Abstract
Epipericardial fat necrosis (EFN), also known as pericardial or mediastinal fat necrosis, has until lately been considered an unusual cause of acute chest pain. Due to increased use of computed tomography (CT) and other imaging techniques, EFN is now believed to be an under-diagnosed cause of acute chest pain. We here present a patient with a short history of acute, left-sided pleuritic chest pain and dyspnoea, with total resolution of symptoms upon few days with nonsteroidal anti-inflammatory drugs (NSAIDs) treatment. Chest X-ray showed a paracardial opacity with ipsilateral pleural effusion, echocardiography revealed features of EFN, and CT scan demonstrated the cardinal lesion of EFN—an ovoid, fat-containing paracardial mass with surrounding inflammatory stranding. There was a near to full radiological resolution in 3 weeks.
Keywords: Epipericardial fat necrosis, mediastinal fat necrosis, pericardial fat necrosis, fat necrosis, chest pain, acute chest pain, pleuritic chest pain
Introduction
Necrosis of the adipose tissue surrounding the heart is regarded as a rare cause of acute chest pain, but several recent reports may indicate that the incidence is higher than previously assumed.
Recent literature most often refers to the condition as epipericardial fat necrosis (EFN), but it is also known as pericardial fat necrosis and mediastinal fat necrosis.1
There seems to be a common consensus in the medical literature that the exact prevalence of EFN is unknown and that EFN often is under-recognized or misdiagnosed.1,2 Today’s understanding of EFN is mostly based on clinical reports containing a single or a limited number of cases. A comprehensive search in English-language medical literature from 2016 yielded only 57 reported cases since the first one in 1957,1 and to our knowledge, the only prevalence-estimate is based on 2 retrospective studies from Brazil demonstrating a prevalence of approximately 2% in patients admitted to the emergency department (ED) with acute atypical chest pain.3,4 Later, the same authors found an increase in presumed EFN diagnosis in their own clinic after disseminating knowledge of the condition and encouraging their radiologists to look for the features of EFN in chest computed tomography (CT) scans taken in the setting of pleuritic chest pain.5
We searched PubMed, Google Scholar, and the data-bases embedded in the publicly funded medical resource Helsebiblioteket.no using different combinations of the terms fat necrosis, epipericardial, pericardial, mediastinal, echocardiography, ultrasound, and echocardiogram as search phrases and identified a limited number of case reports. The diagnosis was mainly based on CT scans. Here, we present a case of EFN with X-ray, CT, echocardiography, and magnetic resonance imaging (MRI).
Case Report
A 22-year-old healthy male was admitted to the ED at Nordland Hospital, Bodø, Norway, with left-sided chest pain intensifying over the last 2 days. At the time of contact with the community emergency primary health system, he felt shortness of breath and was not able to inhale properly due to pain. He had also noticed an increase in resting heart rate. The examining physician found him slightly hypertensive, tachycardic, and tachypnoeic with possibly diminished breath sounds at the affected left side. Point-of-care C-reactive protein (CRP) was 23 mg/L (ref. <5) and an electrocardiogram (ECG) showed sinus rhythm 92 beats per minute, Q-wave in aVF, V3-V6, and peaked T-waves in V2-V6. The patient was referred to a chest X-ray and admitted for further assessment at our ED. The primary suspicion of the referring doctor was pneumothorax.
Upon arrival at hospital, the pain was described as constant ‘stinging’, 7 to 8 on visual analogue 1 to 10 scale (VAS) and worsened severely when laying down and with inspiration, movements which also made the pain radiate to the left shoulder. Apart from this, he did not feel ill, had no cough, fever, or other symptoms. Clinical assessment was normal, and vital measurements (virtually identical to those reported from the referring doctor) are shown in Table 1. CRP and D-dimer were elevated, and venous blood gas revealed a mild, compensated respiratory acidosis as shown in Table 2.
Table 1.
Vital measurements.
| Blood pressure | 159/92 |
| Heart rate, per minute | 102 |
| Respiratory frequency, per minute | 20 |
| Temperature, °C (ear) | 36.6 |
| Peripheral saturation % (without O2) | 97 |
Table 2.
Venous blood sample.
| TnI < 10 (<10) |
| TnI hs < 3 (<47) |
| NT-proBNP 35 ng/l (<85) |
| CRP 31 mg/L (<5) |
| D-dimer 0,8 mg/L (<0.5 mg/L) |
| pH 7.40 (7.35-7.45) |
| pCO2 6.52 kPa (4.70-6.00) |
| 30.3 mmol/L (21.0-27.0) |
| BE 5.4 (−3 to 3) |
| Lactate 0.7 mmol/L (0.5-2.2) |
Abbreviation: CRP, C-reactive protein.
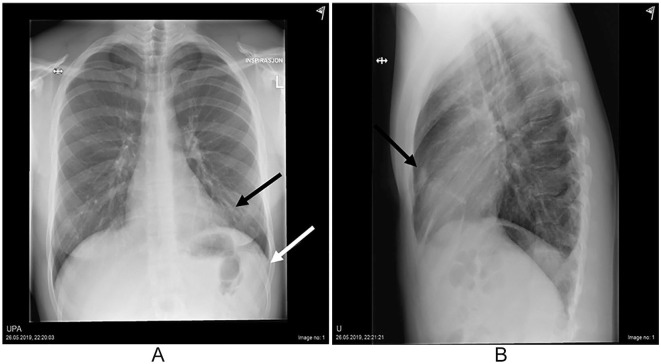
Chest X-ray showed no pneumothorax but a small amount of pleural effusion in the left hemithorax and a subtle ill-defined opacity in the left upper lobe anterior to the heart resulting in loss of the left heart contour on the postero-anterior view (so-called silhouette sign) (Figure 1).
Figure 1.
Opacity in basal part of left lung (black arrow) resulting in diminished left heart border and small amount of pleural effusion (white arrow) (A). Same opacity in sagittal view of chest (B).
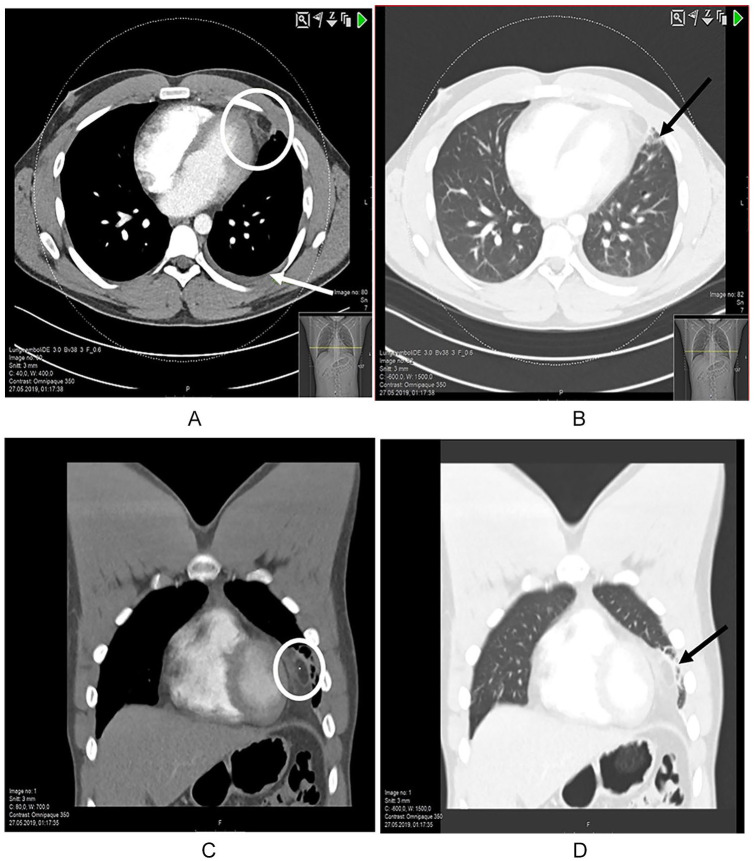
To rule out pulmonary embolism, a chest CT scan was performed. Pulmonary angiogram showed no evidence of embolism, but a focal inflammatory stranding in a pericardial fat layer lateral to the apex of the left ventricle. The inflammation surrounded an ovoid-shaped structure of fat density with striking resemblance to the findings in epiploic appendagitis, a subgroup of intraperitoneal focal fat infarctions.
The finding was accompanied by slight thickening of the adjacent pericardium as well as reactive changes in neighbouring lung parenchyma in terms of a subsegmental atelectasis in lingula and basal parts of the left lung and a small amount of pleural effusion (Figure 2).
Figure 2.
Chest CT scan in axial view with inflammatory stranding around an ovoid structure of fat density (circle), atelectasis in an adjacent part of the left lung and a small amount of left-sided pleural effusion (arrow) (A). Same ovoid structure seen in axial lung window (B) and in coronal views as well (C and D). CT indicates computed tomography.
The radiological findings were consistent with EFN with associated thickening of the adjacent pericardium and associated pleural effusion and atelectasis. Pericarditis was one of the major differentials who should be considered in this situation. The nonspecific X-ray findings did not change the probability regarding pericarditis. However, on the CT exam, one would expect some grade of pericardial effusion and global thickening, or at least more pronounced thickening of pericardium compared to focal changes seen here. EFN was rated as the most probable diagnosis as the CT appearance in total was so typical and almost pathognomonic for this condition.
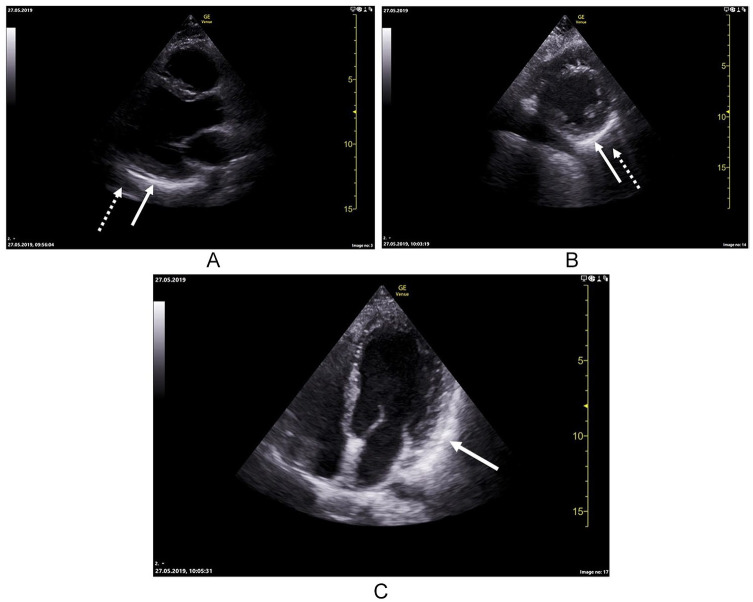
A transthoracic echocardiography (TTE) showed a hypoechogenic area located in front of the left ventricle and possible highly echogenic adjacent pericardium covering the free wall of the left ventricle (Figure 3). There were no other obvious pathological findings. The patient improved rapidly and was discharged from hospital with ibuprofen as needed.
Figure 3.
Parasternal long axis demonstrating highly echogenic pericardium covering the inferolateral wall of the left ventricle (whole arrow) and a hypoechogenic area in front of it (dotted arrow) (A). Parasternal short axis showing the same features as long axis (B). Apical 4-chamber view showing highly echogenic pericardium covering the lateral wall of left ventricle (C).
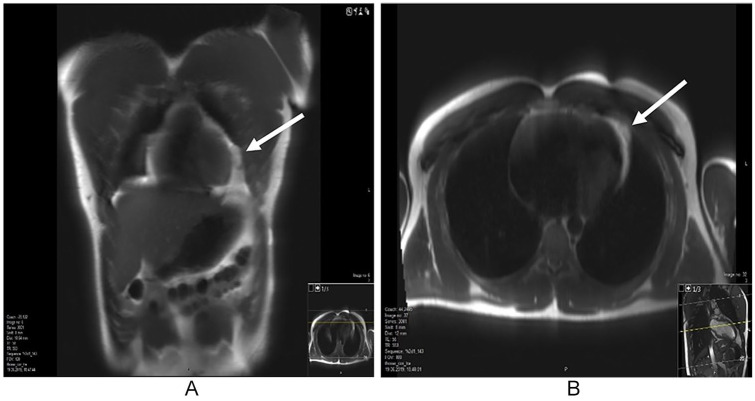
Heart MRI scan performed 3 weeks later showed a normal heart with neither evidence of oedema in the surrounding fat layers, nor pericardial or pleural effusion, just slightly aggravated small vessels in the epicardial fat at the left pericardio-costal angle as the only remnant of inflammation (Figure 4).
Figure 4.
Follow-up study with cardiac MRI 3 weeks after the initial presentation showed slightly coarse pattern of the fat tissue. Shown are coronal (A) and axial (B) T2 views. MRI indicates magnetic resonance imaging.
Discussion
Our case illustrates an archetypical example of EFN.
Presentation is usually left-sided pleuritic chest pain of sudden onset in a healthy person with no obvious triggering event. No age or gender predilection is known to exist.3
Tachycardia, tachypnoea, and diaphoresis may be present, and in some cases, the pain radiates to left shoulder, neck, or back.
Differential diagnoses include pulmonary embolism, pneumothorax, pericarditis, and acute coronary syndrome. In most cases, acute pericarditis will be the most relevant differential diagnosis as clinical presentation can be similar to EFN and neither blood tests nor ECG can definitely distinguish between them.
Slightly elevated D-dimer, CRP, and/or white blood cell count is relatively common in EFN,2,6-10 which also is true for most of its differentials. Echocardiogram is usually normal, but nonspecific ECG changes such as ST-T wave abnormalities are reported.11 In contrast, in patients with acute pericarditis, typical ECG findings are present in approximately 60% of the cases.12
In terms of nonclassical histories, initial presentations with syncope, haemoptysis, and shock-requiring vasopressors have been described.11 A pericardial friction rub and chest wall tenderness can be present.11 A positive ventilation-perfusion scintigraphy13 has also been reported.
Low specificity of symptoms, clinical findings, and blood tests14 makes diagnostic imaging essential.
Chest X-ray is often normal or reveals only a small pleural effusion.2,15,16 A unspecific opacity near the cardiac silhouette, often with ipsilateral pleural effusion, can be seen in later stages of inflammation.16 Differential diagnoses on X-ray include paracardiac fat pad, pericardial cysts, and mediastinal or pulmonary neoplasm.13
A CT-scan has become the key modality in diagnosing EFN. A clear-cut diagnosis is attainable in most cases as EFN has the quite characteristic appearance of a focal fatty necrosis in the mediastinal paracardiac region, featured as an ovoid, encapsulated fatty mass with varying degrees of stranding in surrounding tissue. Such radiological features associated with acute chest pain are highly indicative of EFN.3,17 A diagnostic triad consisting of this encapsulated fatty lesion with dense strands, pericardial thickening, and chest pain was postulated in 200417 and has later been proven valid.3,4
Diagnosis can be challenging if radiological features are misinterpreted or missed due to subtle forms of inflammation18,19 or when presentation is atypical. Furthermore, CT cannot always distinguish between benign and malignant fatty tumours.16,19 In these cases, contrast-enhanced MRI will better demonstrate the presence of inflammation and help rule out malignancies.18,19 As for CT, MRI findings will vary in correlation with the stages of fat necrosis.18,19
Recommendations for treatment and follow-up include a shorter period of nonsteroidal anti-inflammatory drugs (NSAIDs)3,10,14,20 and a follow-up CT scan in 4 to 8 weeks to confirm healing and definitely exclude malignancies.3,9 Most patients are pain free within the first week1,14,17,21 and serial CT has shown partial or full resolution after 2 to 12 weeks.8,9,11,22,23 Relapses are sometimes seen,17,19,21,22 but reports of these are rare and prognosis is usually very good. Surgery may still be indicated in rare cases if diagnostic uncertainties remain or for treatment of persisting, intractable pain.1,13,17,20
The anatomical substrate for EFN is the epipericardial fat pad abutting the cardiophrenic space (eg, the parietal pericardium, the anterior wall of thorax, and the diaphragm).4 Epicardial fat thickness can be evaluated with echocardiography, where the epicardial fat is identified as the echo-free space between the outer wall of the myocardium and the visceral layer of pericardium and its thickness is measured perpendicularly on the free wall of the right ventricle at end-systole in 3 cardiac cycles.24
Two different CT-patterns of EFN have been identified, the most eye-catching being the ovoid fat-lesion with dense stranding (as demonstrated in our case) and the more common the mixed fat-soft tissue mass with little stranding.4 In addition, 3 different locations for the necrotic mass to appear in the cardiophrenic space is reported; in the diaphragmatic fat, in the precordial fat or adherent to the chest wall.4 Pain is always ipsilateral to the lesion.3 EFN predominantly occurs in the left hemithorax,15,17,18,25 although right-sided cases have also been reported.3,11,14,15,20 Small atelectasis and plural lesions can be seen.3,21,26
The aetiology of EFN remains unknown, but it is believed to represent the same entity as the more common various types of intraperitoneal focal fatty necrosis (epiploic appendagitis and omental infarction), fat necrosis in the breast and in subcutaneous tissue as both pathological traits and CT-findings are similar.1,7,17 Major working theories of EFN are necrosis trigged by a Valsalva manoeuvre, torsion of a vascularized fat appendage, or pre-existing abnormalities (lipoma and hamartoma).11,13,21 The latter have been seen in some patients undergoing surgery.1,16,20 Obesity will generally increase the volume of epipericardial fat and was originally suggested as a predisposing factor for EFN. So far, no clear correlation has been observed and the theory is weakened.1,13,21
A Valsalva manoeuvre during straining or heavy lifting will cause increased intrathoracic pressure and rapid changes in intravascular capillary pressure which hypothetically can induce diffuse bleeding with subsequent haemorrhagic necrosis in loosely attached adipose tissue.1,13,16,20 There are no indications that physical activity per se may trigger or cause EFN, but this topic is yet to be explored.
In the few cases performed, TTE in EFN are reported as normal, despite a positive chest CT.3,6,9,10,22 It has been suggested that failure to identify the epipericardial fat pad necrosis in EFN might be due to lung artefacts or the patient being unable to position himself optimally for the examination due to pain.6 An exception was a report where both transthoracic and transoesophageal echocardiography revealed a paracardiac mass with a calcified rim, suggestive of chronic EFN.27
In the EDs, point-of-care ultrasound (POCUS) is rising as a clinical tool allowing immediate patient-care decisions, often using a linear probe assessing symptoms from the chest. Chest wall sonography in the emergency setting of chest pain after ruling out myocardial infarction has been reported.26 Here, the researchers found particular sonographic findings in 4 patients, consisting of hyperechoic nodules in the epipericardial fat surrounded by a hypoechoic halo and increased echogenicity of adjacent adipose tissue. Findings were consistent with EFN on CT and sonographically like other forms of fatty necrosis. As POCUS is an emerging field in medicine and recently included in the Norwegian residence programme, we believe that cases of EFN will be incidentally detected by chest wall ultrasound in the EDs and could result in more ultrasound-based findings positive for EFN, as shown in our patient.
No age predilection is known to exist, but the typical patient has so far been characterized as middle-aged with presentation at 43 to 50 years.3,4,11,14,16 The latest articles in our search (2016-2019) reported in sum slightly younger patients2,9,10,22,23,25,26,28,29 resulting in a mean age of 39.4 years. Lately, reports of EFN in the paediatric population have also emerged.6,30,31
Regarding prevalence, to our knowledge, only 2 Brazilian studies have addressed this question thoroughly. The first study was performed in 2014, with an even more detailed follow-up in 2016.3,4 In sum, more than 11 000 chest CT scans taken in the researchers ED were retrospectively reviewed having EFN in mind.3-5 Around 426 and 926 scans were taken in the setting of acute atypical chest pain in 2014 and 2016, respectively. Among these, 11 and 20 scans were found positive for EFN, yielding an EFN-prevalence of 2.58% and 2.15%. No case of EFN was found in scans taken without record of pain, and every diagnosed case suffered from pleuritic pain. In the 2014 material, only 3 patients (27%) were diagnosed correctly with EFN. After an educational intervention among their radiologists, the authors found the number of EFN cases more than doubled.5
These results caused the authors to conclude that EFN is an underdiagnosed and overlooked condition in the EDs owing to unfamiliarity of the diagnosis amid physicians and radiologists.
Conclusions
The purpose of our case report is to highlight the diagnosis of EFN, as this is probably overlooked among emergency physicians. Our patient demonstrates every clinico-radiological classical trait of EFN and is a good example for educational purposes.
We also report a positive echocardiography, which is not described in acute EFN earlier. It is important to recognize the sonographic features to give the correct patient care.
Our literature search indicates that a number of patients with EFN are undiagnosed if further CT evaluation is not performed if serious causes of chest pain are ruled out. It also leaves the impression that recent case reports more often involve younger patients, including children.
Acknowledgments
The authors thank Petter Myren, MD, for initial assessment of the patient and later providing general support in the writing process and Silje Kariin Rånes Edvardsen, MD, for contributing to patient workup.
Footnotes
Funding:The author(s) received no financial support for the research, authorship, and/or publication of this article.
Declaration of conflicting interests:The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: S.G.M. primarily designed and drafted the paper. P.B. wrote the radiologic content of the case report and attained the images. K.T.L. revised the paper. All authors have read and approved the final version.
Disclosure and Ethics: The patient have read the article, images included, and provided written informed consent for its publication.
ORCID iDs: Synnøve Gjelsten Mortensen  https://orcid.org/0000-0002-1529-2865
https://orcid.org/0000-0002-1529-2865
Knut Tore Lappegård  https://orcid.org/0000-0002-9976-7791
https://orcid.org/0000-0002-9976-7791
References
- 1. Fred HL. Invited commentary: epipericardial fat necrosis: a unique clinicoradiologic disease. Proc (Bayl Univ Med Cent). 2016;29:434-435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Nguyen DN, Tran CD, Rudkin SM, Mueller JS, Hartman MS. Epipericardial fat necrosis: uncommon cause of acute pleuritic chest pain. Radiol Case Rep. 2018;13:1276-1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. de Souza Giassi K, Costa AN, Bachion GH, et al. Epipericardial fat necrosis: an underdiagnosed condition. Br J Radiol. 2014;87:20140118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. de Souza Giassi K, Costa AN, Bachion GH, Kairalla RA, Filho JRP. Epipericardial fat necrosis: who should be a candidate? Am J Roentgenol. 2016;207:773-777. [DOI] [PubMed] [Google Scholar]
- 5. de Souza Giassi K, Costa AN, Kairalla RA, Parga Filho., JR. Epipericardial fat necrosis: increasing the rate of diagnosis by disseminating knowledge within a single institution. Radiol Bras. 2018;51:62-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bolourchi M, Renjen P, Kovanlikaya A, et al. Epipericardial fat pad necrosis – a rare cause of chest pain in an adolescent [published online ahead of print December 21, 2018]. Pediatr Emerg Care. doi: 10.1097/PEC.0000000000001716. [DOI] [PubMed] [Google Scholar]
- 7. Coulier B. Epipericardic fat necrosis: CT diagnosis. JBR-BTR. 2010;93:317-318. [DOI] [PubMed] [Google Scholar]
- 8. Shah AH, Bogale V, Hurst D, dePrisco G. Epipericardial fat necrosis as a cause of acute chest pain. Proc (Bayl Univ Med Cent). 2016;29:432-433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Simon C, Lorek C, Boularan C, Fontaine-Delaruelle C. A 35-year-old woman with acute pleuritic chest pain and an unusual mediastinal opacity. Chest. 2019;155:e17-e20. [DOI] [PubMed] [Google Scholar]
- 10. Friedman Y, Gayer G, Margolin M, Kneller A, Mouallem M. Epipericardial fat necrosis. Isr Med Assoc J. 2018;5:327-328. [PubMed] [Google Scholar]
- 11. Baig A, Campbell B, Russell M, Singh J, Borra S. Epicardial fat necrosis: an uncommon etiology of chest pain. Cardiol J. 2012;19:424-428. [DOI] [PubMed] [Google Scholar]
- 12. Adler Y, Charron P, Imazio M, et al. 2015 ESC guidelines for the diagnosis and management of pericardial diseases: the Task Force for the Diagnosis and Management of Pericardial Diseases of the European Society of Cardiology (ESC)Endorsed by: the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J. 2015;36:2921-2964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lacasse M-C, Prenovault J, Lavoie A, Chartrand-Lefebvre C. Pericardial fat necrosis presenting as acute pleuritic chest pain. J Emerg Med. 2013;44:e269-e271. [DOI] [PubMed] [Google Scholar]
- 14. Carvalho NB, de Paula Lopes e Silva N, Pereira PPN, Morganti AV, Silva SL, Rodrigues AS. Epipericardial fat necrosis. An important differential diagnosis of chest pain. Int J Cardiovasc Sci. 2017;30:91-94. [Google Scholar]
- 15. de Souza Giassi K, Costa AN, Apanavicius A, Bachion GH, Musolino RS, Kairalla RA. Epipericardial fat necrosis: an unusual cause of chest pain. J Bras Pneumol. 2013;39:627-629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fred HL. Pericardial fat necrosis: a review and update. Tex Heart Inst J. 2010;37:82-84. [PMC free article] [PubMed] [Google Scholar]
- 17. Pineda V, Caceres J, Andreu J, Vilar J, Domingo ML. Epipericardial fat necrosis: radiologic diagnosis and follow-up. Am J Roentgenol. 2005;185:1234-1236. [DOI] [PubMed] [Google Scholar]
- 18. Unal E, Karcaaltincaba M, Akpinar E, Ariyurek OM. The imaging appearances of various pericardial disorders. Insights Imaging. 2019;10:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lee HH, Ryu DS, Jung SS, Jung SM, Choi SJ, Shin DH. MRI findings of pericardial fat necrosis: case report. Korean J Radiol. 2011;12:390-394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bhatt MY, Martínez-Jiménez S, Rosado-de-Christenson ML, Watson KR, Walker CM, Kunin JR. Imaging manifestations of mediastinal fat necrosis. Case Rep Radiol. 2013;2013:323579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Runge T, Greganti MA. Epipericardial fat necrosis – a rare cause of pleuritic chest pain: case report and review of the literature. Arch Med Sci. 2011;7:337-341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ali A, Kabeer A, Menon A, et al. A rare cause of dyspnea: pericardial epiploic appendigitis. Am J Resp Crit Care. 2019;199:A3197. [Google Scholar]
- 23. Ferretti GR, Rigaud D. Acute chest pain related to pericardial fat necrosis. Can Respir J. 2016;2016:1948325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Iacobellis G, Willens HJ. Echocardiographic epicardial fat: a review of research and clinical applications. J Am Soc Echocardiogr. 2009;22:1311-1319. [DOI] [PubMed] [Google Scholar]
- 25. Mohamed Hoesein FA, Swaans M, van Es HW. A 39-year-old otherwise healthy female with acute chest pain. Heart. 2016;102:540. [DOI] [PubMed] [Google Scholar]
- 26. Diaz J, Alegria J, Perez D, Medina C. Epipericardial fat necrosis: sonographic findings and their correlation with computed tomography. J Ultrasound Med. 2016;35:2279-2283. [DOI] [PubMed] [Google Scholar]
- 27. Lee BY, Song KS. Calcified chronic pericardial fat necrosis in localized lipomatosis of pericardium. Am J Roentgenol. 2007;188:W21-W24. [DOI] [PubMed] [Google Scholar]
- 28. Cho S, Kubota K, Inoue Y, et al. 胸痛で救急外来を受診したepipericardial fat necrosisの1例 [Epipericardial fat necrosis with chest pain in the emergency department: a case report]. Nihon Kyukyu Igakukai Zasshi/J Jpn Assoc Acute Med. 2019;30:920-925. [Google Scholar]
- 29. Celikkanat S, Hamcan S, Bozlar U, Tasar M. Epipericardial fat necrosis clinically mimicking pulmonary embolism: computed tomographic angiography findings. Am J Emerg Med. 2016;34:2056.e2055-2056.e2056. [DOI] [PubMed] [Google Scholar]
- 30. Aiga S, Hosoya Y, Nozaki T, Matsusako M. Epipericardial fat necrosis: rare cause of chest pain in children. Pediatr Int. 2018;60:767-768. [DOI] [PubMed] [Google Scholar]
- 31. Artunduaga M, Fuqua BL, Pierry C, Soto Giordani GA, Roman-Colon AM. Imaging diagnosis of epipericardial fat necrosis in children. Pediatr Radiol. 2020;50:285-288. [DOI] [PubMed] [Google Scholar]