Abstract
The aim of this study was to examine the effect of electrical muscle stimulation on muscle atrophy caused by neuromyelitis optica. Two neuromyelitis optica patients with flaccid paralysis participated. The participants underwent a general rehabilitation program (transfer, balance, and gait training) and electrical muscle stimulation on the quadriceps femoris muscle of their paralyzed side. The change in the thickness of the vastus lateralis muscle ranged from −0.6 to 1.4 mm. These changes were minimal and did not indicate muscle atrophy. These findings suggest that in addition to general physiotherapy, electrical muscle stimulation seems safe and feasible in the acute phase of neuromyelitis optica.
Keywords: Neuromyelitis optica, muscle atrophy, rehabilitation, electrical muscle stimulation
Introduction
Neuromyelitis optica (NMO) is a disorder characterized by repeated exacerbation and remission of severe optic neuritis and transverse myelitis.1 Transverse myelitis often leads to flaccid paralysis along the area of neurarchy. Rehabilitation is one treatment for NMO.2 However, there are few reports regarding rehabilitation for NMO, and no published studies have focused on the effect of electrical muscle stimulation (EMS) in individuals with NMO. Thus, the aim of this study was to examine the effect of EMS on NMO-related muscle atrophy in two patients with flaccid paralysis. We hypothesized that EMS of the muscle affected by NMO-related flaccid paralysis can reduce muscle atrophy.
Cases
Two NMO patients admitted to Hiroshima University Hospital for the treatment of re-emerging symptoms participated in this study. The patients were positive for serum anti-aquaporin-4 antibody (AQP-4). They had unilateral flaccid paralysis, and muscle strength on the paralyzed side was graded 0 or 1 with manual muscle testing (MMT). MMT characterizes muscle strength using a rating scale from 0 to 5.3 Their characteristics are summarized in Table 1 and are described in detail below.
Table 1.
The characteristics of two patients.
| Case | A | B | ||
|---|---|---|---|---|
| Age (years) | 43 | 26 | ||
| Sex | Female | Male | ||
| Height (cm) | 162.5 | 179.8 | ||
| Weight (kg) | 42.2 | 90.2 | ||
| Admission (days) | 49 | 23 | ||
| Medication | Steroid pulse therapy, PE therapy, IVIg therapy | Steroid pulse therapy, PE therapy | ||
| Thickness of the VL muscle (mm) (paralyzed/non-paralyzed side) | Before | 22.0/21.5 | 26.1/26.6 | |
| After | 22.5/22.9 | 25.5/26.1 | ||
| ADL | BI | Before | 35 | 55 |
| After | 85 | 90 | ||
| FIM | Before | 85 | 94 | |
| After | 112 | 117 | ||
PE: plasma exchange; IVIg: intravenous immunoglobulin; VL: vastus lateralis; ADL: activity of daily living; BI: Barthel index; FIM: Functional Independence Measure.
Each patient underwent a general rehabilitation program that was performed by physical therapist and involved transfer, balance, and gait training. Transfer training was based on moving from the sitting to the standing position. Balance training included single-leg standing posture and standing on an unstable surface. Gait training included walking on an uneven ground and floor using a knee ankle foot orthosis and parallel bars. In addition to general physiotherapy, we performed EMS at 20 Hz with a monophasic square wave pulse with a duration of 250 µs, an on time of 5 s, an off time of 2 s, no rise time, and decay using an EMS device (AUTO Tens PRO Rehabili Unit; Homer Ion Co., Ltd, Tokyo, Japan). Belt electrodes were used for skeletal muscle stimulation. The electrodes were wrapped around the waist, distal parts of thigh, and crus of the paralyzed side (Figure 1). The stimulation intensity was individually set to the maximal level without discomfort in each patient. EMS was performed in a lying position for 20 min once per day. Participants were instructed not to actively contract muscles during the stimulation.
Figure 1.
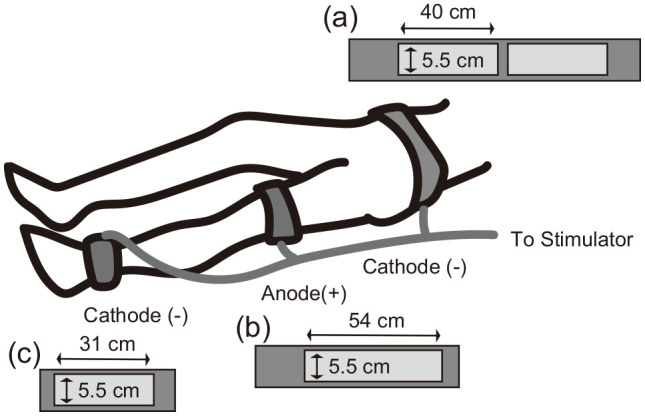
Belt electrodes were wrapped around the waist, distal parts of the thigh, and crus of the paralyzed side. The waist electrode had a width of 5.5 cm and a length of 40 cm twice (a), the thigh electrode had a width 5.5 cm and a length 54 cm (b), and the crus electrode had a width 5.5 cm and a length 31 cm (c).
We evaluated the thickness of the patients’ vastus lateralis (VL) muscle. We measured the thickness of the VL muscle and the subcutaneous tissue at the center of the line between the superior lateral edge of the patella and greater protuberance on ultrasound using a linear array probe with a frequency of 7.5 MHz (Noblus; Hitachi Aloka Medical, Tokyo, Japan).4 Both subjects underwent the ultrasound examination in a seated position. During measurement, both the hip and knee extension angles were fixed at 90°. In addition, as an accessory evaluation, we assessed the subjects’ activities of daily living (ADL) level using the Barthel index (BI) and the Functional Independence Measure (FIM). All procedures were performed by the same investigator at admission and at discharge.
No adverse events due to EMS were observed in either participant. The affected lower limb of both patients remained paralyzed (e.g. grade 0 or 1 on the MMT).
Case A
The patient was a 43-year-old female who was diagnosed with NMO 6 years previously. She was admitted for treatment for exacerbation of sensory disorder, paralysis, and bladder/rectal disorder. She received steroid pulse therapy for 3 days after admission. On the 5th day of hospitalization, she could not speak clearly. Plasma exchange (PE) therapy was performed five times from the 9th to the 19th day, and intravenous immunoglobulin (IVIg) therapy was performed from the 21st to the 25th day. A second course of steroid pulse therapy was performed from the 30th to the 32nd day of hospitalization. Physiotherapy began on the 2nd day and ended on the 48th day. She was hospitalized at our institution for 49 days and then transferred to a rehabilitation hospital. The patient performed physiotherapy (40 min) and underwent EMS intervention (20 min) once per day, five times per week, for a total of 34 times.
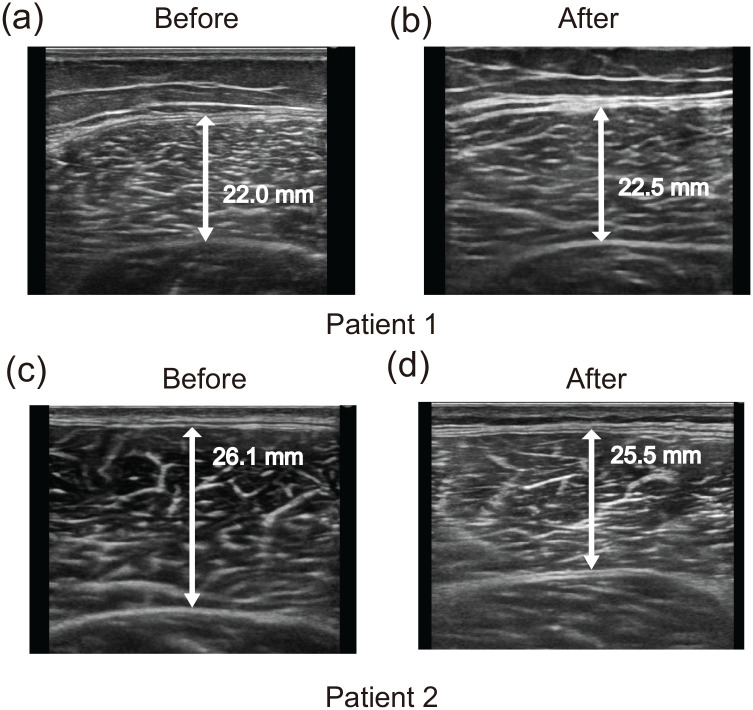
The thickness of the VL muscle was increased by 0.5 mm on the paralyzed side and by 1.4 mm on the non-paralyzed side (Table 1 and Figure 2(a) and (b)). The BI and FIM scores showed improvement (from 35 to 85 and from 85 to 112, respectively; Table 1).
Figure 2.
Ultrasound images of the vastus lateralis muscle in each patient before and after the intervention. Before images (a) and (c) were obtained at the first rehabilitation session, and after images (b) and (d) were obtained at the last rehabilitation session for each patient.
Case B
The patient was a 26-year-old male who was diagnosed 2 months previously. Before admission to our hospital, he had received steroid pulse therapy two times at another hospital. PE therapy was performed five times from the 9th to the 18th day of hospitalization. Physiotherapy began on the 8th day and ended on the 22nd day. The patient was hospitalized at our institution for 22 days and then transferred to a rehabilitation hospital. The patient performed physiotherapy (40 min) and underwent the EMS intervention (20 min) once per day, five times per week, for a total of 11 times.
The thickness of the VL muscle decreased by 0.6 mm on the paralyzed side and by 0.5 mm on the non-paralyzed side (Table 1 and Figure 2(c) and (d)). The BI and FIM scores improved (from 55 to 90 and from 94 to 117, respectively; Table 1).
Discussion
This is the first report to examine the effect of EMS on muscles with flaccid paralysis caused by NMO. The thickness of the VL muscle was minimally changed after physiotherapy and EMS treatment. These findings suggest that in addition to general physiotherapy, EMS intervention seems safe and feasible in the acute phase of NMO.
NMO is characterized by the rapid development and recurrence of exacerbation and remission. We believe that after the neuritis is addressed, the recovery of muscle strength with the reduction of paralysis contributes to the early reacquisition of movement. Therefore, it is useful to minimize subsequent muscle atrophy during the paralysis phase.
There are many reports regarding muscle disuse atrophy. Factors that contribute to muscle disuse atrophy include bed rest,5,6 immobilization,7 peripheral nerve or spinal cord injury,8,9 and stroke.10 Reports have indicated that muscle atrophy can occur even in healthy people5–7 and young people.7 In addition, these changes may last only 5–10 days.5–7 In this study, the subjects had flaccid paralysis, and their hospitalization period ranged from 20 to 49 days, and each patient had a paralyzed lower limb (e.g. MMT 0 or 1). Therefore, the patients in this report were considered to have a high risk of muscle atrophy. A study on EMS intervention for Guillain-Barré syndrome, a demyelinating disease similar to NMO, has recently been reported.11 Harbo et al.11 reported that EMS intervention tended to inhibit muscle mass loss more than no intervention (p = 0.08) and seemed safe and feasible in the early phase of Guillain-Barré syndrome. EMS has been found effective for muscle atrophy caused by bed rest,12 immobilization,7 and nerve injury.8,9 In this study, the subjects showed minimal change in the thickness of the VL muscle. The results of this report support previous findings8,11,13 and suggest that the use of EMS to treat muscles with paralysis seems safe and feasible in the early phase of NMO.
Conclusion
In conclusion, we report the application of EMS intervention in acute-phase NMO patients. No adverse events due to EMS were observed in either participant, and minimal changes in the thickness of the VL muscle were observed. EMS intervention may be a safe and effective intervention in the acute phase of NMO.
Acknowledgments
The authors thank the NMO patients for their commitment to the study.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical approval: This study was conducted with the approval of the Hiroshima University’s Committee of Ethics in Research (no. E-1171-1).
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Informed consent: Written informed consent was obtained from the patients for publication of this case report.
ORCID iD: Yuichi Nishikawa  https://orcid.org/0000-0002-0088-8447
https://orcid.org/0000-0002-0088-8447
References
- 1. Patterson SL, Goglin SE. Neuromyelitis optica. Rheum Dis Clin North Am 2017; 43: 579–591. [DOI] [PubMed] [Google Scholar]
- 2. Suo DM, Liu LL, Jia K, et al. Multidisciplinary rehabilitation for adults with neuromyelitis optica spectrum disorders: a pilot study. J Rehabil Med 2019; 51: 692–697. [DOI] [PubMed] [Google Scholar]
- 3. Hislop H, Montgomery J. Daniels and Worthingham’s muscle testing: techniques of muscle examination. 7th ed. New York: Saunders, 2002. [Google Scholar]
- 4. Nishikawa Y, Watanabe K, Takahashi T, et al. Sex differences in variances of multi-channel surface electromyography distribution of the vastus lateralis muscle during isometric knee extension in young adults. Eur J Appl Physiol 2017; 117(3): 583–589. [DOI] [PubMed] [Google Scholar]
- 5. Kortebein P, Ferrando A, Lombeida J, et al. Effect of 10 days of bed rest on skeletal muscle in healthy older adults. JAMA 2007; 297: 1772–1774. [DOI] [PubMed] [Google Scholar]
- 6. Ferrando AA, Paddon-Jones D, Hays NP, et al. EAA supplementation to increase nitrogen intake improves muscle function during bed rest in the elderly. Clin Nutr 2010; 29(1): 18–23. [DOI] [PubMed] [Google Scholar]
- 7. Dirks ML, Wall BT, Snijders T, et al. Neuromuscular electrical stimulation prevents muscle disuse atrophy during leg immobilization in humans. Acta Physiol 2014; 210(3): 628–641. [DOI] [PubMed] [Google Scholar]
- 8. Marqueste T, Alliez JR, Alluin O, et al. Neuromuscular rehabilitation by treadmill running or electrical stimulation after peripheral nerve injury and repair. J Appl Physiol 2004; 96: 1988–1995. [DOI] [PubMed] [Google Scholar]
- 9. Kim SJ, Roy RR, Zhong H, et al. Electromechanical stimulation ameliorates inactivity-induced adaptations in the medial gastrocnemius of adult rats. J Appl Physiol 2007; 103: 195–205. [DOI] [PubMed] [Google Scholar]
- 10. Scherbakov N, von Haehling S, Anker SD, et al. Stroke induced Sarcopenia: muscle wasting and disability after stroke. Int J Cardiol 2013; 170: 89–94. [DOI] [PubMed] [Google Scholar]
- 11. Harbo T, Markvardsen LK, Hellfritzsch MB, et al. Neuromuscular electrical stimulation in early rehabilitation of Guillain-Barré syndrome: a pilot study. Muscle Nerve 2019; 59(4): 481–484. [DOI] [PubMed] [Google Scholar]
- 12. Gerovasili V, Stefanidis K, Vitzilaios K, et al. Electrical muscle stimulation preserves the muscle mass of critically ill patients: a randomized study. Crit Care 2009; 13(5): R161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Silva PE, de Cássia Marqueti R, Livino-de-Carvalho K, et al. Neuromuscular electrical stimulation in critically ill traumatic brain injury patients attenuates muscle atrophy, neurophysiological disorders, and weakness: a randomized controlled trial. J Intensive Care 2019; 7: 59. [DOI] [PMC free article] [PubMed] [Google Scholar]