Abstract
Background
The Infectious Diseases Society of America influenza guidelines no longer require fever as part of their influenza case definition in patients requiring hospitalization. However, the impact of fever or lack of fever on clinical decision-making and patient outcomes has not been studied.
Methods
We conducted a retrospective review of adult patients admitted to our tertiary health service between April 2016 and June 2019 with laboratory-confirmed influenza, with and without fever (≥37.8ºC). Patient demographics, presenting features, and outcomes were analyzed using Pearson’s chi-square test, the Wilcoxon rank-sum test, and logistic regression.
Results
Of 578 influenza inpatients, 219 (37.9%) had no fever at presentation. Fever was less likely in individuals with a nonrespiratory syndrome (adjusted odds ratio [aOR], 0.44; 95% CI, 0.26–0.77), symptoms for ≥3 days (aOR, 0.53; 95% CI, 0.36–0.78), influenza B infection (aOR, 0.45; 95% CI, 0.29–0.70), chronic lung disease (aOR, 0.55; 95% CI, 0.37–0.81), age ≥65 (aOR, 0.36; 95% CI, 0.23–0.54), and female sex (aOR, 0.69; 95% CI, 0.48–0.99). Patients without fever had lower rates of testing for influenza in the emergency department (64.8% vs 77.2%; P = .002) and longer inpatient stays (median, 2.4 vs 1.9 days; P = .015). These patients were less likely to receive antiviral treatment (55.7% vs 65.6%; P = .024) and more likely die in the hospital (3.2% vs 0.6%; P = .031), and these differences persisted after adjustment for potential confounders.
Conclusions
Absence of fever in influenza is associated with delayed diagnosis, longer length of stay, and higher mortality.
Keywords: diagnosis, fever, influenza, influenza-like illness
Prompt recognition of influenza in patients who require admission to the hospital is important to allow initiation of targeted antiviral therapy and minimize the risks of transmission to staff and other patients. However, influenza can be challenging to diagnose due to the range of clinical manifestations and considerable symptomatic overlap with other conditions [1–3]. To assist with the targeting of diagnostic investigations, hospitals may routinely test patients meeting a clinical case definition.
Case definitions for influenza may vary between institutions but typically require fever in addition to respiratory or systemic symptoms. The widely used Centers for Disease Control and Prevention (CDC) definition of influenza-like illness (ILI) is a fever of 37.8°C or higher in conjunction with cough and/or sore throat. However, the clinical manifestations of influenza infection in comorbid, elderly, and immunosuppressed individuals are less likely to include fever [4–6]. Including fever as an essential element of a case definition may therefore lower its sensitivity, leading to missed diagnoses and delays to treatment [7].
In this study, we investigated patient factors and clinical outcomes in adults hospitalized with influenza infection with and without fever at the time of arrival.
METHODS
Setting
This retrospective cohort study was conducted at The Royal Melbourne Hospital, a single-center, 571-bed tertiary-referral health service in metropolitan Melbourne. The study site sees ~80 000 emergency presentations per year, leading to 40 000 admissions. Influenza testing is performed in the hospital pathology laboratory by respiratory virus polymerase chain reaction (PCR). During the study period, local policy recommended respiratory PCR testing for patients presenting with ILI (Supplementary Box 1). However, patients need not have met a strict case definition to be tested, and investigation for influenza could occur at the discretion of the treating physician. Point-of-care testing was not in use during the study period.
Study Population
Adult inpatients with laboratory-confirmed influenza between January 2016 and June 2019 who had been previously identified as part of the Influenza Complications Action Network (FluCAN) program were considered for inclusion. FluCAN is a surveillance program that operates at 16 health services across Australia between April and October, collecting clinical information on inpatients with influenza.
Study inclusion criteria were (1) admission to the hospital via the emergency department (ED) AND (2) influenza A or B identified by respiratory PCR testing AND (3) recruited to the FluCAN cohort (noting that patients were not included in FluCAN if they presented out of the influenza season or had been discharged from the hospital before being identified). Exclusion criteria were (1) respiratory PCR test performed >72 hours after arrival OR (2) admission following interhospital transfer. Exclusion criteria were to avoid inclusion of patients with nosocomial infection, which may have different clinical characteristics at the time of hospital presentation.
Data Collection and Definitions
Detailed clinical information for each patient regarding presenting syndrome, comorbidities, chest x-ray findings, and antiviral therapy is collected as part of FluCAN recruitment. Additional information including ED observations, microbiological test timing, length of stay, discharge diagnoses, and complications were extracted from hospital databases and linked using unique patient identifiers. Patient comorbidities were considered present if captured by the FluCAN standard collection instrument [8] or ICD-10 coding algorithms for Charlson Comorbidity Index (CCI) score [9]. Patients at increased risk for influenza complications (age >65, pregnant, Aboriginal or Torres Strait Islander, aged care resident, immunosuppressed, obese or underlying cardiorespiratory or neurological disease) were defined as per the Australian Therapeutic Guidelines: Antibiotic [10].
Patients were categorized based on the presence or absence of fever at the time of hospital presentation. Fever was defined as a temperature of ≥37.8°C recorded at any time while still in the ED. For patients arriving via the ED but transferred directly to the intensive care unit (ICU), the highest temperature recorded in the first 4 hours after ICU admission was used.
Demographics, comorbidities, and outcomes were compared between patients with and without fever at presentation. Outcomes considered were whether respiratory PCR testing occurred in the ED (ie, before patient transfer to an inpatient ward), testing delay (the number of hours between patient arrival and receipt of a respiratory swab at the microbiology laboratory), ED diagnosis (influenza vs noninfluenza), length of stay, oseltamivir treatment, ICU admission, organ failure (kidney injury, shock, or respiratory failure), death, and re-presentation within 30 days.
Statistical Analysis
Categorical variables are summarized with counts and percentages, and continuous variables with means and standard deviations or medians and interquartile ranges, as appropriate. Differences between patients with and without fever were evaluated using Pearson’s chi-square test or the Fisher exact test for categorical variables and the Wilcoxon rank-sum test for continuous variables. Hypothesis testing was conducted at the 5% significance level.
Multivariate logistic regression analysis was performed to investigate patient factors associated with fever at the time of hospital presentation. The following clinically relevant variables were chosen a priori: age (<65 or ≥65 years), sex, the presence or absence of specific comorbidities (cardiac, respiratory, neurological, renal, or liver disease, immunosuppression, diabetes, malignancy), current smoking status, duration of illness before hospital presentation (<3 days or ≥3 days), respiratory vs nonrespiratory presenting syndrome, the presence of consolidation on chest x-ray, and influenza virus type identified. Variables with a P value of <.1 on univariate analysis were subsequently included in a multivariate model. A subgroup analysis was performed for patients for whom detailed vaccination history was available.
Age and duration of illness were treated as categorical variables in the above analysis. A threshold of 65 years for age was chosen based on its established increase in risk of influenza complications [10]. A threshold of 3 days for duration of illness was chosen based on published data regarding mean temperatures in influenza infection over time [11]. Potential associations of presenting temperature with age and symptom duration as continuous variables were investigated separately using scatterplots with linear model fitting.
Multivariate regression was used to evaluate the impact of fever on outcomes after adjusting for potential confounders. For each of the outcomes—(1) respiratory PCR testing in the ED, (2) receipt of oseltamivir, (3) ICU admission, (4) 30-day re-presentation, and (5) mortality—separate models were built, with each model including the following independent variables: age (<65 years or ≥65 years), sex (male or female), CCI score, risk factors for influenza complications (present or absent), immunosuppression (present or absent), and duration of illness (<3 days or ≥3 days).
Analysis was performed using the statistical computing language R, version 3.6.1 [12].
Melbourne Health Human Research Ethics Committee approval was obtained before commencement, (QA2019071). A waiver of consent was provided for the overall study, noting that all patients recruited to FluCAN had provided individual verbal consent for data collection [8].
RESULTS
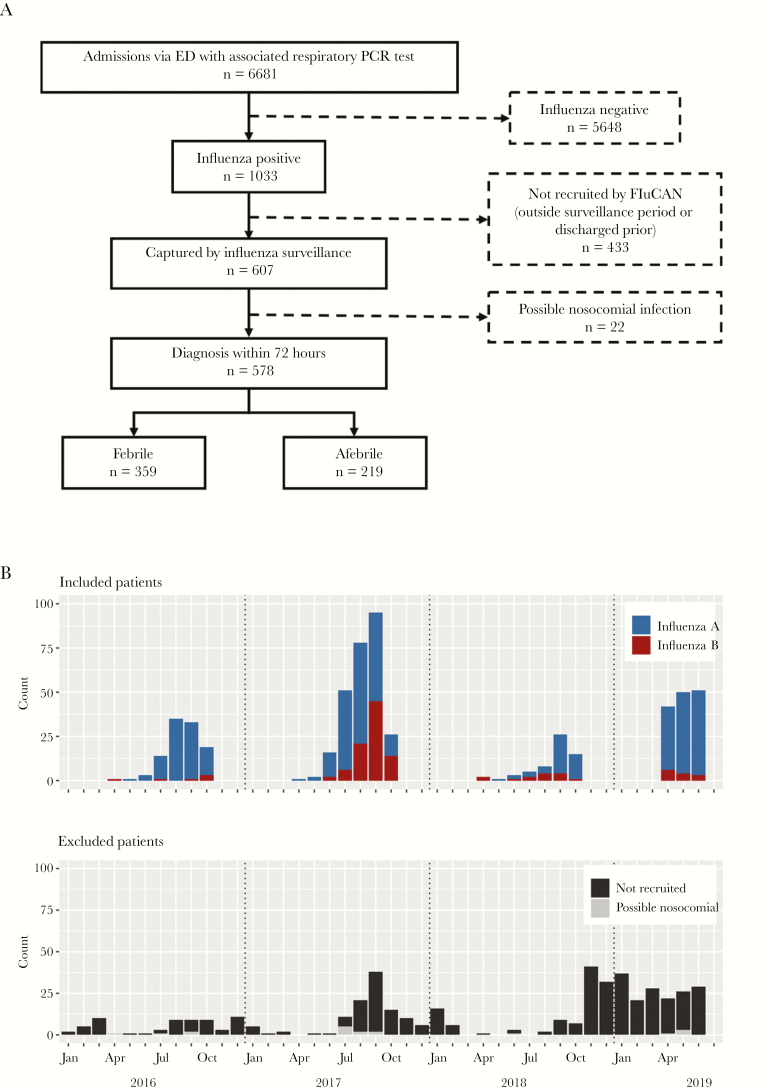
Of 1033 patients with influenza during the study period, 578 were included (Figure 1A). The 2017 influenza season contributed the largest number of patients to the study, with ~40% of the total influenza A patients and 73% of the total influenza B patients recruited from this year (Figure 1B).
Figure 1. .
Inclusion and exclusion of the patient cohort. A, Consort diagram. B, Number of patients included and excluded in each month during the study period (January 2016—June 2019) according to influenza subtype and reason for exclusion. Abbreviations: ED, emergency department; PCR, polymerase chain reaction.
Of the 455 patients excluded, 22 had suspected nosocomial influenza, 236 arrived out of season, and 197 were discharged before they could be recruited. Notably, the median hospital length of stay for this last group was 12 hours, compared with a median of 50 hours for patients who were recruited. Excluded patients were on average younger (median age, 53 vs 66 years; P < .001) and less likely to have 1 or more medical comorbidities (28% vs 43%; P < .001) but had similar rates of fever (60% vs 62%; P = .531).
Of the 578 included patients, 359 (62.1%) had a temperature ≥37.8°C recorded while in the ED. There were slight annual variations, with 62%, 59%, 67%, and 66% of patients presenting with fever in 2016, 2017, 2018, and 2019, respectively.
Patients without fever were on average older, had higher CCI scores, and had higher rates of chronic cardiorespiratory disease (Table 1). They were more likely to present later in the illness course and with a nonrespiratory syndrome. Although influenza A was the most common viral subtype in both groups, those without fever were more likely to have influenza B infection.
Table 1. .
Number (Percentage) of Hospitalized Influenza Patients With and Without Fever According to Virus Type, Age, Sex, Comorbid Conditions, and Features of Clinical Presentation
| Total n = 578 |
Fever n = 359 |
No Fever n = 219 |
P Value | |
|---|---|---|---|---|
| Virus | ||||
| Influenza A | 457 | 301 (65.9) | 156 (34.1) | <.001 |
| Influenza B | 121 | 58 (47.9) | 63 (52.1) | |
| Age | ||||
| Age <65 y | 275 | 206 (74.9) | 69 (25.1) | <.001 |
| Age 65–80 y | 157 | 81 (51.6) | 76 (48.4) | |
| Age >80 y | 146 | 72 (49.3) | 74 (50.7) | |
| Sex | ||||
| Male | 284 | 189 (66.5) | 95 (33.5) | .038 |
| Female | 294 | 170 (57.8) | 124 (42.2) | |
| Charlson Comorbidity Index score | ||||
| CCI 0 | 337 | 231 (68.5) | 106 (31.5) | <.001 |
| CCI 1–2 | 187 | 103 (55.1) | 84 (44.9) | |
| CCI >2 | 54 | 25 (46.3) | 29 (53.7) | |
| Comorbiditiesa | ||||
| Respiratory disease | 181 | 91 (50.3) | 90 (49.7) | <.001 |
| Diabetes | 151 | 86 (57.0) | 65 (43.0) | .155 |
| Malignancy | 58 | 31 (53.4) | 27 (46.6) | .197 |
| Liver disease | 22 | 13 (59.1) | 9 (40.9) | .824 |
| Cardiac disease | 181 | 100 (55.2) | 81 (44.8) | .028 |
| Neurological disease | 111 | 61 (55.0) | 50 (45.0) | .105 |
| Renal disease | 75 | 39 (52.0) | 36 (48.0) | .071 |
| Immunosuppression | 94 | 55 (58.5) | 39 (41.5) | .503 |
| Pregnant | 5 | 5 (100) | 0 (0) | .162 |
| Current smoker | 66 | 42 (63.6) | 24 (36.4) | .891 |
| Risk factors for complications | 407 | 234 (57.5) | 173 (42.5) | .001 |
| Presenting features | ||||
| Respiratory illness | 506 | 324 (64.0) | 182 (36.0) | .017 |
| Nonrespiratory illness | 72 | 35 (48.6) | 37 (51.4) | |
| Symptom duration 0–2 d | 224 | 162 (72.3) | 62 (27.7) | <.001 |
| Symptom duration >2 d | 354 | 197 (55.6) | 157 (44.4) | |
| Chest x-ray consolidationb | 87 | 57 (65.5) | 30 (34.5) | .555 |
Data are presented as No. (%).
Abbreviations: CCI, Charlson Comorbidity Index; ICD-10, International Classification of Diseases, Tenth Revision.
aComorbidities were defined as present if captured by the FluCAN standard collection instrument [8] or ICD-10 coding algorithms for Charlson Comorbidity Index score [9].
bBased on formal radiologist report of imaging.
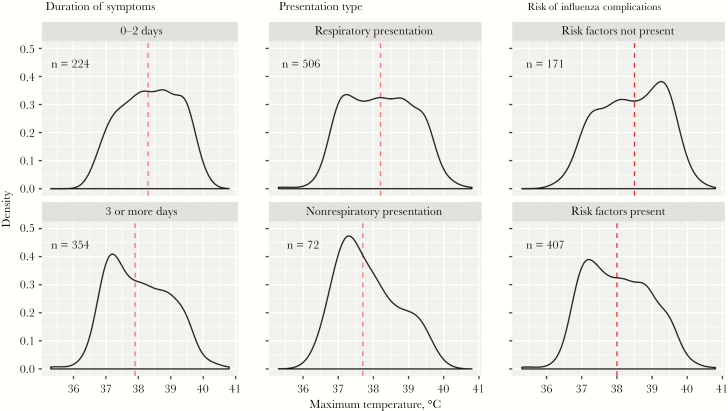
Median maximum temperatures were lower in individuals with symptoms for 3 or more days, a nonrespiratory syndrome, and specific risk factors for complications of influenza (Figure 2). When treated as a continuous variable, maximum temperature was negatively correlated with age and symptom duration (Supplementary Figure 1).
Figure 2. .
Density plot of maximum temperature recorded on presentation for patients requiring hospitalization with influenza, grouped by duration of symptoms, presentation type, and presence or absence of risk factors for influenza complications. Median temperature is indicated by the dashed line. Maximum temperature is defined as the highest temperature recorded at any time while in the emergency department or, for patients transferred directly to the intensive care unit, in the first 4 hours after arrival.
Influenza patients who presented without fever had a longer median time to diagnostic testing and were less likely to have this testing completed before leaving the ED (Table 2). Median hospital length of stay was also 12 hours longer when compared with influenza patients with fever. There was no difference in median ED stay (total time spent in the emergency department) for patients with and without fever (Table 2).
Table 2. .
Differences in Clinical Management and Outcomes in Patients Hospitalized With Influenza With and Without Fever at the Time of Arrival
| Febrile Group n = 359 |
Afebrile Group n = 219 |
P Value | |
|---|---|---|---|
| Testing and diagnosis | |||
| ED stay,a median (IQR), h | 5.9 (3.8–11.2) | 5.9 (3.8–9.3) | .683 |
| Diagnostic testing in EDb | 277 (77.2) | 142 (64.8) | .002 |
| Testing delay,c median (IQR), h | 2.9 (1.3–6.1) | 3.7 (2.0–8.4) | .001 |
| ED diagnosis of influenza | 81 (22.6) | 22 (10.0) | <.001 |
| Outcomes | |||
| Length of stay, median (IQR), d | 1.9 (1.0–4.0) | 2.4 (1.3–4.5) | .015 |
| Oseltamivir treatment | 235 (65.5) | 122 (55.7) | .024 |
| Intensive care unit stayd | 28 (7.8) | 19 (8.7) | .828 |
| Organ failuree | 51 (14.2) | 40 (18.3) | .237 |
| In-hospital mortality | 2 (0.6) | 7 (3.2) | .031 |
| 30-d re-presentation | 29 (8.1) | 19 (8.7) | .922 |
Data are presented as No. (%) unless otherwise indicated.
Abbreviations: ED, emergency department; ICU, intensive care unit; IQR, interquartile range; PCR, polymerase chain reaction.
aTotal time spent in the emergency department.
bRespiratory PCR testing performed before patient transfer from ED to inpatient ward.
cThe time from patient presentation to receipt of a respiratory specimen by the microbiology laboratory.
dRequirement for ICU support at any time during hospital admission.
eRespiratory failure, shock, or acute kidney injury diagnosed during hospital stay.
Although the afebrile cohort was on average older and more likely to have specific risk factors for complications of influenza, they were also less likely to receive targeted antiviral therapy. The mortality rate—though low overall—was higher in patients without fever. There were no statistically significant differences in other markers of disease severity such as organ failure or ICU admission.
Multivariate Analysis
The results of univariate and multivariate analysis are shown in Table 3. Fever was less likely in patients over 65 years of age (adjusted odds ratio [aOR], 0.36; 95% CI, 0.23–0.54) and those with symptoms for 3 or more days at the time of presentation (aOR, 0.53; 95% CI, 0.36–0.78). There were also lower rates of fever in those with a history of chronic respiratory disease (aOR, 0.55; 95% CI, 0.37–0.81) and influenza B infection (aOR, 0.45; 95% CI, 0.29–0.70). Fever was more likely in individuals who presented with a typical respiratory manifestation of influenza compared with those with a nonrespiratory presentation (aOR, 2.25; 95% CI, 1.29–3.92).
Table 3. .
A, Univariate Analysis of Patient Factors Associated With Fever at the Time of Hospital Presentation With Influenza. B, Variables With P <.1 Were Subsequently Included in Multivariate Model
| A, Univariate Analysis | B, Multivariate Analysis | |||
|---|---|---|---|---|
| OR (95% CI) | P Value | aOR (95% CI) | P Value | |
| Demographics | ||||
| Age ≥65 y | 0.34 (0.24–0.49) | <.001 | 0.36 (0.23–0.54) | <.001 |
| Female sex | 0.69 (0.49–0.97) | .031 | 0.69 (0.48–0.99) | .046 |
| Comorbiditiesa | ||||
| Cardiac disease | 0.66 (0.46–0.94) | .022 | 1.11 (0.71–1.73) | .65 |
| Respiratory disease | 0.49 (0.34–0.7) | <.001 | 0.55 (0.37–0.81) | .003 |
| Neurological disease | 0.69 (0.46–1.05) | .085 | 0.99 (0.62–1.57) | .958 |
| Renal disease | 0.62 (0.38–1.01) | .055 | 0.98 (0.56–1.71) | .939 |
| Immunosuppression | 0.84 (0.53–1.31) | .432 | ||
| Liver disease | 0.88 (0.37–2.09) | .766 | ||
| Malignancy | 0.67 (0.39–1.16) | .154 | ||
| Diabetes | 0.75 (0.51–1.09) | .129 | ||
| Current smoker | 1.08 (0.63–1.83) | .786 | ||
| Presenting features | ||||
| Respiratory illness | 1.88 (1.15–3.09) | .013 | 2.25 (1.29–3.92) | .004 |
| Symptom duration ≥3 d | 0.48 (0.34–0.69) | <.001 | 0.53 (0.36–0.78) | .001 |
| Influenza B infection | 0.48 (0.32–0.72) | <.001 | 0.45 (0.29–0.70) | <.001 |
| Chest x-ray consolidationb | 1.19 (0.74–1.92) | .478 | ||
An OR <1 indicates reduced odds of having fever.
Abbreviations: aOR, adjusted odds ratio; ICD-10, International Classification of Diseases, Tenth Revision; OR, odds ratio.
aComorbidities were defined as present if captured by the FluCAN standard collection instrument [8] or ICD-10 coding algorithms for Charlson Comorbidity Index scores [9].
bBased on formal radiologist imaging report.
After adjusting for the potential confounding effects of age, comorbid conditions, and illness duration, patients presenting without fever were still less likely to have diagnostic testing performed in the ED (aOR, 0.51; 95% CI, 0.34–0.76), less likely to receive antiviral therapy (aOR, 0.51; 95% CI, 0.34–0.75), and more likely to die in the hospital (aOR, 6.36; 95% CI, 1.22–33.2) (Table 4). There was no statistical difference in rates of ICU admission or re-presentation.
Table 4.
Odds Ratios of Secondary Outcomes for Influenza Patients who Present Without Fever After Adjustment for Age (<65 Years or ≥65 Years), Sex, Charlson Comorbidity Index Score, Risk Factors for Influenza Complications, Immunosuppression, and Duration of Illness (<3 Days or ≥3 Days)
| Outcomes | aOR (95% CI) | P Value |
|---|---|---|
| Diagnostic testing in EDa | 0.51 (0.34–0.76) | <.001 |
| Oseltamivir treatment | 0.51 (0.34–0.75) | <.001 |
| Intensive care unit stayb | 1.13 (0.59–2.16) | .709 |
| 30-d re-presentation | 0.97 (0.51–1.84) | .927 |
| In-hospital mortality | 6.36 (1.22–33.2) | .028 |
An OR <1 indicates less likely to have a specified outcome. Full models are included in Supplementary Tables 1–5.
Abbreviations: aOR, adjusted odds ratio; ED, emergency department; ICU, intensive care unit; PCR, polymerase chain reaction.
aRespiratory PCR testing performed before patient transfer from emergency department to inpatient ward.
bRequirement for ICU support at any time during hospital admission.
Of the 578 patients included in the study, detailed information regarding prior influenza vaccination was available for 262. On univariate analysis, current vaccination was associated with lower rates of fever at presentation (OR, 0.47; 95% CI, 0.29–0.79). However, the vaccinated cohort was significantly older than those who were unvaccinated (median, 78 vs 54 years), and this difference in fever rates between the groups did not persist after correction for age.
DISCUSSION
Many patients who require hospitalization with influenza will present without fever as part of their clinical syndrome. In this setting, the use of traditional case definitions may lead to delayed or missed diagnoses. Here we outline a range of clinical factors impacting the likelihood of fever in influenza that clinicians should consider when formulating a diagnosis. While we have found evidence to support some of these associations in the existing literature, we believe that this is the first study to compile these findings. Most significantly, here we add to existing knowledge by demonstrating the impact of presenting temperature in influenza on diagnosis, clinical management, and a range of patient outcomes.
It is well established that fever is a less prominent feature of influenza in older and immunosuppressed individuals [5, 6]. As we have shown here—and consistent with the natural history of influenza infection—fever is more likely to be absent in individuals who present later in the disease course. Our study also suggests lower rates of fever in females, those who present with a nonrespiratory syndrome, patients who have underlying chronic lung disease, and those with influenza B infection.
While differences in the endocrine and immunological responses between men and women are recognized, our overall understanding of gender-specific differences in the response to influenza infection is limited [13]. However, both increased vaccine uptake [14] and efficacy [15] have been reported for females and could be contributing factors.
The observed lower rates of fever in patients with underlying respiratory disease are consistent with a previous case–control study of 369 patients from the United States [16] that demonstrated poor sensitivity of ILI for asthmatic patients with influenza, largely due to the absence of fever. The authors in this study speculated about the potential modulatory effects of steroids in this group. The same authors also reported a poor sensitivity for ILI in hospitalized patients with other chronic conditions where a high proportion were using medications with antipyretic effects [4]. The impact of smoking is less clear. Although a previous study of 158 Chinese health care workers with influenza suggested higher rates of fever in smokers, we did not find such an association here [17].
Patients with influenza B infection were significantly less likely to present with fever. Although influenza A and B are associated with comparable clinical outcomes in the inpatient setting [18], there may be intrinsic differences in these infections that impact rates of fever and health care attendance.
A Japanese study of 196 patients who presented to the hospital in the first 3 days of influenza infection found higher mean temperatures in patients with H3N2 infection compared with H1N1 or influenza B [19]. Although viral subtyping was not performed in our study, H3N2 is known to have been predominant in 3 out of the 4 influenza seasons and could therefore contribute to the differences between our influenza A and influenza B patients. Additionally, differences in rates of health care attendance in the first few days of illness (when fever is most likely to be present) have been reported between influenza A and influenza B [20]. In our cohort, patients with influenza A were twice as likely to present within the first 3 days of illness (43.3% vs 21.5%).
Of concern, individuals who would benefit most from treatment may be at greatest risk of being misdiagnosed using current case definitions. Patients with risk factors for complications of influenza had both a lower median maximum temperature than those without (Figure 2) and lower rates of fever (Table 1). Previous research would support this finding. In a prospective cohort study of 270 patients with specific risk factors for complications, the CDC ILI definition had a sensitivity of 31% for identifying patients with influenza, despite the use of either fever or respiratory complaint as inclusion criteria [3]. In our cohort, the sensitivity was 56% if using a fever at any time in the ED and 30% if using just the triage temperature as in the aforementioned study.
Our study suggests that the presence or absence of fever at the time of hospital presentation has an impact on clinical decision-making regarding diagnosis and treatment. Documented fever was associated with earlier testing for influenza and an increased likelihood that testing was completed before leaving the ED. The overall rate of ED diagnosis of influenza was low when compared with the proportion who underwent testing in the ED, suggesting that results were not always available before patient transfer to an inpatient ward. However, patients with fever were relatively more likely to have been correctly diagnosed (22% vs 10%), which may be explained by earlier testing in this group or increased clinician confidence in making a clinical diagnosis when fever is present.
Patients without fever were less likely to receive antiviral therapy despite being more likely to have risk factors for influenza complications. Overall, oseltamivir use was low, given recommendations for treatment in any hospitalized influenza patient regardless of duration of symptoms [21]. However, in-hospital prescription of antivirals varies widely in the literature from 19.5% to 93.2% [22–27]. Persisting misconceptions around the benefits of treatment beyond 48 hours may be a factor [23].
Patients presenting without fever were hospitalized longer (median length of stay, 2.4 vs 1.9 days; P = .015) and were more likely to die in the hospital even after controlling for confounders (Table 4). There was no statistically significant difference in other markers of disease severity such as organ failure, ICU admission, or 30-day representation. While delayed diagnosis may explain part of the increased mortality in our afebrile group, there may be other factors not accounted for here. The presence or absence of fever could reflect the robustness of the immune response to infection and may therefore be a prognostic marker. In the setting of sepsis, for example, failure to develop fever has been shown to be more common in individuals who die compared with survivors [28]. Alternatively, there may be differences in health care–seeking behavior, with patients without fever waiting longer before presenting to the hospital [29].
There are some limitations to our study. This was a single-center study, and testing practice may vary between institutions. The retrospective nature means that there may be other confounders not identified. Case detection was reliant on clinician-initiated testing, and there may be individuals with influenza who were never identified. As we have not examined the medical record for each patient in detail, we cannot comment on the impact of patient-reported fever. Patients who remained in the hospital longer were more likely to be recruited, and therefore our results may be biased toward more severe infections. Finally, as the primary data collection for our study was limited to the typical influenza season in Australia, the results may not be generalizable to “out-of-season” infections.
Despite the limitations of classic ILI definitions for case identification in the hospital setting, our study suggests that the presence or absence of fever continues to influence clinical decision-making, with potential downstream effects for a range of patient outcomes.
The recently updated Infectious Diseases Society of America guidelines for seasonal influenza have de-emphasized the importance of fever in screening decisions and now recommend testing for all patients admitted to the hospital with a respiratory illness during influenza season [21]. Our research supports this change. Given the implications for diagnosis, treatment, and survival in patients with influenza, it is important to ensure that this change in guidelines translates into a change in clinical practice.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
The authors would like to thank Khanh Ho, data analyst at Royal Melbourne Hospital.
Financial support. S.Y.C.T. was supported by an Australian National Health and Medical Research Council Career Development Fellowship (#1145033).
Potential conflicts of interest. All authors: no reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Monmany J, Rabella N, Margall N, et al. Unmasking influenza virus infection in patients attended to in the emergency department. Infection 2004; 32:89–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Miller MR, Peters TR, Suerken CK, et al. Predictors of influenza diagnosis among patients with laboratory-confirmed influenza. J Infect Dis 2015; 212:1604–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dugas AF, Valsamakis A, Atreya MR, et al. Clinical diagnosis of influenza in the ED. Am J Emerg Med 2015; 33:770–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Babcock HM, Merz LR, Fraser VJ. Is influenza an influenza-like illness? Clinical presentation of influenza in hospitalized patients. Infect Control Hosp Epidemiol 2006; 27:266–70. [DOI] [PubMed] [Google Scholar]
- 5. Falsey AR, Baran A, Walsh EE. Should clinical case definitions of influenza in hospitalized older adults include fever? Influenza Other Respir Viruses 2015; 9(Suppl 1):23–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Apewokin S, Vyas K, Lester LK, et al. Influenza A outbreak in an ambulatory stem cell transplant center. Open Forum Infect Dis 2014; 1:XXX–XX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hartman L, Zhu Y, Edwards KM, et al. Underdiagnosis of influenza virus infection in hospitalized older adults. J Am Geriatr Soc 2018; 66:467–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kelly PM, Kotsimbos T, Reynolds A, et al. FluCAN 2009: initial results from sentinel surveillance for adult influenza and pneumonia in eight Australian hospitals. Med J Aust 2011; 194:169–74. [DOI] [PubMed] [Google Scholar]
- 9. Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care 2005; 43:1130–9. [DOI] [PubMed] [Google Scholar]
- 10.Therapeutic Guidelines Limited. Treatment of influenza for individual benefit. In: eTG complete (antibiotic). 2019. Available at: https://tgldcdp.tg.org.au.acs.hcn.com.au/viewTopic?topicfile=influenza&guidelineName=Antibiotic#toc_d. Accessed 12 March 2019. [Google Scholar]
- 11. Lau LL, Cowling BJ, Fang VJ, et al. Viral shedding and clinical illness in naturally acquired influenza virus infections. J Infect Dis 2010; 201:1509–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. R Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2019. Available at: https://www.R-project.org/. Accessed 20 July 2019. [Google Scholar]
- 13. Gabriel G, Arck PC. Sex, immunity and influenza. J Infect Dis 2014; 209(Suppl 3):S93–9. [DOI] [PubMed] [Google Scholar]
- 14.Australian Institute of Health and Welfare. 2009 Adult Vaccination Survey. Canberra, Australia: Australian Institute of Health and Welfare; 2011. Available at: https://www.aihw.gov.au/. Accessed 5 April 2020. [Google Scholar]
- 15. Chambers C, Skowronski DM, Rose C, et al. Should sex be considered an effect modifier in the evaluation of influenza vaccine effectiveness? Open Forum Infect Dis 2018; 5:XXX–XX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Babcock HM, Merz LR, Dubberke ER, Fraser VJ. Case-control study of clinical features of influenza in hospitalized patients. Infect Control Hosp Epidemiol 2008; 29:921–6. [DOI] [PubMed] [Google Scholar]
- 17. Chughtai AA, Wang Q, Dung TC, Macintyre CR. The presence of fever in adults with influenza and other viral respiratory infections. Epidemiol Infect 2017; 145:148–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Su S, Chaves SS, Perez A, et al. Comparing clinical characteristics between hospitalized adults with laboratory-confirmed influenza A and B virus infection. Clin Infect Dis 2014; 59:252–5. [DOI] [PubMed] [Google Scholar]
- 19. Kaji M, Watanabe A, Aizawa H. Differences in clinical features between influenza A H1N1, A H3N2, and B in adult patients. Respirology 2003; 8:231–3. [DOI] [PubMed] [Google Scholar]
- 20. Irving SA, Patel DC, Kieke BA, et al. Comparison of clinical features and outcomes of medically attended influenza A and influenza B in a defined population over four seasons: 2004–2005 through 2007–2008. Influenza Other Respir Viruses 2012; 6:37–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Uyeki TM, Bernstein HH, Bradley JS, et al. Clinical practice guidelines by the Infectious Diseases Society of America: 2018 update on diagnosis, treatment, chemoprophylaxis, and institutional outbreak management of seasonal influenza. Clin Infect Dis 2018; 68:e1–e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. McGeer A, Green KA, Plevneshi A, et al. ; Toronto Invasive Bacterial Diseases Network Antiviral therapy and outcomes of influenza requiring hospitalization in Ontario, Canada. Clin Infect Dis 2007; 45:1568–75. [DOI] [PubMed] [Google Scholar]
- 23. Herman M, Smieja M, Carruthers S, Loeb M. Oseltamivir use amongst hospitalized patients infected with influenza. Influenza Other Respir Viruses 2014; 8:547–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Appiah GD, Chaves SS, Kirley PD, et al. Increased antiviral treatment among hospitalized children and adults with laboratory-confirmed influenza, 2010–2015. Clin Infect Dis 2017; 64:364–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lindegren ML, Griffin MR, Williams JV, et al. Antiviral treatment among older adults hospitalized with influenza, 2006–2012. PLoS One 2015; 10:e0121952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Parkash N, Beckingham W, Andersson P, Kelly P, Senanayake S, Coatsworth N. Hospital-acquired influenza in an Australian tertiary centre 2017: a surveillance based study. BMC Pulm Med 2019; 19:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Macesic N, Kotsimbos TC, Kelly P, Cheng AC. Hospital-acquired influenza in an Australian sentinel surveillance system. Med J Aust 2013; 198:370–2. [DOI] [PubMed] [Google Scholar]
- 28. Knaus WA, Sun X, Nystrom O, Wagner DP. Evaluation of definitions for sepsis. Chest 1992; 101:1656–62. [DOI] [PubMed] [Google Scholar]
- 29. Sopirala MM, Haas DM, Ali NA, et al. Effect of fever on hospital presentation, diagnosis, and treatment in patients with H1N1/09 influenza. J Hosp Med 2013; 8:20–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.