Summary
Clostridioides difficile, an obligate anaerobic bacterium, causes infections leading to prolonged diarrhea. The bacterium produces dormant spores that can withstand an aerobic environment, resulting in easy environmental transfer. Here, we present a convenient sporulation and purification protocol that can be practiced in any lab setting using a portable anaerobic glove bag. This protocol also optimizes existing cell growth methods and presents a detailed trouble shooting guide.
This protocol is a modification of those previously reported by Edwards and McBride (2016) and Shen et al. (2016).
Graphical Abstract
Highlights
-
•
Easy preparation of C. difficile spores for in vitro/in vivo study
-
•
Strain sporulation and spore germination can be done in any lab with anaerobic bag
-
•
Detailed purification steps
-
•
Detailed trouble shooting guide alleviates common issues
Clostridioides difficile, an obligate anaerobic bacterium, causes infections leading to prolonged diarrhea. The bacterium produces dormant spores that can withstand an aerobic environment, resulting in easy environmental transfer. Here, we present a convenient sporulation and purification protocol that can be practiced in any lab setting using a portable anaerobic glove bag. This protocol also optimizes existing cell growth methods and presents a detailed trouble shooting guide.
Before You Begin
Preparing Reagents
Timing: 6 h
-
1.
Prepare Brain Heart Infusion (BHI) + Taurocholic acid (TCA) plates. (plates should be less than 2 months old)
-
2.
Prepare 70:30 sporulation plates. (plates should be less than 1 week old)
-
3.
Prepare 50% (w/v) sucrose solution in distilled water.
-
4.
Prepare 10% (w/v) solution of taurocholic acid in distilled water.
-
5.
Prepare 10% (w/v) solution of L-cysteine in distilled water.
-
6.
Prepare 10% bovine serum albumin in 1× phosphate buffered saline.
-
7.
Prepare Spilfyter® bag:
CRITICAL: An anaerobic environment must be maintained when working with vegetative C. difficile cells or germinating spores. A Spilfyter® bag (Methods Video S1) is used to achieve an anerobic environment while streaking plates and transferring cultures (see Methods Video S2). All materials necessary for spore production are placed inside the bag, the bag is flushed with N2 several times, filled with N2, and sealed. Plates are pre-reduced and incubated anaerobically inside a BD GasPak™ EZ Anaerobe Container System containing a GasPak™ EZ Anaerobic Sachet to generate a CO2 and H2 atmosphere. Aseptic techniques must be practiced at all times to reduce the possibility of contamination.
In our protocol a Spilfyter® bag is used to achieve the anerobic environment. The bag is flushed and filled with nitrogen gas prior to use.
The streaking of plates and transferring cultures must be done in the Spilfyter® bag.
Key Resources Table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Chemicals, Peptides, and Recombinant Proteins | ||
| Taurocholic Acid - 10% solution (w/v) in ddH2O | Cayman Chemical | 16215 |
| L-cysteine - 10% solution (w/v) in ddH2O | Alfa Aesar | A10435 |
| Tris base | Thermo Fisher | BP152-5 |
| Bovine Serum Albumin | Sigma Aldrich | A4612 |
| Other | ||
| Refrigerated centrifuge | Eppendorf | 5810R |
| Swinging bucket rotor | Eppendorf | A-4-62 |
| Spilfyter® bag | Grainger | 3NPA2 |
| GasPak™ EZ Anaerobic containers | BD | 260671 |
| GasPak™ EZ Anaerobic sachets | BD | 260001 |
Step-by-Step Method Details
Recipe for 1 L BHI +TCA Medium Germination Plates (Makes 40–25 mL Plates)
| Reagent | Amount |
|---|---|
| BHI broth base | 37 g |
| Yeast extract | 5 g |
| Agar | 15 g |
| Taurocholic acid 10% w/v | 10 mL |
| L-cysteine 10% w/v | 10 mL |
Note: Taurocholic acid and L-cysteine are separately sterilized by passage through Millipore 0.2 μm filters and added to autoclaved medium cooled to approximately 65°C. Plates are stored at 4°C for up to 2 months.
Recipe for 1 L 70:30 Medium for Sporulation Plates (Makes 28–35 mL Plates)
| Reagent | Amount |
|---|---|
| Bacto Peptone | 63 g |
| Proteose Peptone | 3.5 g |
| Ammonium sulfate | 0.7 g |
| Tris base | 1.06 g |
| BHI broth base | 11.1 g |
| Yeast extract | 1.5 g |
| Agar | 15 g |
| L-cysteine 10% w/v | 3 mL |
Note: See Fimlaid et al. (2013) for details on 70:30 medium
Note: L-cysteine is sterilized by passage through a 0.2 μm Millipore filter and added to autoclaved medium cooled to approximately 65°C. Plates are stored at 4°C for up to 1 week.
Isolation of Single Colonies from C. difficile Spores
Timing: 1 h
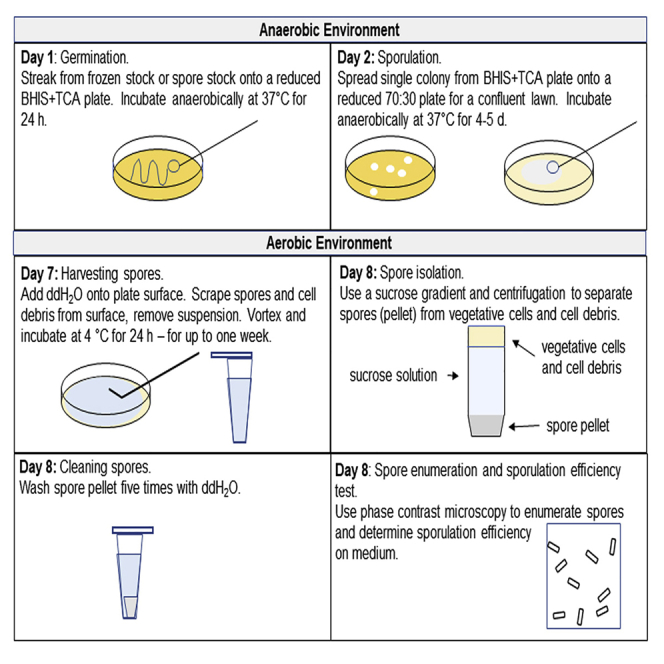
C. difficile spores are germinated on BHI+TCA plates to provide single colonies of vegetative cells.
CRITICAL: All plates must be pre-reduced in an anaerobic environment, such as that provided by a BD GasPak™ EZ Incubation System containing a GasPak™ anerobic sachet, for at least 2 h prior to use. See Before You Begin section for complete details.
Note, if starting from frozen C. difficile spore stocks (Sorg and Dineen, 2009), follow steps 1–3; for liquid stock, follow steps 4–7.
-
1.
In an anaerobic environment, place approximately 20 μL of frozen spore stock on BHI+TCA plate with a sterile inoculating loop.
-
2.
Streak plates for single colony isolation.
-
3.
Incubate plates anaerobically at 37°C for 1 to 2 days.
Alternatives: from liquid C. difficile spore stock
-
4.
Prepare a 1:10 dilution of spore stock in sterile ddH2O (final volume will depend on how many plates will be streaked).
-
a.
Heat spores in a water bath at 65°C for 20 min to prepare spores for germination.
-
b.
Vortex heated spore dilution.
-
5.
In an anaerobic environment, pipette 20 μL of heated spore stock dilution onto BHI+TCA plates.
-
6.
Streak for isolation of single colonies with a sterile inoculating loop.
-
7.
Incubate plates anaerobically at 37°C for 1–2 days.
Note: Excessive heat will lead to degradation of the spore coat, resulting in decreased germination. Do not let water bath exceed 65°C to prevent destroying spores.
Note: This method also works well when using frozen vegetative stock cultures.
Note: For best results use a C. difficile strain known to be an efficient spore former. The C. difficile strain ATCC 1382 can be used as a positive control during experimentation.
Note:C. difficile spores are hydrophobic and anionic and will adhere to plastic surfaces such as pipette tips and microcentrifuge tubes. To retain as many spores as possible, vortex before pipetting, and pipette up and down several times when transferring spores or making dilutions. Store spores in glass vials or tubes to minimize adhesion.
Note: To achieve an anerobic environment while working with spore cultures, a Spilfyter® bag is flushed with N2 several times, filled with N2 and sealed. All materials necessary are placed inside the bag before flushing with N2. Plates are incubated anaerobically in a BD GasPak™ EZ Anaerobic Container System with an anaerobic atmosphere created by using a GasPak™ EZ Anaerobic Sachet.
Sporulation of C. difficile Vegetative Cells
Timing: 1 h
A single vegetative C. difficile colony is streaked onto a 70:30 sporulation plate and incubated anaerobically to encourage vegetative cells to sporulate.
CRITICAL: All plates must be pre-reduced in an anaerobic environment, such as that provided by a BD GasPak™ EZ Incubation System containing a GasPak™ anerobic sachet, for at least 2 h prior to use. See Before You Begin section for complete details.
-
8.
In an anaerobic environment, using a sterile inoculating loop, pick one vegetative colony from the BHI+TCA plate and transfer to a 70:30 sporulation plate.
-
9.
Streak the colony down the center of the plate.
-
10.
Spread the colony for confluent growth over the entirety of the plate with a sterile inoculating loop.
-
11.
Incubate plates anaerobically at 37°C for 4–5 days.
Note: In our hands, most strains produce more spores on 70:30 sporulation medium than on BHI medium plates.
Note: Strains do not need to be precultured in liquid medium prior to inoculation onto 70:30 sporulation medium. A single colony from a BHIS+TCA plate can be transferred to sporulation plate and spread-inoculated for confluent growth.
Note: Different strains will require different incubation times on the 70:30 plates for optimal spore production.
Note: Three 70:30 sporulation plates generally produce ~ 1 mL of final purified spore preparation containing 1 × 109 to 4 × 109 spores/mL, depending on the strain.
Note: Sporulation of cells after growth on 70:30 (or other) medium can be directly assessed by examination of cells via phase contrast microscopy. Cells do not need to be immobilized prior to examination.
Harvesting C. difficile Spores from Sporulation Plates
Timing: 1–2 h
Spores are scraped from the surface of a 70:30 sporulation plates and incubated at 4°C to encourage spore release from mother cells.
Note: At this point, vegetative cells have sporulated and spores can be exposed to an aerobic environment. Continue work in a BSL2 Safety Cabinet using aseptic techniques.
-
12.
Pipette 1.5 mL sterile ddH2O onto the surface of the 70:30 sporulation plate containing confluent cell growth.
-
13.
Using a sterile inoculating loop, scrape cell debris and spores free from surface of plate.
-
14.
Tilt plate to one side to collect suspended spore suspension and cell debris.
-
15.
Pipette solution with cell debris and spores into a sterile 1.5 mL microcentrifuge tube.
-
16.
Vortex to resuspend.
-
17.
Repeat steps 12–16 for each 70:30 sporulation plate used.
-
18.
Incubate at 4°C for 24 h up to 1 week to facilitate spore release from mother cells.
Note: Cells do not need to be incubated at −20°C in step 18 to release spores.
Note: Centrifugation and washing of spores in ice-cold ddH2O before incubation at 4°C can result in the spores clumping together.
Note: The use of ethanol (final concentration = 47.5%) to kill or lyse vegetative cells is described in previously published protocols. At this stage, ethanol treatment will not significantly increase numbers of isolated spores and will result in cell clumping and production of excessive cell debris, limiting spore recovery. The ethanol step should not be used if a sucrose gradient is used for purification (see below).
Note: Different C. difficile strains sporulate to different degrees. To determine the degree of sporulation in a strain, enumerate spores before purification with sucrose gradient.
Isolation and Purification of C. difficile Spores
Timing: 3–4 h
Use of a density gradient to remove cell debris and vegetative cells and retain spores. Spore pellet is washed to remove sucrose.
Note: The following steps can continue be done aerobically because the spores are aerotolerant.
Note: Work in a BSL2 safety cabinet using aseptic technique.
-
19.
Pre-cool the swinging bucket rotor to 4°C in the centrifuge instrument.
-
20.
Fill 15 mL conical tubes with 10 mL 50% (w/v) sucrose solution.
-
21.
Remove 1.5 mL microcentrifuge tubes with spores and cell debris from 4°C storage and vortex to resuspend.
Note: Do not wash spores in ice-cold ddH2O before or after sucrose gradient centrifugation.
-
22.
Using a pipette, carefully layer about 1.5 mL of spore prep onto the top of the 10 mL of 50% w/v sucrose solution in the 15 mL conical tubes.
-
a.
Three tubes containing spore preps can be combined and 1.5 mL applied to each of two 15 mL conical tubes containing sucrose solution.
-
23.
Centrifuge 15 mL conical tubes at 3,600 rpm for 20 min to separate the cell debris from the spores. The cell debris will remain at the top of the 50% w/v sucrose suspension, while the heavier spores will form a pellet at the bottom of the conical tube.
-
24.
Carefully pipette upper cell debris layer on top of the sucrose solution and at the interface.
-
25.
Use a pipettor to remove the remaining sucrose solution without disturbing the spore pellet at the bottom of the conical tube.
-
26.
Resuspend the spore pellet in 500 μL sterile room temperature ddH2O in the 15 mL conical tubes.
-
27.
Combine resuspended spore pellets from two 15 mL conical tubes into one 1.5 mL microcentrifuge tube and vortex.
-
28.
Place 1.5 mL tubes in microcentrifuge and centrifuge 14,000 × g for 5 min at room temperature.
-
29.
Remove supernatant with pipette, resuspend spore pellet in 1 mL sterile ddH2O, and vortex.
Note: If spore pellet is difficult to resuspend, pipette up and down to encourage resuspension. Only use room temperature sterile ddH2O, use of ice-cold water can make the pellet difficult to resuspend.
-
30.
Centrifuge at 14,000 × g for 5 min.
-
31.
Wash pellet as described above for a total of 5 washes.
-
32.
After the final wash, resuspend spore pellet to a final volume of 1 mL with sterile ddH2O.
-
33.
Store purified spore preparation at 4°C in a glass vial.
Direct Enumeration of Spores
Timing: 1 h
A phase contrast microscope is used to enumerate and determine purity of spore preparation (Figure 1).
Note: If there are too many spores to count in a 1:10 dilution, further dilute and adjust calculations accordingly.
Note: If clumps of spores are seen via phase contrast microscopy, add 1% BSA in PBS as indicated in the Troubleshooting section below. Diluting in PBS alone may also help with the aggregation issue.
-
34.
Make a 1:10 dilution of spores in sterile ddH2O.
-
35.
Vortex to distribute spores.
-
36.
Place 5 μL of dilution on a Petroff-Hauser counting chamber (depth 0.02 mm).
-
37.
Place a drop of immersion oil on top of the cover slip.
-
38.
Using phase contrast microscopy and 100× magnification lens, count spores, vegetative cells, and spores in mother cells.
-
a.
Spores will be small, phase-bright rods, vegetative cells will be long, phase-dark rods, and spores in mother cells with be rods with a bright oval at the end.
-
39.
Calculate spores per mL:
-
b.
-
40.
Calculate spore preparation purity
-
c.
Figure 1.
Image of Partially Purified Spores from C. difficle Strain RPRE2
Light micrograph produced on a Zeiss Standard 15 microscope by using phase contrast microscopy. Each side of the small squares (in Neubauer ruling) is μm in length. The entire image was slightly darkened to enhance contrast for printing.
Enumeration of Viable Spores
Timing: 2 h
To determine the number of viable spores per mL of spore stock.
Note: Dilution factors for spore stocks depend on the viability and number of spores. Countable plates contain between 30 and 300 colonies. Plating a range of dilutions is necessary to obtain countable plates.
Note: BHI+TCA plates must be pre-reduced in an anaerobic environment such as a BD GasPak™ EZ Incubation System containing a GasPak™ anerobic sachet for at least 2 hr prior to use.
Note: To achieve an anerobic environment while working with cultures spores, a Spilfyter® bag is flushed with N2 several times, filled with N2 and sealed. All materials necessary are placed inside the bag before flushing with N2. Plates are incubated anaerobically in a BD GasPak™ EZ Anaerobic Container System with an anaerobic atmosphere created by using a GasPak™ EZ Anaerobic Sachet.
-
41.
Make serial dilutions of spore stock in sterile ddH2O in 1.5 mL microcentrifuge tubes.
-
a.
10−1, 10−2, 10−3, etc.
-
42.
Heat the centrifuge tubes containing spore dilutions in a water bath at 65°C for 20 min.
-
43.
In an anaerobic environment, plate 100 μL of spore dilution on reduced BHI + TCA plates and spread evenly over the entirety of the plate with a sterile inoculating loop.
-
44.
Incubate anaerobically at 37°C for 1–2 days.
-
45.
Count the number of colonies on plates that have between 30 and 300 colonies.
-
46.
Determine the number of colony forming units (CFUs):
Expected Outcomes
High quality purified spore preparations should be >99% spores.
Three 70:30 sporulation plates should yield spore stock between 1 × 109 spores/mL and 4 × 109 spores/mL depending on strain.
This protocol has been used successfully to produce spore preps for the following C. difficile strains: ATCC 1382, UK1, RPRE2, RPRE5-3, RPRE6-6, RPO1-15, RPO2-5 and other locally obtained strains in our lab.
Limitations
Spore yield is dependent on the C. difficile strain, medium used and incubation time under anaerobic conditions. These conditions will need to be operationally determined for other strains. A positive control strain, such as ATCC 1382 should be used initially to verify media and methods.
Troubleshooting
Problem
While some strains will sporulate, as evidenced by phase contrast microscopy, they may not be efficient in releasing spores from mother cells.
Potential Solution
-
1.
Scrape cells from sporulation plates, centrifuge and wash cells in room temperature ddH2O, and incubate at 4°C for 24 h up to 1 week to facilitate spore release from mother cells.
-
2.
Do not use chloroform addition (1:25) or 1 mg/mL lysozyme in an attempt to improve spore yield as it will lead to increased cell debris without substantially improving yield. .
Problem
After washing with ddH2O, some strains of C. difficile spores may clump together making them difficult to suspend and enumerate.
Potential Solution
-
1.
Washing with ice-cold ddH2O can contribute to clumping. Use room temperature ddH2O.
-
2.
Make a 10% solution of bovine serum albumin (BSA) in phosphate buffered saline (PBS).
-
3.
Add the 10% BSA in PSB solution to the purified spore preparation to a final concentration of 1%.
-
4.
Vortex.
Note: Addition of BSA in PBS at a 1% final concentration does not affect the outcome of germination and growth assays.
Problem
Some strains of C. difficile do not sporulate to a significant degree.
Potential Solution
-
1.
Try both 70:30 and BHI plates as sporulation media.
-
2.
Make sure BHI plates contain 25 mL and 70:30 plates contain 35 mL of media.
-
a.
BHI is a richer medium than 70:30 and plates do not require more than 25 mls for effective sporulation
-
3.
Increase the number of 70:30 plates pooled together to produce each 1 ml of final spore prep. In this protocol, three 70:30 plates are used to produce 1 ml of final spore prep.
-
4.
Mother cells can be induced to release spores by incubation at 4°C or freezing at −15°C to −25°C.
Resource Availability
Lead Contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Michael Sadowsky, sadowsky@umn.edu;
Materials Availability
This study did not generate any new materials.
Data and Code Availability
This protocol did not generate any new data or code.
Acknowledgments
This work was supported, in part, by the Office of the Assistant Secretary of Defense for Health Affairs, under Investigator Initiated Research Awards W81XWH-17-1-0635 (P.I.D.) and W81XWH-17-1-0636 (A.K.) and by grant 5U01HL127479-03 from the NIH, under the MIN-REACH Research and Evaluation Hub. We would like to thank Alexa Weingarden and Raymond Erickson for helpful discussions and John Ferguson for help with images.
Author Contributions
A.K., P.D., and M.S. conceived of the study and directed the experiments, M.W. and C.E. did the experiments, M.W. and M.S. wrote the manuscript, and A.K., P.D., and C.E. edited the manuscript.
Declaration of Interests
The authors declare no competing interests.
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.xpro.2020.100071.
Contributor Information
Melissa Weldy, Email: weld0002@umn.edu.
Michael J. Sadowsky, Email: sadowsky@umn.edu.
References
- Edwards A.N., McBride S.M. Isolating and purifying Clostridium difficile spores. Methods Mol. Biol. 2016;1476:117–128. doi: 10.1007/978-1-4939-6361-4_9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fimlaid K.A., Bond J.P., Schutz K.C., Putnam E.E., Leung J.M., Lawley T.D., Shen A. Global analysis of the sporulation pathway of Clostridium difficile. PLoS Genet. 2013;9:e1003660. doi: 10.1371/journal.pgen.1003660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen A., Fimlaid K.A., Pishdadian K. Inducing and quantifying Clostridium difficile spore formation. Methods Mol. Biol. 2016;1476:129–142. doi: 10.1007/978-1-4939-6361-4_10. [DOI] [PubMed] [Google Scholar]
- Sorg J.A., Dineen S.S. Laboratory maintenance of Clostridium difficile. Curr. Protoc. Microbiol. 2009 doi: 10.1002/9780471729259.mc09a01s12. Chapter 9:Units 9A.1.1-9A.1.10. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
In our protocol a Spilfyter® bag is used to achieve the anerobic environment. The bag is flushed and filled with nitrogen gas prior to use.
The streaking of plates and transferring cultures must be done in the Spilfyter® bag.
Data Availability Statement
This protocol did not generate any new data or code.



