Abstract
Increased bacterial translocation (BT) across the gut barrier due to greater intestinal permeability (IP) is seen across a range of conditions, including alcohol-related liver disease (ArLD). The phenomenon of BT may contribute to both the pathogenesis and the development of complications in ArLD. There are a number of methods available to assess IP and in this review we look at their various advantages and limitations. The knowledge around BT and IP in ArLD is also reviewed, as well as the therapeutic strategies currently in use and in development.
Keywords: alcohol-related liver disease, bacterial translocation, cirrhosis, gut barrier, intestinal permeability
Introduction
Liver disease is a significant burden on global healthcare with approximately 2 million attributable deaths worldwide per year.1 Over 75 million people globally have been diagnosed with alcohol-use disorder, which puts them at risk of alcohol-related liver disease (ArLD). Studies have shown that only around a third of those consuming harmful amounts of alcohol will develop significant fibrosis, and 1 in 10 will develop cirrhosis.2,3 The pathogenesis of ArLD is multifactorial and is influenced by individual metabolic, genetic and comorbidity variables as well as environmental factors4 One of these causative mechanisms may be the increased passage of bacteria or bacterial products into the systemic circulation – a process known as bacterial translocation (BT) – due to increased permeability of the intestinal barrier.5 An increase in bacterial products entering the liver via the portal circulation with an increase in pro-inflammatory cytokine release from Kupffer cell stimulation leads to further hepatocellular injury. Assessment of intestinal permeability (IP) can be performed by direct functional assessment of the intestinal barrier using non-absorbable probes, usually oligosaccharides. Alternatively, surrogate markers of barrier function can be measured including systemic biomarkers or bacterial products. Identification of this pathway in the development of ArLD opens up the possibility of therapeutic intervention. In this article, we review the current knowledge around BT and IP in ArLD, including methods of assessment and therapeutic intervention that are in clinical use or under development. The therapeutic strategies discussed include manipulation of the gut microbiome through antibiotic decontamination, use of probiotics and faecal microbiota transplantation (FMT).
The gut microbiome and bacterial translocation
The gut microbiome consists of approximately 1014 microorganisms with up to 1000 different microbial species.6 This multitude of organisms performs many roles, including metabolising indigestible compounds, supplying vitamins, protecting the gut against pathogen colonisation and contributing to the host immune system.7 The majority of bacteria that reside in the human gut belong to either the Bacteroidetes or Firmicutes phyla.
BT is a physiological process that has been detected in up to 5% of the population with microbiological methods available at that time and plays a part in maintaining host immunity by presenting small amounts of bacteria and bacterial components to the reticuloendothelial system in the liver.8 It was first described in 1979 by Berg and colleagues. They cultured viable enteric bacteria, predominantly Escherichia coli and lactobacilli, from the mesenteric lymph nodes (MLN) of mice.9 This concept has now been extended to include the passage of non-viable bacterial products and fragments.10 These products include DNA; lipopolysaccharide (LPS): large molecules found on the outer membrane of Gram-negative bacteria; peptidoglycan: polymer that plays a key structural role in Gram-positive bacteria cell walls; lipoteichoic acid (LTA): a key constituent of Gram-positive bacterial cell walls; and flagellin: the protein that forms the main component of a bacterial flagellum.
By contrast, ‘pathological BT’ has been defined as a ‘sustained increase in quantity (rate and/or degree) of BT.10 Studies have shown that pathological BT occurs in conditions such as intestinal obstruction, inflammatory bowel disease (IBD), malignancy, Alzheimer’s disease, depression and liver disease.11,12 It has been shown to occur in up to 25–30% of patients with Child-C cirrhosis.13
The Enterobacteriaceae family (E. coli, Klebsiella spp.), enterococci and streptococci spp. are the most prevalent organisms seen in BT in humans.14 A study in mice demonstrated that Gram-negative bacteria translocated in large numbers to the MLN whereas Gram-positive and obligate anaerobic bacteria translocated at much lower levels.15 Another study also showed that some species of E. coli are able to translocate more effectively than others.16 This difference is thought to be due to an enhanced ability to adhere to the intestinal mucosa.
Healthy intestinal barrier
The intestinal barrier between the gut lumen and the systemic circulation is comprised of a number of entities that act to allow controlled absorption of nutrients while maintaining a barrier to the majority of enteric pathogens including bacteria, fungi and viruses.
The mucus barrier is subdivided into two layers: an inner sterile layer attached to the epithelium and a looser outer layer that is colonised by bacteria. The latter is composed of glycoproteins known as mucins (Muc), which are primarily made up of O-linked oligosaccharides (O-glycans). These mucins are secreted by intestinal goblet cells and either form a gel (Muc2, Muc5AC and Muc6) or remain as membrane-bound glycoproteins (Muc1, Muc3-4, Muc12-13 and Muc17.17,18 Muc2 is the most abundant mucin in the small intestine and colon.17 The mucus layer varies in thickness throughout the gut, with the thickest layer seen in the colon, which also has the highest density of microbes.18 It also plays an important role in maintaining the integrity of the intestinal barrier and protection from microbial pathogens. Several experiments have shown the importance of the mucosal layer, and of Muc2 in particular, against infectious diseases. Muc2-deficient mice were shown to be more susceptible to infection by Salmonella spp. and Citrobacter rodentium.19,20
There are a group of proteins, including defensins and regenerating islet-derived proteins, known as antimicrobial proteins (AMPs) that also play a role in mucosal defence against bacteria. They are secreted into the gut by Paneth cells at the bottom of each intestinal crypt. These Paneth cells are directly stimulated by bacteria and bacterial products, such as LPS. These are known collectively as pathogen-associated molecular patterns (PAMPs) and they activate toll-like receptors on the surface of Paneth cells.20 The importance of these cells in barrier defence has been demonstrated by showing an increase in BT to MLNs in a study of Paneth cell-deficient mice,21 which corresponds with previous mice studies showing that Paneth cell α-defensins protect against pathogenic Salmonella strains.22
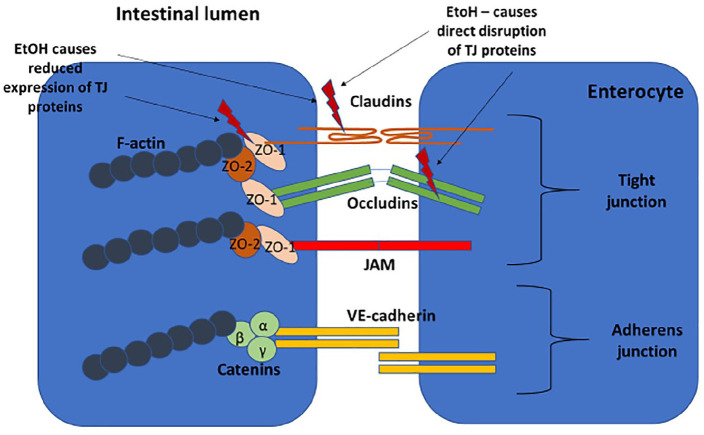
The spaces between intestinal epithelial cells are composed of a cluster of proteins that make up the apical junction complex (Figure 1). This structure comprises the tight junction (TJ) – made up of the transmembrane proteins occludin, claudin and junctional adhesion molecules – as well as the cytoplasmic components and zonula occludens proteins that connect the transmembrane proteins to the F-actin in the cytoskeleton.23 The adherens junction sits below the TJ and consists of the transmembrane protein E-cadherin and cytoplasmic catenin proteins that connect to the F-actin.24 Contraction of actomyosin microfilaments in the cytoplasm opens the intercellular space.
Figure 1.
Apical junctional complex: TJ consisting of transmembrane proteins (claudin, occludin, JAM) and cytoplasmic molecules (ZO-1, ZO-2). Adherens junction sits below the TJ and also consists of transmembrane (VE-cadherin) and cytoplasmic (catenin) component proteins. The cytoplasmic molecules connect to F-actin, which contracts to open the paracellular space.
JAM, junctional adhesional molecule; TJ, tight junction; VE-cadherin, vascular endothelial cadherin; ZO-1, ZO-2, zonula-occludens 1 and 2.
Molecules cross the intestinal barrier via one of two pathways: transcellular and paracellular. Transcellular transport entails passage of molecules through cells and takes place via simple passive diffusion, active transport via ATP-driven efflux pumps and endocytosis. These processes mediate the transport of high-molecular-weight antigens (>600 Da) as well as food proteins. Antigens can also cross the epithelium through specialised microfold (M) cells located in Peyer’s patches, which are located predominantly in the small intestine. Passage between the epithelial cells is known as paracellular permeability and is tightly regulated by the apical junction complex.25 This facilitates the transport of small molecules, solutes and ions while restricting the passage of larger molecules such as bacteria and smaller bacterial products such as LPS.26
Mechanisms of damage to gut barrier in ArLD
Increased intestinal permeability in ArLD
Alcohol has a direct toxic effect on the intestinal mucosa in both acute and chronic consumption. Acute ingestion of high levels of alcohol causes epithelial cell damage and death in mice.27 There is limited data on the amount of alcohol needed to cause increased IP. A study of patients with alcohol dependence found increased IP (as measured by 51Cr-EDTA) in a subset of these patients but there was no correlation with the amount of alcohol consumed.28 Experiments on Caco-2 monolayers have shown that alcohol directly disrupts TJ proteins and reduces expression of ZO-1 and claudin-1.29 Alcohol is metabolised to acetaldehyde by alcohol dehydrogenase (expressed on epithelial cells and produced by the microbiota) and this has also been shown to disrupt the TJs in Caco-2 cell monolayers.30 Both animal models and studies in humans have shown an increase in the mucus layer in response to alcohol.31 This increased thickness may limit the antimicrobial properties of the mucus and thus increase BT.
Structural and functional changes have also been documented in patients with cirrhosis that are thought to reduce intestinal mucosal integrity. One study showed significantly reduced expression of the TJ proteins occludin and claudin-1 in patients with cirrhosis compared with healthy controls, with greater reduction in protein expression in patients with decompensated compared with compensated cirrhosis.32
A number of studies have reported changes in intestinal microcirculation in patients with portal hypertension, which has been linked to mucosal congestion, oedema and dilatation of intercellular spaces.26,27 These structural changes may contribute to altered mucosal integrity leading to increased IP, but definitive evidence of this potential mechanism is awaited.33
The disruption of circadian rhythms by alcohol and the subsequent disturbance of gut barrier function has also been suggested as a mechanism for increased IP. In heavy alcohol consumption, alcohol is also metabolised by the cytochrome p450 enzyme Cyp2e1, which produces reactive oxygen species and other products that induce oxidative stress.34 Cyp2e1 is also expressed on intestinal epithelial cells and these Cy2e1-generated products may contribute to increased IP by direct disruption of the intestinal epithelium or oxidative stress-induced cellular signalling which alters TJ protein regulation.35 A study on Caco-2 cells showed that alcohol exposure increased oxidative stress and caused monolayer dysfunction, as measured by reduced transepithelial resistance as well as an increase in the levels of circadian clock proteins PER2 and CLOCK.36 These effects were not seen when the monolayers were pre-treated with an antioxidant scavenger.
Changes in the intestinal microbiota community in ArLD
The majority of the studies in cirrhosis have focussed on small intestinal bacterial overgrowth (SIBO). SIBO is characterised by an increase in the number and/or abnormal type of bacteria in the small intestine with >105 colony-forming units (CFUs) of bacteria cultured from jejunal aspirates the traditional gold standard of diagnosis, although this test has largely been replaced by breath testing.37
Alcohol use also predisposes patients to developing SIBO. A retrospective study of 196 patients found moderate alcohol to be a strong risk factor for developing SIBO with a prevalence of 58% compared with 38.9% of abstainers (p = 0.008).38 This risk has also been shown in animal models, which revealed an altered microbiota in response to alcohol with a decrease in Firmicutes and an increase in Bacteroidetes.39 A recent review of studies in humans found that, overall, all patients with cirrhosis, including those who drink alcohol, had an increase in Gammaproteobacteria and bacilli, whereas only those who drank showed a depletion of clostridia.40
Altered intestinal motility may also play a role in the pathogenesis of SIBO in cirrhosis by leading to delayed intestinal transit time, which predisposes patients to developing SIBO. A study of proximal small bowel manometry showed significantly prolonged intestinal motor complexes in patients with cirrhosis compared with healthy controls.41 Further studies have shown delayed intestinal transit in patients with cirrhosis and have correlated it with increasing severity of liver disease.42,43 Lichtman and colleagues developed an animal model to show a link between induced SIBO and hepatic inflammation in rats.44 They showed significantly increased hepatic injury and mortality in rats with SIBO from self-filling blind jejunal loops compared with those with self-emptying blind loops.
A recent meta-analysis of studies of SIBO in cirrhosis showed an overall prevalence of 40.8% compared with 10.7% in healthy controls.45 There was no significant difference in prevalence of SIBO amongst patients according to aetiology of cirrhosis. The prevalence of SIBO according to severity of liver disease was analysed in 12 studies that found that the prevalence was higher in decompensated cirrhosis (CTP B and C) compared with compensated (CTP A) and higher in CTP Class C than Class B.
Two studies within a recent meta-analysis looked at BT in conjunction with SIBO in cirrhosis. They found that SIBO was an independent risk factor for BT, with 31.1% of patients with cirrhosis and SIBO having systemic bacterial DNA compared with only 4.8% of patients with cirrhosis and no SIBO.46,47 This correlates with previous animal models that showed a positive correlation between BT and SIBO. However, as up to 50% of animals with SIBO did not subsequently have BT, this work suggested that SIBO is only one of many mechanisms that lead to BT.48
A recent review of the literature on the changes to the intestinal microbiota in alcoholic liver disease concluded that the alterations in gut microbiota were associated with increased IP and gut barrier breakdown.49 However, as the authors point out, neither the causal relationship nor the full clinical implications of these phenomena have been fully established and further studies are required.
Immune dysfunction in ArLD and its role in BT
In cirrhosis, there are a number of changes to almost all parts of the immune system, which not only reduce its capability of dealing with BT but also contribute to the intestinal barrier damage.50 There is significant dysfunction of the reticuloendothelial system as well as increased portosytemic shunting, which leads to reduced clearance of BT.51,52 There is also reduced chemotaxis, bacterial phagocytosis and monocyte activation in cirrhosis compared with controls.53
The intestinal immune system, comprising the gut-associated lymphoid tissue (GALT) as well as parts of the adaptive and innate immune systems, is also affected in cirrhosis. The increase in BT seen in cirrhosis has been associated with an increase in the number of activated inflammatory cells such as monocytes, dendritic cells and T cells.54–56 These cells produce greater amounts of pro-inflammatory cytokines (such as TNF-α, IFN-γ, IL-6) within the intestinal mucosa causing increased intestinal inflammation.54–56 Studies in animal models of cirrhosis have also shown reduced production of antimicrobial molecules from Paneth cells including α-defensins and RegIII proteins, which are involved in gut microbial homeostasis.39,57
Evidence that this pro-inflammatory intestinal milieu leads to increased IP, and thus further BT, comes from a study of patients with non-alcoholic fatty liver disease (NAFLD) and ArLD. Activated macrophages in the intestinal mucosa correlated with levels of lipopolysaccharide binding protein (LBP) and LPS in patients with decompensated cirrhosis.58 There was also altered TJ protein expression with decreased ZO-1 expression in decompensated cirrhosis.
Clinical consequences of BT in ArLD
Bacterial infections in patients with ArLD represent a significant burden in terms of morbidity and mortality.51,59 The commonest infections in patients with cirrhosis are spontaneous bacterial peritonitis (SBP), urinary tract infections, soft tissue infections and pneumonia.40 The increased translocation of pathogenic bacterial strains from the gut to the systemic system is thought to be one of the causative factors behind these infections (see Figure 2). Further evidence for the importance of BT in the development of infection in patients with cirrhosis was demonstrated in a study where fluorescently labelled E. coli was orally administered to rats with cirrhosis. The labelled bacteria were shown to translocate not just to the MLNs but also to the ascitic fluid,57 thus showing a model for developing SBP.
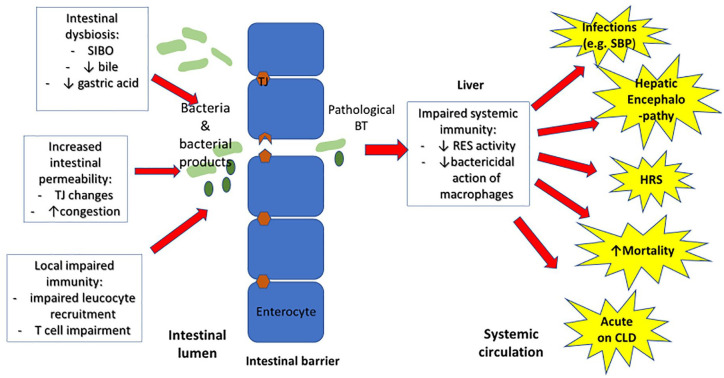
Figure 2.
Mechanisms and consequences of BT in liver disease: changes to the intestinal microbiota, intestinal permeability and local immune responses lead to increased BT. In combination with reduced systemic immunity in the liver (reduced RES and macrophage activity), these changes may lead to complications of liver disease including increased infections such as SBP, acute decompensation of cirrhosis, HRS, HE and increased mortality.
BT, bacterial translocation; CLD, chronic liver disease; HE, hepatic encephalopathy; HRS, hepatorenal syndrome; RES, reticuloendothelial system; SBP, spontaneous bacterial peritonitis; SIBO, small intestinal bacterial overgrowth.
There have been several association studies linking increased gut permeability and BT with liver disease in both human and animal models.60,61 An early study in patients with ArLD showed a positive association between endotoxin (LPS) levels and severity of ArLD.62 Alcohol fed to rats with liver disease leads to high levels of endotoxin and also correlates with the severity of liver disease.63 It is also established that alcohol in combination with endotoxin leads to more severe liver damage than either factor alone through damaging pro-inflammatory processes.64
There is also some evidence from animal models where oral administration of non-absorbable antibiotics or lactobacillus reduces endotoxin levels and hepatic injury in alcohol-fed rats.65,66 A study by Keshavarzian and co-workers indicates a causal role for BT by showing that alcohol-induced increases in gut permeability and subsequent BT precede the development of alcoholic steatohepatitis in rats.67
One of the most compelling causative arguments linking BT with liver disease has been a study by Llopis and colleagues, who looked at patients with acute alcoholic hepatitis (AAH). AAH is one of the most severe forms of ArLD with a 28-day mortality of nearly 20%.68 This study showed that patients with AAH had a specific bacterial community that was not present in patients with a similar alcohol intake but without AAH. Furthermore, this specific microbiota composition from patients with severe AAH, when transplanted into mice, was able to induce a much more severe pattern of liver inflammation compared with mice transplanted with non-AAH microbiota.69
Further evidence for BT-induced liver disease comes from a recent paper that identified cytolysin, a specific exotoxin secreted by Enterococcus faecalis, as a causative factor of hepatocyte inflammation and death in severe AAH.70 AAH patients with cytolysin-positive E. faecalis had an increased mortality as well as increased severity of liver inflammation. Mice transplanted with cytolysin-positive stool developed more severe liver injury, steatosis and fibrosis than those mice colonised with cytolysin-negative stool.
The influence of the gut microbiota and BT on the brain was studied by Leclercq and co-workers who showed that alcohol-dependent patients who had increased gut permeability (as measured by 51Cr-EDTA vide infra) not only had altered gut microbiota but this change was associated with higher scores of anxiety, alcohol craving and depression.28 This measured state implies a role for the microbiota and the gut-brain axis in modulating behaviour such as alcohol-dependence.
Methods to assess intestinal permeability
Numerous methods are available to assess IP – both directly and indirectly – these are summarised in Table 1.
Table 1.
Advantages and disadvantages of methods of assessing IP.
| Method | Pros | Cons |
|---|---|---|
| Direct measures of IP | ||
| Dual sugar probes (e.g. lactulose/mannitol) | Gold-standard method Use of two sugars controls for non-mucosal factors (altered renal function/gastric emptying/intestinal transit) |
Metabolised by colonic bacteria – valid assessment of small bowel IP only Large volume to drink for unwell patients Time-consuming experiment |
| 51Cr-EDTA, PEG | Not metabolised by colonic bacteria – assess whole intestine | Single probe |
| FITC-dextran | Measured from whole blood – urine collection not needed | Single probe Fluorescein has a similar fluorescence to bilirubin. |
| Transcutaneous fluorescence | Non-invasive – no collection of blood or urine samples | Technique under development – not fully validated in humans |
| Indirect measures of IP | ||
| Serum LPS | Easily available assay | Short half-life |
| Serum LPS-binding protein | Longer half-life | Acute phase protein – increased in infective episodes |
| Bacterial cultures | Gold-standard method | Poor sensitivity Lag time of several days to grow bacteria Fastidious organisms difficult to culture |
| Serum bacterial DNA | PCR technique much more sensitive than cultures Quicker technique than culture |
Limited data linking bDNA with increased IP |
| Intestinal fatty-acid binding protein | Easily available assay run on serum sample | Data links iFABP to epithelial damage rather than increased IP |
| Zonulin | Correlation between increased IP as measured by dual sugar probe | Questions over assay validity |
bDNA, bacterial DNA; FITC-dextran, fluorescein isothiocyanate conjugated dextran (a fluorescently labelled inert polysaccharide); IP, intestinal permeability; LPS, lipopolysaccharide; PCR, polymerase chain reaction; PEG, polyethylene glycol.
Direct measures of intestinal permeability
Single probe measurement of IP is performed by quantifying the excretion into urine of a non-metabolised probe that passes across the intestinal barrier. A probe such as lactulose (342 Da) crosses the intestinal barrier via the paracellular route and, in healthy individuals, only a very small amount should cross into the systemic system to be excreted in the urine. Alternative probe molecules can be used; these include polyethylene glycol (PEG) and 51Cr-labelled ethylenediaminetetraacetic acid (EDTA). These are neither produced endogenously nor metabolised by colonic bacteria, and thus may provide a more accurate picture of whole intestinal permeability compared with oligosaccharides, which are metabolised by colonic bacteria.7151Cr-EDTA crosses the intestinal barrier paracellularly, and its excretion in urine is thought to represent TJ disruption,72 but it has also been shown to be affected by both renal function and tissue distribution.73 PEG is available in multiple different molecular weights that can be administered simultaneously to improve sensitivity. Importantly, using a single probe to measure IP means that the measurement is susceptible to pre-mucosal factors such as altered gastric emptying or intestinal transit and renal clearance.74
The use of two probes was therefore proposed in order to mitigate for these non-mucosal factors.75 The current gold-standard method of direct assessment of IP involves ingestion of two sugars, commonly lactulose and mannitol, followed by collection of urine over 24 h. Increasingly 13C mannitol or rhamnose is used to avoid ‘contamination’ of the test by inadvertent mannitol consumption in food or medication, which can affect its baseline excretion. The smaller molecule, usually mannitol (182Da), crosses the intestinal barrier freely via the transcellular pathway and acts as a control. Both lactulose and mannitol are thought to be affected in the same way by factors such as dysbiosis, altered motility, metabolism by colonic bacteria and renal function.71 Analysis is performed by liquid chromatography plus mass spectrometry or high-pressure liquid chromatography alone.26
The ratio of excreted lactulose to mannitol is taken as an indicator of the permeability of the small intestinal barrier via the paracellular route. The optimal time period for urine collection to minimise individual subject variation and to evaluate the small intestine has been shown to be 2.5– 4 h post ingestion.76
Increased IP, as measured by dual sugar probes, has been demonstrated in patients with cirrhosis, where higher IP correlates with increasing severity of liver disease.77,78 This was also shown by Vogt and colleagues; however, they found that IP was not a predictor of infection free-survival or survival overall.79
Dextran molecules have also been used to assess IP. Dextrans are inert polysaccharides available in varying sizes (3 kDa to 2000 kDa) that can be conjugated to fluorophores. Following oral administration of fluorescently labelled dextrans, blood, urine or tissue samples (typically collected 1–4 h after administration) can be analysed by a fluorimeter or microscope to assess the degree to which the probe has passed the intestinal barrier. While this approach is not yet approved for clinical use, fluorescein isothiocyanate conjugated dextran (FITC-dextran) has been used in research studies for this purpose in both human and animal permeability studies.80–82
Another fluorescence-based approach is also under development to allow non-invasive assessment of IP without the need for urine/blood sample collection. Dorshow and colleagues reported quantification of IP in rats based on transcutaneous detection of the fluorescence signal from two orally administered fluorescent dyes (MB-401 and MB-302 – fluorophores with comparable molar masses to lactulose and mannitol). In this study, fluorescence was detected using a non-invasive fibre-optic probe (removing the requirement to collect urine or blood samples), with measurements made through the ears of the anaesthetised rats.83 A similar method is currently being deployed by our group in a first-in-human trial to assess transcutaneous fluorescence spectroscopy as a tool for clinical assessment of IP.84 This trial is focussed primarily on the use of fluorescein as the fluorescent contrast agent, which is used widely in the clinic for other diagnostic procedures. In addition, this technique has the advantages of providing automated data collection and analysis, potentially allowing faster, more flexible and cheaper diagnoses when compared against the alternative approaches discussed previously.
Indeed, most of the previously mentioned tests are time-consuming and there are other limitations as well, including the lack of control probe for tests other than lactulose/mannitol. The subject is required to fast overnight before ingesting the sugar probe(s) which are usually dissolved in water (e.g. 5 g lactulose, 2 g mannitol in 100 ml water) and required to drink a further litre of water during the test to ensure adequate urine output.74 Performing these tests on acutely unwell patients with severe liver disease who may struggle to consume large volumes of liquid is difficult. Using systemic markers of bacterial translocation as an indirect assessment of IP is therefore an attractive alternative.
Indirect measures of IP
The presence of the endotoxin LPS, one of the major components of the outer membrane of Gram-negative bacteria, has been used as a measure of systemic bacterial load. LPS can be measured in serum by the limulus amoebocyte lysate assay (LAL test). The assay was first developed in the 1970s and, although the sensitivity of this test has improved with the development of a chromogenic assay,85 there remain a number of limitations with using this method on whole blood in humans. The major problems with this assay include the necessity of an endotoxin-specific chromogenic substrate, lack of consensus over standard-curve preparation and the need to eliminate plasma inhibitors from the endotoxin assay.86,87 In addition to these issues, LPS has a short half-life of only 2–3 h, which limits its practical use.
LBP is an alternative marker of bacterial translocation. It is an acute phase protein that is synthesised by hepatocytes in response to the presence of LPS and bacteria in the bloodstream. It binds to bacterial LPS and facilitates its transfer to cell receptors such as CD14, which stimulates an inflammatory response leading to cytokine release from liver macrophages. It has a much longer half-life (2–3 days) than LPS.
A study of 102 cirrhotic patients88 found elevated LBP levels in those with non-infected ascites compared with those without ascites, suggesting increased BT in patients with portal hypertension. Higher LBP levels in these patients correlated with higher TNF-α, IL-6 and sCD14 levels, which then normalised after 4 weeks of norfloxacin treatment suggesting that enteric bacteria may be involved in this process. A further study of 84 cirrhotic patients by the same group showed that LBP level was the only factor associated with severe bacterial infection in a multivariate analysis,89 implicating BT in the development of infectious complications in cirrhosis.
Measuring bacterial load by culturing bacteria directly from the blood or other bodily compartment is the gold standard of detection; however, the limitations include poor sensitivity of this method, the lag time to grow organisms and the fastidious nature of many bacteria.90 Polymerase chain reaction (PCR) amplification techniques have thus been used to try and directly detect bacterial DNA (bDNA) in the systemic circulation. The method entails extracting and purifying the DNA from whole blood samples before using quantitative real-time PCR to detect the bacterial load. The primers used target the highly conserved 16S rRNA gene present in all bacterial genes91; however, there is a limitation to using this gene as it is not a single copy gene, and results may vary by an order of magnitude, depending on the target species.
Several studies have linked serum bDNA with other markers of BT or indirect markers of IP. These include a correlation between bDNA and serum endotoxin after binge drinking.92 Several studies have also correlated serum and/or ascitic bDNA with inflammatory markers or cytokines in patients with liver disease93–95; however, the evidence that links systemic bDNA with measures of increased IP is more limited.
A small study of seven patients with Child Pugh A or B cirrhosis found serum and ascitic fluid bDNA in only one patient although this patient did have the highest IP as assessed by the lactulose/mannitol test.96 All patients showed altered IP compared with a control group of 14 healthy volunteers. Serum bDNA will not pick up leucocyte-associated bDNA thus whole blood DNA detection may be a more sensitive technique. Whole blood bDNA in the STOPAH trial was detectable in the majority of AH patients and correlated with infectious complications.68
Zonulin, a 47 kDa protein discovered by Fasano and colleagues,97 has shown some potential as a biomarker of IP. Experiments in vitro have shown that it is involved in the disassembly of TJ proteins in non-human primate samples and induces increased IP on murine samples.97–99 The same group also performed murine studies that showed an increase in IP (as measured by dual sugar probes) with exposure to zonulin. It has subsequently been used as an indirect measure of gut permeability in a variety of disease models including autoimmune disease, inflammatory bowel disease and alcohol use disorder.100–102 However, the diagnostic validity of the assay has recently been called into question, which may limit its future potential.103,104
Intestinal fatty-acid binding protein (IFABP) is a cytosolic protein located on small bowel enterocytes that are released into the systemic circulation following damage to the mucosal epithelium.105 A small study of 18 patients found that increased iFABP correlated with increased IP (as measured by urinary lactose/rhamnose) following strenuous exercise.106 There are few studies in liver disease although one study of patients with chronic viral hepatitis found higher levels of both LPS and iFABP in these patients compared with controls.107 A correlation with between iFABP and increased IP has been shown in other conditions, including sickle cell disease,108 although again LPS is used as a surrogate marker of BT and increased IP.
Measurement of faecal albumin has also been used as an alternative marker for IP as a healthy intestinal barrier that should prevent the movement of albumin from the blood to interstitial spaces and the intestinal lumen.109 An animal model showed increased faecal albumin, which correlated with increased IP as measured by FITC-dextran, in alcohol-fed mice compared with controls.31 However, one of the limitations of this test in liver disease is that it can be confounded by low serum albumin levels, which are often a feature of cirrhosis.109
Therapeutic interventions to reduce BT in liver disease
Prophylactic antibiotics in liver disease are given to those patients who are at high risk of developing SBP infection.110 The use of poorly absorbable antibiotics, such as norfloxacin, is a strategy to try and selectively decontaminate the bowel of pathogenic Gram-negative bacteria. An animal model of rats with cirrhosis treated with norfloxacin showed reduced rates of bacterial peritonitis but no change in BT overall.111 The major disadvantage to the widespread use of prophylactic antibiotics though is the rise of multidrug-resistant strains of pathogens and the increasing risk these pose to patients with cirrhosis.112 An early study to examine the effect of paromomycin (a non-absorbable, broad-spectrum antibiotic) on endotoxin levels in patients with ArLD did not demonstrate any significant change in endotoxin or liver function tests after 4 weeks.113
The use of the non-absorbable antibiotic rifaximin as an alternative to lactulose for hepatic encephalopathy has been established for some time,114 with good efficacy and with low risk of developing multidrug resistant infection.112 Recently, it has also been tested as an alternative to norfloxacin as primary or secondary prophylaxis for SBP, with one recent RCT showing significantly lower rates of SBP in patients on rifaximin for secondary prophylaxis compared with patients on norfloxacin.115 A recent meta-analysis found that rifaximin was a reasonable alternative to norfloxacin for SBP prevention in patients with hepatitis C cirrhosis.116 The overall evidence, however, remains limited and further evidence is awaited on the broader role of rifaximin in preventing bacterial infections in patients with cirrhosis.
The use of probiotics and prebiotics to influence gut microbiota has gained increasing traction in recent years. Prebiotics are non-digestible compounds, typically fibres, that are used as a substrate for beneficial bacteria. Probiotics are live microorganisms with intended beneficial properties that are added to the gut microbiota.117 These beneficial properties can include preventing mucosal colonisation by pathogenic species and modulation of the local immune response.118
The results from both animal and human studies have been mixed. A recent review found that some studies showed administration of lactobacilli alone or in combination with bifidobacteria species were effective at reducing BT (as measured by endotoxin) in rat models of cirrhosis,119,120 but others showed no discernible benefit.121 A similar picture is seen in randomised placebo-controlled studies in patients with cirrhosis where probiotics have shown a reduction in infection,118 gut bacterial profile and BT.122,123
Overall, however, the impact of pro and prebiotics on outcomes in cirrhosis are fairly limited, with one recent RCT showing no benefit of probiotics on infection rate or mortality.124 There may be a number of reasons behind this, including failure of most probiotics to reach the intestine due to inactivation in the stomach,125 or intestinal dysmotility or they may simply be delivered in insufficient numbers to compete with the increased numbers of pathogenic bacteria seen in cirrhosis.
Transplanting faeces from healthy donors in order to restore the gut microbiota is now an established treatment for recurrent Clostridioides difficile infection and is approved by the National Institute for Health and Care Excellence (NICE) in the UK.95 However, there is increasing interest in the use of FMT outside this indication. In a small safety study of 20 patients, the safety of FMT plus broad-spectrum antibiotics was examined versus usual standard of care (lactulose and rifaximin) for hepatic encephalopathy. Improved cognition and microbial diversity, but no change in model for end-stage liver disease (MELD) score were reported, although the use of antibiotics as well as FMT in the treatment group could have contributed to some of these changes.126
A further small trial by the same group looked at changes in microbiota composition in decompensated cirrhosis due to antibiotics, and whether these changes could be improved with FMT. They administered 5 days of broad-spectrum antibiotics (ciprofloxacin 500 mg twice daily, amoxicillin 500 mg thrice daily, metronidazole 500 mg thrice daily) followed by FMT to 10 patients with decompensated cirrhosis and compared this intervention with 10 patients with decompensated cirrhosis on lactulose and rifaximin. They showed that the reduced bacterial diversity after antibiotic administration was reversed by FMT.127
A prospective feasibility study (PROFIT Trial) of FMT in cirrhosis is currently underway in the UK. This trial does not involve pre-administration of broad-spectrum antibiotics, unlike other studies, and also delivers the FMT directly to the small bowel (via nasojejunal tube). The trial is aiming to assess not only the safety and tolerability of administering FMT to patients with cirrhosis, but also to try and quantify the effects through measuring changes to the recipient stool microbiome, plasma endotoxin and bacterial DNA levels as well as inflammatory cytokines.128
There is also growing interest in using FMT in AAH. A small pilot study of eight steroid-ineligible patients with AAH treated with FMT for 7 days via nasogastric tube showed it to have reduced mortality compared with unmatched historical controls together with reductions seen in the abundance of some pathogenic microbiota species.129
The recent study by Duan and colleagues used bacteriophage to specifically target the cytolysin-producing bacterium E. faecalis, which has been shown to induce more severe liver injury in AAH, in mice colonised by faecal bacteria from patients with severe AAH.70 The phage treatment was able to reverse the hepatocyte injury and steatosis induced in these mice.
Conclusion
The role of increased intestinal permeability and bacterial translocation in the development of liver disease and its complications has been of interest for some time. Its full role in the pathogenesis of liver cirrhosis, its potential for therapeutic manipulation and our ability to accurately measure it are not yet fully realised and clearly require further study. The gold-standard measurement of IP in humans is the use of dual sugar probes but this method has several limitations, not least of all the practicality of performing this on acutely unwell hospitalised patients. Markers such as bacterial DNA, zonulin, LBP and iFABP are all promising but they remain indirect measures of IP. The data regarding therapeutic intervention in this area remains similarly incomplete with many questions about the roles of both probiotics and antibiotic decontamination still to be answered. However, the potential therapeutic role of FMT in decompensated liver cirrhosis and especially in hard-to-treat conditions such as AAH is an exciting avenue of future research.
Footnotes
Conflict of interest statement: The authors declare that there is no conflict of interest.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: We would like to acknowledge support from the Imperial Biomedical Research Centre and the MRC Precision Medicine grant Minimising Mortality in Alcoholic Hepatitis.
ORCID iD: Nikhil Vergis  https://orcid.org/0000-0002-9815-0917
https://orcid.org/0000-0002-9815-0917
Contributor Information
Charlotte Skinner, Department of Metabolism, Digestion and Reproduction, St Mary’s Hospital Campus, Imperial College London, London, UK.
Alex J. Thompson, Department of Surgery & Cancer, St. Mary’s Hospital Campus, Imperial College London, London, UK
Mark R. Thursz, Department of Metabolism, Digestion and Reproduction, St Mary’s Hospital Campus, Imperial College London, London, UK
Julian R. Marchesi, Department of Metabolism, Digestion and Reproduction, St Mary’s Hospital Campus, Imperial College London, London, UK
Nikhil Vergis, Department of Metabolism, Digestion and Reproduction, St Mary’s Hospital Campus, Imperial College London, W2 1NY, UK.
References
- 1. Asrani SK, Devarbhavi H, Eaton J, et al. Burden of liver diseases in the world. J Hepatol 2019; 70: 151–171. [DOI] [PubMed] [Google Scholar]
- 2. Bellentani S, Saccoccio G, Costa G, et al. Drinking habits as cofactors of risk for alcohol induced liver damage. Gut 1997; 41: 845–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Becker U, Deis A, Sorensen TI, et al. Prediction of risk of liver disease by alcohol intake, sex, and age: a prospective population study. Hepatology 1996; 23: 1025–1029. [DOI] [PubMed] [Google Scholar]
- 4. Stickel F, Hampe J. Genetic determinants of alcoholic liver disease. Gut 2012; 61: 150–159. [DOI] [PubMed] [Google Scholar]
- 5. Schnabl B, Brenner DA. Interactions between the intestinal microbiome and liver diseases. Gastroenterology 2014; 146: 1513–1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tremaroli V, Bäckhed F. Functional interactions between the gut microbiota and host metabolism. Nature 2012; 489: 242–249. [DOI] [PubMed] [Google Scholar]
- 7. Yu LCH, Wang JT, Wei SC, et al. Host-microbial interactions and regulation of intestinal epithelial barrier function: from physiology to pathology. World J Gastrointest Pathophysiol 2012; 3: 27–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sedman PC, Macfie J, Sagar P, et al. The prevalence of gut translocation in humans. Gastroenterology 1994; 107: 643–649. [DOI] [PubMed] [Google Scholar]
- 9. Berg RD, Garlington AW. Translocation of certain indigenous bacteria from the gastrointestinal tract to the mesenteric lymph nodes and other organs in a gnotobiotic mouse model. Infect Immun 1979; 23: 403–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wiest R, Lawson M, Geuking M. Pathological bacterial translocation in liver cirrhosis. J Hepatol 2014; 60: 197–209. [DOI] [PubMed] [Google Scholar]
- 11. Nagpal R, Yadav H. Bacterial translocation from the gut to the distant organs: an overview. Ann Nutr Metab 2017; 71: 11–16. [DOI] [PubMed] [Google Scholar]
- 12. Balzan S, Quadros CDA, Cleva RD, et al. Bacterial translocation: overview of mechanisms and clinical impact. J Gastroenterol Hepatol 2007; 22: 464–471. [DOI] [PubMed] [Google Scholar]
- 13. Cirera I, Bauer TM, Navasa M, et al. Bacterial translocation of enteric organisms in patients with cirrhosis. J Hepatol 2001; 34: 32–37. [DOI] [PubMed] [Google Scholar]
- 14. O’Boyle CJ, MacFie J, Mitchell CJ, et al. Microbiology of bacterial translocation in humans. Gut 1998; 42: 29–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Steffen EK, Berg RD, Deitch EA. Comparison of translocation rates of various indigenous bacteria from the gastrointestinal tract to the mesenteric lymph node. J Infect Dis 1988; 157: 1032–1038. [DOI] [PubMed] [Google Scholar]
- 16. Ljungdahl M, Lundholm M, Katouli M, et al. Bacterial translocation in experimental shock is dependent on the strains in the intestinal flora. Scand J Gastroenterol 2000; 35: 389–397. [DOI] [PubMed] [Google Scholar]
- 17. Johansson MEV, Phillipson M, Petersson J, et al. The inner of the two Muc2 mucin-dependent mucus layers in colon is devoid of bacteria. Proc Natl Acad Sci 2008; 105: 15064–15069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Johansson MEV, Hansson GC. Immunological aspects of intestinal mucus and mucins. Nat Rev Immunol 2016; 16: 639–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zarepour M, Bhullar K, Montero M, et al. The mucin Muc2 limits pathogen burdens and epithelial barrier dysfunction during Salmonella enterica serovar Typhimurium colitis. Infect Immun 2013; 81: 3672–3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bergstrom KSB, Kissoon-Singh V, Gibson DL, et al. Muc2 protects against lethal infectious colitis by disassociating pathogenic and commensal bacteria from the colonic mucosa. PLoS Pathog 2010; 6: e1000902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Vaishnava S, Behrendt CL, Ismail AS, et al. Paneth cells directly sense gut commensals and maintain homeostasis at the intestinal host-microbial interface. Proc Natl Acad Sci 2008; 105: 20858–20863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Salzman NH, Ghosh D, Huttner KM, et al. Protection against enteric salmonellosis in transgenic mice expressing a human intestinal defensin. Nature 2003; 422: 522–526. [DOI] [PubMed] [Google Scholar]
- 23. Anderson JM, Itallie CMV. Physiology and function of the tight junction. Cold Spring Harb Perspect Biol 2009; 1: a002584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hartsock A, Nelson WJ. Adherens and tight junctions: structure, function and connections to the actin cytoskeleton. Biochim Biophys Acta 2008; 1778: 660–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Suzuki T. Regulation of intestinal epithelial permeability by tight junctions. Cell Mol Life Sci 2013; 70: 631–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bischoff SC, Barbara G, Buurman W, et al. Intestinal permeability – a new target for disease prevention and therapy. BMC Gastroenterol 2014; 14: 189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lippai D, Bala S, Catalano D, et al. Micro-RNA-155 deficiency prevents alcohol-induced serum endotoxin increase and small bowel inflammation in mice. Alcohol Clin Exp Res 2014; 38: 2217–2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Leclercq S, Matamoros S, Cani PD, et al. Intestinal permeability, gut-bacterial dysbiosis, and behavioral markers of alcohol-dependence severity. Proc Natl Acad Sci 2014; 111: E4485–E4493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wang Y, Tong J, Chang B, et al. Effects of alcohol on intestinal epithelial barrier permeability and expression of tight junction‑associated proteins. Mol Med Rep 2014; 9: 2352–2356. [DOI] [PubMed] [Google Scholar]
- 30. Dunagan M, Chaudhry K, Samak G, et al. Acetaldehyde disrupts tight junctions in Caco-2 cell monolayers by a protein phosphatase 2A-dependent mechanism. Am J Physiol-Gastrointest Liver Physiol 2012; 303: G1356–G1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hartmann P, Chen P, Wang HJ, et al. Deficiency of intestinal mucin-2 ameliorates experimental alcoholic liver disease in mice. Hepatol Baltim Md 2013; 58: 108–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Assimakopoulos SF, Tsamandas AC, Tsiaoussis GI, et al. Altered intestinal tight junctions’ expression in patients with liver cirrhosis: a pathogenetic mechanism of intestinal hyperpermeability. Eur J Clin Invest 2012; 42: 439–446. [DOI] [PubMed] [Google Scholar]
- 33. Reiberger T, Ferlitsch A, Payer BA, et al. Non-selective betablocker therapy decreases intestinal permeability and serum levels of LBP and IL-6 in patients with cirrhosis. J Hepatol 2013; 58: 911–921. [DOI] [PubMed] [Google Scholar]
- 34. Lu Y, Cederbaum AI. CYP2E1 potentiation of LPS and TNFα-induced hepatotoxicity by mechanisms involving enhanced oxidative and nitrosative stress, activation of MAP kinases, and mitochondrial dysfunction. Genes Nutr 2010; 5: 149–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Forsyth CB, Voigt RM, Keshavarzian Ali Intestinal CYP2E1: a mediator of alcohol-induced gut leakiness. Redox Biol 2014; 3: 40–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Davis IV, BT, Voigt RM, Shaikh M, et al. CREB protein mediates alcohol-induced circadian disruption and intestinal permeability. Alcohol Clin Exp Res 2017; 41: 2007–2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Stefano MD, Quigley EMM. The diagnosis of small intestinal bacterial overgrowth: two steps forward, one step backwards? Neurogastroenterol Motil 2018; 30: e13494. [DOI] [PubMed] [Google Scholar]
- 38. Gabbard SL, Lacy BE, Levine GM, et al. The impact of alcohol consumption and cholecystectomy on small intestinal bacterial overgrowth. Dig Dis Sci 2014; 59: 638–644. [DOI] [PubMed] [Google Scholar]
- 39. Yan AW, Fouts DE, Brandl J, et al. Enteric dysbiosis associated with a mouse model of alcoholic liver disease. Hepatol Baltim Md 2011; 53: 96–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Engen PA, Green SJ, Voigt RM, et al. The gastrointestinal microbiome. Alcohol Res Curr Rev 2015; 37: 223–236. [PMC free article] [PubMed] [Google Scholar]
- 41. Chesta J, Defilippi C, Defilippi C. Abnormalities in proximal small bowel motility in patients with cirrhosis. Hepatology 1993; 17: 828–832. [PubMed] [Google Scholar]
- 42. Nagasako CK, de Oliveira Figueiredo MJ, de Souza Almeida JR, et al. Investigation of autonomic function and orocecal transit time in patients with nonalcoholic cirrhosis and the potential influence of these factors on disease outcome. J Clin Gastroenterol 2009; 43: 884. [DOI] [PubMed] [Google Scholar]
- 43. Chander Roland B, Garcia-Tsao G, Ciarleglio MM, et al. Decompensated cirrhotics have slower intestinal transit times as compared with compensated cirrhotics and healthy controls. J Clin Gastroenterol 2013; 47: 888. [DOI] [PubMed] [Google Scholar]
- 44. Lichtman SN, Sartor RB, Keku J, et al. Hepatic inflammation in rats with experimental small intestinal bacterial overgrowth. Gastroenterology 1990; 98: 414–423. [DOI] [PubMed] [Google Scholar]
- 45. Maslennikov R, Pavlov C, Ivashkin V. Small intestinal bacterial overgrowth in cirrhosis: systematic review and meta-analysis. Hepatol Int 2018; 12: 567–576. [DOI] [PubMed] [Google Scholar]
- 46. Wang J, Chen M, Sun G, et al. Small bowel bacterial overgrowth and endotoxemia in cirrhosis. Zhonghua Nei Ke Za Zhi 2002; 41: 459–461. [PubMed] [Google Scholar]
- 47. Jun DW, Kim KT, Lee OY, et al. Association between small intestinal bacterial overgrowth and peripheral bacterial DNA in cirrhotic patients. Dig Dis Sci 2010; 55: 1465–1471. [DOI] [PubMed] [Google Scholar]
- 48. Guarner C, Runyon BA, Young S, et al. Intestinal bacterial overgrowth and bacterial translocation in cirrhotic rats with ascites. J Hepatol 1997; 26: 1372–1378. [DOI] [PubMed] [Google Scholar]
- 49. Stärkel P, Leclercq S, Timary P de, et al. Intestinal dysbiosis and permeability: the yin and yang in alcohol dependence and alcoholic liver disease. Clin Sci 2018; 132: 199–212. [DOI] [PubMed] [Google Scholar]
- 50. Wiest R, Garcia-Tsao G. Bacterial translocation (BT) in cirrhosis. Hepatology 2005; 41: 422–433. [DOI] [PubMed] [Google Scholar]
- 51. Bunchorntavakul C, Chamroonkul N, Chavalitdhamrong D. Bacterial infections in cirrhosis: a critical review and practical guidance. World J Hepatol 2016; 8: 307–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Tandon P, Garcia-Tsao G. Bacterial infections, sepsis, and multiorgan failure in cirrhosis. Semin Liver Dis 2008; 28: 26–42. [DOI] [PubMed] [Google Scholar]
- 53. Bonnel AR, Bunchorntavakul C, Reddy KR. Immune dysfunction and infections in patients with cirrhosis. Clin Gastroenterol Hepatol 2011; 9: 727–738. [DOI] [PubMed] [Google Scholar]
- 54. Muñoz L, Albillos A, Nieto M, et al. Mesenteric Th1 polarization and monocyte TNF-α production: first steps to systemic inflammation in rats with cirrhosis. Hepatology 2005; 42: 411–419. [DOI] [PubMed] [Google Scholar]
- 55. Muñoz L, Borrero MJ, Ubeda M, et al. Interaction between intestinal dendritic cells and bacteria translocated from the gut in rats with cirrhosis. Hepatology 2012; 56: 1861–1869. [DOI] [PubMed] [Google Scholar]
- 56. Úbeda M, Muñoz L, Borrero MJ, et al. Critical role of the liver in the induction of systemic inflammation in rats with preascitic cirrhosis. Hepatology 2010; 52: 2086–2095. [DOI] [PubMed] [Google Scholar]
- 57. Teltschik Z, Wiest R, Beisner J, et al. Intestinal bacterial translocation in rats with cirrhosis is related to compromised paneth cell antimicrobial host defense. Hepatology 2012; 55: 1154–1163. [DOI] [PubMed] [Google Scholar]
- 58. Du Plessis J, Vanheel H, Janssen CEI, et al. Activated intestinal macrophages in patients with cirrhosis release NO and IL-6 that may disrupt intestinal barrier function. J Hepatol 2013; 58: 1125–1132. [DOI] [PubMed] [Google Scholar]
- 59. Bartoletti M, Giannella M, Lewis RE, et al. Bloodstream infections in patients with liver cirrhosis. Virulence 2016; 7: 309–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Keshavarzian A, Holmes EW, Patel M, et al. Leaky gut in alcoholic cirrhosis: a possible mechanism for alcohol-induced liver damage. Am J Gastroenterol 1999; 94: 200–207. [DOI] [PubMed] [Google Scholar]
- 61. Bode C, Kugler V, Bode JC. Endotoxemia in patients with alcoholic and non-alcoholic cirrhosis and in subjects with no evidence of chronic liver disease following acute alcohol excess. J Hepatol 1987; 4: 8–14. [DOI] [PubMed] [Google Scholar]
- 62. Bigatello LM, Broitman SA, Fattori L, et al. Endotoxemia, encephalopathy, and mortality in cirrhotic patients. Am J Gastroenterol 1987; 82: 11–15. [PubMed] [Google Scholar]
- 63. Nanji AA, Khettry U, Sadrzadeh SM, et al. Severity of liver injury in experimental alcoholic liver disease. Correlation with plasma endotoxin, prostaglandin E2, leukotriene B4, and thromboxane B2. Am J Pathol 1993; 142: 367–373. [PMC free article] [PubMed] [Google Scholar]
- 64. Bhagwandeen BS, Apte M, Manwarring L, et al. Endotoxin induced hepatic necrosis in rats on an alcohol diet. J Pathol 1987; 152: 47–53. [DOI] [PubMed] [Google Scholar]
- 65. Adachi Y, Moore LE, Bradford BU, et al. Antibiotics prevent liver injury in rats following long-term exposure to ethanol. Gastroenterology 1995; 108: 218–224. [DOI] [PubMed] [Google Scholar]
- 66. Nanji AA, Khettry U, Sadrzadeh SM. Lactobacillus feeding reduces endotoxemia and severity of experimental alcoholic liver (disease). Proc Soc Exp Biol Med 1994; 205: 243–247. [DOI] [PubMed] [Google Scholar]
- 67. Keshavarzian A, Farhadi A, Forsyth CB, et al. Evidence that chronic alcohol exposure promotes intestinal oxidative stress, intestinal hyperpermeability and endotoxemia prior to development of alcoholic steatohepatitis in rats. J Hepatol 2009; 50: 538–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Thursz MR, Richardson P, Allison M, et al. Prednisolone or Pentoxifylline for alcoholic hepatitis. N Engl J Med 2015; 372: 1619–1628. [DOI] [PubMed] [Google Scholar]
- 69. Llopis M, Cassard AM, Wrzosek L, et al. Intestinal microbiota contributes to individual susceptibility to alcoholic liver disease. Gut 2016; 65: 830–839. [DOI] [PubMed] [Google Scholar]
- 70. Duan Y, Llorente C, Lang S, et al. Bacteriophage targeting of gut bacterium attenuates alcoholic liver disease. Nature 2019; 575: 505–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Galipeau HJ, Verdu EF. The complex task of measuring intestinal permeability in basic and clinical science. Neurogastroenterol Motil 2016; 28: 957–965. [DOI] [PubMed] [Google Scholar]
- 72. Miele L, Valenza V, La Torre G, et al. Increased intestinal permeability and tight junction alterations in nonalcoholic fatty liver disease. Hepatology 2009; 49: 1877–1887. [DOI] [PubMed] [Google Scholar]
- 73. Bjarnason I, O’Morain C, Levi AJ, et al. Absorption of 151chromium-labeled ethylenediaminetetraacetate in inflammatory bowel disease. Gastroenterology 1983; 85: 318–322. [PubMed] [Google Scholar]
- 74. Mishra A, Makharia GK. Techniques of functional and motility test: how to perform and interpret intestinal permeability. J Neurogastroenterol Motil 2012; 18: 443–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Menzies IS. Transmucosal passage of inert molecules in health and disease. In: Skadhauge E, Heintze K. (eds) Intestinal absorption and secretion, Lancaster: MTP Press, 1984, pp.527–543. [Google Scholar]
- 76. Sequeira IR, Lentle RG, Kruger MC, et al. Standardising the lactulose mannitol test of gut permeability to minimise error and promote comparability. PLoS One 2014; 9: e99256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Pascual S, Such J, Esteban A, et al. Intestinal permeability is increased in patients with advanced cirrhosis. Hepatogastroenterology 2003; 50: 1482–1486. [PubMed] [Google Scholar]
- 78. Liboredo JC, Vilela EG, Ferrari MDLDA, et al. Nutrition status and intestinal permeability in patients eligible for liver transplantation. J Parenter Enter Nutr 2015; 39: 163–170. [DOI] [PubMed] [Google Scholar]
- 79. Vogt A, Reuken PA, Stengel S, et al. Dual-sugar tests of small intestinal permeability are poor predictors of bacterial infections and mortality in cirrhosis: a prospective study. World J Gastroenterol 2016; 22: 3275–3284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Wheeler PG, Menzies IS, Creamer B. Effect of hyperosmolar stimuli and coeliac disease on the permeability of the human gastrointestinal tract. Clin Sci Mol Med 1978; 54: 495–501. [DOI] [PubMed] [Google Scholar]
- 81. Weström BR, Svendsen J, Ohlsson BG, et al. Intestinal transmission of macromolecules (BSA and FITC-Labelled Dextrans) in the neonatal pig. Neonatology 1984; 46: 20–26. [DOI] [PubMed] [Google Scholar]
- 82. Ekström G, Weström B, Telemo E, et al. The uptake of fluorescein-conjugated dextran 70,000 by the small intestinal epithelium of the young rat and pig in relation to macromolecular transmission into the blood. J Dev Physiol 1988; 10: 227–233. [PubMed] [Google Scholar]
- 83. Dorshow RB, Hall-Moore C, Shaikh N, et al. Measurement of gut permeability using fluorescent tracer agent technology. Sci Rep 2017; 7: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Thompson A. Non-invasive transcutaneous spectroscopy for the assessment of gut permeability, Protocol Version 4.1, ClinicalTrials.gov – NCT03434639, https://clinicaltrials.gov/ProvidedDocs/39/NCT03434639/Prot_SAP_004.pdf (2018).
- 85. Iwanaga S, Morita T, Harada T, et al. Chromogenic substrates for horseshoe crab clotting enzyme. Its application for the assay of bacterial endotoxins. Haemostasis 1978; 7: 183–188. [DOI] [PubMed] [Google Scholar]
- 86. Fukui H, Brauner B, Bode JC, et al. Chromogenic endotoxin assay in plasma. Selection of plasma pretreatment and production of standard curves. J Clin Chem Clin Biochem 1989; 27: 941–946. [DOI] [PubMed] [Google Scholar]
- 87. Tsuji K, Martin PA, Gaunnac GL. Recovery of endotoxin from human plasma by acid oxidative treatments as monitored by an automated microtiter plate-chromogenic substrate Limulus amebocyte lysate (LAL) assay method. Prog Clin Biol Res 1987; 231: 443–457. [PubMed] [Google Scholar]
- 88. Albillos A, Hera A, de la González M, et al. Increased lipopolysaccharide binding protein in cirrhotic patients with marked immune and hemodynamic derangement. Hepatology 2003; 37: 208–217. [DOI] [PubMed] [Google Scholar]
- 89. Albillos A, de-la-Hera A, Alvarez-Mon M. Serum lipopolysaccharide-binding protein prediction of severe bacterial infection in cirrhotic patients with ascites. Lancet 2004; 363: 1608–1610. [DOI] [PubMed] [Google Scholar]
- 90. Gaibani P, Mariconti M, Bua G, et al. Development of a broad-range 23S rDNA real-time PCR assay for the detection and quantification of pathogenic bacteria in human whole blood and plasma specimens. BioMed Res Int 2013; 2013: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Patel A, Harris KA, Fitzgerald F. What is broad-range 16S rDNA PCR? Arch Dis Child - Educ Pract 2017; 102: 261–264. [DOI] [PubMed] [Google Scholar]
- 92. Bala S, Marcos M, Gattu A, et al. Acute binge drinking increases serum endotoxin and bacterial DNA levels in healthy individuals. PLoS One 2014; 9: e96864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Caro E, Francés R, Zapater P, et al. Grade of soluble inflammatory response is mainly affected by circulating bacterial DNA concentrations in cirrhosis. Liver Int 2016; 36: 1473–1480. [DOI] [PubMed] [Google Scholar]
- 94. González-Navajas JM, Bellot P, Francés R, et al. Presence of bacterial-DNA in cirrhosis identifies a subgroup of patients with marked inflammatory response not related to endotoxin. J Hepatol 2008; 48: 61–67. [DOI] [PubMed] [Google Scholar]
- 95. Frances R. Bacterial DNA activates cell mediated immune response and nitric oxide overproduction in peritoneal macrophages from patients with cirrhosis and ascites. Gut 2004; 53: 860–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Thalheimer U, De Iorio F, Capra F, et al. Altered intestinal function precedes the appearance of bacterial DNA in serum and ascites in patients with cirrhosis: a pilot study. Eur J Gastroenterol Hepatol 2010; 22: 1228–1234. [DOI] [PubMed] [Google Scholar]
- 97. Fasano A, Not T, Wang W, et al. Zonulin, a newly discovered modulator of intestinal permeability, and its expression in coeliac disease. Lancet 2000; 355: 1518–1519. [DOI] [PubMed] [Google Scholar]
- 98. Wang W, Uzzau S, Goldblum SE, et al. Human zonulin, a potential modulator of intestinal tight junctions. J Cell Sci 2000; 113: 4435–4440. [DOI] [PubMed] [Google Scholar]
- 99. Tripathi A, Lammers KM, Goldblum S, et al. Identification of human zonulin, a physiological modulator of tight junctions, as prehaptoglobin-2. Proc Natl Acad Sci U S A 2009; 106: 16799–16804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Fasano A. Zonulin, regulation of tight junctions, and autoimmune diseases. Ann N Y Acad Sci 2012; 1258: 25–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Caviglia GP, Dughera F, Ribaldone DG, et al. Serum zonulin in patients with inflammatory bowel disease: a pilot study. Minerva Med 2019; 110: 95–100. [DOI] [PubMed] [Google Scholar]
- 102. Donnadieu-Rigole H, Pansu N, Mura T, et al. Beneficial effect of alcohol withdrawal on gut permeability and microbial translocation in patients with alcohol use disorder. Alcohol Clin Exp Res 2018; 42: 32–40. [DOI] [PubMed] [Google Scholar]
- 103. Scheffler L, Crane A, Heyne H, et al. Widely used commercial ELISA for human Zonulin reacts with complement C3 rather than preHaptoglobin2. bioRxiv 2017; 157578. [Google Scholar]
- 104. Ajamian M, Steer D, Rosella G, et al. Serum zonulin as a marker of intestinal mucosal barrier function: may not be what it seems. PLoS One 2019; 14: e0210728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Thuijls G, Wijck K, Grootjans J, et al. Early diagnosis of intestinal ischemia using urinary and plasma fatty acid binding proteins. Ann Surg 2011; 253: 303–308. [DOI] [PubMed] [Google Scholar]
- 106. March DS, Marchbank T, Playford RJ, et al. Intestinal fatty acid-binding protein and gut permeability responses to exercise. Eur J Appl Physiol 2017; 117: 931–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Sandler NG, Koh C, Roque A, et al. Host response to translocated microbial products predicts outcomes of patients with HBV or HCV infection. Gastroenterology 2011; 141: 1220–1230e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Dutta D, Methe B, Amar S, et al. Intestinal injury and gut permeability in sickle cell disease. J Transl Med 2019; 17: 183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Wang L, Llorente C, Hartmann P, et al. Methods to determine intestinal permeability and bacterial translocation during liver disease. J Immunol Methods 2015; 421: 44–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Fernández J, Tandon P, Mensa J, et al. Antibiotic prophylaxis in cirrhosis: good and bad. Hepatology 2016; 63: 2019–2031. [DOI] [PubMed] [Google Scholar]
- 111. Runyon BA, Squier S, Borzio M. Translocation of gut bacteria in rats with cirrhosis to mesenteric lymph nodes partially explains the pathogenesis of spontaneous bacterial peritonitis. J Hepatol 1994; 21: 792–796. [DOI] [PubMed] [Google Scholar]
- 112. Tandon P, Delisle A, Topal JE, et al. High prevalence of antibiotic-resistant bacterial infections among patients with cirrhosis at a US liver center. Clin Gastroenterol Hepatol 2012; 10: 1291–1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Bode C, Schäfer C, Fukui H, et al. Effect of treatment with paromomycin on endotoxemia in patients with alcoholic liver disease—a double-blind, placebo-controlled trial. Alcohol Clin Exp Res 1997; 21: 1367–1373. [PubMed] [Google Scholar]
- 114. Zullo A, Hassan C, Ridola L, et al. Rifaximin therapy and hepatic encephalopathy: pros and cons. World J Gastrointest Pharmacol Ther 2012; 3: 62–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Praharaj D, Taneja S, Duseja A, et al. Randomized control trial of rifaximin and norfloxacin in primary and secondary prophylaxis of spontaneous bacterial peritonitis (SBP) in cirrhotic patients. J Clin Exp Hepatol 2017; 7: S71. [Google Scholar]
- 116. Sidhu GS, Go A, Attar BM, et al. Rifaximin versus norfloxacin for prevention of spontaneous bacterial peritonitis: a systematic review. BMJ Open Gastroenterol 2017; 4: e000154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Yan K, Garcia-Tsao G. Novel prevention strategies for bacterial infections in cirrhosis. Expert Opin Pharmacother 2016; 17: 689–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Rayes N, Seehofer D, Hansen S, et al. Early enteral supply of lactobacillus and fiber versus selective bowel decontamination: a controlled trial in liver transplant recipients. Transplantation 2002; 74: 123–127. [DOI] [PubMed] [Google Scholar]
- 119. Chiva M, Soriano G, Rochat I, et al. Effect of Lactobacillus johnsonii La1 and antioxidants on intestinal flora and bacterial translocation in rats with experimental cirrhosis. J Hepatol 2002; 37: 456–462. [DOI] [PubMed] [Google Scholar]
- 120. Sánchez E, Nieto JC, Boullosa A, et al. VSL#3 probiotic treatment decreases bacterial translocation in rats with carbon tetrachloride-induced cirrhosis. Liver Int 2015; 35: 735–745. [DOI] [PubMed] [Google Scholar]
- 121. Bauer TM, Fernández J, Navasa M, et al. Failure of Lactobacillus spp. to prevent bacterial translocation in a rat model of experimental cirrhosis. J Hepatol 2002; 36: 501–506. [DOI] [PubMed] [Google Scholar]
- 122. Lata J, Novotný I, Príbramská V, et al. The effect of probiotics on gut flora, level of endotoxin and Child-Pugh score in cirrhotic patients: results of a double-blind randomized study. Eur J Gastroenterol Hepatol 2007; 19: 1111–1113. [DOI] [PubMed] [Google Scholar]
- 123. Liu Q, Duan ZP, Ha DK, et al. Synbiotic modulation of gut flora: effect on minimal hepatic encephalopathy in patients with cirrhosis. Hepatol Baltim Md 2004; 39: 1441–1449. [DOI] [PubMed] [Google Scholar]
- 124. Pande C, Kumar A, Sarin SK. Addition of probiotics to norfloxacin does not improve efficacy in the prevention of spontaneous bacterial peritonitis: a double-blind placebo-controlled randomized-controlled trial. Eur J Gastroenterol Hepatol 2012; 24: 831–839. [DOI] [PubMed] [Google Scholar]
- 125. Fredua-Agyeman M, Gaisford S. Comparative survival of commercial probiotic formulations: tests in biorelevant gastric fluids and real-time measurements using microcalorimetry. Benef Microbes 2014; 6: 141–151. [DOI] [PubMed] [Google Scholar]
- 126. Bajaj JS, Kassam Z, Fagan A, et al. Fecal microbiota transplant from a rational stool donor improves hepatic encephalopathy: a randomized clinical trial. Hepatology 2017; 66: 1727–1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Bajaj JS, Kakiyama G, Savidge T, et al. Antibiotic-associated disruption of microbiota composition and function in cirrhosis is restored by fecal transplant. Hepatology 2018; 68: 1549–1558. [DOI] [PubMed] [Google Scholar]
- 128. Woodhouse CA, Patel VC, Goldenberg S, et al. PROFIT, a PROspective, randomised placebo controlled feasibility trial of Faecal mIcrobiota Transplantation in cirrhosis: study protocol for a single-blinded trial. BMJ Open 2019; 9: e023518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Philips CA, Pande A, Shasthry SM, et al. Healthy donor fecal microbiota transplantation in steroid-ineligible severe alcoholic hepatitis: a pilot study. Clin Gastroenterol Hepatol 2017; 15: 600–602. [DOI] [PubMed] [Google Scholar]