Summary
This protocol is a procedure for establishment and culture of cancer and non-cancer organoids using tissues from biliary tract carcinoma (BTC) patients. These BTC organoids can be used for various biological analyses and drug screening. One challenge in establishing and culturing BTC organoids is non-cancer cells contaminating surgically resected tumor tissues form organoids concurrently with cancer organoids. Careful validation that the established organoids are cancer-derived is important.
For complete details on the use and generation of this protocol, please refer to Saito et al. (2019) in the journal Cell Reports.
Graphical Abstract

Highlights
-
•
A procedure for establishment and culture of organoids using tissues from BTC patients
-
•
Patient-derived BTC organoids can be used for biological analyses and drug screening
-
•
Non-cancer cells in tumor tissues grow and overcome cancer cells to form organoids
-
•
Careful validation that the established organoids are cancer-derived is important
This protocol is a procedure for establishment and culture of cancer and non-cancer organoids using tissues from biliary tract carcinoma (BTC) patients. These BTC organoids can be used for various biological analyses and drug screening. One challenge in establishing and culturing BTC organoids is non-cancer cells contaminating surgically resected tumor tissues form organoids concurrently with cancer organoids. Careful validation that the established organoids are cancer-derived is important.
Before You Begin
Tissue Sample Preparation
For organoid culture, we use cancer and non-cancer tissue specimens obtained from patients who had been diagnosed preoperatively as having biliary tract carcinomas (BTCs) and underwent surgical resection. Macroscopic appearance of a surgically resected specimen obtained from a patient with intrahepatic cholangiocarcinoma (IHCC) is shown in Figure 1.
CRITICAL: Obtaining fresh and high-quality tissue samples is most important for success in establishing organoids.
-
•
Contact pathologists in your hospital and discuss tissue sampling in advance.
-
•
We usually transfer resected tissues in a 100 mm sterile dish on ice and subject them to organoid culture immediately.
Alternatives: If you cannot process immediately, resected tissues should be transferred in a cryotube without cryoprotectant and stored in dry ice or liquid nitrogen. We have established organoids using resected tissues stored in liquid nitrogen for several months.
-
•
For BTCs, the surgically resected tissue samples are small, especially gallbladder cancer (GBC) and bile duct cancer (BDC).
Note: in case of BTCs, the surgically resected tissue samples are small (especially with GBC and BDC). As the priority for hospitals is clinicopathological diagnosis, this means that the samples available for research can be even more limited. This may be one of the reasons for low success rate of BTC organoids.
-
•
Using these remaining specimens of primary tissues, you can establish organoids, extract DNA for whole exome sequencing (WES) and RNA for transcriptome analysis, and perform histological examination for the primary tissues.
Figure 1.
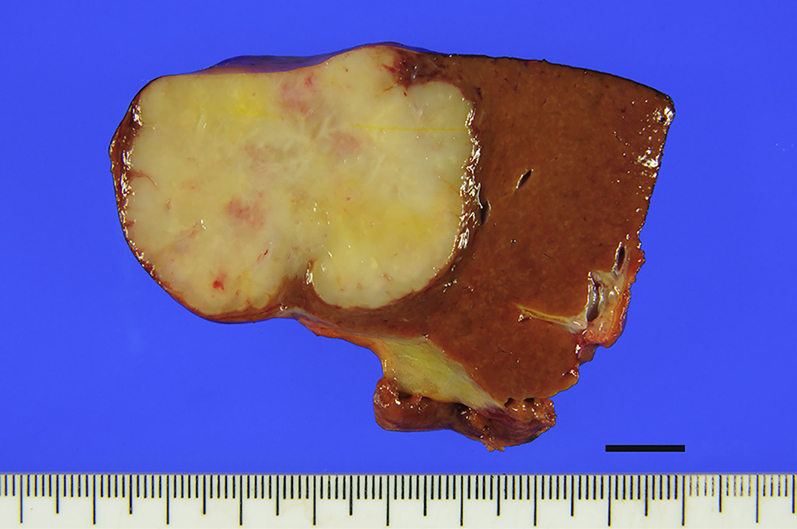
Macroscopic Appearance of a Surgically Resected IHCC Specimen
Scale bar, 10 mm.
Key Resources Table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Biological Samples | ||
| Human biliary tract carcinoma tissue samples | This study | N/A |
| Chemicals, Peptides, and Recombinant Proteins | ||
| Advanced DMEM/F12 | Thermo Fisher Scientific | Cat# 12634010 |
| GlutaMAX Supplement | Thermo Fisher Scientific | Cat# 35050061 |
| HEPES | Thermo Fisher Scientific | Cat# 15630080 |
| Penicillin-Streptomycin | Thermo Fisher Scientific | Cat# 15140122 |
| B-27 Supplement | Thermo Fisher Scientific | Cat# 17504044 |
| N-2 Supplement | Thermo Fisher Scientific | Cat# 17502048 |
| Nicotinamide | Sigma-Aldrich | Cat# N0636 |
| N-Acetyl-L-cysteine | Sigma-Aldrich | Cat# A9165 |
| [Leu15]-Gastrin I human | Sigma-Aldrich | Cat# G9145 |
| Recombinant Mouse EGF | Thermo Fisher Scientific | Cat# PMG8043 |
| Y-27632 | Wako | Cat# 253-00513 |
| Forskolin | Tocris Bioscience | Cat# 1099 |
| A83-01 | Tocris Bioscience | Cat# 2939 |
| R-spondin 1 expressing 293T cell line | Merck Millipore | Cat# SCC111 |
| Matrigel (growth factor reduced, phenol red-free) | Corning | Cat# 356231 |
| Dispase II | Thermo Fisher Scientific | Cat# 17105041 |
| Collagenase type XI | Sigma-Aldrich | Cat# C7657 |
| TrypLE Express | Thermo Fisher Scientific | Cat# 12605028 |
| Recovery™ Cell Culture Freezing Medium | Thermo Fisher Scientific | Cat# 12648010 |
Materials and Equipment
Digestion Buffer
| Dulbecco’s modified Eagle medium (DMEM) |
| 2.5%v/v fetal bovine serum |
| 0.0125% dispase type II (Thermo Fisher Scientific) |
| 0.0125% collagenase type XI (Sigma-Aldrich) |
This buffer is stored at 4°C and is warmed at 24-26°C before use.
Culture Media
| Advanced DMEM/F12 (Thermo Fisher Scientific) |
| supplemented with Glutamax |
| 10 mM HEPES, penicillin/streptomycin |
| 1x N2 supplement |
| 1x B27 supplement |
| 50 ng/mL EGF |
| 1.25 mM N-acetylcysteine |
| 10 nM gastrin |
| 10 mM nicotinamide |
| R-spondin 1∗∗ |
| 5 μM A83-01 |
| 10 μM forskolin |
| 10 μM Y-27632 |
This medium is stored at 4°C and is warmed at 24-26°C before use.
10% conditioned medium from R-spondin 1 expressing 293T cell line
Matrigel
| Make 1 mL aliquots of Matrigel and store them at −20°C. |
| We thaw them on ice ∼2 h before use. |
Step-By-Step Method Details
Establishing Organoids Using Cancer Tissues
TIMING: ∼2 h
-
1.
Cut tissue samples into small pieces (∼10-30 mg) using a disposable sterile scalpel (Figure 2).
Figure 2.
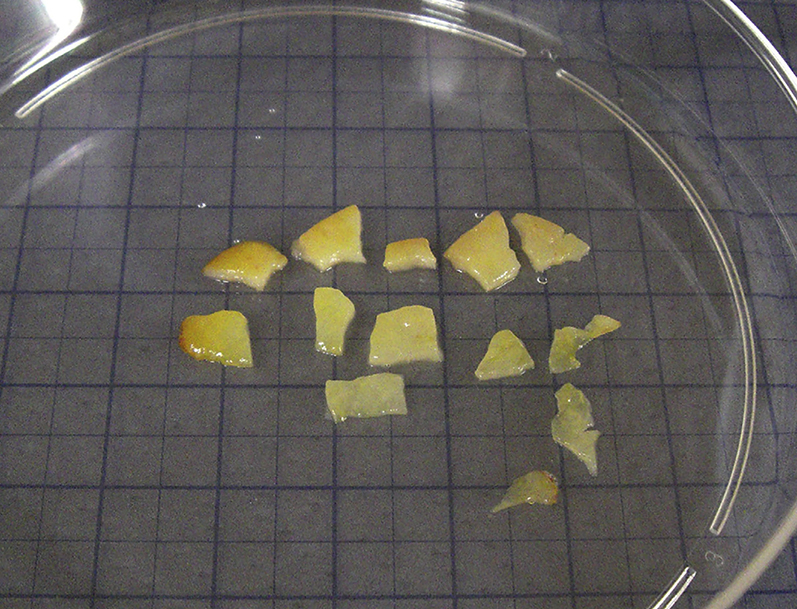
Small Pieces of Resected Tumor Tissues
-
2.
Transfer one of the cut tissues into a 1.5 mL Eppendorf tube and then add digestion buffer (∼200 μL). Mince the tissue using sterile ophthalmic scissors in the tube. The other pieces of tissue can be utilized for extraction of DNA and RNA or histology.
-
3.
Add digestion buffer (∼800 μL) and incubate at 37°C for 1 h using a shaking heat block (∼800 rpm).
Alternatives: A shaking water bath can be used instead, but we have had cell culture contamination by using a shaking water bath several times. Thus, we highly recommend the shaking heat block.
PAUSE POINT: You can leave the experiments for 1 hour.
-
4.Settle under gravity for 1 min and collect supernatant in another 1.5 mL Eppendorf tube.
-
a.Optional: the pellet can be used for re-process for a second organoid culture establishment by repeating steps from 1c. We have succeeded in establishment of BTC organoids by a second establishment once, whereas a first establishment was failed.
-
a.
-
5.
Centrifuge the collected supernatant at ∼500 g for 3 min at 24-26°C.
-
6.
Remove and discard supernatant and wash pellet with 1 mL PBS.
-
7.
Centrifuge at ∼500 g for 3 min at 24-26°C.
-
8.
Remove supernatant and add Matrigel on ice. Remove all supernatant (PBS) as possible, as remaining of PBS results in dilution of Matrigel. Matrigel should be ice-cold. We usually add 20 μL Matrigel for approximately 5 X 103 cells. Cells and Matrigel should be mixed well by pipetting several times in the tube on ice. Avoid bubbles in Matrigel when pipetting.
-
9.
Plate cells suspended in Matrigel on a culture plate. We usually plate ∼20 μL suspension of cells and Matrigel in a 48-well plate to form dome (Figure 3). Any types of plates can be used, as no specific treatment on a plate is necessary. Incubate at 37°C for 15 min to solidify Matrigel dome.
Figure 3.

Matrigel Dome
-
10.
Overlay culture medium and incubate at 37°C in a standard incubator (5% CO2). Medium should be prewarmed at 24-26°C before use. We usually add 250 μL culture medium in a 48-well plate for initial plating. After 3-4 days of culture, we add more 250 μL culture medium (total 500 μL). You can observe the growth of organoids after ∼5 days.
Passage of Organoids
TIMING: ∼1 h
We typically incubate organoids for 7-10 days between initial plating and passage.
-
1.
Remove culture medium.
-
2.
Add 500 μL TrypLE Express (Thermo Fisher Scientific) per well at 24-26°C and mix with Matrigel dome well by pipetting several times to generate a single cell suspension.
-
3.
Incubate at 37°C for 15 min.
-
4.
Collect in 1.5 mL Eppendorf tube and centrifuge at ∼2000 g for 3 min at 24-26°C.
-
5.
Remove and discard supernatant. Matrigel is disintegrated and removed.
-
6.
Wash pellet with 1 mL PBS.
-
7.
Centrifuge at ∼2000 g for 3 min at 24-26°C.
-
8.
Remove supernatant and add Matrigel on ice as described in step 1 h.
-
9.
Plate cells suspended in Matrigel on a culture plate and incubate to allow Matrigel to solidify, overlay culture medium and incubate at 37°C as described in steps 1i and 1j.
-
10.
We typically passage BTC organoids once every 7-10 days and split cells at ratios 1:5-1:10. We have maintained BTC organoids stably for > 1 year without morphologic alterations.
Note: Passage interval depends on the growth of cells. When organoids are confluent in Matrigel (i.e., there is no space for organoids growing), they should be passaged.
-
11.
Once organoids can be cultured stably and passaged several times, they can be used for analysis and/or experimentation.
Quality Control of BTC Organoids
When establishing organoids using surgically resected BTC tissue specimens, careful validation that the established organoids are cancer-derived is important. For example, you should check the following points.
-
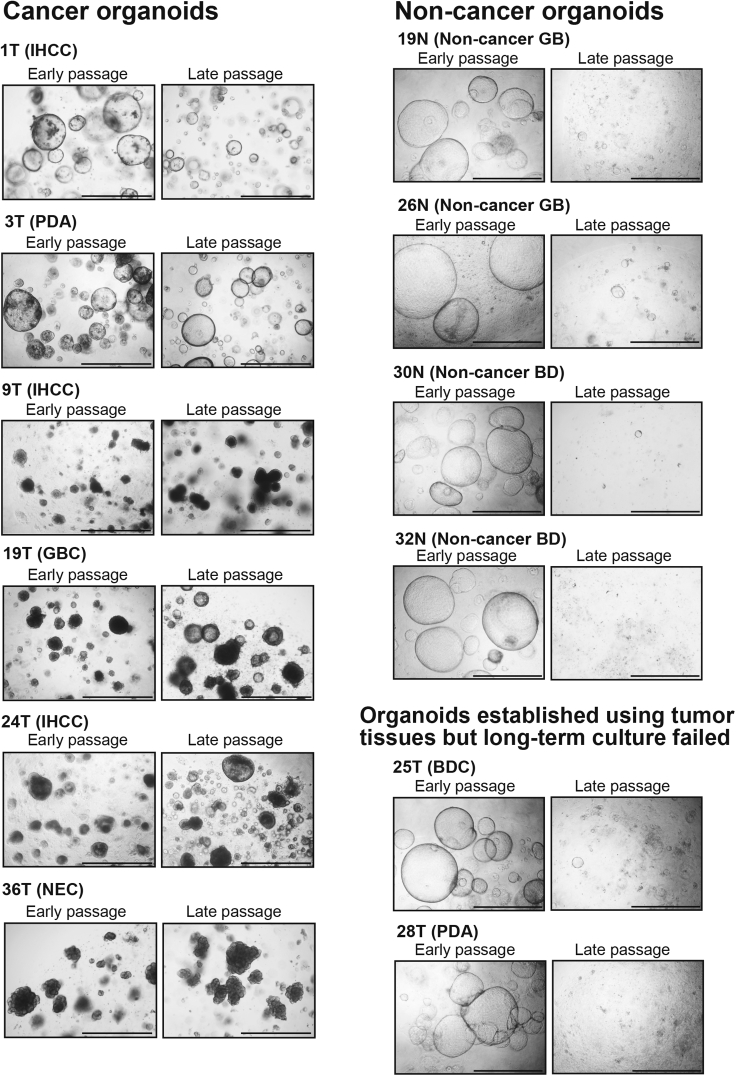
1.
Cancer organoids and non-cancer organoids exhibit completely different morphology as shown in Figure 4. Non-cancer organoids show a simple large cystic morphology with a thin monolayer, whereas cancer organoids exhibit irregular morphologies including a cystic structure and a solid structure that recapitulate the pathological features of the original primary BTCs.
Figure 4.
Morphologic Features of Cancer and Non-cancer Organoids
Scale bars, 1000 μm. IHCC, intrahepatic cholangiocarcinoma; PDA, pancreatic ductal adenocarcinoma; GBC, gallbladder cancer; NEC, neuroendocrine carcinoma; BDC, bile duct cancer.
-
2.
Cancer organoids can be cultured stably for > 1 year. There is no significant difference in the morphological changes in cancer organoids between early (< 2 months) and late (> 4 months) passages (Figure 4). On the other hand, non-cancer organoids show more elevated proliferation activity at the early stage than cancer organoids, but cease proliferation at around passage 15 as shown in Figures 4 and 5. See also Figure 3 in our original paper (Saito et al., 2019).
Figure 5.
Culture Courses of Cancer and Non-cancer Organoids
-
3.
Non-cancer organoids for which long-term culture is not possible do not harbor any driver gene mutations, whereas cancer organoids for which long-term culture is possible harbor mutations of driver genes such as TP53 and KRAS. See Figure 5D in our original paper (Saito et al., 2019).
The success rates for establishment of organoids derived from BTCs in our study (Saito et al., 2019) were relatively low (IHCC: 50%, GBC: 20%) in comparison to those for colon cancer organoids described previously (van de Wetering et al., 2015). We consider that establishment of organoids using BTC tissues is relatively difficult for the following reasons:
-
1.
The size of surgically resected BTC tissue samples is generally small (especially GBC and BDC).
-
2.
BTC tissues contain a number of stromal cells such as fibroblasts, blood vessels and immune cells other than cancer cells. Thus, it is often difficult to resect substantial amounts of BTC cells without contamination by non-cancer cells.
-
3.
The frequency of driver gene mutations such as TP53 and KRAS in BTCs is relatively low in comparison to other cancers such as colorectal cancer (Jain et al., 2016).
Expected Outcomes
Once you verify the established organoids are cancer-derived by morphology, culture course and driver gene mutation, these BTC organoid lines can be maintained for a long-term (> 1 year) by passage once every 7-10 days. There is variability in the growth rate of organoids from different individuals. See Figures 3 in our original paper (Saito et al., 2019).
These BTC organoid lines can be cryopreserved in liquid nitrogen. After step 2g, remove supernatant and add 500 μL Recovery Cell Culture Freezing Medium. Cells suspended in the medium can be transferred in a cryotube and stored in liquid nitrogen for a long time (> 1 year). Cells can be recovered after cryopreservation by thawing followed by steps 2d-2i.
You can extract DNA, RNA and protein from these BTC organoids for DNA sequencing, quantitative PCR, microarray, RNA-seq and western blotting, respectively. You can also utilize these BTC organoids for immunohistochemistry with paraffin-embedded sections and drug screening as described in our original paper (Saito et al., 2019). You can observe biological similarity between primary tissues and established organoids by immunohistochemistry, exome and transcriptome analyses.
Limitations
One limitation in establishing and culturing BTC organoids is contamination of non-cancer cells. Organoids established using tumor tissues sometime show a morphology similar to non-cancer organoids and fail to undergo long-term expansion (see Figure 4). Non-cancer cells contaminating surgically resected tumor tissues form organoids concurrently with cancer organoids. Organoids derived from non-cancer tissues show more robust proliferation activity at the early stage of culture than cancer organoids. Non-cancer cells contaminating surgically resected tumor tissues grow predominantly and overcome cancer cells to form organoids, and no cancer organoids form at all (Saito et al., 2019). Recent studies have also reported a similar phenomenon in the establishment of colon and liver cancer organoids (Broutier et al., 2017, van de Wetering et al., 2015).
To avoid this problem, we recommend quality control of organoids described above, i.e., (1) morphology, (2) culture course, (3) driver gene mutation. Careful validation that the established organoids are cancer-derived is important.
Troubleshooting
Problem
Low success rate
Potential Solutions
The success rate for establishment of organoids derived from BTCs with TP53 and KRAS mutations could be improved by the addition of nutlin-3a and removal of EGF from the culture medium to inhibit contamination by non-cancer cells, as described previously (Seino et al., 2018).
Cancer organoids and non-cancer organoids exhibit completely different morphology as shown in Figure 4. Non-cancer organoids show a simple large cystic morphology with a thin monolayer, whereas cancer organoids exhibit irregular morphologies including a cystic structure and a solid structure that recapitulate the pathological features of the original primary BTCs. We are successful in separation of cancer organoids by picking them up using a pipette under a microscope. To increase the success rate for establishment of cancer organoids, it may be beneficial to pick up and isolate only cancer organoids from contaminated non-cancer organoids under a microscope. This may be an easy and effective method for establishing cancer organoids without contaminating non-cancer organoids.
Acknowledgments
This work was supported by a Keio University Special Grant-in-Aid for Innovative Collaborative Research Projects (to Y.S.) and JSPS KAKENHI grant number JP17H03592 (to Y.S.).
Author Contributions
Y.S. and H.S. designed and conducted the experiments, analyzed the data, and wrote the manuscript. T.M. performed the organoid culture.
Declaration of Interests
The authors declare no competing interests.
References
- Broutier L., Mastrogiovanni G., Verstegen M.M., Francies H.E., Gavarró L.M., Bradshaw C.R., Allen G.E., Arnes-Benito R., Sidorova O., Gaspersz M.P. Human primary liver cancer-derived organoid cultures for disease modeling and drug screening. Nat. Med. 2017;23:1424–1435. doi: 10.1038/nm.4438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain A., Kwong L.N., Javle M. Genomic Profiling of Biliary Tract Cancers and Implications for Clinical Practice. Curr. Treat. Options Oncol. 2016;17:58. doi: 10.1007/s11864-016-0432-2. [DOI] [PubMed] [Google Scholar]
- Saito Y., Muramatsu T., Kanai Y., Ojima H., Sukeda A., Hiraoka N., Arai E., Sugiyama Y., Matsuzaki J., Uchida R. Establishment of Patient-Derived Organoids and Drug Screening for Biliary Tract Carcinoma. Cell Rep. 2019;27:1265–1276.e4. doi: 10.1016/j.celrep.2019.03.088. [DOI] [PubMed] [Google Scholar]
- Seino T., Kawasaki S., Shimokawa M., Tamagawa H., Toshimitsu K., Fujii M., Ohta Y., Matano M., Nanki K., Kawasaki K. Human Pancreatic Tumor Organoids Reveal Loss of Stem Cell Niche Factor Dependence during Disease Progression. Cell Stem Cell. 2018;22:454–467.e6. doi: 10.1016/j.stem.2017.12.009. [DOI] [PubMed] [Google Scholar]
- van de Wetering M., Francies H.E., Francis J.M., Bounova G., Iorio F., Pronk A., van Houdt W., van Gorp J., Taylor-Weiner A., Kester L. Prospective derivation of a living organoid biobank of colorectal cancer patients. Cell. 2015;161:933–945. doi: 10.1016/j.cell.2015.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]