Abstract
Trichloroethylene exposure is a major risk factor for pulmonary veno-occlusive disease. We demonstrated that trichloroethylene alters the endothelial barrier integrity, at least in part, through vascular endothelial (VE)-Cadherin internalisation, and suggested that this mechanism may play a role in the development of pulmonary veno-occlusive disease.
Keywords: trichloroethylene, pulmonary veno-occlusive disease, endothelial permeability
To the Editor,
Pulmonary veno-occlusive disease (PVOD) is a rare but severe form of pulmonary hypertension (PH) characterised by a progressive pulmonary venular and arterial obstruction associated with a pulmonary capillary proliferation. Compared with pulmonary arterial hypertension (PAH), the clinical course of PVOD is usually more aggressive and is characterised by poor response to PAH therapy. The use of pulmonary vasodilators can be associated with the development of life-threatening pulmonary oedema, and lung transplantation remains the preferred therapy for eligible PVOD patients.1 PVOD can be idiopathic, heritable (loss-of-function biallelic mutations in EIF2AK4 gene), drugs and toxins induced (alkylating agents, organic solvents) and associated to connective tissue disease (especially systemic-sclerosis).2 PVOD is significantly associated with occupational exposure to organic solvents and especially trichloroethylene (TCE).3,4 A significant exposure to TCE was found in 42% of patients with PVOD.4 In this study, histological specimens were available for analysis in seven PVOD patients, including one with a history of positive exposure to TCE. This patient demonstrated venular, capillary and arterial lesions that were typical of PVOD. Despite the fact that TCE exposure is a major risk factor for PVOD, nothing is known about mechanisms leading to the development of the disease in this context.
Several pathophysiological mechanisms involving TCE have been described in other field. In oncology, case-control studies have shown a positive association between exposure to TCE and development of renal carcinomas.5 The genotoxic nature of TCE metabolites eliminated by the kidneys could explain this increased risk of cancer. Exposure to TCE is also a risk factor for systemic sclerosis. TCE was shown to be responsible for dysimmunity with an increase in the Th17/Treg ratio potentially associated with the development of the autoimmune response.6 TCE is also a source of oxidative stress at the cellular level with the production of reactive oxygenated species (ROS) and nitrogen. Mice exposed to TCE by inhalation present lung lesions with Club cell vacuolation, thickening of pulmonary capillaries and increased oxidised glutathione as a marker of oxidant stress.7 It has also been shown a vascular toxicity of TCE responsible for the production of ROS by endothelial cells.8
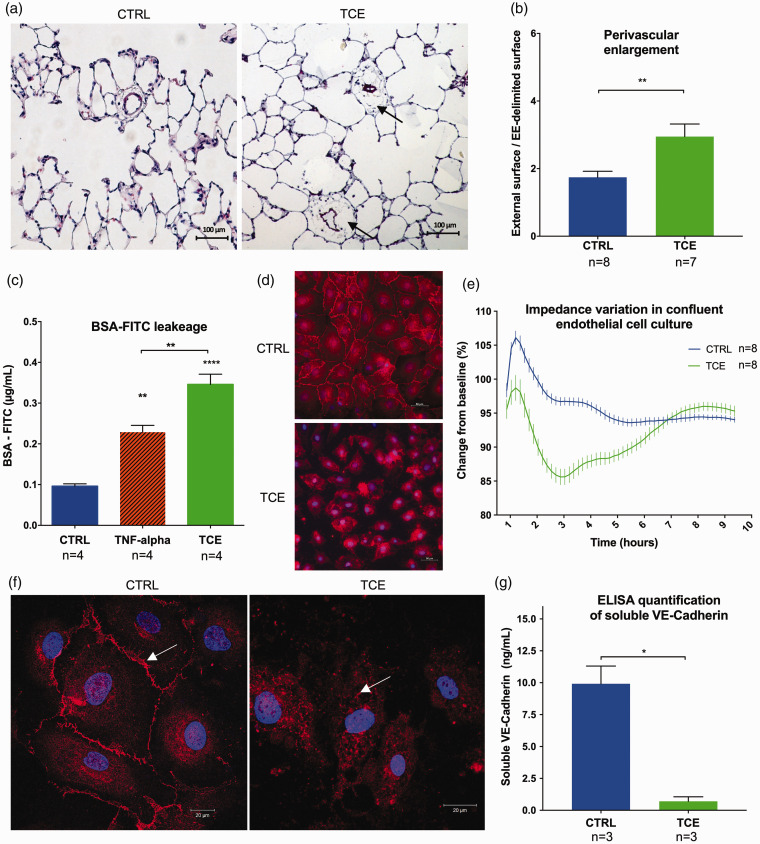
The aim of our study was to describe the effects of TCE exposure on pulmonary vascular and endothelial function. In vivo experiments were conducted on four-week-old Wistar rats (100–200 g). Eight rats were administered a single dose of TCE (2000 mg/kg, i.p.) in olive oil (volume of injection: 500 µL) as previously described.9 The control group (eight rats) was treated with equivalent volumes of the vehicle. They were followed for 35 days and haemodynamic studies were conducted before sacrifice. Under these conditions, no PH or right ventricular hypertrophy was observed after exposure to TCE. However, it seems elusive to establish an animal model of PVOD because of the very long TCE exposure duration in human (>10 years). Nevertheless, histological analysis of the lungs revealed a significant enlargement of the perivascular space of small distal vessels (diameter ≤50 µm) (Fig. 1a and b) in the rats exposed to TCE compared to the control group (p = 0.0092). This prolonged vascular effect, 35 days after a single exposure to TCE, may be due to the lipophilic nature of the compound. It may remain in fat-rich tissues with subsequent low-level chronic release, but we did not address this point in our study.
Fig. 1.
Trichloroethylene (TCE)-induced endothelial dysfunction with an increase of vascular permeability. (a) Lung histology 35 days after TCE exposure in rats shows perivascular enlargement (arrows) of pulmonary small distal vessels (diameter ≤50 µm) (Hematoxylin and Eosin stains). (b) Quantification of the perivascular enlargement in control and TCE-exposed lungs. Twenty consecutive fields at 20× magnification were analysed per rat in a blinded fashion. We measured the external surface delimited by the outer edge of the adventitia, and the vascular surface delimited by the external elastica (EE-delimited surface) and assessed the perivascular enlargement through the ratio external surface/EE-delimited surface. (c) BSA-FITC leakage through confluent monolayers of pulmonary endothelial cells is increased after TCE exposure. (d) Immunofluorescence analysis against VE-Cadherin (red) and nuclei (blue): cells exposed to TCE lose adherent junction of VE-Cadherin. (e) Curve of electric impedance (iCELLigence) of confluent monolayers of pulmonary endothelial cells after complete medium change (T0) with TCE (green) or without (blue): TCE induces a reversible decline of electric impedance. (f) Confocal microscopy analysis shows that endothelial cells exposed to TCE lose membrane localisation of VE-Cadherin which is internalised into the cytoplasm. (g) ELISA quantification of soluble VE-Cadherin (reflecting cell membrane expression) in cell culture supernatants of pulmonary endothelial cells with or without 2 h of TCE exposure. A decrease of soluble VE-Cadherin is observed after TCE exposure. Data are presented as mean ± SEM. *p < 0.05, **p < 0.01, ****p < 0.0001.
To explain this perivascular enlargement after TCE exposure, we hypothesised that TCE favours pulmonary endothelial permeability as a step in the pathophysiology of PVOD development.
In vitro studies were conducted on human pulmonary micro-vascular endothelial cell (HMVEC) exposed to TCE (1000 ppm), TNF-alpha (10 ng/mL) as positive control or regular medium in control group. Similar dose of TCE have already been used for experiment on endothelial cells.8 Using calibration curves, we measured the concentrations of TCE in the medium using gas-chromatography linked mass-spectrometry. Due to TCE high volatility, steady state was measured 15 min after solubilisation of 1000 ppm in the medium, showing actual concentrations of 5 to 10 ppm.
We performed a permeability study using Transwell® assay. Fluorescein isothiocyanate labelled bovine serum albumin (BSA-FITC) was added in the media of confluent HMVEC monolayer grown on the upper compartment of Transwell® inserts (0.4 µm pore size). Endothelial permeability was assessed by measuring the FITC fluorescence in the lower compartment of the Transwell® after 4 h of TCE or TNF-alpha exposure (Fig. 1c). A significant increase in transendothelial passage of BSA-FITC was observed in the wells containing TCE compared to those exposed to regular medium (control p < 0.0001) or TNF-alpha (p = 0.0018).
To observe the effect of TCE at the cellular level, we conducted immunofluorescent studies on HMVEC confluent cultures. We focussed on adherent junction of vascular endothelial (VE)-Cadherin that can be regulated to control endothelial barrier integrity. Cell cultures were exposed or not to TCE as described above. VE-Cadherin-based intercellular adherent junctions were visualised after immunostaining and imaging with epifluorescence microscopy (Fig. 1d). Unexposed control cells had a pavement pattern and were linked together with VE-Cadherin-based adherent junction. After exposure to TCE, adherent junctions were lost and intercellular spaces became prominent.
The iCELLigence™ real-time cell analyzer (RTCA) allows the label-free real-time monitoring of cultured cell behaviour. The RTCA system uses microelectrode biosensor array that measures impedance when cells adhere to the microelectrodes.10 When cells reach confluence, the impedance curve show a plateau. Apoptosis, loss of adherence and opening of endothelial barrier induce a decrease in impedance. Exposure to TCE decreased impedance values of confluent HMVEC from the first to the sixth hour (Fig. 1e). This transitory decrease in electrical impedance is interpreted as a transient breakdown of the endothelial barrier without cell viability compromise.
Immunostaining and confocal microscopy analyses revealed that confluent control HMVEC express VE-Cadherin at the cell membrane. After TCE exposure, this pattern is lost and VE-Cadherin displays a cytoplasm location (Fig. 1f). Soluble VE-Cadherin (sVE-Cadherin) reflects membrane expression of the protein that is released into the medium after cleavage by proteases at basal level. Hence, we quantified sVE-Cadherin concentration in HMVEC supernatant as a surrogate of VE-Cadherin membrane expression using ELISA kit (Quantikine® ELISA, R&D Systems, Minneapolis). Exposure to TCE was associated with a significant decrease in sVE-Cadherin levels (p = 0.0125), as compared to control cells (Fig. 1f). Interestingly, massive endothelial disruption and damage in sepsis has already been associated with decreased sVE-Cadherin level due to VE-Cadherin internalisation.11 In this line, the loss of membrane expression, the cytoplasmic relocation of VE-Cadherin and the drop in sVE-Cadherin levels suggest a regulation of the endothelial permeability by TCE through VE-Cadherin internalisation.
We demonstrated in this work that TCE caused a breakdown of the endothelial barrier in vitro. Increase in endothelial permeability may explain histological observations made in vivo on the rat model exposed to TCE, where perivascular oedema was found at the pulmonary level.
Increase in endothelial permeability has been described as a mechanism involved in the development of PH. Dasatinib exposure is a cause of drug-induced PAH that induces lung vascular dysfunction with an increase in vascular permeability.12 Alteration of vascular permeability and endothelial barrier function has also been described after cigarette smoke exposure.13 In a case-control study comparing PVOD patients with PAH, all patients with significant exposure to TCE had concurrent tobacco exposure.4 Tobacco and solvent exposures may have a potentiator effect for the development of PVOD, with similar pathogenic mechanisms. In case of breakdown of the endothelial barrier, connective tissue and underlying smooth muscle cells are exposed to circulating growth factors leading to uncontrolled proliferation and remodelling.14 Increase in endothelial permeability is also associated with the recruitment of inflammatory cells involved in the initial development of vascular remodelling observed in PAH.15 Further studies should focus on the molecular mechanisms involved in endothelial permeability dysregulation induced by TCE.
In conclusion, this study indicates that TCE alters the endothelial barrier integrity, at least in part, through VE-Cadherin internalisation, and suggests that this mechanism may play a role in the development of PVOD.
Conflict of interest
J.C. and M.R. report grants from Fondation du Souffle, during the conduct of the study. M.H. reports personal fees from Actelion, grants and personal fees from Bayer, grants and personal fees from GSK, personal fees from Merck, personal fees from United Therapeutics, outside the submitted work. G.M., M.K.N., M.Q., C.R.-M., P.D., F.L, M.C.V, S.C.-K. and F.P. have nothing to disclose.
Funding
G.M. is 2017 Laureate of Fonds de Recherche en Santé Respiratoire et de la Fondation du Souffle. J.C. and M.R. received also grant from Fondation du Souffle and Fonds de Recherche en Santé Respiratoire. This study was supported by LabEx LERMIT, Laboratory of Excellence in Research on Medication and Innovative Therapeutics.
References
- 1.Montani D, Lau EM, Dorfmüller P, et al. Pulmonary veno-occlusive disease. Eur Respir J 2016; 47: 1518–1534. [DOI] [PubMed] [Google Scholar]
- 2.Simonneau G, Montani D, Celermajer DS, et al. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur Respir J 2019; 53: 1801913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Montani D, Girerd B, Jaïs X, et al. Clinical phenotypes and outcomes of heritable and sporadic pulmonary veno-occlusive disease: a population-based study. Lancet Respir Med 2017; 5: 125–134. [DOI] [PubMed] [Google Scholar]
- 4.Montani D, Lau EM, Descatha A, et al. Occupational exposure to organic solvents: a risk factor for pulmonary veno-occlusive disease. Eur Respir J 2015; 46: 1721–1731. [DOI] [PubMed] [Google Scholar]
- 5.Raaschou-Nielsen O, Hansen J, McLaughlin JK, et al. Cancer risk among workers at Danish companies using trichloroethylene: a cohort study. Am J Epidemiol 2003; 158: 1182–1192. [DOI] [PubMed] [Google Scholar]
- 6.Li S, Yu Y, Yang P, et al. Trichloroethylene alters Th1/Th2/Th17/Treg paradigm in mice: a novel mechanism for chemically induced autoimmunity. Int J Toxicol 2018; 37: 155–163. [DOI] [PubMed] [Google Scholar]
- 7.Giovanetti A, Rossi L, Mancuso M, et al. Analysis of lung damage induced by trichloroethylene inhalation in mice fed diets with low, normal, and high copper content. Toxicol Pathol 1998; 26: 628–635. [DOI] [PubMed] [Google Scholar]
- 8.Ou J, Ou Z, McCarver DG, et al. Trichloroethylene decreases heat shock protein 90 interactions with endothelial nitric oxide synthase: implications for endothelial cell proliferation. Toxicol Sci 2003; 73: 90–97. [DOI] [PubMed] [Google Scholar]
- 9.Forkert PG, Forkert L. Trichloroethylene induces pulmonary fibrosis in mice. Can J Physiol Pharmacol 1994; 72: 205–210. [DOI] [PubMed] [Google Scholar]
- 10.Quatredeniers M, Nakhleh MK, Dumas SJ, et al. Functional interaction between PDGFβ and GluN2B-containing NMDA receptors in smooth muscle cell proliferation and migration in pulmonary arterial hypertension. Am J Physiol Lung Cell Mol Physiol 2018; 316: L445–L455. [DOI] [PubMed] [Google Scholar]
- 11.Ebihara I, Hirayama K, Nagai M, et al. Soluble vascular endothelial-cadherin levels in patients with sepsis treated with direct hemoperfusion with a polymyxin B-immobilized fiber column. Ther Apher Dial 2014; 18: 272–278. [DOI] [PubMed] [Google Scholar]
- 12.Phan C, Jutant E-M, Tu L, et al. Dasatinib increases endothelial permeability leading to pleural effusion. Eur Respir J 2018; 51: 1701096. [DOI] [PubMed] [Google Scholar]
- 13.Rounds S and Lu Q. Cigarette smoke alters lung vascular permeability and endothelial barrier function (2017 Grover Conference Series). Pulm Circ 2018; 8: 2045894018794000. [DOI] [PMC free article] [PubMed]
- 14.Burton VJ, Ciuclan LI, Holmes AM, et al. Bone morphogenetic protein receptor II regulates pulmonary artery endothelial cell barrier function. Blood 2011; 117: 333–341. [DOI] [PubMed] [Google Scholar]
- 15.Huertas A, Perros F, Tu L, et al. Immune dysregulation and endothelial dysfunction in pulmonary arterial hypertension: a complex interplay. Circulation 2014; 129: 1332–1340. [DOI] [PubMed] [Google Scholar]