Abstract
Background:
Current health systems do not effectively address all aspects of chronic care. For better self-management of disease, kidney patients have identified the need for improved health care information, interaction with health care providers, and individualization of care.
Objective:
The Triple I study examined challenges to exchange of information, interaction between patients and health care providers and individualization of care in in-center hemodialysis with the aim of identifying the top 10 challenges that individuals on in-center hemodialysis face in these 3 areas.
Design:
We employed a sequential mixed methods approach with 3 phases:
1. A qualitative study with focus groups and interviews (Apr 2017 to Aug 2018);
2. A cross-sectional national ranking survey (Jan 2019 to May 2019);
3. A prioritization workshop using a modified James Lind Alliance process (June 2019)
Setting:
In-center hemodialysis units in 7 academic centers across Canada: Vancouver, Calgary, Edmonton, Winnipeg, Ottawa, Montreal, and Halifax.
Participants:
Individuals receiving in-center hemodialysis, their caregivers, and health care providers working in in-center hemodialysis participated in each of the 3 phases.
Methods:
In Phase 1, we collected qualitative data through (1) focus groups and interviews with hemodialysis patients and their caregivers and (2) individual interviews with health care providers and decision makers. Participants identified challenges to in-center hemodialysis care and potential solutions to these challenges. In Phase 2, we administered a pan-Canadian cross-sectional ranking survey. The survey asked respondents to prioritize the challenges to in-center hemodialysis care identified in Phase 1 by ranking their top 5 topics/challenges in each of the 3 “I” categories. In Phase 3, we undertook a face-to-face priority setting workshop which followed a modified version of the James Lind Alliance priority setting workshop process. The workshop employed an iterative process incorporating small and large group sessions during which participants identified, ranked, and voted on the top challenges and innovations to hemodialysis care. Four patient partners contributed to study design, implementation, analysis, and interpretation.
Results:
Across the 5 participating centers, we conducted 8 focus groups and 44 interviews, in which 113 participants identified 45 distinct challenges to in-center hemodialysis care. Subsequently, completion of a national ranking survey (n = 323) of these challenges resulted in a short-list of the top 30 challenges. Finally, using small and large group sessions to develop consensus during the prioritizing workshop, 38 stakeholders used this short-list to identify the top 10 challenges to in-center hemodialysis care. These included individualization of dialysis-related education; improved information in specific topic areas (transplant status, dialysis modalities, dialysis-related complications, and other health risks); more flexibility in hemodialysis scheduling; better communication and continuity of care within the health care team; and increased availability of transportation, financial, and social support programs.
Limitations:
Participants were from urban centers and were predominately English-speaking. Survey response rate of 31.5% in Phase 2 may have led to selection bias. We collected limited information on social determinants of health, which could confound our results.
Conclusion:
Overall, the challenges we identified demonstrate that individualized care and information that improves interaction with health care providers is important to patients receiving in-center hemodialysis. In future stages of this project, we will aim to address these challenges by trialing innovative patient-centered solutions.
Trial Registration:
Not applicable.
Keywords: hemodialysis, end-stage kidney disease, quality of care, patient-oriented research, patient engagement, patient-centred care
Abrégé
Contexte:
Les systèmes de santé actuels ne traitent pas efficacement tous les aspects des soins aux malades chroniques. Pour mieux autogérer la maladie, les patients atteints de néphropathies expriment un besoin de personnalisation des soins et d’informations de santé facilitant les interactions avec leurs soignants.
Objectif:
L’étude Triple I s’est penchée sur l’échange d’information, l’interaction entre les patients et les soignants et la personnalisation des soins en hémodialyse en center. Nous souhaitions cerner les dix principaux défis auxquels font face les patients dans ces trois secteurs.
Type d’étude:
Nous avons procédé en trois phases selon une approche séquentielle à méthodes mixtes:
1. étude qualitative avec groupes échantillons et entretiens individuels (avril 2017 à août 2018);
2. sondage de classement transversal au niveau national (janvier à mai 2019);
3. atelier consacré à la définition des priorités utilisant une version modifiée du James Lind Alliance process (juin 2019)
Cadre:
Les unités d’hémodialyse de sept centres hospitaliers universitaires à travers le Canada (Vancouver, Calgary, Edmonton, Winnipeg, Ottawa, Montréal et Halifax).
Participants:
Des patients hémodialysés en centre, leurs soignants et des fournisseurs de soins travaillant dans les unités d’hémodialyse ont participé à chacune des trois phases.
Méthodologie:
Au cours de la phase 1, nous avons recueilli des données qualitatives par l’entremise 1) de groupes échantillons et d’entretiens avec des patients hémodialysés et leurs soignants, et 2) d’entretiens individuels avec des fournisseurs de soins et des décideurs. Les participants ont mis en évidence les défis liés aux soins d’hémodialyse en centre et les possibles solutions à ceux-ci. Pour la phase 2, nous avons procédé à un sondage de classement transversal pancanadien où les répondants devaient classer par ordre de priorité les difficultés recensées au cours de la phase 1. Les répondants devaient classer leurs cinq principaux défis dans chacune des trois catégories établies lors de la phase 1. La phase 3 a consisté en un atelier d’établissement des priorités selon une version modifiée du processus de la James Lind Alliance. Pour cet atelier, nous avons utilisé un processus itératif comportant des séances en petits et grands groupes au cours desquelles les participants ont identifié, classé et voté sur les principaux défis et innovations en matière de soins d’hémodialyse. Quatre patients partenaires ont contribué à la conception de l’étude, à sa mise en œuvre, de même qu’à l’analyse et à l’interprétation des résultats.
Résultats:
Dans les cinq sites ayant participé à la phase 1, nous avons mené 8 groupes de discussion et 44 interviews au cours desquels 113 participants ont mentionné 45 défis distincts liés aux soins d’HD en centre. Par la suite (phase 2), la complétion d’un sondage de classement national (n=323) de ces défis a mené à une liste restreinte de 30 difficultés. Puis, lors de l’atelier visant le dégagement d’un consensus (phase 3), 38 intervenants ont utilisé cette courte liste lors de séances en petits et grands groupes pour s’entendre sur les 10 principaux défis des soins d’hémodialyse en centre. Cette courte liste incluait notamment des besoins pour i) une éducation personnalisée sur la dialyse; ii) des informations de meilleure qualité sur certains sujets précis (transplantation, modalités de dialyse, complications liées à la dialyse et autres risques pour la santé); iii) plus de flexibilité dans les horaires de dialyse; iv) une meilleure communication et continuité dans les soins au sein des équipes soignantes; et v) une plus grande disponibilité des programmes de transport, de soutien financier et de soutien social.
Limites:
La majorité des participants provenait de centres urbains et s’exprimait en anglais. Le taux de réponse au sondage de la phase 2 était de 31,5 %, ce qui pourrait avoir entraîné des biais de sélection. Nous avons recueilli peu d’information sur les déterminants sociaux de santé, ce qui pourrait brouiller nos résultats.
Conclusion:
Dans l’ensemble, les enjeux soulevés démontrent que l’individualisation des soins et l’échange d’informations facilitant les interactions avec les fournisseurs de soins sont importants pour les patients hémodialysés en centre. Pour la suite de ce projet, nous tenterons de surmonter ces défis par l’expérimentation de solutions innovantes axées sur les patients.
Introduction
With advances in acute care in recent decades, the landscape of health and health care needs has changed dramatically. Most of the North Americans aged 40 years and greater have at least one chronic illness (many have 3 or more), and 10% to 15% have chronic kidney disease (CKD).1-3 Chronic health conditions are still predominately cared for within the hospital system, yet this system was designed to care for acute illnesses and is not structured to effectively address all aspects of care required for chronic conditions.4,5 In addition, health information is now widely available to the public and individuals with CKD are keen to take increasing responsibility for self-managing their health.6-8 New technologies make it possible for patients to communicate with their health professionals and manage their own health information. Innovative health care models designed to encourage self-management and provide patient-centered care for individuals with chronic disease are needed to meet the needs of patients with kidney disease.4,9,10
A focus on patient-centered care and informed decision-making has become increasingly important in nephrology.11-13 A Patient-Oriented Research meeting including 52 participants from across Canada was organized by a national steering committee in Montreal in 2014. In this planning meeting, participants developed the framework to establish a national patient-oriented research network in CKD and prioritized research questions through consensus by patients, caregivers, clinicians, and health care decision makers. At this meeting, kidney patients identified that they wanted research to answer the following questions: “Can my care be more about me and my needs?”; “How can I get more information about my health, so that I can better manage my condition?”; and “What model of care will best deliver evidence-based personalized care?”14 Three broad areas of innovation were conceived based on these patient priorities: Information, Interaction, and Individualization.
Using this framework, the Triple I study was designed to characterize the perceptions of people treated with in-center hemodialysis (HD), regarding how their care could be improved by innovations related to “Information” (providing patients with information about their health and their health care),”Interaction” (how health care providers interact with patients), and “Individualization” (customization or adaptation of a patient’s care to reflect their individual circumstances, values and preferences). This multi-center project consists of 2 waves with multiple phases within each wave.
Wave 1 of the Triple I project aimed to identify the top 10 challenges to address in in-center HD care within the 3 “I” categories (Information, Interaction, Individualization).
Method
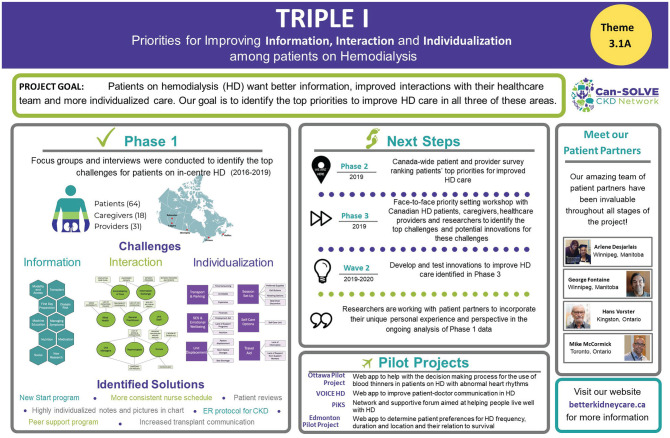
In this multi-center, pan-Canadian study, we employed a sequential mixed methods approach through multiple phases, each with equal priority, to determine the top 10 priorities for improving care for individuals receiving in-center HD as related to information, interaction, and individualization. The 3 phases of Wave 1 (Figure 1) were as follows:
Figure 1.
Triple I project overview.
Phase 1: a qualitative study with focus groups and interviews;
Phase 2: a cross-sectional national ranking survey; and
Phase 3: a prioritizing workshop using a modified James Lind Alliance process.15
The study was approved by the research ethics boards at the main study sites (University of Calgary [REB16-2136] and University of Manitoba [HS20494 [H2017:049] and HS22255 [H2018:411]]) and all subsites (University of Ottawa, University of Alberta, Dalhousie University, University of British Columbia, and Center Hospitalier de l’Université de Montréal). Informed consent was obtained from all participants.
Patient Engagement
The Triple I project is part of the Canadians Seeking Solutions to Overcome Chronic Kidney Disease (Can-SOLVE CKD) research program, a network with the vision of improving outcomes and optimizing care for Canadians with or at high risk for CKD through patient-oriented research.16 The central tenet of Can-SOLVE is the involvement of people with experience living with kidney disease throughout all stages of the research process, from protocol development to interpretation of results. The Triple I project steering committee includes 4 patient partners, 2 of whom act as project co-leads, with a variety of experience relating to in-center HD. Each patient partner participated in and contributed to all stages of the study and all played an important role in ensuring that the perspectives of individuals with first-hand knowledge of HD were incorporated throughout the project.
Phase 1: Focus Groups and Interviews to Identify Challenges to HD Care
In Phase 1, we explored the challenges to HD care and potential solutions to these challenges as identified by individuals receiving in-center HD, their caregivers, and health care providers with experience working in HD. During this phase, we collected qualitative data by means of (1) focus groups and interviews with HD patients and their caregivers and (2) individual interviews with health care providers and decision makers. We recruited participants between April 17, 2017 and August 1, 2018. Potential participants were identified and initially approached by HD unit staff. During the recruitment visit, research staff also asked patients whether they had a caregiver who may be interested in participating. Health care providers from across Canada with HD expertise were invited by email request and/or in person to participate in semi-structured interviews. They were recruited using a purposive approach supplemented by snowball sampling. Focus groups and interviews took place in Calgary, Edmonton, Winnipeg, Halifax, and Ottawa from May 24, 2017 to August 16, 2018. Two experienced researchers facilitated the focus groups and conducted the individual interviews to maintain consistency.
Potential solutions to challenges generated by patients and caregivers helped to inform the questions we asked during interviews with health care providers. This iterative approach helped health care providers and decision makers consider patient-important solutions and thereby identify any system-related facilitators or barriers that could be relevant to implementation. Transcripts from the focus groups and interviews were initially analyzed using conventional content analysis. The goal of conventional content analysis is to avoid using preconceived categories, and instead to allow categories and category names to emerge directly from the data. In this type of analysis, researchers immerse themselves in the data to allow for new insights to become apparent.17 Challenges, solutions to challenges, facilitators, and barriers to care identified during focus groups and interviews were coded using NVivo Pro Version 11 (QSR International Pity Ltd).18 Detailed methodology and in-depth qualitative analysis of the Phase 1 data will be reported separately.
Phase 2: Nationwide Ranking Survey of Challenges Identified in Focus Groups and Interviews
In Phase 2, we administered a pan-Canadian cross-sectional survey, which aimed to confirm impressions and expand on data collected from the interviews and focus groups in Phase 1 by attempting to reach a more diverse sample of participants. On the survey, we listed the challenges to in-center HD care identified in Phase 1 and asked respondents to prioritize these challenges. The respondents were asked to rank their top 5 topics/challenges from most to least important by writing a number from 1 to 5 beside their top 5 choices (1 = most important; 5 = least important). They followed the same procedure for each of 3 categories: Information, Interaction, and Individualization. We also included open-ended questions on the survey where participants listed additional issues and potential solutions to the top challenges that they identified on their survey (Supplemental Appendix 1). We piloted the survey from December 15, 2018 to January 31, 2019 with 10 Phase 1 participants and then modified the survey based on participant feedback.
Survey recruitment was performed at 6 sites across Canada: Winnipeg, Calgary, Edmonton, Halifax, Vancouver, and Montreal. In view of the disproportionate representation of Indigenous peoples on HD,19 an Indigenous research assistant encouraged the involvement of Indigenous participants at the Winnipeg site. Surveys translated into French and simple Chinese were available for administration at the Montreal and Vancouver sites, respectively. Surveys were available for completion as per participant preference in paper or electronic format. The online version of the survey was made available to all HD patients and providers across Canada from January 31 to May 20, 2019 through posting on the Triple I website (www.betterkidneycare.ca), and mentions on the Chronic Disease Innovation Centre at Seven Oaks General Hospital and Can-SOLVE CKD Network Twitter pages. A member of the research staff at each of the 6 Triple I study sites approached potential participants during HD to complete the survey. Health care providers were sent a link to their institutional e-mail address to complete the online version of the survey on the Triple I website. Consent for all participants was implied by completion of the survey which included a cover letter indicating the nature of implied consent. All responses were anonymous.
We entered all completed and partially completed hard copy surveys into an online Research Electronic Data Capture (REDCap) database housed at the University of Manitoba.20 Surveys completed electronically were directly entered into the REDCap database at time of completion. Total score and adjusted score weighted by the number of options to rank in each Triple I category were calculated for each challenge listed on the survey. Subsequently, the adjusted score for each challenge was used to complete a short list of the top 30 issues/challenges. Potential solutions to the challenges that respondents listed on the survey were coded and collated for use in future phases of the project. In a subset of incorrectly completed surveys, participants ranked ALL priorities from 1 to 5, not only their top 5. For these surveys, we calculated the number of 1s and number of 5s indicated for each Triple I category to identify the highest and lowest priority challenges. Results were compared to the final list of top challenges from the correctly completed surveys to evaluate any important differences; as none were found, the full sample size was used to generate results. To assess generalizability, we compared differences in the top 30 challenges shortlist by ethnicity, geographic region, and sex. We did this by identifying challenges short-listed in the demographic subgroups that were not included in the final shortlist of top 30 challenges. We also assessed whether challenges identified in our final top 10 challenges were included in the shortlists of these demographic subgroups. Data were analyzed using Microsoft Excel 2019 (Excel version 16.22, Microsoft Corp) and SAS Studio version 3.71 (SAS Institute, Inc., Cary, NC, USA).
Phase 3: Face to Face Priority Setting Workshop to Identify the Top 10 Challenges to HD Care
In Phase 3, we undertook a face-to-face priority setting workshop involving multiple stakeholders with in-center HD experience (patients, caregivers, health care providers, researchers, and policy/decision makers) from across Canada. Research staff from all 7 Triple I Sites (Winnipeg, Calgary, Edmonton, Halifax, Montreal, Ottawa, and Vancouver) recruited participants for the meeting. HD unit staff, social workers, and patient partners identified patients and caregivers from the HD units at their respective Triple I sites. Indigenous patients were purposively sampled at the Winnipeg site to ensure Indigenous representation at the workshop. Team members at all Triple I sites also identified health care providers, researchers, and policy/decision makers. The face-to-face workshop followed a modified version of the James Lind Alliance15 priority setting workshop process and took place in Winnipeg, Manitoba on June 15, 2019. A Can-SOLVE CKD Patient Council Lead reviewed the workshop structure and plan and attended the meeting, as did a member of the Can-SOLVE Indigenous Peoples’ Research Council.16 An Indigenous wisdom keeper opened the day by welcoming participants with an opening prayer.
Workshop participants were provided with the shortlist of 30 challenges to HD care generated in Phase 2 of the project for review prior to the workshop. The workshop involved an initial session with all participants to provide background, an outline, goals for the day and ground rules. Participants were then divided into groups of 8-10 people in which they spent an hour discussing the top 30 challenges generated from Phase 2. To minimize bias, the 30 challenges were presented as a general list, and purposefully were not presented in ranked order. After a short break, participants returned to the same groups and spent an hour discussing and ranking their top 10 challenges. Trained facilitators were present in each group to help generate discussion and consensus. A list of the top 18 priorities was subsequently created by aggregating the top 10 lists from each group. All participants came together in a larger group to discuss the aggregate rankings, provide perspectives, and give additional background to rankings from individual groups’ discussions and ask questions for clarification.
Following this large group meeting, participants were separated into new groups of 8 to 10 people and each group once again identified the top 10 challenges to HD care from the shortlist of 18 challenges generated earlier in the day. The top 10 challenges identified by each group were once again aggregated and the top 13 challenges identified by this analysis were presented to the entire group. Ties were agreed on by discussion and ultimately a vote. Consolidation and consensus of priorities was achieved through whole group discussion.
Results
Phase 1: Focus Groups and Interviews
One hundred thirteen people from 5 urban Canadian centers with direct in-center HD experience participated in the focus groups and interviews. Focus groups (Winnipeg, n = 29; Halifax, n = 19; Edmonton, n = 17; Calgary, n = 13; Ottawa, n = 4) included 47 patients (21 females) and 18 caregivers (13 females). The mean age of focus group participants was 61 (±15) years (range: 22-93 years) and mean time on in-center HD was 4.7 (±5.6) years (range: 3 months-34 years). We conducted interviews with 17 patients and 31 health care providers (77% female): 14 nephrologists, 11 nurses, 2 pharmacists, 2 kinesiologists, and 2 dieticians. On average, health care providers had been in practice for 13.2 years (±5.6 years) ranging from 1 to 40 years.
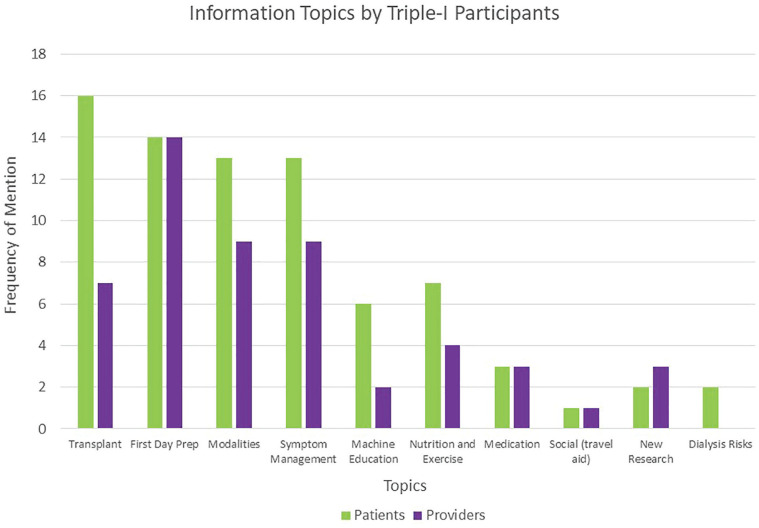
Participants identified a total of 45 challenges in the 3 Triple I categories during the focus groups and interviews: 18 challenges for Information, 16 challenges for Interaction, and 11 challenges for Individualization. The challenges and priorities identified across different regions of Canada were generally similar, as were those identified by patients/caregivers and health care providers (Figure 2). However, patients indicated more challenges with information regarding transplantation, whereas health care providers identified that provision of information regarding new research could be improved. Detailed qualitative analysis will be reported separately.
Figure 2.
Challenges to information exchange by participant type.
Phase 2: Nationwide Ranking Survey
A total of 1026 individuals were approached to complete surveys at participating HD units. We received and analyzed 323 surveys. In 53 questionnaires, ranking was completed incorrectly, but was still interpretable and included in the analysis as described in the Methods section. Most of the respondents (79%) were patients (n = 224) and 56% had been receiving HD for between 1 and 5 years. Other respondents included 6 caregivers (2%), 2 individuals who identified as both caregiver and patient (0.7%), and 51 (18%) health care providers (10 nephrologists, 29 nurses, 4 social workers, 2 pharmacists, 1 nurse practitioner, 1 support staff, 1 occupational therapist, 2 kinesiologists, and 1 other). More male (61%) than female patients completed the demographic questions, but most health care respondents were female (73%). While 45% of health care providers were 35 to 49 years old, patients and caregivers were generally older with 41% being 65 to 79 years old. Forty respondents did not provide any demographic data. Additional demographic data can be found in Table 1.
Table 1.
Demographics of Respondents to Phase 2 Ranking Survey.
| All (n = 283) | Patient and caregivers (n = 232) | Health care providers (n = 51) | |
|---|---|---|---|
| Sex (n) | |||
| Female | 128 (45) | 91 (39) | 37 (73) |
| Male | 153 (54) | 141 (61) | 12 (24) |
| Gender fluid, nonbinary/2-spirited | 0 | 0 | 0 |
| Prefer not to answer/missing | 2 (1) | 0 | 2 (4) |
| No demographics provided | 40 | ||
| Age (n) | |||
| 18-34 | 23 (8) | 11 (5) | 12 (24) |
| 35-49 | 55 (19) | 32 (14) | 23 (45) |
| 50-64 | 88 (31) | 75 (32) | 13 (25) |
| 65-79 | 96 (34) | 94 (41) | 2 (4) |
| 80+ | 18 (6) | 18 (8) | 0 |
| Prefer not to answer/missing | 3 (1) | 2 (1) | 1 (2) |
| No demographics provided | 40 | ||
| Ethnicity (n) | |||
| White | 166 (59) | 136 (59) | 30 (59) |
| Canadian Indigenous | 37 (13) | 35 (15) | 2 (4) |
| East Asian | 7 (2) | 4 (2) | 3 (6) |
| South Asian | 25 (9) | 20 (9) | 5 (10) |
| African-Canadian/Caribbean-Canadian | 11 (4) | 11 (5) | 0 |
| Other | 17 (6) | 13 (6) | 4 (8) |
| Prefer not to answer/missing | 20 (7) | 13 (6) | 7 (14) |
| No demographics provided | 40 | ||
| Location (n) | |||
| Atlantic | 38 (13) | 34 (15) | 4 (8) |
| Québec | 29 (10) | 27 (12) | 2 (4) |
| Ontario | 5 (2) | 4 (2) | 1 (2) |
| Prairies | 203 (72) | 164 (71) | 39 (76) |
| British Columbia | 7 (2) | 2 (1) | 5 (10) |
| Territories | 0 | 0 | 0 |
| Prefer not to answer/missing | 1 (0) | 1 (0) | 0 |
| No demographics provided | 40 | ||
| Daily use of technology (n) | |||
| Yes | 211 (75) | 160 (69) | 51 (100) |
| No | 67 (24) | 67 (29) | 0 |
| Not sure | 1 (0) | 1 (0) | 0 |
| Prefer not to say/missing | 4 (1) | 4 (2) | 0 |
| No demographics provided | 40 | ||
| Social media use (n) | |||
| Daily | 103 (36) | 72 (31) | 31 (61) |
| 1-3 times per week | 20 (7) | 14 (6) | 6 (12) |
| 1-3 times per month | 11 (4) | 10 (4) | 1 (2) |
| Rarely | 31 (11) | 26 (11) | 5 (10) |
| Never | 107 (38) | 100 (43) | 7 (14) |
| Not sure | 1 (0) | 1 (0) | 0 |
| Prefer not to say/missing | 10 (4) | 9 (4) | 1 (2) |
| No demographics provided | 40 | ||
Note. Data are presented as n (%).
A shortlist of the top 30 challenges to HD care identified by the ranking survey is presented in Table 2. Almost half (47%) of these top 30 challenges were related to information that patients and caregivers receive. When we compared the top 30 challenges by ethnicity, sex, and geographic region, we identified challenges included in the shortlists of these subgroups that were missing from the final top 30 shortlist that we used in the prioritizing workshop. Respondents who identified as white ranked “individualized set-up of bed or chair” and “more information about the dialysis machine” in the top 30 challenges, and respondents who identified as non-white ranked “access to employment resources” in the top 30. Male respondents ranked “more information on the HD machine” in the top 30, and female-identifying respondents ranked “information on exercise,” “individualized set-up of bed/chair and machine,” “access to employment resources,” and “appropriate doctor for patient concerns” in the top 30. Challenges not included in the final top 30 shortlist by geographic region are shown in Table 3. In addition, the final top 10 challenges identified in the prioritizing workshop were generally included in the 30 challenges shortlist of all demographic subgroups listed above. Exceptions were Quebec for which final Challenges 4 and 7 were not included and BC for which final Challenge 9 was not included.
Table 2.
Top 30 Challenges Identified From Phase 2 Survey.
| Challenges identified | Total Scorea | Adjusted scoreb b | Rank for section | Total rank |
|---|---|---|---|---|
| Information | ||||
| Better information about transplant status | 379 | 379 | 1 | 1 |
| More information about the health risks and other conditions association with HD | 337 | 337 | 2 | 2 |
| More information about what can go “wrong” during HD | 261 | 261 | 3 | 7 |
| Better information (more frequent, clearer, better timing) about the pros and cons of different dialysis modalities | 249 | 249 | 4 | 9 |
| More information about how to manage HD symptoms | 242 | 242 | 5 | 10 |
| Timing, frequency, and amount of information being received should be individualized | 232 | 232 | 6 | 12 |
| More information on research and advances in kidney health | 227 | 227 | 7 | 14 |
| More information about traveling while on HD | 221 | 221 | 8 | 16 |
| More information about financial support and managing financial issues | 220 | 220 | 9 | 17 |
| Better information (more frequent, clearer, better timing) about the pros and cons of different HD access types | 217 | 218 | 10 | 18 |
| More information about medications and their side effect from prescribers in HD | 205 | 205 | 11 | 19 |
| More information about what to expect on the first day of HD | 180 | 180 | 12 | 24 |
| Better information (more and better timing) about nutrition and diet | 178 | 178 | 13 | 25 |
| More information on social programs for people on HD | 152 | 152 | 14 | 27 |
| Interaction | ||||
| Have HD nurses who are familiar with patients’ health details | 335 | 298 | 1 | 3 |
| Patients have enough time with or the ability to access the rounding nephrologist when needed | 329 | 293 | 2 | 5 |
| Improved communication between patient and/or health care providers (within HD unit, but also with specialists, transplant and family physicians) | 317 | 282 | 3 | 6 |
| Improve continuity of care by having same staff for patients during each HD session | 289 | 257 | 4 | 8 |
| HD nurses have specialized experience/training | 261 | 232 | 5 | 11 |
| It is frustrating for patients when they are told to see a family physician about health concerns they bring up in HD | 256 | 228 | 6 | 13 |
| Patients have enough time with or the ability to access nursing/allied health staff | 249 | 222 | 7 | 15 |
| Information about a patients’ care is complete and available in the HD chart | 165 | 147 | 8 | 29 |
| Physicians have access to all the information they need to take care of patients in the HD unit | 158 | 141 | 9 | 30 |
| Individualization | ||||
| Availability of flexible, reliable, and affordable transportation to/from HD | 483 | 295 | 1 | 4 |
| Availability of several chair/bed options in each unit | 325 | 198 | 2 | 20 |
| Patients’ care plan considers finances and there is access to resources for people with low income | 316 | 193 | 3 | 21 |
| Privacy in the HD unit to allow for comfortable discussions of sensitive or private issues | 315 | 192 | 4 | 22 |
| More flexibility to change HD spots/schedule | 307 | 187 | 5 | 23 |
| Access to social programs for people on HD | 279 | 170 | 6 | 26 |
| Access to exercise/biking program specifically designed for HD | 243 | 148 | 7 | 28 |
Note. HD = hemodialysis.
From survey.
Adjusted by the number of options to rank.
Table 3.
Challenges Ranked Top 30 by Geographic Location Missing From Overall Top 30.
| Location | Challenge |
|---|---|
| Atlantic | Understanding which physician is most appropriate to see to deal with patient concerns |
| Sometimes it seems that no one working in HD cares or is interested in patients’ issues | |
| Fewer differences in HD health care providers’ opinions for patient care plans | |
| The way the HD unit is organized and run helps patients and HCP get the information they need to optimize care | |
| Quebec | Understanding which physician is most appropriate to see to deal with patient concerns |
| Fewer differences in HD health care providers’ opinions for patient care plans | |
| The way the HD unit is organized and run helps patients and HCP get the information they need to optimize care | |
| More information on the role of exercise on HD and exercise programs | |
| HD machine and chair/bed set-up and positioning should be individualized | |
| Patients have access to HD unit managers and the managers seem connected to the unit | |
| Prairies | Same as Top 30 Challenges |
| Ontario | Sometimes it seems that no one working in HD cares or is interested in patients’ issues |
| More information on the role of exercise on HD and exercise programs | |
| HD machine and chair/bed set-up and positioning should be individualized | |
| Patients have access to HD unit managers and the managers seem connected to the unit | |
| More information about self-care and opportunities to do self-care in HD | |
| Sometimes patients may not want to actively participate in their care | |
| BC | Understanding which physician is most appropriate to see to deal with patient concerns |
| The way the HD unit is organized and run helps patients and HCP get the information they need to optimize care | |
| More information on the role of exercise on HD and exercise programs | |
| HD machine and chair/bed set-up and positioning should be individualized | |
| More information about self-care and opportunities to do self-care in HD | |
| Different format or way of receiving information in HD. | |
| Access to employment aid and resources for people on HD. | |
| More detailed information on how to care for my HD access |
Note. HD = hemodialysis; BC = British Columbia.
Phase 3: Priority Setting Workshop
Thirty-eight people with in-center HD experience from diverse geographic regions in Canada attended the priority setting workshop on June 15, 2019. Participants were 63% female (n = 24) and included 10 patients, 4 Triple-I patient partners, 3 caregivers, 4 nurses, 3 nephrologists, 3 social workers, 5 policy/decision makers, 2 researchers, 1 pharmacist, 2 Can-SOLVE leaders, and 1 Indigenous elder, with some participants playing multiple roles. Participants were from diverse geographic regions of Canada (Nova Scotia 21%; Quebec 16%; Ontario 8%; Manitoba 37%; Alberta 16%; British Columbia 2%). The “Timing, frequency, and amount of information being received should be individualized (specific to each patient)” was identified as the highest priority at this workshop.
In the final discussion of the day, the top 13 priorities identified by the small group ranking exercises were presented and discussed. As a result of this discussion, Priority 11, “Information available in the patient’s chart should be complete,” was added to Priority 2, “Continuity of care” to create “Improve continuity of care in HD and ensure that information about a patient’s care is complete in their chart.” In addition, Priority 12, “Availability of financial resources” was combined with Priority 5, “Improve availability of affordable transportation to and from hemodialysis,” and broadened to encompass multiple financial challenges that are frequently identified in HD to create a modified Priority 5, “More information and access to financial resources and support including availability of flexible, reliable and affordable transport to/from hemodialysis, housing and nutrition/diet.”
Table 4 presents the final rankings for the “Top 10 challenges to address in in-centre hemodialysis care” that were identified at the workshop. In addition to identifying the top 10 challenges, there was consensus that it is imperative to consider and incorporate cultural sensitivity into any solutions addressing the top 10 challenges in HD care.
Table 4.
Top 10 Challenges to Address in In-centre Hemodialysis Care.
| Top 10 challenges to address in in-center hemodialysis care | |
|---|---|
| 1 | Timing, frequency, and amount of information being received should be individualized (specific to each patient) |
| 2 | Improve continuity of care in hemodialysis and info about a patient’s care is complete in their chart |
| 3 | Improve the way information is communicated between health care providers and patients |
| 4 | It’s frustrating for patients when they are told to see a family physician about health concerns they bring up in hemodialysis |
| 5 | More information and access to financial resources and support including availability of flexible, reliable and affordable transport to/from hemodialysis, housing and nutrition/diet |
| 6 | More flexibility to change hemodialysis spots/schedule |
| 7 | Better information about the pros and cons of different dialysis modalities |
| 8 | More information about health risks and other conditions associated with hemodialysis |
| 9 | Better information about transplant status |
| 10 | More information and access to social programs for people on hemodialysis |
Discussion
The Triple I Study focuses on improving patient-centered care in the areas of Information, Interaction, and Individu-alization within the in-center HD setting. In Wave 1 of this project, we adapted the James Lind Alliance methodology15 to identify specific challenges to address in these 3 areas as identified by patients, their caregivers, and health care providers. The top 10 challenges that we identified through this process included lack of individualization of the provision of dialysis-related information and education; improved information in areas that are important to patients (transplant status, dialysis modalities, dialysis-related complications, and other health risks in HD); flexibility in the HD scheduling, communication, and continuity of care within the health care team; and availability of social and peer support programs.
Improved communication between physicians and patients as well as between providers for better continuity of care was a key theme in the challenges that we identified (Challenges 2, 3, and 4). The importance of improving communication during health care interactions and the role of inadequate communication in contributing to delays in care and adverse health outcomes has previously been identified in individuals with chronic diseases and specifically in end-stage kidney disease (ESKD) by the Coalition for Supportive Care of Kidney Patients (CSCKP), a coalition of patients, providers, and decision makers working to provide better quality care for individuals with ESKD in the United States.4,9 Additionally, in a Canadian research priority-setting exercise, improving communication between patients and health care providers was the top research uncertainty identified for patients on or nearing dialysis.8
Lifestyle, social, and psychological factors have been identified as important aspects of care in the long-term management of chronic diseases.4 In addition, lifestyle, psychosocial aspects, and maximizing quality of life were identified as important factors in choosing research priorities for patients across all stages of CKD in previous studies performed in diverse geographical regions.8,21-23 Tong et al22 and Urquhart-Secord et al21 found that patients prioritized research questions related to maximizing quality of life and reducing complications of dialysis and CKD. Using an international Delphi survey administered to 1181 patients, caregivers, and health care providers from 73 countries, Evangelidis et al identified a consensus-based list of priority outcomes to be used in future HD trials. These outcomes, including vascular access problems, dialysis adequacy, fatigue, cardiovascular disease, and mortality, are different from the challenges we identified.23 However, patient comments and discussions in previous workshops suggest that patients viewed dialysis adequacy as relating to quality of life, ie, “dialysis that is adequate for enabling patients to feel well” rather than related to urea kinetics.24 In addition, outcomes related to lifestyle and well-being were rated as more important by patients/caregivers than health care professionals.23 The importance of a focus on lifestyle and well-being in clinical care are supported by the challenges we identified in the need for flexibility in HD scheduling, social support programs, and access to financial assistance and social programs (Challenges 5, 6, and 10).
We identified several challenges that related to education and information provision (Challenges 1, 7, 8, and 9). Similarly, improved content and timing of information and improving the ways in which information was provided were identified as social science research priorities in a qualitative study of dialysis and transplant patients in the Netherlands.25 The prioritization of cardiovascular outcomes in the international Delphi study of priority outcomes in HD supports the need we identified for more information regarding health risks and other conditions associated with HD. Similar to findings in our study, the need for better information on renal replacement modalities has been identified previously by the CSCKP, whose specific focus was ensuring that the option of conservative care was included during discussions about modality choice.9 Interestingly, the need for improved information regarding transplant status (Challenge 9) has not been identified in previous priority setting exercises.
To our knowledge, there are no other published patient-oriented studies investigating the challenges to in-center HD care in this manner. Previous studies have focused on prioritizing research outcomes and uncertainties rather than identifying challenges to patient-centered care and have not focused on in-center HD, but rather have included the general CKD population or individuals on all dialysis modalities. A 2015 systematic review by Tong et al7 identified 16 studies investigating research priorities for patients with kidney disease, 5 of which included priorities for dialysis and 3 for HD specifically. As this review focussed on research priorities rather than challenges to care, it is not surprising that the themes identified were different from those in our study.
Although physicians and health care providers have previously identified challenges to information, individualization, and interaction as barriers to effective chronic care, this project was the first to demonstrate that patients and their caregivers also identify these challenges as key to improving the quality of care. In contrast, previous studies have focussed on improving the relevance of research questions rather than clinical care. The CSCKP has previously identified the importance of enhanced information, interaction in terms of shared decision making, and individualization of care in ESKD. We expanded the findings of these investigators by identifying specific challenges within these 3 areas that are important to patients and their caregivers and should be prioritized to improve patient-centered care in HD and increase the engagement of individuals on HD in self-management.
Strengths of our study include use of a priority setting methodology that has been previously used successfully with individuals at various stages of CKD.15 In addition, our steering committee was composed of a diverse group of individuals with in-center HD experience including patients, nephrologists, researchers, and policy makers from across Canada. Importantly, our study methodology was guided by patient partners and results were driven primarily by the information and opinions that we collected from a group of patients from 7 geographically diverse HD centers who were representative of the overall HD population in Canada, their caregivers, and health care providers.26 This strengthens the generalizability of our findings to HD units across Canada. We also translated our survey into French and Chinese to address the cultural diversity of members of the HD community in various centers in Canada (Montreal and Vancouver). Finally, to address concerns around cultural sensitivity, each phase of the study engaged members of the Indigenous community from the Can-SOLVE Indigenous People’s Engagement Council (IPERC), our steering committee includes 2 Indigenous patient partners, and later phases of our study have incorporated an Indigenous research assistant to ensure Indigenous representation and cultural safety in all study meetings and interactions.
The inclusion of only English-speaking participants in the focus groups and priority setting workshop is a limitation of our study. Although the Phase 2 survey was available in French, English, and Chinese, individuals who were not fluent in these languages were excluded, which may limit the generalizability of results in specific cultural contexts. Although other HD surveys have identified similar response rates, our response rate of 31.5% is low compared to that obtained in the general population. Non-responders in previous surveys in the HD population have been significantly different from responders in terms of sex, age, race, and education; thus introducing the possibility of selection bias.27 This is particularly important to acknowledge, as reasons for not participating in the ranking survey are unknown.
Moreover, participants in our priority setting workshop were predominately from in-center HD units in large urban centers and this excluded perspectives from individuals dialyzing in smaller centers and more rural settings. In addition, the results of our study reflect the perspectives of individuals on in-center HD and cannot be translated directly to individuals on home modalities. However, many of the themes identified in the Triple I project could be used as a starting point to explore challenges to care in home dialysis modalities. We collected limited information regarding social determinants of health in our study, a potential confounder of our findings. Importantly, the consensus-based methodology we used to identify the top 10 challenges did not allow us to assess whether there was important regional variation in the ranking or selection of these challenges. Similarly, we were not able to assess whether the ranking of the challenges varied according to other patient characteristics such as age or sex. However, we used a rigorous methodology based on work by the James Lind Alliance and we believe that our top 10 list reflects the consensus of the workshop participants who were represented in diverse geographic regions in Canada. Finally, our study focussed on challenges to HD care in Canadian settings and as such, findings may be limited to the Canadian context. However, similarities in the priorities identified in our study and other international studies suggest that our findings may be relevant in HD settings outside of Canada.22,25 In fact, a recent systematic review that included 260 qualitative studies from around the world noted several similar challenges to our study when assessing the effect of burden of treatment on capacity for CKD self-management. Identified challenges included issues with communication, lack of continuity of care, insufficient information that was difficult to comprehend regarding CKD, financial insecurity, lack of social support, and lack of reliable transportation to and from HD.28
Our findings have both clinical and research implications. Clinicians can begin to explore solutions to the challenges we have identified in their individual HD units to improve patient-centered care. Future research should focus on the development and implementation of innovative solutions to the challenges that we identified. Importantly, such solutions have the potential to improve health status and well-being in patients receiving in-center HD. In this regard, as Wave 2 of the Triple I study begins, we will investigate candidate solutions to the highest priority challenges identified in Wave 1. We recognize that multiple factors such as HD unit funding and staff resources, regional differences in HD care models, technology acceptance and literacy rates, population characteristics and system policies and regulations can all play a role in the ability to implement solutions to the challenges we have identified in individual HD units or renal programs. To facilitate widespread implementation, as we move forward with Wave 2 of the Triple I project, we are working with renal programs across the country to develop solutions that can be adapted to accommodate HD units with diverse needs and characteristics. Potential solutions will be collated, reviewed and refined by the national Triple I steering committee. Once consensus is achieved, several solutions will be evaluated across Triple I study sites in Canada.
In conclusion, using comprehensive patient-centered methodology and a diverse sample of individuals with HD experience, we identified the top challenges to address within in-center HD care. Future stages of the project will aim to improve the quality of care in HD by addressing these challenges using innovative patient-centered solutions that facilitate self-management and engagement of patients and caregivers in their care.
Supplemental Material
Supplemental material, Triple_I_Protocol_Manuscript_Appendix_Phase_2_Ranking_Survey for A Mixed Method Investigation to Determine Priorities for Improving Information, Interaction, and Individualization of Care Among Individuals on In-center Hemodialysis: The Triple I Study by Krista Rossum, Juli Finlay, Michael McCormick, Arlene Desjarlais, Hans Vorster, George Fontaine, Melanie Talson, Priscila Ferreira Da Silva, Kaytlynn V. Soroka, Rachelle Sass, Matthew James, Allison Tong, Claire Harris, Yuriy Melnyk, Manish M. Sood, Neesh Pannu, Rita S. Suri, Karthik Tennankore, Stephanie Thompson, Marcello Tonelli and Clara Bohm in Canadian Journal of Kidney Health and Disease
Footnotes
Ethics Approval and Consent to Participate: The study was approved by the research ethics boards at the main study sites (University of Calgary [REB16-2136] and University of Manitoba [HS20494 [H2017:049] and HS22255 [H2018:411]]) and all subsites (University of Ottawa, University of Alberta, Dalhousie University, University of British Columbia, and Center Hospitalier de l’Université de Montréal). Informed consent was obtained from all participants.
Consent for Publication: All authors consent to publication.
Availability of Data and Materials: The data and materials are available from corresponding author upon reasonable request.
Authors Contributions: K.R., J.F., M.M., A.D., H.V., G.F., M.T., K.S., M.J., A.T., C.H., M.S., N.O., R.S.S., K.T., C.T., and C.B. contributed to research idea and study design. K.R., J.F., A.D., G.F., M.T., P.F.D.S., K.S., C.H., Y.M., M.M.S., N.P., and R.S. contributed to data acquisition. K.R., J.F., M.M., A.D., H.V., G.F., M.T., P.F.D.S., K.S., R.S., C.T., and C.B. contributed to data analysis and interpretation. J.F., C.T., and C.B. were involved in supervision or mentorship. M.M., A.D., H.V., and G.F. were involved as patient partners. Each author contributed important intellectual content during manuscript drafting or revision, accepts personal accountability for the author’s own contributions, and agrees to ensure that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: K.R., J.F., M.M., A.D., H.V., G.F., M.T., P.F.D.S., K.S., M.J., A.T., C.H., Y.M., N.P., S.T., C.T., and C.B. declare that they have no relevant financial interests. Dr. Sood has received speaker fees from AstraZeneca. Dr. Tennankore has received advisory board and/or consulting fees from AstraZeneca, Otsuka, Jansen, and Baxter and unrestricted investigator-initiated grant funding from Astellas and Otsuka.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work is a project of the Canadians Seeking Solutions and Innovations to Overcome Chronic Kidney Disease (Can-SOLVE CKD) Network, which is supported by the Canadian Institutes of Health Research (CIHR) under Canada’s Strategy for Patient-Oriented Research Grant 20R26070. Dr. Tonelli is supported by a Foundation grant from the CIHR. Dr. Suri is supported by a Junior 2 Clinician Research Scholar award from the Fonds de Recherche—Santé (Quebec). Dr. Bohm is supported by the Manitoba Medical Services Foundation F.W. Du Val Clinical Research Professorship.
ORCID iDs: Rita S. Suri  https://orcid.org/0000-0002-0519-3927
https://orcid.org/0000-0002-0519-3927
Clara Bohm  https://orcid.org/0000-0001-7710-7162
https://orcid.org/0000-0001-7710-7162
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Arora P, Vasa P, Brenner D, et al. Prevalence estimates of chronic kidney disease in Canada: results of a nationally representative survey. CMAJ. 2013;185(9):E417-E423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Centers for Disease Control and Prevention. Prevalence of CKD stages 1988-1994 to 2015-2016. https://nccd.cdc.gov/CKD/detail.aspx?Qnum=Q8. Published 2020. Accessed August 15, 2020.
- 3. Chronic diseases in America. https://www.cdc.gov/chronicdisease/resources/infographic/chronic-diseases.htm. Published 2020. Accessed August 15, 2020.
- 4. Clarke JL, Bourn S, Skoufalos A, Beck EH, Castillo DJ. An Innovative approach to health care delivery for patients with chronic conditions. Popul Health Manag. 2017;20(1):23-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Siu AL, Spragens LH, Inouye SK, Morrison RS, Leff B. The ironic business case for chronic care in the acute care setting. Health Aff. 2009;28(1):113-125. [DOI] [PubMed] [Google Scholar]
- 6. Hemmelgarn BR, Pannu N, Ahmed SB, et al. Determining the research priorities for patients with chronic kidney disease not on dialysis. Nephrol Dial Transplant. 2017;32(5):847-854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tong A, Chando S, Crowe S, et al. Research priority setting in kidney disease: a systematic review. Am J Kidney Dis. 2015;65(5):674-683. [DOI] [PubMed] [Google Scholar]
- 8. Manns B, Hemmelgarn B, Lillie E, et al. Setting research priorities for patients on or nearing dialysis. Clin J Am Soc Nephrol. 2014;9(10):1813-1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. O’Hare AM, Armistead N, Schrag WL, Diamond L, Moss AH. Patient-centered care: an opportunity to accomplish the “Three Aims” of the National Quality Strategy in the Medicare ESRD program. Clin J Am Soc Nephrol. 2014;9(12):2189-2194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. America CoQoHi . Crossing the Quality Chasm: A New Health System for the 21st Century. Washington, DC: National Academy of Sciences; 2001. [PubMed] [Google Scholar]
- 11. Williams AW, Dwyer AC, Eddy AA, et al. Critical and honest conversations: the evidence behind the “Choosing Wisely” campaign recommendations by the American Society of Nephrology. Clin J Am Soc Nephrol. 2012;7(10):1664-1672. [DOI] [PubMed] [Google Scholar]
- 12. Jun M, Manns B, Laupacis A, et al. Assessing the extent to which current clinical research is consistent with patient priorities: a scoping review using a case study in patients on or nearing dialysis. Can J Kidney Health Dis. 2015;2:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Barnieh L, Jun M, Laupacis A, Manns B, Hemmelgarn B. Determining research priorities through partnership with patients: an overview. Semin Dial. 2015;28(2):141-146. [DOI] [PubMed] [Google Scholar]
- 14. Listening, Learning and Leading: A Strategic Planning Meeting to Identify Priorities for Research for Canadians With Kidney Disease. Canadians Seeking Solutions and Innovation to Overcome Chronic Kidney Disease Network. Montreal: Canadian Society of Nephrology; 2014. [Google Scholar]
- 15. Cowan K, Oliver S. The James Lind Alliance Guidebook. Oxford, England: James Lind Alliance; 2016. [Google Scholar]
- 16. Levin A, Adams E, Barrett BJ, et al. Canadians Seeking Solutions and Innovations to Overcome Chronic Kidney Disease (Can-SOLVE CKD): form and function. Can J Kidney Health Dis. 2018;5:1-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hsieh H, Shannon S. Three approaches to qualitative content analysis. Qual Health Res. 2005;15(9):1277-1288. [DOI] [PubMed] [Google Scholar]
- 18. Nvivo Qualitative Data Analysis. 11 ed. Chadstone, VIC, Australia: QSR International Pty Ltd; 2012. [Google Scholar]
- 19. Canadian Institute for Health Information (CIHI). End-stage Renal Disease among Aboriginal Peoples in Canada: Treatment and Outcomes. Ottawa, ON: CIHI; 2013. [Google Scholar]
- 20. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377-381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Urquhart-Secord R, Craig JC, Hemmelgarn B, et al. Patient and caregiver priorities for outcomes in hemodialysis: an international nominal group technique study. Am J Kidney Dis. 2016;68(3):444-454. [DOI] [PubMed] [Google Scholar]
- 22. Tong A, Sainsbury P, Carter SM, et al. Patients’ priorities for health research: focus group study of patients with chronic kidney disease. Nephrol Dial Transplant. 2008;23(10):3206-3214. [DOI] [PubMed] [Google Scholar]
- 23. Evangelidis N, Tong A, Manns B, et al. Developing a set of core outcomes for trials in hemodialysis: an international Delphi survey. Am J Kidney Dis. 2017;70:464-475. [DOI] [PubMed] [Google Scholar]
- 24. Tong A, Manns B, Hemmelgarn B, et al. Establishing core outcome domains in hemodialysis: report of the standardized outcomes in nephrology-hemodialysis (song-HD) consensus workshop. Am J Kidney Dis 2017;69(1):97-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Schipper K, Abma TA. Coping, family and mastery: top priorities for social science research by patients with chronic kidney disease. Nephrol Dial Transplant. 2011;26(10):3189-3195. [DOI] [PubMed] [Google Scholar]
- 26. Canadian Institute for Health Information (CIHI). Treatment of End-stage Organ Failure in Canada, Canadian Organ Replacement Register, 2009 to 2018: End-stage Kidney Disease and Kidney Transplants—Data Tables. Ottawa, ON: CIHI; 2019. [Google Scholar]
- 27. Dad T, Tighiouart H, Fenton JJ, et al. Evaluation of non-response to the In-Center Hemodialysis Consumer Assessment of Healthcare Providers and Systems (ICH CAHPS) survey. BMC Health Services Research. 2018;18(1):790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Roberti J, Cummings A, Myall M, et al. Work of being an adult patient with chronic kidney disease: a systematic review of qualitative studies. BMJ Open. 2018;8(9):e023507. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Triple_I_Protocol_Manuscript_Appendix_Phase_2_Ranking_Survey for A Mixed Method Investigation to Determine Priorities for Improving Information, Interaction, and Individualization of Care Among Individuals on In-center Hemodialysis: The Triple I Study by Krista Rossum, Juli Finlay, Michael McCormick, Arlene Desjarlais, Hans Vorster, George Fontaine, Melanie Talson, Priscila Ferreira Da Silva, Kaytlynn V. Soroka, Rachelle Sass, Matthew James, Allison Tong, Claire Harris, Yuriy Melnyk, Manish M. Sood, Neesh Pannu, Rita S. Suri, Karthik Tennankore, Stephanie Thompson, Marcello Tonelli and Clara Bohm in Canadian Journal of Kidney Health and Disease