Abstract
Aims:
Within the past few years, there has been tremendous growth in clinical trials of chimeric antigen receptor (CAR) T-cell therapies. Unlike those of many small-molecule pharmaceuticals, CAR T-cell therapy clinical trials are fraught with risks due to the use of live cell products. The aim of this study is to reach a consensus with experts on the most relevant set of risks that practically occur in CAR T-cell therapy clinical trials.
Methods:
A Delphi method of consensus development was used to identify the risks in CAR T-cell therapy clinical trials, comprising three survey rounds. The expert panel consisted of principal investigators, clinical research physicians, members of institutional ethics committees, and Good Clinical Practice managers.
Results:
Of the 24 experts invited to participate in this Delphi study, 20 participants completed Round 1, Round 2, and Round 3. Finally, consensus (defined as >80% agreement) was achieved for 54 risks relating to CAR T-cell clinical trials. Effective interventions related to these risks are needed to ensure the proper protection of subject health and safety.
Conclusion:
The Delphi method was successful in gaining a consensus on risks relevant to CAR T-cell clinical trials in a geographically diverse expert association. It is hoped that this work can benefit future risk-based quality management in clinical trials and can potentially promote the better development of CAR T-cell therapy products.
Keywords: CAR T-cell therapy, clinical trial, Delphi study, risk identification, subject protection
Introduction
As a revolutionary medical technology, chimeric antigen receptor (CAR) T-cell therapy holds substantial promise to provide a cure for diseases that often lack practical and realistic treatment options.1,2 Within the past decade, considerable attention and large investments have poured into the cellular immunotherapy industry all over the world, which has resulted in tremendous growth in clinical studies in cellular immunotherapy.3 In China, the development of CAR T-cell therapy clinical studies has flourished with the stimulation of supportive government policies in recent years.4 In 2017, the number of Chinese clinical studies of CAR T-cell therapies surpassed that of the United States, as posted on ClinicalTrials.gov.5 In addition, since the National Medical Products Administration (NMPA) issued Guidance for research and evaluation of cellular therapy products in 2017, 15 investigational new drug CAR T-cell therapy registered clinical trials have been approved as of January 2020.
Unlike many small-molecule pharmaceuticals, CAR T-cell therapy is a class of living cell products, and the clinical trial process is complex due to the heterogeneity of starting materials, the limitation of quality indicators, the lack of long-term safety evaluation methods and so on, which pose a potential severe risk to subjects.6,7 Further, a failure to meet certain requirements of quality management in clinical trials, including those relating to the eligibility of institutions and researchers and SOP (standard operating procedure) as well as ethical review compliance, also compounds the potential risk. Therefore, the International Conference on Harmonization, the United States, and the European Union have issued relevant guidelines to encourage a series of risk-based quality management strategies for sponsors to ensure subjects’ safety and data credibility in clinical trials.8–10 The new Chinese Drug Administration Law (2019 edition) also required risk-based quality management in drug clinical trials.
Given the potential risk of CAR T-cell therapy, the adoption of risk-based quality management is paramount to provide assurance that the rights, safety and well-being of subjects are protected. To our knowledge, no study focusing on risk identification in CAR T-cell therapy clinical trials has been reported. The aim of this study is to reach a consensus with experts on the most relevant set of risks that are likely to occur in CAR T-cell therapy clinical trials from the perspective of subject protection. The conclusion reached in this study is likely to be influential on future risk-based quality management in CAR T-cell therapy clinical trials, and it may also promote the further development of CAR T-cell therapy products in China.
Methods
Study structure
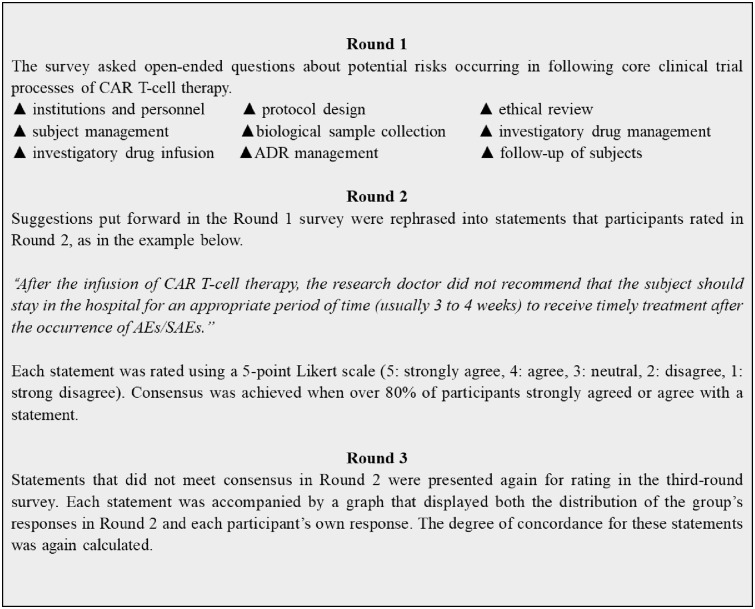
The Delphi method is a popular research method that has been successfully used by large numbers of experts across diverse locations and has proven to be a reliable measurement instrument in developing new concepts and setting the direction of future-oriented research.11–13 The Delphi method has been used to establish consensus across a range of subject areas, including in the field of public health and clinical trials.14,15 In this study, a three-round Delphi process was used to identify risks that likely occur in clinical trials of CAR T-cell therapy that also have the potential to cause great harm to the safety of the subject (Figure 1). Prior to the Delphi process, a brainstorming session and literature review were conducted to identify the core clinical trial processes that involved potential risks.
Figure 1.
Overview of the Delphi process.
ADR, adverse drug reaction; AE, adverse event; CAR, chimeric antigen receptor; SAE, serious adverse event.
In the first round, participants were asked open-ended questions designed to elicit a wide range of potential risks in the core clinical trial processes of CAR T-cell therapy. These responses were then collated by the researchers for further second- and third-round questionnaires that comprised a series of statements that participants were required to rank or rate according to their level of agreement using a five-point Likert scale (“5: strongly agree”, “4: agree”, “3: neutral”, “2: disagree”, “1: strongly disagree”). Participants were asked to consider their previous answers in light of the group’s responses and the comments that had been made throughout the Delphi survey. If they wished to, they could change their answers and make any further comments.
Participant selection
In Delphi exercises, a minimum of 12 respondents is generally considered to be sufficient to enable a consensus to be achieved, and larger sample sizes can provide diminishing returns regarding the validity of the findings.16 Nevertheless, Delphi sample groups depend more on group dynamics in reaching a consensus than on statistical power.17,18
It was not until the end of 2017 that the NMPA issued technical guidelines for clinical trials of cellular therapies, and these guidelines have greatly accelerated the progress of clinical trials on cellular immunotherapy and stem cell therapy in China. Before this, the clinical study of cell therapy was mainly conducted in the form of clinical research, which did not have the purpose of collecting data for market authorization and does not require strict compliance with good clinical practice (GCP) and good manufacturing practice. Therefore, there are few clinical trials of CAR T-cell therapy being conducted in China, and all of them are still in early stages. In this study, we wish to harness the opinions of a group of participants who have rich experience in CAR T-cell therapy clinical research and are currently involved in CAR T-cell therapy clinical trials. These experts include principal investigators, clinical research physicians, members of institutional ethics committees (IECs), and GCP experts. Finally, 24 experts were invited, 20 agreed to take part in our study via email, and four participants were both clinical research physicians and members of an IEC.
Data collection and analysis
Nine core clinical trial processes were identified for investigation through a brainstorming session and literature review: institutions and personnel, protocol design, ethical review, clinical subject management, biological sample collection, investigatory drug management, investigatory drug infusion, adverse drug reaction (ADR) management and the follow-up of subjects. Qualtrics survey software was used for the design and distribution of all questionnaires online, and all participants were given a code that made their answers identifiable to the researcher across questionnaire rounds. Participants were asked to complete each questionnaire within 2 weeks. Email reminders were sent at 1 week and 2 days prior to the questionnaire deadline.
Round 1 questionnaire
In the first-round questionnaire, open-ended questions designed to elicit a wide range of specific manifestations of risk were posed. At the end of the open-ended questions, a blank space was provided for any further comments or information participants wished to provide. All answers received in the first round were recorded as statements of potential risk based on the specific nature of the participant’s response; similar suggestions were collapsed into one statement that retained the intended meaning. Where there was any uncertainty about whether the comments were referring to the same risk, or there was a subtle difference, comments were kept as separate statements.
Round 2 questionnaire
The second-round questionnaire comprised 59 statements generated from the answers and comments provided by the participants in the first round. Space was also provided below each statement for any further comments that participants wished to make. Participant ratings were entered into SPSS data analysis software, and the frequency of each type of rating for each statement was calculated.
Round 3 questionnaire
The third-round questionnaire was used to assess 15 statements that had not met consensus in the second round. Consensus was defined as agreement with a statement (agree or strongly agree) by 80% of participants. There is no set level of agreement for a consensus in Delphi studies, with the level selected by the researcher often being dependent on the number of participants and the priorities of the study.19 In this study, 80% was chosen to reflect the smaller overall number of participants and the desire to represent the panel’s views as accurately as possible. When the third-round questionnaires were received, participants’ ratings for each of the 15 statements were again entered into SPSS, and the frequency of each response was calculated to identify any statements that had now reached consensus.
Result and discussion
In this study, we focused on only the topics related to the quality risk management of CAR T-cell products clinical trials. Therefore, other topics related to whole lifecycle management of CAR T-cell products were not included in this Delphi study.
Of the 24 experts invited to participate in this Delphi study, 20 completed Round 1, Round 2, and Round 3. Participant demographics are shown in Table 1. The participants’ mean age was 41 years across the three rounds, and approximately three-quarters of participants resided in the Shanghai and Beijing metro areas. This is likely because the majority of sites conducting clinical trials of CAR T-cell therapy in China are located in these two cities. The majority of respondents were senior academics, had doctoral degrees and had been working in the field of CAR T-cell therapy clinical research for ⩾3 years.
Table 1.
Demographic characteristics of Delphi participants.
| Rounds 1, 2, 3 (N = 20) | |
|---|---|
| Gender | |
| Male | 5 |
| Female | 15 |
| Mean age | 41 |
| Province | |
| Shanghai | 11 |
| Beijing | 4 |
| Zhejiang | 3 |
| Jiangsu | 1 |
| Tianjin | 1 |
| Current role | |
| Director physician | 5 |
| Director pharmacist | 1 |
| Associate director physician | 4 |
| Doctor-in-charge | 4 |
| Pharmacist-in-charge | 4 |
| Senior engineer | 2 |
| Education | |
| Doctoral degree | 13 |
| Master’s degree | 7 |
| Years working in the field | |
| 10+ years | 3 |
| 6–9 years | 6 |
| 3–5 years | 7 |
| 1–2 years | 4 |
| The number of patients cared by each participant | |
| 1–19 | 6 |
| 20–49 | 9 |
| 50–100 | 3 |
| 100–250 | 2 |
Table 2 shows the 59 statements collected in Round 1 and the group responses to each risk. By Round 2, consensus was achieved for 44 out of 59 statements. Fifteen statements needed a third round before consensus was reached. Finally, 54 risks were identified by experts in Round 3, and five were either not expected to occur or were not expected to pose a significant risk to subjects. Notably, this is not to say that there are no other risks other than those identified here in CAR T-cell therapy clinical trials; rather, the risks discussed here are considered to be more likely to occur in clinical trials and may pose a greater safety threat to trial subjects.
Table 2.
Responses of the expert panel in the three-round Delphi study.
| Summary of risk statements collected in Round 1 | Round 2 |
Round 3 |
||||
|---|---|---|---|---|---|---|
| Agree | M | SD | Agree | M | SD | |
| 1. Institutions and personnel | ||||||
| 1.1 The sponsor does not meet the basic requirements to conduct the clinical trial (such as lack of funds, inadequate production, analysis and quality control teams). | 100% | 4.60 | 0.50 | |||
| 1.2 The medical institution does not meet the basic requirements to conduct the clinical trial (such as personnel, venue, technology, emergency equipment). | 95% | 4.45 | 0.60 | |||
| 1.3 The research team has not previously been or is less involved in clinical trials of cell therapy. | 75% | 4.35 | 0.88 | 95% | 4.55 | 0.52 |
| 1.4 The research team is not trained adequately in CAR T-cell therapy clinical trials. | 90% | 4.15 | 0.59 | |||
| 1.5 There is an inadequate number of medical experts with rich experience in clinical CAR T-cell therapy trials on the IEC. | 75% | 4.10 | 0.72 | 95% | 4.25 | 0.67 |
| 1.6 The research team is not equipped with a clinical research nurse with relevant experience. | 70% | 3.90 | 0.72 | 65% | 3.45 | 0.78 |
| 1.7 There is no qualified safety assessment team to ensure the safety of the subjects in the clinical research and to provide suggestions for decision-making. | 80% | 4.15 | 0.75 | |||
| 2. Protocol design | ||||||
| 2.1 When a first-in-human clinical trial involves vulnerable populations (such as children, the cognitively impaired, or end-stage patients), the research protocol and safety monitoring plan in the trial are not rigorously and meticulously justified. | 95% | 4.50 | 0.61 | |||
| 2.2 The determination of inclusion, exclusion, and withdrawal criteria is not clear or scientific. | 100% | 4.75 | 0.44 | |||
| 2.3 In the early dose exploration test, the appropriate observation period is not set according to the characteristics of cell therapy. | 95% | 4.45 | 0.60 | |||
| 2.4 In the dose exploration test, the dosage regimen is not reasonably determined based on changes in temporary drug properties. | 55% | 3.25 | 0.75 | 35% | 2.85 | 0.83 |
| 2.5 There is no detailed and operable emergency plan for the failure to manufacture investigatory cell therapy products. | 60% | 3.40 | 0.94 | 45% | 3.10 | 0.85 |
| 2.6 The definition of dose-limiting toxicity is not justified clearly and scientifically. | 95% | 4.55 | 0.76 | |||
| 2.7 The determination of the clinical trial end point (ORR/PFS/OS) is not justified clearly and scientifically. | 90% | 4.40 | 0.68 | |||
| 2.8 The follow-up plan is not consistent with the specific characteristics of CAR T-cell therapy products. | 100% | 4.45 | 0.51 | |||
| 2.9 The sponsor did not effectively communicate with NMPA experts on the issues of safety, effectiveness, and subject protection when developing the clinical trial protocol. | 70% | 3.90 | 0.85 | 85% | 4.20 | 0.75 |
| 3. Ethical review | ||||||
| 3.1 The IEC does not develop ethical review guidelines or SOPs for reviewing CAR T-cell therapy products. | 70% | 3.80 | 1.01 | 90% | 4.15 | 0.96 |
| 3.2 The IEC does not adequately evaluate the safety and effectiveness of CAR T-cell therapy drugs based on evidence from preclinical studies. | 95% | 4.50 | 0.61 | |||
| 3.3 When conducting an ethical review of high-risk clinical trials of CAR T-cell therapy, no external professional consultants are contacted to justify the scientific nature of the research protocol. | 95% | 4.40 | 0.75 | |||
| 3.4 The IEC does not comprehensively review the compensation plans in the event of adverse drug events for the subjects. | 70% | 3.95 | 0.89 | 90% | 4.40 | 0.76 |
| 3.5 The IEC does not comprehensively review the content of the informed consent and how it was obtained from subjects. | 90% | 4.30 | 0.86 | |||
| 3.6 The IEC does not follow up on approved clinical trials. | 100% | 4.50 | 0.51 | |||
| 4. Clinical subject management | ||||||
| 4.1 Recruitment information that has not been approved by the IEC is released. | 100% | 4.50 | 0.51 | |||
| 4.2 The sponsor does not purchase insurance for the subjects. | 95% | 4.50 | 0.76 | |||
| 4.3 The subjects are not screened consistently with the previously established criteria of inclusion and exclusion. | 95% | 4.60 | 0.75 | |||
| 4.4 Informed consent is obtained after the formal clinical trials begin. | 100% | 4.70 | 0.47 | |||
| 4.5 The content of informed consent is not fully understood by the subjects, or subjects are not given sufficient time for decision-making. | 75% | 3.90 | 0.79 | 85% | 4.10 | 0.72 |
| 4.6 Informed consent is not obtained from the subjects if the study protocol changes. | 95% | 4.30 | 0.73 | |||
| 4.7 When subjects are members of vulnerable populations, they (or their guardians or authorized clients) are not informed in detail on the risks and benefits of clinical trials when obtaining informed consent. | 100% | 4.55 | 0.51 | |||
| 4.8 After the infusion of CAR T-cell therapy products, research doctors do not recommend that the subjects should stay in the hospital for an appropriate period of time (usually 3–4 weeks) in order to receive timely treatment after the occurrence of AEs/SAEs. | 70% | 4.05 | 0.83 | 90% | 4.35 | 0.77 |
| 5. Biological sample collection | ||||||
| 5.1 Failure to control radiotherapy, chemotherapy regimens, or washout periods before apheresis results in the impairment of target T-cell function and vitality. | 90% | 4.45 | 0.83 | |||
| 5.2 The routine complete blood and lymphocyte ratio of subjects is not fully evaluated before apheresis. | 100% | 4.75 | 0.44 | |||
| 5.3 Improper temporary storage of collected biological samples or long storage times result in damage to target T-cell function and vitality. | 100% | 4.70 | 0.47 | |||
| 5.4 Cell collection personnel in medical institutions do not obtain relevant training or health examination certificates. | 75% | 4.10 | 0.79 | 95% | 4.50 | 0.61 |
| 5.5 No backup of collected biological samples and produced cell preparations is held. | 100% | 4.60 | 0.50 | |||
| 6. Investigatory drug management | ||||||
| 6.1 The effect of cell freezing medium on the function and vitality of resuscitated cells is not verified in the preclinical study. | 100% | 4.75 | 0.44 | |||
| 6.2 Transportation conditions of CAR T-cell products are unsuitable. | 100% | 4.75 | 0.44 | |||
| 6.3 No specific department or personnel is responsible for receiving investigatory cell preparations. | 100% | 4.55 | 0.51 | |||
| 6.4 The quality inspection report of CAR T-cell therapy is not checked carefully when receiving the investigatory CAR T-cell product from the sponsor. | 100% | 4.55 | 0.60 | |||
| 6.5 The environmental cleanliness where the investigatory cell preparations are processed in the medical institution does not meet the relevant requirements. | 70% | 4.20 | 1.11 | 55% | 3.35 | 0.89 |
| 6.6 The storage of investigatory CAR T-cell preparations in the medical institution is not managed and recorded in accordance with regulatory requirements. | 100% | 4.60 | 0.50 | |||
| 7. Investigatory drug infusions | ||||||
| 7.1 In the clinical trial of CAR T-cell therapy for hematological tumors, standard pretreatment processes (bridging chemotherapy and lymphodepletion) are not conducted before infusion. | 90% | 4.60 | 0.82 | |||
| 7.2 Safety evaluation for patients is not conducted 1 day before or on the day of infusion. | 95% | 4.65 | 0.75 | |||
| 7.3 Researchers do not inspect the cell product packaging for damage, seepage, etc. | 70% | 3.90 | 1.17 | 55% | 3.55 | 1.08 |
| 7.4 Product numbers and information are not carefully checked when administering to subjects. | 95% | 4.70 | 0.73 | |||
| 7.5 First-aid measures are not accessible in a timely manner in the case of severe allergic reactions, infusion reactions, hypotension, and other reactions during the infusion. | 95% | 4.70 | 0.73 | |||
| 7.6 The dose of the cell therapy preparation for infusion is not calculated based on the weight of the subject on the day of infusion. | 65% | 3.95 | 0.94 | 90% | 3.45 | 0.58 |
| 7.7 The investigatory cell products are not infused as soon as possible after cell resuscitation. | 95% | 3.70 | 0.92 | |||
| 7.8 The time taken to infuse the cell product is so long that it is likely to affect T-cell function and vitality. | 95% | 4.60 | 0.60 | |||
| 8. ADR management | ||||||
| 8.1 Researchers do not fully assess and anticipate the possibility of adverse reactions. | 100% | 4.50 | 0.51 | |||
| 8.2 Emergency measures for adverse reactions are not prepared in advance, or these emergency measures cannot be accessed quickly. | 100% | 4.70 | 0.47 | |||
| 8.3 First-line researchers are not trained in clinical manifestations, monitoring, management, etc. of adverse reactions of cell therapy drugs. | 100% | 4.75 | 0.44 | |||
| 8.4 Adverse reaction occurrence in the subject is not observed for a period of time after apheresis. | 80% | 4.55 | 0.69 | |||
| 8.5 Subject vital signs and their changes are not monitored continuously during the cell therapy product infusion. | 100% | 4.75 | 0.44 | |||
| 8.6 The formulation of AE and SAE response plans does not refer to the latest literature recommendations and foreign guidelines on adverse reaction treatment. | 100% | 4.35 | 0.49 | |||
| 8.7 When SAEs occur, the IEC does not intervene in time with corresponding measures. | 70% | 4.15 | 0.88 | 90% | 4.30 | 0.61 |
| 9. Following up of subjects | ||||||
| 9.1 For subjects who voluntarily dropped out in the middle of the trial, the research doctor does not contact the subject and provide treatment recommendations for adverse reactions when necessary. | 95% | 4.30 | 0.57 | |||
| 9.2 For subjects who completed a clinical trial and left the site, the researchers do not maintain necessary contact with the subject for follow-up. | 100% | 4.55 | 0.51 | |||
| 9.3 For cell products that are expected to have long-term viability, long-term follow-up programs are not conducted. | 100% | 4.50 | 0.51 | |||
Statements in bold did not reach consensus in this study.
AE, adverse event; CAR, chimeric antigen receptor; PFS, progression-free survival; IEC, independent ethics committee; NMPA, National Medical Products Administration; ORR, objective response rate; OS, overall survival; SAE, serious adverse event; SOP, standard operating procedure.
Experience relating to CAR T-cell therapy product clinical trials in China and abroad demonstrates that the products are genetically modified and may cause adverse reactions such as cytokine release syndrome (CRS), neurotoxicity, tumor lysis syndrome, and macrophage activation syndrome. Furthermore, with the application of gene editing technologies such as CRISPR/Cas9 in the field of immune cell therapy, new products and technologies have also brought many potential safety risks to patients, such as off-target effects and tumorigenicity.20 The safety risk control plan is one of the most critical considerations in the design and conduct of a CAR T-cell therapy clinical trial. It is necessary to correctly identify potential risks in CAR T-cell therapy clinical trials to create effective safety risk control plans. To our knowledge, this is the first study providing insight into expert opinion on the potential subject safety risks in clinical CAR T-cell therapy trials. Based on a three-round Delphi process, 54 risks in nine core clinical trial processes were identified based on the experts’ opinions. Quality management for CAR T-cell therapy clinical trials should include effective interventions for these identified risks to maximize the protection of subject safety.
Considering that CAR T-cell therapy is generally considered high risk, it is necessary to ensure the accessibility of emergency measures, the support of relevant departments such as the intensive care unit, and effective and standardized training for researchers (Risk Statements 1.3, 1.4, 8.2, 8.4). The training should be put in place to inform researchers and other staff about the risks, clinical manifestations, the management of CRS, and neurotoxicity observed with CAR T-cell treatment. Especially in early clinical research, medical institutions and researchers should be selected with cell therapy experience and the ability to diagnose and manage adverse reactions (Risk Statements 1.2–1.4). In addition, a safety assessment team composed of multidisciplinary and experienced experts should be established to discuss the safety of subjects in clinical trials and to provide suggestions for decision-making (Risk Statement 1.7).
While the desire to access potentially therapeutic interventions is completely understandable, scientific research on cell-based therapy is at an early stage, and its potential value is far from fully understood.21 Therefore, it is imperative for the IEC to completely evaluate the risk-efficiency profile of cell-based therapies before conducting a formal clinical trial (Risk Statements 3.2–3.4). Furthermore, the IEC should conduct a comprehensive assessment of the qualifications of the researchers and the laboratory conditions (Risk Statement 3.1). There is a risk that investigators may heed patients and their families’ wishes to access beneficial treatments and exaggerate the therapeutic benefits of cell-based therapy while avoiding mentioning side effects when obtaining informed consent.22 In addition, the IEC should focus on reviewing compliance with the informed consent procedure (Risk Statements 3.5, 3.6). In the process of obtaining informed consent, applicants and researchers should ensure that subjects fully understand the risks of cell therapy clinical trials and should neither overstate the clinical benefits of cell therapy nor give too brief a description of the risk of adverse reactions (Risk Statements 4.5–4.7). The IEC also needs to conduct in-process supervision of CAR T-cell therapy clinical trials to determine a reasonable frequency of follow-up review based on the risk of the products (Risk Statement 4.7). Given that clinical trials of CAR T-cell therapies tend to be multicenter trials, the establishment of regional ethics committees was also suggested by the expert panel to integrate and share regional resources. This committee would instruct and supervise in areas of research protocol, informed consent and the compliance and timeliness of trial processes.
In the clinical trial protocol, the inclusion and exclusion criteria are not only the threshold for subjects to participate in clinical trials but also ideally a comprehensive evaluation of the subject’s risk tolerance. For clinical trials of CAR T-cell therapy products, normal treatment should be carried out in relapsed or refractory patients for whom there are no effective CAR T-cell treatment methods. Therefore, the definition of “relapsed”, “refractory”, and “lack effective treatment methods” should be clear and scientific, and subjects should be recruited in strict accordance with the pre-established inclusion and exclusion criteria (Risk Statements 2.2, 4.3). Normally, it takes time for potential ADRs to manifest themselves after immune cell product infusion. Therefore, sufficient observation intervals (at least 2 weeks) should be established according to the characteristics of cell products when conducting dose exploration studies to minimize the safety risk to subjects in clinical trials23 (Risk Statement 2.3). Moreover, the responsibilities of the sponsors and the investigators should be clearly defined, and the safeguards for the significant risk to the subject should be specified (Risk Statements 8.1–8.3). In addition, the formation of a safety risk control plan should ideally refer to the clinical consensus or the latest authoritative research results in China and abroad (Risk Statement 8.6). It is important to ensure that the patient and family are given wallet cards to remind them about the signs and symptoms of adverse reactions such as CRS and neurotoxicity, which require immediate medical attention. Clinical experts in China and abroad have a relatively clear understanding of grading and controlling serious ADRs, such as CRS, neurotoxicity, and macrophage activation syndrome.24–26 For example, verifying tocilizumab (two doses) is ordered and available for administration before a dose of CAR T-cell product is administered according to the risk evaluation and mitigation strategy of Food and Drug Administration-approved Yescarta and Kymriah.27,28
In contrast to small-molecule drugs, cell therapy products are living cell preparations, and the process of collection of biological samples and the management and infusion of investigatory drugs in CAR T-cell clinical trials introduce more risks. In order to avoid manufacturing failure of CAR T-cell therapy products as much as possible, the routine complete blood and lymphocyte ratio of subjects should be fully evaluated before apheresis (Risk Statement 5.2). The main thing that makes cell therapy more complex from a supply chain perspective is the requirement to maintain the drug at the appropriate temperature throughout the chain of custody, from manufacturing to short-term storage, to long-term storage, and then finally out to the clinical sites for dosing. Therefore, fast cold chain transportation and cryogenic warehouse should be available for shipment and conservation of cell products (Risk Statement 6.2, 6.6). Additionally, the investigatory cell products should be infused as soon as possible after cell resuscitation, and the time taken to infuse investigatory drugs should be controlled properly to maintain cell activity as well as avoid infusion reactions (Risk Statements 7.7–7.8).
ADR data should be fully collected, and the risk control plan should be updated continually during the clinical research phase. Vital signs of subjects should be monitored continuously, and changes should be noted for timely detection of the occurrence of adverse events (AEs)/serious AEs (SAEs), both after apheresis and during the cell therapy product infusion (Risk Statements 8.4–8.5). After the infusion of the CAR T-cell therapy product, research doctors should recommend that the subjects stay in the hospital for an appropriate period of time (usually 3–4 weeks) to receive timely treatment in the event of an occurrence of AEs/SAEs. (Risk Statement 4.8). The short-term safety follow-up time can be reasonably set up according to the previous pharmacokinetic data (Risk Statements 9.1–9.2). For example, treatment can be followed up to the time when the product is not detected in vivo or 2 years after the infusion, whichever is longer.29 In addition, since CAR T-cell therapy products are genetically modified, which introduces the associated risk of tumorigenicity, the subject should be observed for delayed AEs for as long as 15 years following exposure to the investigational CAR T-cell therapy product30,31 (Risk Statement 9.3).
Strengths and limitations
This study highlights the identification process of major risks in CAR T-cell therapy clinical trials. An important strength of this study is the tailored and iterative study design. Extensive literature review and a face validity check on risks in CAR T-cell therapy clinical trails have contributed to the validity of the study, as well as pilot-testing of the surveys. Furthermore, our research is focused on identifying the most likely potential risks, which was strengthened by recruiting an expert team in the fields of in CAR-T clinical trials and patient care. Moreover, our study did include face-to-face consultations with experts between the three rounds of Delphi study so as to discuss ratings, investigate areas of disagreement, and gain more in-depth insights. Although the research design and execution were rigorous, some limitations still exist. First, the sample size in this study was small, and it would be of benefit in future studies to recruit a larger number of experts to ensure depth and any diversity in their responses. However, it has been suggested that sample size may not be the major concern in Delphi studies and that comparable reliability may be obtained with both fewer and greater numbers of participants.32 Second, since the identification of risks is a relatively subjective process, only major risks that are likely to occur in CAR T-cell therapy clinical trials were identified by the expert panel in this study, and some important risks in the clinical trials may have been missed. Finally, the severity of each risk to the safety of subjects was not weighted in this study. In the future, our research will weigh the severity of these risks and use these risk indicators to conduct risk assessments of CAR T-cell therapy clinical trials.
Conclusion
China has great demand for CAR T-cell therapy products and has made efforts to accelerate their development. In view of the high risk of CAR T-cell therapy products, the safety of subjects should be given priority, and reasonable scientific risk-based quality management in clinical trials should be developed. In this study, 54 risks were identified by an expert panel, and effective interventions for these risks would maximize the protection of subject safety. Our work could potentially benefit future risk-based quality management in CAR T-cell therapy clinical trials, which may promote the development of CAR T-cell therapy products.
Footnotes
Conflict of interest statement: The authors declare that there is no conflict of interest.
Ethics statement: According to the World Medical Association Declaration of Helsinki, ethical approval is needed for any research that involves human participants, their tissue, and/or data to ensure that the dignity, rights, safety and well-being of all participants are the primary consideration of the research project. In this study, a Delphi method was used to reach a consensus among experts on the most relevant set of risks in CAR T-cell therapy clinical trials, which is not a medical research involving subjects. Therefore, ethics approval was not required in this study.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Natural Science Foundation of China (grant numbers 81972392), Shanghai Municipal Health Commission (201840306), Shanghai Municipal Education Commission (HJW-R-2019-66-19) and Clinical Science and Technology Innovation Program of Shanghai Shen Kang Hospital Development Center (SHDC12017629).
Contributor Information
Weijia Wu, Department of Clinical Pharmacy and Pharmaceutical Management, School of Pharmacy, Fudan University, Shanghai, China.
Yan Huo, National Institution of Food and Drug Control, National Medical Products Administration, Beijing, China.
Xueying Ding, Engineering Technology Research Center of Cell Therapy and Clinical Translation, Shanghai Science and Technology Committee; Clinical Research Center, Shanghai General Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China.
Yuhong Zhou, Department of Medical Oncology, Zhongshan Hospital, Fudan University, Shanghai, China.
Shengying Gu, Clinical Research Center, Shanghai General Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China.
Yuan Gao, Department of Clinical Pharmacy and Pharmaceutical Management, School of Pharmacy, Fudan University, Pudong District, Shanghai, 200433, China.
References
- 1. Schneider CK, Salmikangas P, Jilma B, et al. Challenges with advanced therapy medicinal products and how to meet them. Nat Rev Drug Discov 2010; 9: 195–201. [DOI] [PubMed] [Google Scholar]
- 2. Buzhor E, Leshansky L, Blumenthal J, et al. Cell-based therapy approaches: the hope for incurable diseases. Regen Med 2014; 9: 649–672. [DOI] [PubMed] [Google Scholar]
- 3. Tang J, Hubbard-Lucey VM, Pearce L, et al. The global landscape of cancer cell therapy. Nat Rev Drug Discov 2018; 17: 465–466. [DOI] [PubMed] [Google Scholar]
- 4. Cheng B, Lu S-L, Fu X-B. Regenerative medicine in China: main progress in different fields. Mil Med Res 2016; 3: 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gou L, Gao J, Yang H, et al. The landscape of CAR T-cell therapy in the United States and China: a comparative analysis. Int J Cancer 2019; 144: 2043–2050. [DOI] [PubMed] [Google Scholar]
- 6. Brudno JN, Kochenderfer JN. Toxicities of chimeric antigen receptor T cells: recognition and management. Blood 2016; 127: 3321–3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bailey AM, Arcidiacono J, Benton KA, et al. United States food and drug administration regulation of gene and cell therapies. Adv Exp Med Biol 2015; 871: 1–29. [DOI] [PubMed] [Google Scholar]
- 8. European Medicines Agency. Guideline on safety and efficacy follow-up and risk management of advanced therapy medicinal products, https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-safety-efficacy-follow-risk-management-advanced-therapy-medicinal-products_en.pdf (2008, accessed 10 December 2019).
- 9. ICH Q9: quality risk management, https://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Quality/Q9/Step4/Q9_Guideline.pdf (2005, accessed 10 December 2019).
- 10. Food and Drug Administration. Guidance for industry: oversight of clinical investigations-a risk-based approach to monitoring, https://www.fda.gov/regulatory-information/search-fda-guidance-documents/oversight-clinical-investigations-risk-based-approach-monitoring (2008, accessed 10 December 2019).
- 11. Mackway-Jones K, Carley S. An international expert Delphi study to determine research needs in major incident management. Prehosp Disaster Med 2012; 27: 351–358. [DOI] [PubMed] [Google Scholar]
- 12. Manca DP, Varnhagen S, Brett-MacLean P, et al. Rewards and challenges of family practice: web-based survey using the Delphi method. Can Fam Physician 2007; 53: 278–286, 277. [PMC free article] [PubMed] [Google Scholar]
- 13. Rowe G, Wright G. The Delphi technique as a forecasting tool: issues and analysis. Int J Forecast 1999; 15: 353–375. [Google Scholar]
- 14. Korpershoek YJ, Bruins Slot JC, Effing TW, et al. Self-management behaviors to reduce exacerbation impact in COPD patients: a Delphi study. Int J Chron Obstruct Pulmon Dis 2017; 12: 2735–2746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gillies K, Skea ZC, MacLennan SJ, et al. Determining information for inclusion in a decision-support intervention for clinical trial participation: a modified Delphi approach. Clin Trials 2013; 10: 967–976. [DOI] [PubMed] [Google Scholar]
- 16. Vogel C, Zwolinsky S, Griffiths C, et al. A Delphi study to build consensus on the definition and use of big data in obesity research. Int J Obes (Lond) 2019; 43: 2573–2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Slade SC, Dionne CE, Underwood M, et al. Standardised method for reporting exercise programmes: protocol for a modified Delphi study. BMJ Open 2014; 4: e006682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Okoli C, Pawlowski SD. The Delphi method as a research tool: an example, design considerations and applications. Inf & Manag 2004; 42: 15–29. [Google Scholar]
- 19. Hasson F, Keeney S, McKenna H. Research guidelines for the Delphi survey technique. J Adv Nurs 2000; 32: 1008–1015. [PubMed] [Google Scholar]
- 20. Ureña-Bailén G, Lamsfus-Calle A, Daniel-Moreno A, et al. CRISPR/Cas9 technology: towards a new generation of improved CAR-T cells for anticancer therapies. Brief Funct Genomics. Epub ahead of print 17 December 2019. DOI: 10.1093/bfgp/elz039. [DOI] [PubMed] [Google Scholar]
- 21. Sugarman J, Barker RA, Kerridge I, et al. Tackling ethical challenges of premature delivery of stem cell-based therapies: ISSCR 2018 annual meeting focus session report. Stem Cell Reports 2018; 11: 1021–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Barker RA, Carpenter MK, Forbes S, et al. The challenges of first-in-human stem cell clinical trials: what does this mean for ethics and institutional review boards? Stem Cell Reports 2018; 10: 1429–1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gao JC, Huang YH, Wang HH, et al. Several considerations on the related issues of clinical trial design of chimeric antigen receptor T lymphocytes in the treatment of lymphohematopoietic malignancies. Chin J Cancer Biother 2019; 26: 833–836. (Chinese). [Google Scholar]
- 24. Acharya UH, Dhawale T, Yun S, et al. Management of cytokine release syndrome and neurotoxicity in chimeric antigen receptor (CAR) T cell therapy. Expert Rev Hematol 2019; 12: 195–205. [DOI] [PubMed] [Google Scholar]
- 25. Neelapu SS, Tummala S, Kebriaei P, et al. Chimeric antigen receptor T-cell therapy—assessment and management of toxicities. Nat Rev Clin Oncol 2018; 15: 47–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ge YQ, Zhu XM, Lu Y, et al. Cytokine release syndrome in patients with B-cell acute lymphocytic leukaemia during CAR—T therapy: nursing care. J Nur Sci 2019; 34: 38–40. (Chinese). [Google Scholar]
- 27. Food and Drug Administration. YESCARTA (axicabtagene ciloleucel), https://www.fda.gov/vaccines-blood-biologics/cellular-gene-therapy-products/yescarta-axicabtagene-ciloleucel (2017, accessed 30 January 2020).
- 28. Food and Drug Administration. KYMRIAH (tisagenlecleucel), https://www.fda.gov/vaccines-blood-biologics/cellular-gene-therapy-products/kymriah-tisagenlecleucel (2017, accessed 28 January 2020).
- 29. Food and Drug Administration. Guidance for industry: gene therapy clinical trials-observing subjects for delayed adverse events, https://www.fda.gov/regulatory-information/search-fda-guidance-documents/gene-therapy-clinical-trials-observing-subjects-delayed-adverse-events (2006, accessed 11 December 2019).
- 30. European Medicines Agency. Guideline on follow-up of patients administered with gene therapy medicinal products, https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-follow-patients-administered-gene-therapy-medicinal-products_en.pdf (2009, accessed 11 December 2019).
- 31. Food and Drug Administration. Guidance for industry: long term follow-up after administration of human gene therapy products, https://www.fda.gov/regulatory-information/search-fda-guidance-documents/long-term-follow-after-administration-human-gene-therapy-products (2020, accessed 11 January 2020).
- 32. Murphy MK, Black NA, Lamping DL, et al. Consensus development methods, and their use in clinical guideline development. Health Technol Assess 1998; 2: i–iv, 1–88. [PubMed] [Google Scholar]