 06005 |
| Gentle Cell Dissociation Reagent | STEMCELL technologies | 07174 |
| N2 supplement 50X | Thermo Fisher Scientific | 17502048 |
| B27 Supplement 100X | Thermo Fisher Scientific | 17504044 |
| N-acetylcysteine | Sigma-Aldrich | A0737 |
| 20X PBST | Thermo Fisher Scientific | 28352 |
| 1 M HEPES PH 7.4 | Fisher Scientific | NC0470071 |
| 5 M Sodium Chloride | Fisher Scientific | NC1177728 |
| Critical Commercial Assays | ||
| Luciferase Assay System | Promega | E1500 |
| Streptavidin (SA) biosensors | PALL ForteBio | 18-5020 |
| NEBuilder HiFi DNA Assembly Cloning Kit | New England Biolabs | E5520S |
| cOmplete® His-tag purification resin | Sigma-Aldrich | 5893801001 |
| CaptivA Protein A affinity resin | Repligen | CA-PRI-0005 |
| Experimental Models: Cell Lines | ||
| Expi293F cells | Thermo Fisher Scientific | A14527 |
| HEK293 STF cells | ATCC | CRL-3249 |
| Mouse Intestinal Organoids | STEMCELL Technologies | 70931 |
| Recombinant DNA | ||
| pcDNA3.1(+) | Thermo Fisher Scientific | V79020 |
| Software and Algorithms | ||
| Prism 8 | Graphpad | https://www.graphpad.com/scientific-software/prism/ |
| Octet Data Acquisition 9.0 | PALL ForteBio | http://mdc.custhelp.com/app/answers/detail/a_id/20503/∼/octet-software-version-and-download-request |
| Octet Data Analysis 9.0 | PALL ForteBio | http://mdc.custhelp.com/app/answers/detail/a_id/20503/∼/octet-software-version-and-download-request |
| Other | ||
| Gravity flow column (Poly-Prep® Chromatography Columns) | Bio-Rad | 7311550 |
| Superdex 200 Increase 10/300 GL | GE Healthcare Life Sciences | 28990944 |
| Matrigel | Corning | CB-40230C |
| Thomson Instrument Company OPTIMUM GROWTH FLASK 125ML | Fisher Scientific | NC0694198 |
| Falcon 96-well clear flat bottom TC-treated Culture Microplate | Corning | 353072 |
| White 96-Well Plates | Thermo Fisher Scientific | 436110 |
RESOURCE AVAILABILITY
Lead Contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Yang Li (yang@surrozen.com).
Materials Availability
All unique/stable reagents generated in this study are available from the Lead Contact with a completed Materials Transfer Agreement.
Data and Code Availability
This study did not generate any unique datasets or code.
MATERIALS AND EQUIPMENT
Reagents
| Solution | Amounts of Chemicals | Final Volume |
|---|---|---|
| 0.2 M dibasic sodium phosphate | 35.61 g Na2HPO4·2H20 | 1 L (in Milli Q water) |
| 0.2 M monobasic sodium phosphate | 27.6 g NaH2PO4·H20 | 1 L (in Milli Q water) |
| 0.2 M Phosphate stock buffer pH 8.0 | 947 mL 0.2 M dibasic sodium phosphate + 53 mL 0.2 M monobasic sodium phosphate |
1 L. Filter, store at 20–25°C. |
| 1 M Imidazole stock solution pH 8.0 | 68.08 g Imidazole to 800 mL of Milli Q water. Adjust pH to 8.0 with 1 M HCl. | 1 L (in Milli Q water). Filter, store at 4°C. |
| Buffer A 50 mM Na-phosphate pH 8.0, 300 mM NaCl |
250 mL 0.2 M phosphate stock buffer pH 8.0 + 60 mL 5 M NaCl | 1 L (in Milli Q water). Store at 4°C. |
| Buffer B 50 mM Na-phosphate pH 8.0, 300 mM NaCl, 200 mM imidazole |
250 mL 0.2 M phosphate stock buffer pH 8.0 + 60 mL 5 M NaCl + 200 mL 1 M imidazole stock solution | 1 L (in Milli Q water). Store at 4°C. |
| Protein A elution buffer 100 mM glycine pH3.3 |
7.5 g glycine to 800 mL Milli Q water. Adjust pH to 3.3 with 1 M HCl. | 1 L (in Milli Q water). Filter, store at 20–25°C. |
| Neutralization buffer 1M Tris-HCl pH8.0, 1.5 M NaCl |
121.14 g Tris + 87.6 g NaCl in 800 mL Milli Q water. Adjust pH to 8.0 with 6 M HCl. | 1 L (in Milli Q water). Filter, store at 20–25°C. |
| TBS 50 mM Tris-HCl pH 7.4, 150 mM NaCl |
6.05 g Tris + 8.76 g NaCl in 800 mL Milli Q water. Adjust pH to 7.4 with 1 M HCl. | 1 L (in Milli Q water). Filter, store at 4°C. |
| 3X FLAG peptide stock solution (5 mg/mL) | 25 mg 3X FLAG peptide | 5 mL (in TBS). Aliquot and store at −20°C. |
| 3X FLAG peptide elution solution (150 μg/mL) | 0.15 mL 3X FLAG peptide stock solution | 5 mL (in TBS). Aliquot and store at −20°C. |
| 1x HBS buffer 20 mM HEPES pH 7.4, 150 mM NaCl |
20 mL 1 M HEPES pH 7.4 + 30 mL 5 M NaCl | 1 L (in Milli Q water). Store at 4°C. |
| 1X lysis reagent | 30 mL Luciferase Cell Culture Lysis 5X Reagent from Luciferase Assay System | 150 mL (in Milli Q water). Store at 4°C. |
| 3 mM IWP2 | 50 mg IWP2 | 35.6 mL (in DMSO). Aliquots and store at −20°C. |
| 1X PBST | 50 mL 20X PBST | 1 L (in Milli Q water). Store at 20–25°C. |
| Octet buffer (BSA-PBST) | 5 g bovine serum albumin (BSA) | 1 L (in 1X PBST). Filter and degas, store at 4°C. |
Equipment
-
•
FPLC: AKTA pure 25 L1, GE Healthcare Life Sciences
-
•
Incubator shaker: Multitron Pro, 25 mm, Infors HT
-
•
Plate reader: SpectraMax Paradigm Multi-mode Detection Platform, Molecular Devices
-
•
Microscope: Leica DMI 4000B, Leica Microsystems
-
•
Biolayer interferometry (BLI) assay equipment: Octet RED96 system, ForteBio
STEP-BY-STEP METHOD DETAILS
This protocol is divided into four main sections. Since the sources of binders can be diverse, and there are many well-established methodologies to identify antibody or various other scaffold-based binders, the protocols described here start after the identification and selection of appropriate binders. The protocols start with the Molecular Cloning section where a few representative formats are described to generate active WNT mimetics. This is followed by Protein Expression and Purification section. In this section, a critical step to profile the activities of protein of interest across a SEC column is discussed. This is then followed by Protein-Protein Interactions section to characterize the binding between WNT mimetic molecules and their receptors. The protocols end with a functional assay to further characterize and understand the biological activities of the WNT mimetics. Other critical considerations, limitations, and troubleshooting are also discussed. It is also important to highlight that alternatives to reagents or methods described in these protocols can also be utilized. For example, different scaffolds or binders, different expression cell lines or systems, different purification resins, biophysical characterization methods, or organoid systems can be used, as long as the general principles of the protocols are followed.
Molecular Cloning of WNT Mimetics
Once the appropriate binders have been selected, the first step in generating a WNT mimetic is to assemble the binders into bi- or multi-specific formats. The assembled molecule should include one or more FZD binding arms as well as one or more LRP binding arms. Binding to either FZD or LRP alone does not result in WNT mimetic activity. Many different formats could be utilized for this purpose; only the 1:1 bispecific tandem scFv, 2:2 tetravalent bispecific scFv, and 1+1:1+1 tetravalent multi-specific scFv formats will be described here (Chen et al., 2020).
Molecular Cloning of 1:1 Bivalent Bispecific Tandem scFv-His6 Molecules
Timing: 4 days
-
1.
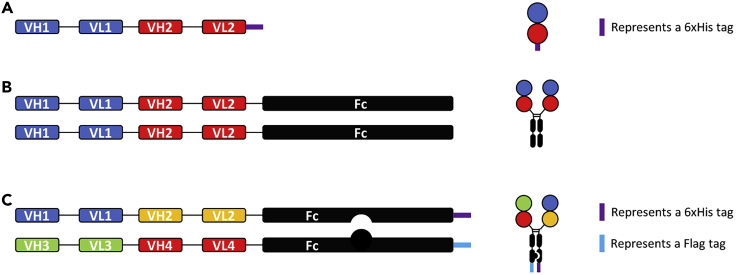
Design the 1:1 bivalent bispecific tandem scFv-His6 molecules as depicted in Figure 1A. For more information, please also see Chen et al., 2020.
Figure 1.
Diagram of Various WNT Mimetic Molecule Constructs
(A) 1:1 bivalent bispecific tandem scFv-His6 format. Each circle represents an scFv domain. The purple thick line at the end of the molecule represents a 6xHis tag.
(B) 2:2 tetravalent bispecific tandem scFv-Fc format.
(C) 1+1:1+1 tetravalent multi-specific tandem scFv-Fc format.
Note: Adding a 6xHis tag to the C-terminus of the bivalent bispecific tandem scFv will simplify the protein purification process. It is important to add a flexible 15-mer linker such as (G4S)3 between the VH and VL for each of the scFv sequences. It is also important to consider a flexible linker between the two scFvs, however the optimal linker length will need to be determined experimentally (Chen et al., 2020).
-
2.
Synthesize the fragment as a gBlock.
-
3.
Clone the gBlock into the pcDNA3.1(+) expression vector:
-
a.
Aliquot 5 μL of 2X NEBuilder HiFi DNA Assembly Master Mix into a PCR tube.
-
b.
Add 100 ng gel purified linearized pcDNA3.1(+) vector to that PCR tube.
-
c.
Add the required amount of gBlock fragment (molar ratio of fragment:vector = 5:1) to that PCR tube.
-
d.
Add nuclease free water to reach a final reaction volume of 10 μL.
-
e.
Mix gently, then allow reaction to proceed at 50°C for 60 min.
-
f.
Transform 2 μL of the assembled products into NEB 5-alpha Competent cells according to the manufacture’s transformation protocol.
Note: gBlock can be ordered from GenScript or Integrated DNA Technologies (IDT). Alternative cloning methodologies, such as PCR based methods, can also be used.
Note: Treatment of the linearized pcDNA3.1(+) vector by Alkaline Phosphatase, Calf Intestinal (CIP) can reduce the background of the cloning from the vector self-ligation.
Alternatives: Besides the NEB 5-alpha Competent cells, other competent cells such as Top10 can also be used for the transformation.
Molecular Cloning of 2:2 Tetravalent Bispecific Tandem scFv-Fc Molecules
Timing: 4 days
-
4.
Design the 2:2 tetravalent bispecific tandem scFv-Fc molecule as depicted in Figure 1B and see Chen et al., 2020.
-
5.
Synthesize the tandem scFv-Fc fragment and assemble as described in Step 3 of Molecular Cloning of 1:1 Bivalent Bispecific Tandem scFv-His6 Molecules.
Molecular Cloning of Asymmetric 1+1:1+1 Tetravalent Multi-specific Tandem scFv-Fc Molecules
Timing: 4 days
-
6.
Design the asymmetric 1+1:1+1 tetravalent multi-specific tandem scFv-Fc molecule as depicted in Figure 1C and see Chen et al., 2020.
-
7.
Synthesize the tandem scFv-Fc fragments and assemble as described in Step 3 of Molecular Cloning of 1:1 Bivalent Bispecific Tandem scFv-His6 Molecules.
Note: Only tandem scFv or tandem scFv-Fc formats are described here to illustrate the principles of generating WNT mimetics. Other formats, such as those reviewed by Brinkmann and Kontermann (2017), can also be utilized. A particular FZD and LRP binder pair may have different preferences for format. The relative orientation between the two binders, attachment sites, and linker lengths between the binders should be explored to achieve optimal activity.
Note: For the formation of Fc heterodimerization, only “knobs-into-holes” is described here as an example. The Fc chain containing the knob (T366W) mutation has a Flag tag (DYKDDDDK) on its C-terminus, and the other Fc chain containing the hole (T366S/L368A/Y407V) mutations has a 6xHis tag on its C-terminus. Other asymmetric Fc heterodimerization architecture can also be utilized (Brinkmann and Kontermann, 2017).
Protein Expression and Purification
Once the DNA constructs are ready, expression, purification, and characterizations come next. This section describes expression, purification, as well as characterization of the purified molecules in a WNT-responsive HEK293 STF reporter assay. Various expression systems could be utilized. The Expi293 based transfection/expression system will be described here. FreeStyle 293 Expression System could be used instead, although the protein expression levels tend to be lower than Expi293 Expression System. Three different purification methods are described here depending on the affinity tags present and the formats used. Activity characterization in a reporter cell, especially a critical step to test all fractions across a purification column, will also be highlighted in this section.
Protein Expression
Timing: 6 days
-
8.
The day before transfection, seed the cells at a density of 2.0 × 106 viable cells/mL and incubate at 37°C in Multitron Pro Incubator shaker rotating at 125 rpm with 8% CO2 and humidity set at 85%.
-
9.
On the day of transfection, determine number and viability of the cells using an automated cell counter.
-
10.
Dilute the cells to 2.95× 106 viable cells/mL with pre-warmed Expi293 Expression Medium.
-
11.
Add 34 mL cell suspension to each sterile, disposable, vented 125-mL Erlenmeyer shaker flask for each 40-mL transfection. Return the cells to the incubator.
-
12.
Meanwhile, for each 40-mL transfection, prepare lipid-DNA complexes as follows:
-
a.
Dilute 40 μg of plasmid DNA in 2 mL Opti-MEM I Reduced Serum Medium. Mix gently.
-
b.
Dilute 108 μL of ExpiFectamine 293 Reagent in 2 mL Opti-MEM I Reduced Serum Medium. Mix gently and incubate for 5 min at 20–25°C.
-
c.
After the 5 min incubation, add the diluted DNA to the diluted ExpiFectamine 293 Reagent to obtain a total volume of 4 mL. Mix gently.
-
d.
Incubate DNA-ExpiFectamine 293 Reagent mixture for 20–30 min at 20–25°C to allow the DNA-ExpiFectamine 293 Reagent complexes to form.
CRITICAL: The incubation time in Step 12 is critical. Do not incubate the ExpiFectamine 293 Reagent and DNA-ExpiFectamine 293 Reagent mixture longer than the time listed. Add DNA-ExpiFectamine 293 Reagent mixture to the flask from Step 11 immediately after Step 12 d is complete.
-
13.
After the DNA-ExpiFectamine 293 Reagent complex incubation is complete, add 4 mL of DNA-ExpiFectamine complex to each shaker flask from Step 11. Gently swirl the flask.
-
14.
Return and incubate the cells in the incubator.
-
15.
Approximately 16–18 h post-transfection, add 200 μL of ExpiFectamine 293 Transfection Enhancer 1 and 2 mL of ExpiFectamine 293 Transfection Enhancer 2 to each flask. The final volume should be approximately 40 mL in each 125-mL flask. Return the cells to the incubator for another three days.
Note: Use aseptic techniques since the cell culture media has no antibiotics and is prone to contamination.
Note: The settings for the shaking speed is dependent on the orbital diameter of the shaker and the size of the flasks. Most incubator shakers for mammalian cell culture have an orbital diameter of 25 mm. If using a shaker with different orbital diameter, the shaking speed needs to be adjusted. The new speed can be calculated using the formula r2= R2x(d1/d2). d1 = the orbital diameter for the old shaker, d2 = the diameter for the new shaker, R = the rpm for the old shaker and r = the rpm for the new shaker.
Note: Use good quality plasmid DNA for transient transfection is important for high expression levels. The EndoFree Plasmid Kit suppliers include Qiagen and Takara Bio, as well as alternative kits from other suppliers can be used. It is important to check the integrity of the purified DNA by agarose gel electrophoresis to ensure plasmid DNA is supercoiled for high transfection efficiency.
Note: After the transfection, the cells can be cultured in the incubator for 4–5 days. It is optional to measure the cell viability on the harvest day. The cell viability varies depending on the transfected and expressed protein, which is usually higher than 60%.
His-Tag Affinity Purification
Timing: 2 days
Purification of the 1:1 tandem scFv-His6 molecules
-
16.
cOmplete® His-tag resin preparation:
-
a.
Transfer the required amount of slurry (0.2 mL resin for a 40 mL transfection sample) into a gravity column.
-
b.
Allow the resin to settle, then let the excess buffer drain through the column by gravity flow.
-
c.
Wash the resin with 5 column volumes Milli Q water.
-
d.
Wash the resin with 5 column volumes Buffer A.
-
17.
Cell culture conditioned media preparation:
-
a.
Transfer the transfected cells to 50 mL conical tubes.
-
b.
Spin down at 4,000 rpm (∼3,500 g) for 30 min at 4°C.
-
c.
Pour supernatant into new, labeled conical tubes.
-
18.
Protein binding and elution:
-
a.
Add the equilibrated resin to the conditioned media (0.2 mL resin for a 40 mL transfection sample).
-
b.
Bind at 20–25°C for 3 h on a Tube Rotator.
-
c.
Allow the resin to settle for 5 min at 4°C.
-
d.
Pellet the resin at 2000 rpm (∼850 g) for 5 min at 4°C.
-
e.
Remove most of the supernatant.
-
f.
Transfer the resin to the gravity flow column and let the conditioned media drain.
-
g.
Wash the resin with 20 column volumes Buffer A.
-
h.
Wash the resin with 20 column volumes washing buffer (Buffer A +5 mM imidazole).
-
i.
Wash the resin with 1 column volume washing buffer (Buffer A +20 mM imidazole).
-
j.
Elute the protein with 5 column volumes Buffer B, collect each elution fraction (200 μL) in prelabelled Eppendorf tubes.
-
k.
Measure the protein concentration of each fraction by measuring the A280 on NanoDrop 2000.
-
l.
Run the fractions on SDS-PAGE gel at both reducing and non-reducing conditions to characterize the eluted proteins.
-
19.
Polish the protein using Superdex 200 Increase 10/300 GL column on AKTA pure:
-
a.
Pool the main fractions from Step 18, usually most proteins are eluted in fractions 2–4.
-
b.
Filter the pooled proteins using a 0.2 μm spin filter.
-
c.
Polish the protein on Superdex 200 Increase 10/300 GL column using 1XHBS as the mobile phase at the flow rate of 0.75 mL/min.
-
d.
Run the protein fractions on SDS-PAGE gel at both reducing and non-reducing conditions to characterize the products.
Note: 0.2 mL resin is usually enough to capture almost all the proteins from a 40 mL transfection since the protein expression level here is in the range of 20–100 mg/mL. Therefore, each elution fraction is 0.2 mL. About 85% of the eluted proteins (mass amount) can be loaded onto the Superdex 200 Increase 10/300 GL column which has the maximal sample loading volume of 500 μL.
Note: The elution volume of the tandem scFv-His6 molecules is usually around 16 mL on Superdex 200 Increase 10/300 GL column (Figure 2A). The aggregates are usually observed but the percentage of the aggregates varies depending on a particular binder and the assembly molecules.
Figure 2.
STF Activities across SEC Fractions
(A–C) L1:5:F1 SEC profiles. (A) Protein trace across SEC. (B) STF activity trace across SEC. (C) Overlay of (A) and (B). Results show that the peak STF activity did not coincide with monomer protein peak, suggesting the monomer is inactive.
(D–F) F1:10:L1:Fc SEC profiles. (D) Protein trace across SEC. (E) STF activity trace across SEC. (F) Overlay of (D) and (E). Results show that the peak STF activity coincided with the monomer protein peak, suggesting the monomer is active (Chen et al., 2020).
CRITICAL: Use ultra-pure imidazole, which has the least absorbance at A280 nm.
CRITICAL: Collect all fractions of the SEC run for assays later.
Protein A Affinity Purification
Timing: 2 days
Purification of the 2:2 tandem scFv-Fc molecules
-
20.
The CaptivA Protein A affinity resin preparation:
The preparation of CaptivA Protein A affinity resin is same as the above described procedure (Step 16) for the cOmplete® His-tag purification resin, except the resin here is washed twice with 1xPBS instead of with Buffer A (Step 16d). 0.2 mL Protein A resin is used for a 40 mL transfection sample.
-
21.
Conditioned media preparation:
The preparation of the conditioned media is same as the above described procedure (Step 17).
-
22.
Protein binding and elution:
-
a.
Binding and washing procedures are the same as the His-Tag Affinity Purification Steps 18 a-g, except 1xPBS is used here to wash the resin, instead of using Buffer A.
-
b.
Elute the protein with 5 column volumes elution buffer (100 mM glycine pH3.3), collect each elution fraction (200 μL) in prelabelled Eppendorf tubes which contain 20 μL neutralization buffer.
-
c.
Invert the Eppendorf tubes gently 3–4 times immediately.
CRITICAL: Invert the Eppendorf tubes gently 3–4 times immediately to mix each elution with neutralization buffer to prevent the reduction or loss of the protein activity in the acidic elution buffer.
-
d.
Measure the protein concentration of each fraction by measuring the A280 on NanoDrop 2000.
-
e.
Run the fractions on SDS-PAGE gel at both reducing and non-reducing conditions to characterize the eluted proteins.
-
23.
Polish the protein using Superdex 200 Increase 10/300 GL column on AKTA pure as Step 19.
Note: The elution volume of the tandem scFv-Fc molecules is usually around 12–13 mL on Superdex 200 Increase 10/300 GL column (Figure 2D), the exact elution volume depends on the protein structure and sequence.
Alternatives: Other Protein A resin such as POROS MabCapture A Select Resin (Cat#82079) from Thermo Fisher Scientific can also be used for the purification.
CRITICAL: Collect all fractions of the SEC run for assays later.
anti-Flag M2 Affinity Purification
Timing: 2 days
Four steps purification (Protein A, His-Tag, anti-Flag M2, and SEC) to purify asymmetric 1+1:1+1 multi-specific tandem scFv-Fc molecules
-
24.
The asymmetric tandem scFv-Fc recombinant proteins are first purified by Protein A resin as described in Steps 20–22.
-
25.
Pool the main eluted protein fractions from Step 24 into the 1.5 mL Eppendorf tube and add 0.2 mL cOmplete® His-tag purification resin, then perform His-Tag Affinity Purification as described in Steps 18 b-l.
-
26.
The anti-Flag M2 Affinity Purification:
-
a.
The anti-Flag M2 affinity resin preparation:
The preparation of anti-Flag M2 affinity resin is same as Step 16, except no Milli Q water wash, and the resin here is washed with 5 column volumes TBS buffer instead of with Buffer A.
-
b.
Protein binding and washing:
Pool the main eluted protein fractions from Step 25 into the 1.5 mL Eppendorf tube and add 0.4 mL anti-Flag M2 affinity resin. Perform binding and washing as Steps 18 b-g, except TBS is used here to wash the resin, instead of using Buffer A.
-
c.
Protein elution:
Elute the protein with 4–5 column volumes 3xFlag peptide elution solution, collect the eluted proteins at 200 μL per fraction in prelabelled individual Eppendorf tubes.
-
d.
Characterize the eluted individual fractions as Steps 18 k-l.
-
27.
Polish the protein using Superdex 200 Increase 10/300 GL column on AKTA pure as Step 19.
Note: The binding capacity of anti-Flag M2 affinity resin is low, therefore typically 0.4 mL resin is needed for the binding to get enough protein for SEC polishing and downstream assays. The eluted protein is usually enriched in three fractions, therefore most protein can be loaded to SEC column without the need to pool all the fractions and concentrate. The elution can be terminated when A280 of the eluted fraction is close to zero.
Alternatives: 3xFlag peptide can also be ordered from other companies such as ThermoFisher Scientific or custom order from the peptide synthesis companies if large amount is needed.
CRITICAL: Use the 3xFlag peptide elution solution to blank the NanoDrop since the elution solution has an absorbance at A280.
CRITICAL: Store the purified proteins from Steps 19, 23 and 27 at 4°C for less than 1 week. If the assays cannot be completed in one week, the proteins should be aliquoted and frozen at −80°C.
CRITICAL: Collect all fractions of the SEC run for assays later.
Super Top Flash (STF) Assay across the SEC Column
Timing: 3 days
Once mimetic molecules are purified via various means, the essential next step is to assess their activities across the SEC column fractions that are saved. The activity assay can be performed on a WNT-responsive cell line such as the HEK293 Super TOP-FLASH (STF) luciferase reporter cells (Janda et al., 2017).
-
28.
Seed 100 μL HEK293 STF cells at a density of 10,000 per well in 96-well plates 24 h prior to adding the assay proteins. IWP2 is added when seeding the cells with the final concentration of 3 μM to inhibit the production of endogenous WNTs.
-
29.
Measure the protein concentration of the peak protein fraction from the SEC column by measuring A280 on NanoDrop. Calculate the volume required from this peak protein fraction to dilute with HEK293 cell culture media to achieve a concentration of 10 nM.
-
30.
Take the same volume from all the other protein fractions and dilute with the same dilution factor with media as in the above step for the peak fraction.
-
31.
After removing 10 μL media from each well, add 10 μL diluted fractions to each well containing the remaining 90 μL medium in the 96-well plates. This will result in equal volumes (but not equal concentrations) for each of the fractions to be tested. Only the peak protein fraction is at 1 nM final concentration.
-
32.
Incubate the plate for 18 h in the cell culture incubator.
-
33.
Remove growth medium from the cultured cells.
-
34.
Dispense 100 μL of 1X lysis reagent into the 96-well plate and incubate for 5 min at 20–25°C on a shaker.
-
35.
Mix 20 μL of cell lysate with 10 μL of Luciferase Assay Reagent.
-
36.
Measure the light produced on the plate reader.
CRITICAL: Ensuring that the activity profile matches the monomer protein peak on the SEC column is an essential step. Generation of agonists is more prone to artifacts. As illustrated in Figures 2A–2C, aggregated protein products may potently stimulate signaling (Chen et al., 2020). Since multiple formats could be utilized to generate WNT mimetics (Chen et al., 2020, Janda et al., 2017, Tao et al., 2019), and the different formats and binders may have different tendencies for aggregation, it is essential to ensure that the observed agonist activities are from the monomer forms of the molecules generated. The typical approach of pooling the monomer peak from the SEC column without thorough examination of the activities from each of the fraction containing different species may lead to false conclusions. As shown by some of the examples here and in Chen et al., 2020, the monomer pools may contain tailing of higher molecular weight species (HMW). If the HMW species are active or highly active, any activity observed in the monomer pool will be complicated by the contribution from the HMW species which may affect consistency and reproducibility. If the monomer is inactive and HMW is active, this will lead to a false conclusion of the molecule described. HMW species may also have altered receptor specificity which further complicates the interpretation from activity assays.
Note: For the STF assay across SEC column fractions, we recommend starting with a concentration of 1 nM equivalent for the protein peak fraction. If saturating STF activities are observed in fractions of highly potent molecules, repeating the STF assay at a lower concentration is necessary. At saturating STF conditions, discerning the peak fraction becomes difficult. In addition, as some mimetics show bell-shaped curves, saturating conditions may not accurately reflect the actual activity peak.
Dose Response STF Assay for a Mimetic Molecule
Timing: 3 days
-
37.
Seed 100 μL HEK293 cells at a density of 10,000 per well in 96-well plates 24 h prior to adding the assay proteins. IWP2 is added when seeding the cells with the final concentration of 3 μM to inhibit the production of endogenous WNTs.
-
38.
Dilute the recombinant WNT mimetics with the HEK293 cell culture media. An example of a dilution series is 100 nM, 10 nM, 1 nM, 0.1 nM, 0.01 nM, 0.001 nM, 0.0001 nM.
-
39.
After removing 10 μL media from each well, add 10 μL diluted WNT mimetics to each well in the 96 well plates to achieve a final assay concentration of 10 nM, 1 nM, 0.1 nM, 0.01 nM, 0.001 nM, 0.0001 nM, 0.00001 nM.
-
40.
Incubate the plate for 18 h in the cell culture incubator.
-
41.
Remove growth medium from the cultured cells.
-
42.
Dispense 100 μL of 1X lysis reagent into the 96-well plate and incubate for 5 min at 20–25°C on a shaker.
-
43.
Mix 20 μL of cell lysate with 10 μL of Luciferase Assay Reagent.
-
44.
Measure the light produced on the plate reader.
Protein-Protein Interactions
To further characterize the assembled molecules, for example, to understand if the geometry affects binding to their respective receptors for further optimization, or in the case of no activity, to ensure the geometry does not impact binding, additional protein-protein interaction assays such as biolayer interferometry (BLI) assay or surface plasmon resonance assay, may be necessary. Here, we used Octet-based BLI assay. The following sections describe a step-binding assay to ensure the assembled molecule can bind to two target receptors simultaneously.
Step-Binding Assay by BLI (Validating the Simultaneous Bindings of the Two Antigens by the Tandem scFv Molecule)
Timing: 30 min
-
49.
Rehydrate streptavidin biosensors (Dip and Read, Pall ForteBio) in a biosensor rack with a 96-well plate containing 200 μL/well of 0.5% (wt/vol) bovine serum albumin (BSA) in PBST for at least 10 min at 20–25°C.
-
50.
Open Data Acquisition 9.0 software (Pall ForteBio) and design the assay protocol with the following time durations:
| Step | Program | Time (s) | Volume (μL) |
|---|---|---|---|
| 1 | Baseline | 60 | 200 |
| 2 | Loading (Biotinylated antigen 1) | 160 | 200 |
| 3 | Baseline | 60 | 200 |
| 4 | Association 1 (tandem scFv) | 300 | 200 |
| 5 | Association 2 (antigen 2) | 300 | 200 |
-
51.
Perform runs with 50 nM biotinylated antigen 1 solution for the loading step, the combination of two association steps with the following design and Octet buffer for the two baseline steps. All proteins are diluted in the Octet buffer.
| Condition | Association 1 | Association 2 |
|---|---|---|
| 1 | Tandem scFv | Antigen 2 in Tandem scFv |
| 2 | Tandem scFv | Tandem scFv |
| 3 | Buffer only | Antigen 2 in Tandem scFv |
| 4 | Buffer only | Tandem scFv |
For example, biotinylated FZD8-CRD as antigen 1, L2:5:F3 or F3:5:L2 as tandem scFv, and LRP6E3E4 as antigen 2, as shown in Figure 4 of Chen et al., 2020.
Note: The protein concentration for loading step needs to be optimized before the assay. The concentrations for both tandem scFv and antigen 2 also need to be determined by 1:1 binding assays. In the assay of Chen et al., 2020, while 50 nM biotinylated FZD8-CRD was used to reach the response of ∼1.5 nm in the sensorgram, 1 μM and 2 μM for L2:5:F3 or F3:5:L2 and LRP6E3E4, respectively, were used.
Note: Use the same concentrations of Tandem scFv as in the “Association 2” step to avoid the possible dissociation of the bound Tandem scFv in “Association 1.” If the tandem scFv binding in “Association 1” is high affinity with no obvious dissociation during washing step, “antigen 2 in buffer” in “Association 2” can be considered instead of “antigen 2 in tandem scFv” to avoid unnecessary consumption of additional protein.
Alternatives: For the step-binding assay, we designed the protein binding orientation as “classical sandwich” method (antigen 1 → tandem scFv → antigen 2). However, “in tandem” binding method (tandem scFv → antigen 1/2 → antigen 2/1) can also be considered. The tandem scFv can be captured on biosensor chip first and both antigens can be added on top sequentially.
-
52.
Collect the step-binding data with Data Analysis 9.0 software (Pall ForteBio).
Note: See Figure 3 for the assay plate design adapted from Figure 4 in Chen et al., 2020.
Figure 3.
Step-Binding Assay by BLI
Sensorgrams (right) are generated by a BLI assay with the designed assay plate (left). Biotinylated FZD8-CRD is captured on the SA-chip surface (Step 2) and L2-F3 is then added (Step 4). The additional binding of LRP6E3E4 on the complex is detected in step 5. Steps 1 and 3 indicate baselines. The protein assemblies on each sensor chip is depicted on the right panel. The assay is adapted from Chen et al., 2020.
Intestinal Organoid Culture and Proliferation Assay
Timing: 7 days
WNT mimetic molecules should be evaluated in additional functional assays in vitro and in vivo. This section describes one such in vitro functional system, mouse intestinal organoids (O'Rourke et al., 2016), for the evaluation of WNT mimetics.
-
53.
Thawing organoids:
-
a.
Warm IntestiCult™ Organoid Growth Medium to 37°C. Pre-warm a 48-well tissue culture plate at 37°C for at least 30 min.
-
b.
Quickly thaw a tube of mouse intestinal organoids by holding the tube in hand.
-
c.
Transfer the organoids in freezing medium into a 15 mL conical tube and add 10 mL DMEM. Spin down organoids at 4°C for 3 min at 150 g. Slowly take out supernatant and repeat the wash with IntestiCult™ Organoid Growth Medium.
-
d.
After spinning down, slowly take out as much supernatant as possible without disrupting the organoid pellet.
-
e.
Resuspend organoids in 200 μL Matrigel on ice, mix gently and quickly dispense 25 μL of the mixture into the center of each well of the pre-warmed 48-well plate. Return the plate to 37°C without shaking and allow Matrigel to solidify for 5 min. A Matrigel dome should form in the center of the well.
-
f.
Gently overlay 300 μL of IntestiCult™ Organoid Growth Medium on top.
-
g.
Incubate organoids at 37°C for 5–7 days before passaging. IntestiCult™ Organoid Growth Medium should be changed every 3–4 days.
Note: After growing for 5–7 days, organoids should reach a good differentiated state with a slight phase-dark lumen and many phase-light finger-like protruding crypts.
-
54.
Passaging organoids:
-
a.
Pre-warm a new 48-well tissue culture plate containing Basal Medium and Gentle Cell Dissociation Reagent to 37°C.
-
b.
Carefully aspirate the IntestiCult™ Organoid Growth Medium from the organoid culture plate without disturbing the Matrigel dome.
-
c.
Apply 200 μL Gentle Cell Dissociation Reagent to each well and incubate at 37°C for 5 min.
-
d.
Collect organoids by gently pipetting up and down a few times and transfer and pool the content of each well to a 15 mL conical tube.
-
e.
Add 10 mL Basal Medium to the tube and spin down organoids at 150 g for 3 min at 4°C.
-
f.
Slowly take out the supernatant without disturbing the organoid pellet. Add appropriate amount of Matrigel to the organoid pellet on ice. Using a P200, gently disrupt organoids into smaller pieces on ice 20 times.
Note: we typically do 1 to 4–6 splits, i.e. 1 well of organoid split to 4–6 wells. For example, in a 1 to 4 split, if starting from 1 well of organoid, 100 μl (4x25 μl) of Matrigel should be added to the resuspend organoids. The degree of organoid dissociation, incubator condition, and the time of media change may all affect how the organoids grow. The appropriate split ratio should be determined empirically by the operator.
-
g.
Quickly dispense 25 μL of the organoid/Matrigel suspension to the center of each well of the pre-warmed 48-well plate and let solidify for 5 min at 37°C.
-
h.
Gently overlay 300 μL of IntestiCult™ Organoid Growth Medium on top.
-
i.
Organoids need to be passaged again after growing at 37°C for 5–7 days if not used for the assay below. IntestiCult™ Organoid Growth Medium should be changed every 3–4 days.
-
55.
Plating organoids for mimetic treatment:
-
a.
Grow and passage organoids as necessary until organoids are in a good differentiated condition and sufficient amount for WNT mimetic treatments. We generally split organoids 1 to 8 to set up for mimetic treatment.
-
b.
Prepare WNT mimetic dilution series as desired in Basal Medium with 1 μM IWP2. Keep the protein dilution series on ice. Thaw a few aliquots of Matrigel on ice.
-
c.
Dissociate organoids.
-
i.
Pre-warm a new 48-well tissue culture plate containing Basal Medium and Gentle Cell Dissociation Reagent to 37°C.
-
ii.
Carefully aspirate the IntestiCult™ Organoid Growth Medium from the organoid culture plate without disturbing the Matrigel dome.
-
iii.
Apply 200 μL Gentle Cell Dissociation Reagent to each well and incubate at 37°C for 5 min.
-
iv.
Collect organoids by gently pipetting up and down a few times and transfer and pool the content of each well to a 15 mL conical tube.
-
v.
Add 10 mL Basal Medium to the tube and spin down organoids at 150 g for 3 min at 4°C.
-
d.
Plate organoids.
-
i.
Slowly take out the supernatant without disturbing the organoid pellet. Depending on the number of organoids, apply Matrigel to the organoid pellet. Using a P200, gently disrupt organoids into smaller pieces on ice 20 times.
Note: Density and starting size of organoids will affect sensitivity of the assay, as intestinal organoids secrete growth factors and help each other grow. At the time of imaging, ∼50 organoids per well is ideal. (Splitting organoid at a ratio of 1 to 8 has been a good starting point for us.) For example, in a 1 to 8 split, if starting from 1 well of organoid, 200 μl (8x25 μl) of Matrigel should be added to the resuspend organoids.
-
ii.
Quickly dispense 25 μL of the organoid/Matrigel suspension to the center of each well of the pre-warmed 48-well plate and let solidify for 5 min at 37°C.
-
iii.
Gently apply 300 μL of Basal Medium with 1 μM IWP2 or the protein dilution serious to the well. Prepare a minimum of 4 wells for each treatment condition for technical repeats.
-
e.
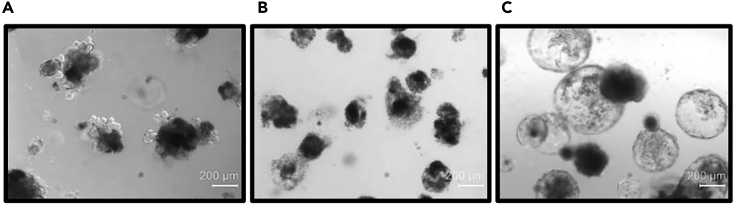
Incubate the plate at 37°C for 3 days and change medium with fresh protein dilutions on day 4. On day 7, image organoids under a transmitted light microscope equipped with phase contrast or DIC. Example images of organoids under various treatments are shown in Figure 4.
Figure 4.
Treating Mouse Intestinal Organoids with WNT Mimetics
(A–C) Images showing mouse intestinal organoids in Basal Medium (A), Basal Medium+IWP2 (B), and Basal Medium+IWP2+mimetic L1-F1-Fc (C) (Chen et al., 2020). Scale bars: 200 μm.
EXPECTED OUTCOMES
Mouse small intestinal organoid culture is a self-sufficient stem cell system. The growth of the organoids depends on endogenous WNTs secreted by Paneth cells located at the tip of the protruding crypts where intestinal stem cells also locate. Without mimetic treatment, epithelial cells proliferate and differentiate into all intestinal epithelial cell types as they migrate up and away from the WNT producing protruding crypt tips. Each organoid usually contains multiple stem cell niches, giving the appearance of multiple protrusions/budding on the organoids. The terminally differentiated cells eventually die and shed into the organoid lumen; the dead cells in the lumen give organoids their dark appearance (Figure 4A). When porcupine inhibitors, such as IWP2, are added to this organoid culture, secretion of endogenous WNTs is inhibited. Organoids degenerate quickly under this condition (Figure 4B). Therefore, under normal published organoid growth conditions, each organoid contains multiple protrusions with dark appearing interiors.
This or other organoid culturing systems can be utilized to assess the potency of WNT mimetics on maintaining and stimulating a WNT-dependent adult stem cell lineage. When the soluble WNT mimetics are added to the organoid culture, with or without IWP2, the WNT gradient produced by the protruding crypts are lost, and all cells in the organoids turn into proliferating stem/precursor cell states and lose the ability to differentiate. This will result in the absence of protrusions/crypts with the round appearance. In addition, in the absence of differentiation and death of terminally differentiated cells shedding into the lumen, the rounded organoids will appear somewhat transparent (Figure 4C). Therefore, the number of protrusions is not a quantitative assessment of the activity of WNT mimetics. However, the ability of mimetics to maintain the hollow sphered organoids correlates with their activity.
The methodologies described here allow for the generation of soluble, long-acting, highly potent WNT mimetic molecules. Such molecules can achieve a pM potency range, several orders of magnitude more potent than endogenous ligands. In addition, the methods allow for the generation of any desired FZD/LRP specificity. Depending on the binding modules selected, especially with antibody-based binders, the assembled molecules can have high expression levels and drug-like properties with potential utility as research tools or therapeutics for diseases where tissue regeneration and repair could be beneficial.
LIMITATIONS
The protocols described here do not include the generation of binders. This can be achieved with several well-established methodologies, including, but not limited to, phage- or yeast-display-based, or in vivo immunization-based campaigns, to identify suitable binders for assembly. In addition, other protein or protein fragments that have affinities toward WNT receptors could also be utilized to generate WNT mimetic molecules. While the methods described here can be used to assemble any combination of binders to achieve any type of specificity, binders with desired profiles will need to be identified first by one of the standard methodologies mentioned above.
A critical step to reduce artifacts is the use of SEC columns to separate the correct product from incorrectly formed species, including aggregates. Good performance of the SEC column and assuring it has the resolution to properly separate different oligomeric species is crucial.
Given that the mimetics generated with the methods described here could achieve pM EC50 values, ensuring the purity of the molecules is crucial for proper activity assessment. For example, in generating various 1+1 hetero-Ig formats (Chen et al., 2020), the final product could contain homodimers of each chain alone. If the homodimers are more active than the heterodimers, any contamination of homodimers could result in inaccurate conclusions. We used three affinity steps, Protein A, His, then Flag, followed by SEC polishing. The intent of these steps is to reduce or eliminate the potential of homo-oligomers contamination. Additional processes (e.g. LC-MS-based quality control or other methods) will be necessary to characterize the purity of the final molecules.
It has been reported in Chen et al., 2020 that different ratios of FZD and LRP binders in the multivalent molecules, for example, 2 FZD/1 LRP, 1 FZD/2 LRP, or even 1 FZD/1 LRP combinations, may all yield different levels of activities. When working with multivalent molecules such as 2 FZD/2 LRP or 2 different FZD binder/2 different LRP binder combinations, it is necessary to test additional control combinations to ensure correct understanding of the active arms within the multivalent molecules. For example, in the F1+F2/2L combination where F1 represents a FZD binder, F2 represents a different FZD binder, and L represents an LRP binder, it is necessary to examine the activity of F1/2L and F2/2L to understand whether the activity of F1+F2/2L comes from a single FZD binding arm or a combination of both F1 and F2 (Chen et al., 2020).
TROUBLESHOOTING
Problem
Low or no WNT activities obtained with the assembled molecules.
Potential Solution
Multivalent format is essential to generate maximal activation of signaling. While the optimal stoichiometry of two FZDs and two LRPs leads to strong activation of WNT/β-catenin signaling, other ratios, such as 2 FZD/1 LRP, 1 FZD/2 LRP, or even 1 FZD/1 LRP combinations, may all yield different levels of activities. In addition to valency requirements, a number of other factors also have the potential to contribute to low or no activity, including affinity, epitope, linker length, relative orientation, geometry, etc.
-
1.
We recommend to first vary the relative orientation and geometry. This can be accomplished, for examples, by reversing the order of the two binders in the tandem scFv format. Alternatively, the binders could be attached to different places on the framework; for example, the dumbbell format described in Chen et al., 2020.
-
2.
If activities are recovered, different linker lengths could be explored next to further optimize the activity.
-
3.
If there is still no activity, a different binder may need to be pursued.
-
4.
Alternatively, combinations with different binders, such as the 1+1 formats described in Chen et al., 2020, could be explored to generate active molecules.
Problem
Low expression of molecules from Expi293 cells.
Potential Solution
While some sequences have inherent difficulties to express, when encountering low expression challenges, a number of approaches would be explored. These include codon optimization, as well as different expression vectors and/or expression systems, such as insect-based expression systems.
Problem
Loss of protein activity after storage at 4°C for more than one week.
Potential Solution
A few proteins become less active after storage at 4°C for more than one week. Therefore, we aliquot all the purified proteins at 50 μL per vial, freeze and store in −80°C. We only use the newly thawed vial when testing the protein activities.
Problem
STF readouts decrease overtime.
Potential Solution
When the HEK293 STF cells have been propagated for about two months, we have observed loss of luciferase units from the STF assay. When this happens, usually a new thaw of HEK293 STF cells will solve this problem.
ACKNOWLEDGMENTS
This work is supported by Surrozen, Inc. The authors thank Wen-Chen Yeh, Trudy Vanhove, Craig Parker, and Lorraine Baer for helpful discussions and for editing the manuscript.
AUTHOR CONTRIBUTIONS
Conceptualization, H.C., C.L., S.L., and Y.L.; Investigation, H.C., C.L., S.L., and Y.L.; Writing – Original Draft, H.C., C.L., S.L., and Y.L.; Writing – Review & Editing, H.C., C.L., S.L., and Y.L.; Funding Acquisition, Y.L.
DECLARATION OF INTERESTS
All authors are current full-time employees and shareholders of Surrozen, Inc., a private biotechnology company incorporated in Delaware. Y.L. is Vice President, Biology at Surrozen, Inc. A patent application is pending for the work described in this manuscript.
References
- Brinkmann U., Kontermann R.E. The making of bispecific antibodies. MAbs. 2017;9:182–212. doi: 10.1080/19420862.2016.1268307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H., Lu C., Ouyang B., Zhang H., Huang Z., Bhatia D., Lee S.J., Shah D., Sura A., Yeh W.C. Development of potent, selective surrogate WNT molecules and their application in defining frizzled requirements. Cell Chem. Biol. 2020;27:598–609. doi: 10.1016/j.chembiol.2020.02.009. [DOI] [PubMed] [Google Scholar]
- Dijksterhuis J.P., Baljinnyam B., Stanger K., Sercan H.O., Ji Y., Andres O., Rubin J.S., Hannoush R.N., Schulte G. Systematic mapping of WNT-FZD protein interactions reveals functional selectivity by distinct WNT-FZD pairs. J. Biol. Chem. 2015;290:6789–6798. doi: 10.1074/jbc.M114.612648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janda C.Y., Dang L.T., You C., Chang J., de Lau W., Zhong Z.A., Yan K.S., Marecic O., Siepe D., Li X. Surrogate Wnt agonists that phenocopy canonical Wnt and beta-catenin signalling. Nature. 2017;545:234–237. doi: 10.1038/nature22306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janda C.Y., Waghray D., Levin A.M., Thomas C., Garcia K.C. Structural basis of Wnt recognition by Frizzled. Science. 2012;337:59–64. doi: 10.1126/science.1222879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusse R., Clevers H. Wnt/beta-catenin signaling, disease, and emerging therapeutic modalities. Cell. 2017;169:985–999. doi: 10.1016/j.cell.2017.05.016. [DOI] [PubMed] [Google Scholar]
- O'Rourke K.P., Ackerman S., Dow L.E., Lowe S.W. Isolation, culture, and maintenance of mouse intestinal stem cells. Bio Protoc. 2016;6 doi: 10.21769/bioprotoc.1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao Y., Mis M., Blazer L., Ustav M.J., Steinhart Z., Chidiac R., Kubarakos E., O'Brien S., Wang X., Jarvik N. Tailored tetravalent antibodies potently and specifically activate Wnt/Frizzled pathways in cells, organoids and mice. Elife. 2019;8:e46134. doi: 10.7554/eLife.46134. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This study did not generate any unique datasets or code.