Abstract
Background:
The condition known as 22q11 microdeletion syndrome has a broad phenotypic spectrum, with many affected individuals experiencing mild-to-moderate immunodeficiency. Currently, there are significant variations in live vaccine practices and immunological testing prior to live vaccine administration due to safety concerns and limited established guidelines.
Methods:
Queensland Children’s Hospital (QCH) Child Development Unit, offers a state-wide 22q11 microdeletion clinic. This is a retrospective single-centre review, capturing the majority of children with 22q11 microdeletion in Queensland, Australia. We describe the live vaccination status of 134 children, age 0 to 18 years under our care between 2000 and 2018, adverse events following immunisation (AEFI) and the proportion of children who received additional pneumococcal coverage. An immunological investigation pathway prior to live vaccine administration is proposed.
Results:
Of the 134 children, 124 were eligible for live vaccinations as per the Australian National Immunisation Program: 82% had received dose one of measles, mumps and rubella (MMR) vaccine, 77% had completed MMR dose two and 66% had completed varicella immunisation. There were no AEFI notifications reported. Of the total sample of children, 18% received a fourth dose of conjugate pneumococcal vaccine (Prevenar 7 or 13) and 16% received a dose of Pneumovax 23 from 4 years of age. Immunology workup practices were demonstrated to vary widely prior to live vaccine administration. Most patients’ immune profiles were consistent with mild-to-moderate immunodeficiency.
Conclusion:
We propose an immunological investigation and vaccination pathway with the aim of providing guidance and consistency to clinicians caring for children with 22q11 microdeletion.
Keywords: 22q11 microdeletion syndrome, DiGeorge syndrome, immunisation, velo-cardiofacial syndrome
Introduction
The incidence of 22q11 microdeletion is likely under-recognised; however, it has been estimated to occur in 1 in 4000 live births.1,2 The majority of patients with 22q11 microdeletion have mild-to-moderate immunodeficiency or immune dysregulation.3,4 This consists of both humoral and cellular compartments.3 Disturbances of pharyngeal arch development associated with 22q11 microdeletion can result in altered thymic development, ranging from absent to normal size.2,4 A broad range of T cell deficiency can be seen, with a minority of patients exhibiting severe T cell immunodeficiency associated with complete athymia.2,5 However, T cell lymphopaenia can be unrelated to thymic size.3 Humoral immunity is also often dysfunctional and likely related to altered T cell numbers and regulatory function.3
The consequences of immune abnormalities in combination with underlying anatomical dysfunction (i.e. eustachian tubes and velopharyngeal insufficiency) are an increased susceptibility to prolonged and recurrent viral infections, superimposed bacterial infections, sinopulmonary infections and vaccine preventable infections.2,4,6,7 As a result, immunisation is an important preventative disease measure in this cohort of patients.
Currently, there are limited studies that have evaluated the safety of live vaccine administration in this cohort of patients. To date, clinical practice has been guided by the few studies that have assessed this as well as extrapolating data from studies performed in human immunodeficiency virus (HIV)-infected individuals.8 As a result, there are currently significant variations in live vaccine practices in this patient cohort as well as the immune workup received prior to live vaccine administration.
Studies evaluating live vaccine administration in patients with 22q11 microdeletion have demonstrated that live vaccinations can be safely administered despite evidence of mild and moderate immunodeficiency.6,7,9–14 Sobh et al. and the Infectious Diseases Society of America (IDSA) guideline recommends that live vaccines can be safely administered in patients with 22q11 microdeletion if the total T cell (CD3) count is more than 0.5 × 109/l, helper T cell (CD4) is more than 0. 5 × 109/l, cytotoxic T cell (CD8) count is more than 0.2 × 109/l and a normal mitogen response has been demonstrated.8,15 Recent thymic emigrant cells (RTE) represent naïve T cells and are evidence of thymic output. Measurement of T cell numbers (CD3 count) may overestimate autologous T cell number and function in the presence of oligoclonal or maternally engrafted T cells. Unless T cell Receptor Excision Circles (TRECs) or RTE are measured, some patients with cellular immunodeficiency may be missed.
Despite evidence of mild or moderate immune dysfunction, there appears not to be an increased risk of adverse events following live immunisations (AEFI), such as vaccine-related disease, in patients with mild-to-moderate immunodeficiency.6,10,12 Immunogenicity has also been demonstrated in patients with 22q11 microdeletion with mild-to-moderate immunodeficiency with no significant differences in seroconversion to tetanus, diphtheria, measles, mumps and rubella vaccine.9–11 Of note, cutaneous granulomas are increasingly noted in multiple different primary immune deficiencies (PID) diagnoses including 22q11 microdeletion.16 Vaccine strain rubella has been reported in cutaneous granulomatous lesions of many of these patients,17 and while not explicitly demonstrated in patients with 22q11 microdeletion, this will require ongoing monitoring and consideration.
The Child Development Unit at the Queensland Children’s Hospital (QCH) cares for the majority of children with 22q11 microdeletion in Queensland and also provides state-wide specialist advice for patients with 22q11 microdeletion. This study describes the immunisation profiles including: current vaccination patterns, immunology workup proceeding live vaccine administration, AEFI and the proportion of children who received additional pneumococcal immunisation. From this information, we propose an immunological investigation pathway prior to live vaccine administration for this cohort of patients to aid clinicians caring for children with 22q11 microdeletion in the community and also in hospital.
Methods
Retrospective review involving all children with 22q11 microdeletion under the care of the Child Development Unit at QCH between 2000 and 2018 (n = 134). Individuals were included if they had a chromosome 22q11.2 microdeletion detected on fluorescence in situ hybridisation (FISH) and/or microarray.
Case records were reviewed for laboratory studies of immune function (lymphocyte subsets, lymphocyte proliferation responses to mitogens, RTE count, tetanus and diphtheria serology), immunisation history and AEFI notifications. Laboratory studies of immune function prior or proximate to timing of live vaccine administration were obtained from Pathology Queensland, a centralised pathology laboratory for all public hospitals in Queensland. In Queensland, AEFI reporting is centralised with all notifications recorded in the Notifiable and Other Conditions (NOCS) database. An AEFI notification was checked for all eligible patients. Immunisation history including live vaccines and additional doses of pneumococcal vaccine were extracted from the Australian Immunisation Register (AIR), a national register that records vaccinations given to people of all ages in Australia. Children were immunised either in the community or in hospital.
The primary outcome measure was live vaccination coverage and timeliness by 12 and 18 months of age as per the Australian National Immunisation Program (NIP) recommendations. Live vaccines recommended on the NIP include measles, mumps and rubella (MMR) and varicella containing vaccines (MMRV or monovalent varicella). A delay was defined as live vaccine administration more than or equal to 6 months following the recommended timeframe as per the Australian NIP.
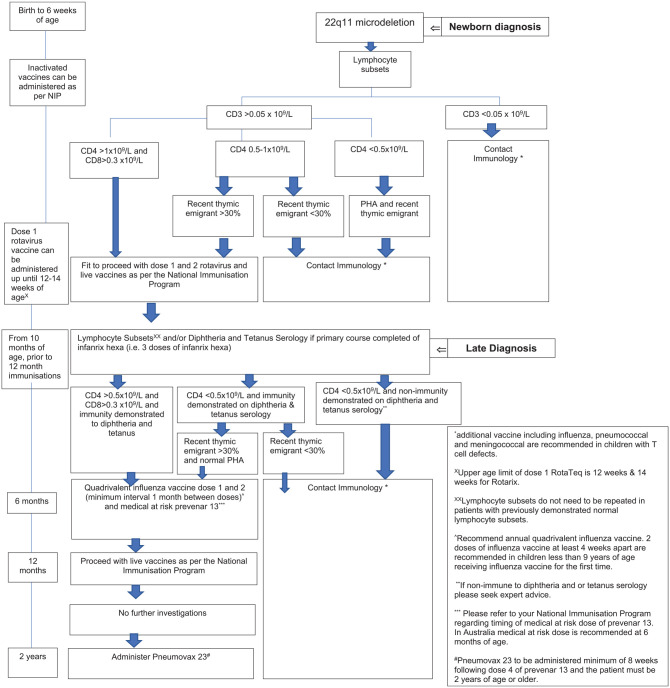
A pathway for immunological investigation and vaccination was developed as part of this study (see Figure 2). Additional pneumococcal immunisation coverage was re-assessed following implementation of a Children Health Queensland Medical at Risk Immunisation Guideline.
Figure 2.
Children Health Queensland 22q11 microdeletion immune investigation and immunisation guideline. An immunological investigation and vaccination pathway for children with 22q11 microdeletion.
PHA, lymphocyte proliferation responses to mitogen.
The need for ethical approval and written informed consent was waived by the Children’s Health Queensland Hospital and Health Service Human Research Ethics Committee on 10 May 2018.
Results
Immunology profile
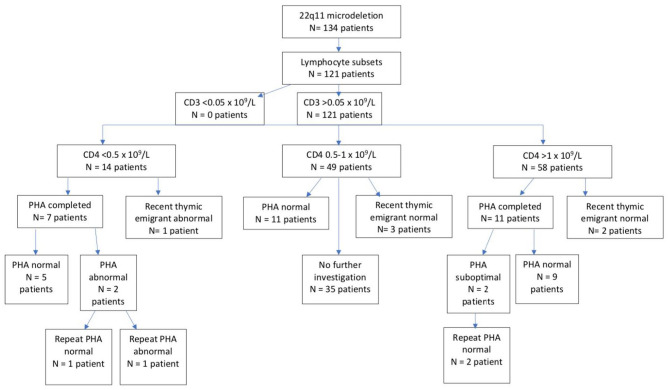
Of the 134 children with 22q11 microdeletion, 121 (90%) children had lymphocyte subsets tested (Figure 1). No children had a CD3 count of less than 0.05 × 109/l, in keeping with severe T cell immunodeficiency; 14 (12%) had a CD4 count of less than 0.5 × 109/l. In those with a CD4 count of less than 0.5 × 109/l, 7 (50%) had lymphocyte proliferation responses to mitogens performed, of which 2 had an abnormal response, with one persistently abnormal response on repeat testing. There was one patient with CD4 count of less than 0.5 × 109/l who had an abnormal RTE population (CD45RA+) of less than 30%.
Figure 1.
Immunology profile in children with 22q11 microdeletion known to QCH.
Abnormal RTE (CD45RA+) <30% total T cell population.
PHA, proliferation responses to mitogens; QCH, Queensland Children’s Hospital; RTE, recent thymic emigrant.
A total of 49 (40%) children had a CD4 count between 0.5 and 1 × 109/l, of which 11 (22%) had normal lymphocyte proliferation responses to mitogens and 3 (6%) demonstrating a normal RTE count. No children with CD4 count between 0.5 and 1 × 109/l had an abnormal proliferation response to mitogens or an abnormal RTE count. Of those with CD4 count of 0.5 to 1 × 109/l, 35 (71%) did not receive further investigation.
Almost half (48%) had a CD4 count more than 1 × 109/l, of which 11 (19%) had further evaluation with lymphocyte proliferation responses to mitogens. Two children (with CD4 count more than 1 × 109/l) had suboptimal results however both responses were normal on final testing. The remaining children (n = 45) with a CD4 count of more than 1 × 109/l did not go on to receive further testing.
Live vaccination coverage and timeliness
The majority of the children had received their live vaccines [102/124 (82%) MMR dose 1 versus 96/124 (77%) MMR dose 2 versus 82/124 (66%) varicella] on time (87% MMR dose 1 versus 76% MMR dose 2 versus 83% varicella) as depicted in Table 1. Due to changes in Australian government legislative criteria, a medical exemption for immunisation is indicated in children who are immunocompromised and unable to receive live vaccines. Of the children who had not completed MMR dose 1 and 2, 11 (9%) and 13 (10%), respectively, had a medical exemption in place. This was similarly seen in the varicella group, whereby 13 (10%) also had a medical exemption completed. The rationale for medical exemption for majority of patients was not apparent, with only 2 appropriately exempted.
Table 1.
Live vaccination coverage and timeliness in children with 22q11 microdeletion (n = 124) known to the QCH.
| MMR dose 1 | MMR dose 2 | Varicella vaccine | |
|---|---|---|---|
| Complete | 102 (82.3%) | 96 (77.4%) | 82 (66.1%) |
| Not complete | 11 (8.9%) | 15 (12.1%) | 29 (23.4%) |
| Medical exemption | 11 (8.9%) | 13 (10.5%) | 13 (10.5%) |
| On time | 87% | 76% | 83% |
| Delayed | 13% | 24% | 17% |
MMR, measles mumps rubella; QCH, Queensland Children’s Hospital.
Only 26/102 (25%) had T cell subsets tested prior to MMR dose 1, 28/96 (29%) prior to MMR dose 2 and 27/82 (33%) prior to varicella vaccine. Of these patients, two (7%) had a CD4 count of less than 0.5 × 109/l and a CD8 count less than 0.3 × 109/l and received MMR dose 1 and 2 and varicella vaccine. For the majority tetanus and diphtheria serology were not tested prior to proceeding to live vaccinations: 16 (16%) completed serology testing prior to MMR dose 1, 18 (19%) prior to MMR dose 2 and 9 (11%) prior to varicella vaccine. Similarly, minority had either lymphocyte proliferation responses to mitogens or RTE evaluation prior to live vaccination, with 15 (15%) completing function testing prior to MMR dose 1, 14 (14%) prior to MMR dose 2 and 11 (13%) prior to varicella vaccination. Variation in immunological workup prior to live vaccine administration could also be related to late diagnosis with approximately 25% of children presenting to our clinic following a late diagnosis.
Adverse events following immunisation
All children with 22q11 microdeletion received their live vaccinations without subsequent adverse reactions. There were no AEFI notifications reported on the NOCS database.
In the two children with CD4 count less than 0.5 × 109/l and CD8 count less than 0.3 × 109/l who received MMR (dose 1 and 2) and varicella, no adverse reaction was recorded in NOCs or medical records. Likewise, in the seven who did not demonstrate immunity to tetanus and or diphtheria, all received MMR (dose 1 and 2) and varicella vaccination without adverse effect. In the one patient with an abnormal mitogen response who received MMR (dose 1 and 2) and varicella, no adverse reaction was recorded.
Additional pneumococcal coverage
Of the 125 eligible children, 22 (18%) received additional conjugate pneumococcal vaccination (Prevenar 7 or 13); 9 (41%) did not go on to receive their additional dose of polysaccharide pneumococcal vaccine (Pneumovax 23). Of the 103 eligible for Pneumovax 23, only 16 (16%) received it from 4 years of age.
Following revision and implementation of Medical At Risk Immunisation Guideline in August 2018, 55 (40% of the 138 children eligible) received their additional doses of conjugate pneumococcal vaccine (Prevenar 13). Of these, 18 (33%) did not go on to receive their additional dose of Pneumovax 23. Of the 110 children eligible for pneumovax 23, 34 (31%) completed their Pneumovax 23 dose from 4 years of age.
Discussion
The live vaccination rates of children with 22q11 microdeletion have been shown to be varied in the literature, with previous reports reporting suboptimal coverage.6,7 It is reassuring to report that most children were up to date with their live vaccines without significant delay. Most childrens’ immune profile was consistent with mild-to-moderate T cell lymphopenia. Despite this, there were no AEFI notifications in this cohort following live vaccinations. This is supportive of previous publications that patients with 22q11 microdeletion and measurable autologous T cells are not at an increased risk of having an adverse reaction to live immunization.7,10–13
Coverage of additional pneumococcal vaccination was low in this cohort. 22q11 microdeletion without cardiac disease is not currently listed as a high-risk medical condition for invasive pneumococcal disease in the Australian Immunisation Handbook. However, it is known that majority of patients with 22q11 microdeletion have mild-to-moderate immune deficiency, an increased susceptibility to sinopulmonary infections and underlying anatomical dysfunction.2,4,6,7 As a result, we recommend additional pneumococcal vaccination in all children with 22q11 microdeletion.
Immunology workup practices were demonstrated to vary widely prior to live vaccine administration. Current IDSA recommendations for vaccination in the immunocompromised host recommend live vaccines can be safely administered in patients with 22q11 microdeletion if the total T cell (CD3) count is more than 0.5 × 109/l, cytotoxic T cell (CD8) count is more than 0.2 × 109/l, and a normal mitogen response is demonstrated. Those not fulfilling this criteria are recommended to avoid all live vaccines.15 We developed a pathway for immunological investigation and vaccination with the aim of providing further guidance and consistency to clinicians caring for children with 22q11 microdeletion both in the community and hospital system to minimise the significant variation in practice as demonstrated by our study. The guideline also incorporates vaccination recommendations including additional doses of pneumococcal and annual influenza vaccine (Figure 2). These recommendations are based on expert opinion and on the available literature.3,8,13,15,18,19
The observational nature of this study poses several limitations. This is a cohort of patients with complex healthcare needs with multiple healthcare providers that contribute to the significant variations in immunological investigations and vaccination practices noted in our results. Underreporting of AEFI to the NOCS database may have also impacted on the rates of AEFI seen in this cohort. Central pathology Queensland provides a state-wide laboratory service; however, private pathology providers were not contacted for the included patients and may have contributed to the variation seen in immunology workup practices due to underreporting.
Given the high risk of natural infection, it is likely the benefits of immunisations with MMR and varicella vaccine outweigh risks associated with vaccination in patients with mild to moderate immune deficiency.6,7 This is also supported by the low rates of AEFI in this patient cohort. As a result, live vaccines can be considered in children with 22q11 microdeletion despite evidence of mild-to-moderate immunosuppression.
Conclusion
Our study aims to provide consistency in immunology workup practices and vaccination of children with 22q11 microdeletion to minimise the significant variations in practice as demonstrated by our study. It also supports the published findings to date that live vaccination can be safely administered in patients with 22q11 microdeletion despite evidence of mild to moderate immunosuppression.6,9–12 Further studies are required to support guidelines for immunology investigation and guidance for live vaccinations in this cohort of patients.6
Acknowledgments
The authors thank Alberto Pinzon, Consultant Paediatric Immunologist, Queensland Children’s Hospital and Katie Cuneen, Paediatric Advanced Trainee, Queensland Children’s Hospital.
Footnotes
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Conflict of interest: The authors declare that there is no conflict of interest.
ORCID iD: Angela Berkhout  https://orcid.org/0000-0001-6173-7768
https://orcid.org/0000-0001-6173-7768
Contributor Information
Angela Berkhout, The Queensland Children’s Hospital Brisbane, Infection Prevention & Managament, 501 Stanley St, South Brisbane, QLD 4101, Australia; School of Clinical Medicine, The University of Queensland, Brisbane, Queensland, Australia.
Kahn Preece, The John Hunter Children’s Hospital, Newcastle, New South Wales, Australia.
Vanil Varghese, The Queensland Children’s Hospital, Brisbane, Queensland, Australia. School of Clinical Medicine, The University of Queensland, Brisbane, Queensland, Australia.
Vinita Prasad, The Queensland Children’s Hospital, Brisbane, Queensland, Australia. School of Clinical Medicine, The University of Queensland, Brisbane, Queensland, Australia.
Helen Heussler, The Queensland Children’s Hospital, Brisbane, Queensland, Australia. School of Clinical Medicine, The University of Queensland, Brisbane, Queensland, Australia.
Julia Clark, The Queensland Children’s Hospital, Brisbane, Queensland, Australia. School of Clinical Medicine, The University of Queensland, Brisbane, Queensland, Australia.
Sophie C. H. Wen, The Queensland Children’s Hospital, Brisbane, Queensland, Australia. School of Clinical Medicine, The University of Queensland, Brisbane, Queensland, Australia
References
- 1. Bassett A, McDonald-McGinn D, Devriendt K, et al. Practical guidelines for managing patients with 22q11.2 deletion syndrome. J Pediatr 2011; 159: 332–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Shprintzen R. Velo-cardio-facial syndrome: 30 years of study. Dev Disabil Res Rev 2008; 14: 3–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sullivan K. Chromosome 22q11.2 deletion syndrome and DiGeorge syndrome. Immunol Rev 2019; 287: 186–201. [DOI] [PubMed] [Google Scholar]
- 4. Davies EG. Immunodeficiency in DiGeorge syndrome and options for treating cases with complete athymia. Front Immunol 2013; 4: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chinen J, Rosenblatt H, Smith OB, et al. Long term assessment of T cell populations in DiGeorge Syndrome. J Allergy Clin Immunol 2003; 111: 573–579. [DOI] [PubMed] [Google Scholar]
- 6. Hofstetter A, Jakob K, Klein N, et al. Live vaccine use and safety in DiGeorge syndrome. Pediatrics 2014; 133: 946–954. [DOI] [PubMed] [Google Scholar]
- 7. Perez E, Bokszczanin A, McDonald-McGinn D, et al. Safety of live viral vaccines in patients with chromosome 22q11.2 deletion syndrome (DiGeorge syndrome/Velovardiofacial syndrome). Pediatrics 2003; 112: 325–327. [DOI] [PubMed] [Google Scholar]
- 8. Sobh A, Bonilla F. Vaccination in primary immunodeficiency disorders. J Allergy Clin Immunol 2016; 4: 1066–1075. [DOI] [PubMed] [Google Scholar]
- 9. Iroh Tam P-Y, McAllister S. Vaccine responses and immunologic characteristics of pediatric patients with DiGeorge syndrome. Clin Pediatr 2015; 54: 1290–1292. [DOI] [PubMed] [Google Scholar]
- 10. Al-Sukaiti N, Reid B, Lavi S, et al. Safety and efficacy of measles, mumps, and rubella vaccine in patients with DiGeorge syndrome. J Allergy Clin Immunol 2010; 126: 868–869. [DOI] [PubMed] [Google Scholar]
- 11. Azzari C, Gamineri E, Resti M, et al. Safety and immunogenicity of measles-mumps-rubella vaccine in children with congenital immunodeficiency (DiGeorge syndrome). Vaccine 2005; 23: 1668–1671. [DOI] [PubMed] [Google Scholar]
- 12. Moylett E, Wasan A, Noroski L, et al. Live viral vaccines in patients with partial DiGeorge syndrome: clinical experience and cellular immunity. Clinical Immunology 2004; 112: 106–112. [DOI] [PubMed] [Google Scholar]
- 13. Sullivan K, McDonald-McGinn D, Driscoll D, et al. Longnitudinal analysis of lymphocyte function and numbers in the first year of life in chromosome 22q11.2 deletion syndrome (DiGeorge Syndrome/velocardiofacial syndrome). Clin Diagn Lab Immunol 1999; 6: 906–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Miranda M, Martins AT, Carvalho S, et al. Live vaccine in children with DiGeorge/22q11.2 deletion syndrome. Acta Med Port 2019: 514–519. [DOI] [PubMed] [Google Scholar]
- 15. Rubin LG, Levin MJ, Ljungman P, et al. 2013 IDSA clinical practice guidelines for vaccination of the immunocompromised host. Clin Infect Dis 2014; 58: 44–100. [DOI] [PubMed] [Google Scholar]
- 16. Leung JS, Kathleen E, Perelygina L, et al. Prevalence of granulomas in patients with primary immunodeficiency disorders, United States: data from national health care claims and the US immunodeficiency network registry. J Clin Immunol 2018; 38: 717–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Buchbinder D, Hauck F, Albert MH, et al. Rubella virus-associated cutaneous granulomatous disease: a unique complication in immune-deficient patients, not limited to DNA repair disorders. J Clin Immunol 2019; 39: 81–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Australian Technical Advisory Group on Immunisation. Australian Immunisation Handbook, Australian Government De 2018, https://immunisationhandbook.health.gov.au. (2018)[11 November 2019]
- 19. Sullivan K. Live viral vaccines in patients with DiGeorge syndrome. J Clin Immunol 2004; 113: 3. [DOI] [PubMed] [Google Scholar]