Abstract
The worldwide spread of coronavirus disease 2019 (COVID-19), caused by the new severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), was declared a pandemic by the World Health Organization (WHO) in March 2020. According to clinical studies carried out in China and Italy, most patients experience mild or moderate symptoms; about a fifth of subjects develop a severe and critical disease, and may suffer from interstitial pneumonia, possibly associated with acute respiratory distress syndrome (ARDS) and death.
In patients who develop respiratory failure, timely conventional oxygen therapy through nasal catheter plays a crucial role, but it can be used only in mild forms. Continuous positive airway pressure (CPAP) support or non-invasive mechanical ventilation (NIV) are uncomfortable, and require significant man–machine cooperation. Herein we describe our experience of five patients with COVID-19, who were treated with high-flow nasal cannula (HFNC) after failure of CPAP or NIV, and discuss the role of HFNC in COVID-19 patients. Our findings suggest that HFNC can be used successfully in selected patients with COVID-19-related ARDS.
The reviews of this paper are available via the supplemental material section.
Keywords: continuous positive airway pressure, COVID-19, high-flow nasal cannula, respiratory failure, SARS-CoV-2
Introduction
The clinical presentation of the novel coronavirus disease (COVID-19) can vary from asymptomatic or paucisymptomatic to severe forms. Patients usually experience mild or moderate symptoms (80%). However, about 14% of subjects progress to hypoxemic respiratory failure requiring oxygen therapy, and 5% need more advanced respiratory support.1
During the COVID-19 outbreak, the number of patients increased rapidly in a short time, thereby resulting in a deficiency of intensive care physicians. The lack of intensive care unit (ICU) beds or ventilators makes the use of non-invasive ventilation techniques increasingly important.
COVID-19 patients can develop pneumonia characterised by bilateral interstitial infiltrates, possibly leading to ARDS and respiratory failure due to ventilation/perfusion mismatch responsible for shunt effect.1
Patients with acute hypoxemia may experience persistent dyspnea, despite the administration of oxygen flows > 10–15 l/min through a facial mask with reservoir. Under these circumstances, other approaches, such as high-flow nasal cannula (HFNC), continuous positive airway pressure (CPAP) support, or non-invasive mechanical ventilation (NIV), may be useful.2
HFNC oxygen therapy is based on a device capable of providing humidified and heated oxygen at high flows through nasal cannulas. These cannulas can reach a flow of up to 60 l/min at a temperature between 31 and 37°C, and with an absolute humidity of 44 mg H2O/l; FiO2 can range from 21% to 100%.2 The main advantages provided by HFNC include washing of the pharyngeal dead space, reduction of respiratory work, a positive end-expiratory pressure (PEEP) effect, release of a constant inspired oxygen fraction, improvement of mucociliary clearance, and patient comfort.3 It is well known that HFNC can provide a low PEEP, which exerts a beneficial effect on mild-to-moderate respiratory failure.4 Moreover, by delivering humidified and warmed gas through the nasopharynx, HFNC reduces the metabolic work required for gas conditioning.5 Furthermore, HFNC is better tolerated than other ventilatory supports and can decrease the probability of intubation,6 thus improving clinical prognosis in patients with acute respiratory failure.7,8
CPAP respiratory support provides a continuous positive pressure, which, during all breathing phases, is delivered to the airways through external devices applied to patients, who are awake and collaborative. CPAP can supply an early treatment aimed at avoiding intubation, thus providing a valid alternative to invasive ventilation.9
NIV is a ventilatory support technique that is used widely within the therapeutic context of acute respiratory failure. The indication for NIV is based on integration of clinical and blood gas analysis data including signs of respiratory fatigue such as dyspnea, use of accessory respiratory muscles, paradoxical breathing, increased respiratory rate (>25 acts/min), pH < 7.35 (most important parameter), arterial partial pressure of carbon dioxide (PaCO2) > 45 mm Hg or rapid PaCO2 increase (>15–20 mm Hg).10
Although the use of HFNC is suggested for COVID-19-associated acute hypoxemic respiratory failure over non-invasive positive pressure ventilation,11 there are no specific evidence-based guidelines that recommend the most appropriate choice among HFNC, CPAP or NIV.12 Several studies have investigated the efficacy of HFNC in COVID-19 patients, thus finding that this kind of support is a suitable treatment option.7,13–16
Therefore, the aim of our real-life experience is to describe the effects of HFNC in a case series of five COVID-19 patients with ARDS, not responsive to CPAP or NIV.
The COVID-2019 pandemic will be remembered for the rapidity with which it spread, the morbidity and mortality associated with it, and the paucity of evidence-based management guidelines. One of the major concerns of hospitals was to limit spread of infection to health-care workers. Because the virus is spread mainly by respiratory droplets and aerosolised particles, procedures that may potentially disperse viral particles, so-called ‘aerosol-generating procedures’ (AGP), were avoided whenever possible. Included in this category were NIV, HFNC and awake (nonintubated) proning. Accordingly, at many health-care facilities, patients who had increasing oxygen requirements were emergently intubated and mechanically ventilated to avoid exposure to AGP. With experience, physicians realised that mortality of invasively ventilated patients was high and it was not easy to extubate many of these patients. This raised the concern that HFNC and NIV were being underutilised to avoid intubation and to facilitate extubation. In this article, we attempt to separate fact from fiction and perception from reality pertaining to the aerosol dispersion with NIV, HFNC and awake proning. We describe precautions that hospitals and health-care providers must take to mitigate risks with these devices. Finally, we take a practical approach in describing how we use the three techniques, including the common indications, contraindications and practical aspects of application.
Methods
This retrospective observational study was carried out at the Infectious and Tropical Disease Unit (COVID-19 Centre) of Magna Graecia University Hospital of Catanzaro, Italy, from 27 March 2020 to 25 June 2020. Every patient underwent blood gas analysis, chest X-ray integrated by calculation of Brescia Score,17 and high-resolution computerised tomography (HRCT); these evaluations were performed before and after treatment, in order to assess the evolution of lung disease. Conventional oxygen therapy (COT) was performed using either facial mask (up to 5 l/min), mask with reservoir (up to 10 l/min), or Venturi mask (up to 60% FiO2), with the aim of reaching and maintaining a target of peripheral oxygen-saturated haemoglobin fraction (SpO2) > 90%. Surgical masks over nasal cannulas were applied on patient’s mouth and nose, and medical staff wore full personal protective equipment (PPE).
We used CPAP or NIV in patients with acute respiratory failure undergoing oxygen therapy via Venturi mask, if one or more of the following criteria were present: ratio of arterial oxygen partial pressure to fractional inspired oxygen (PaO2/FiO2) < 300 mm Hg with SpO2 less than 92%; respiratory rate (RR) > 28 acts/min; signs of mild respiratory distress, presence of dyspnoea, and contraindications including cardiorespiratory arrest, signs of organ failure, hemodynamic instability, facial trauma, and upper airway obstruction were absent. We started using either CPAP (10 cm H2O; FiO2 up to 60%) or NIV, the latter being applied to the patient with hypoxemic/hypercapnic lung failure and respiratory acidosis, according to a pressure support ventilation (PSV) approach characterised by a PSV of 10–12 cm H2O, associated with a PEEP of 10 cm H2O and a FiO2 up to 60%. A single attempt of CPAP or NIV support lasting a maximum of 1 h was performed.18 CPAP failure was defined on the basis of either insufficient improvement or even worsening of SpO2. After failure of CPAP therapy, we treated all five patients with HFNC, setting the temperature at 31°C, and using a flow of at least 40 l/min and a FiO2 up to 60%, in order to reach and maintain SpO2 within a 94–98% range. In addition, the rate of oxygenation (ROX) index was calculated as SpO2/FiO2 × respiratory rate, according to the method used by Roca et al.19 Negative pressure rooms were not available, so naturally ventilated hospital rooms were used.
Table 1.
Patient characteristics.
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | |
|---|---|---|---|---|---|
| Age, years | 60 | 55 | 62 | 89 | 75 |
| Gender | Female | Male | Female | Female | Male |
| Smoking habit | No | No | No | No | Yes |
| Comorbidities | Hypercholesterolemia | Diabetes, previous TIA, infantile meningitis with cognitive developmental delay, hyperthyroidism, chronic gastritis, chronic liver disease. | Microcytic anaemia, paroxysmal atrial fibrillation. | Arterial hypertension, severe arthrosis. | Idiopathic pulmonary fibrosis, Parkinson’s disease, diabetes mellitus, renal failure, atrial fibrillation. |
| Admission therapy | Statin | Promazine Clothiapine Omeprazole Insulin therapy |
Bisoprolol Pantoprazole |
Pantoprazole Ace-inhibitor Furosemide |
Bisoprolol Ace-inhibitor Nintedanib Insulin therapy |
| Hospitalisation therapy | Hydroxycloroquine Azithromycin Enoxaparin Metilprednisolone Empiric antibiotic therapy Tocilizumab |
Hydroxycloroquine Azithromycin Enoxaparin Metilprednisolone Empiric antibiotic therapy |
Hydroxycloroquine Azithromycin Enoxaparin Metilprednisolone |
Hydroxycloroquine Azithromycin Enoxaparin Metilprednisolone |
Enoxaparin Metilprednisolone Insulin therapy Furosemide Empiric antibiotic therapy |
TIA, transient ischaemic attack.
This study was conducted as a part of routine Good Clinical Practice (GCP) and in accordance with the declaration of Helsinki. The retrospective collection of data was approved by the local Ethical Committee of Calabria Region on 13 May 2020. In addition, informed consent was obtained from all patients.
Descriptive statistics (mean, standard deviation, percentage values) were used to express the categorical and continuous variables. In order to assess the differences observed with regard to relevant parameters recorded before and after HFNC treatment, we analysed PaO2, PaO2/FiO2, and ROX index, whose changes were evaluated by Wilcoxon matched-pairs signed rank test. A p value lower than 0.05 was considered to be statistically significant. Statistical analysis was performed using Prism version 8.2.1 (GraphPad Software Inc., San Diego, CA, USA).
Results
During the study period, a total of 62 patients diagnosed with 59 COVID-19 were admitted. Among 18 subjects who received oxygen supportive therapy, low flow oxygen was insufficient in 5 (27.7%) patients with severe COVID-19 illness, including two men and three women (mean age: 68.2 ± 13.31 years). These five patients suffered from several comorbidities such as hypercholesterolemia, arterial hypertension, diabetes, chronic kidney failure, previous transient ischemic attack (TIA), hyperthyroidism, chronic liver disease, atrial fibrillation and, limited to one single case, pulmonary fibrosis and Parkinson’s disease (Table 1). They were all treated with hydroxychloroquine and azithromycin combination, according to the protocol used by Gautret et al.,20 and with systemic corticosteroids. In three cases, also on the basis of interleukin-6 (IL-6) levels, subcutaneous tocilizumab was administered, as reported elsewhere.21
Table 2.
Radiological features.
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | |
|---|---|---|---|---|---|
| Chest X-ray (BRESCIA SCORE) |
7 | 10 | 11 | 14 | 16 |
| HRCT Image |
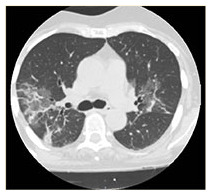
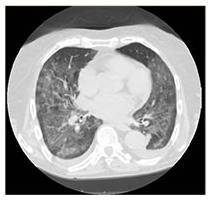
|
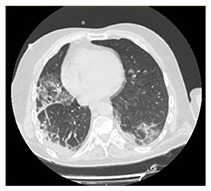
|
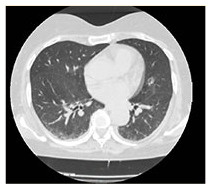
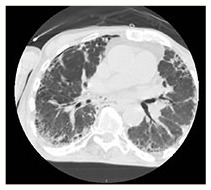
|
|
|
| HRCT Pattern |
COVID-19 interstitial pneumonia with involvement of the middle fields and bi-basal consolidation bands. | Subpleural parenchymal consolidation areas associated with cylindrical traction bronchiectasis; concomitant shaded areas of ground glass, scattered in both lungs. | Subpleural bands characterised by interstitial thickening phenomena from COVID-19 pneumonia, especially detectable in the posterior regions of inferior lobes, bilaterally. | Bilaterally located in lung parenchyma, diffuse signs of ground glass with thickening of the interstitial septa, which configure a crazy paving pattern in COVID-19 pneumonia. | Signs of interstitial disease characterised by subpleural cystic formations with honeycombing aspects, associated with widespread ground glass areas scattered in both lungs. |
COVID-19, Coronavirus disease 2019; HRCT, High-resolution computed tomography.
Chest X-ray images showed interstitial infiltrates characterised by Brescia scores of 7, 10, 11, 14 and 16, respectively. Chest computerised tomography (CT) imaging (Table 2) evidenced the presence of multiple ground-glass opacities in both lungs and in all patients, associated with detection of ‘crazy paving’ pattern in one case. In two patients, subpleural consolidations were present, and in one case CT scans also displayed concomitant honeycombing aspects due to previously diagnosed idiopathic pulmonary fibrosis. SpO2 generally ranged from 75% to 89% before ventilatory therapy.
Table 3.
Respiratory parameters of COVID-19 patients with treated with HFNC.
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | |
|---|---|---|---|---|---|
| Baseline SpO2 (%) | 88 | 87 | 89 | 85 | 75 |
| Baseline ABG analysis | pH 7.49 PCO2 32 mm Hg PO2 54 mm Hg HCO3– 24 mmol/l |
pH 7.50 PCO2 31 mm Hg PO2 56 mm Hg HCO3– 25 mmol/l |
pH 7.48 PCO2 34 mm Hg PO2 55 mm Hg HCO3– 26 mmol/l |
pH 7.50 PCO2 33 mm Hg PO2 41 mm Hg HCO3– 30 mmol/l |
pH 7.33 PCO2 66 mm Hg PO2 38 mm Hg HCO3– 41 mmol/l |
| Baseline PaO2/FiO2 |
257 | 266 | 261 | 195 | 180 |
| SpO2 (%) during CPAP or NIV |
87 | 85 | 89 | 83 | 81 |
| Oxygen flow rate (l/min) | 40 | 50 | 40 | 45 | 60 |
| SpO2 (%) after HFNC |
98 | 97 | 96 | 96 | 94 |
| ABG analysis after HFNC | pH 7.45 PCO2 32 mm Hg PO2 75.5 mm Hg HCO3– 23 mmol/l |
pH 7.46 PCO2 34 mm Hg PO2 75.7 mm Hg HCO3– 24 mmol/l |
pH 7.45 PCO2 37 mm Hg PO2 72 mm Hg HCO3– 26 mmol/l |
pH 7.47 PCO2 33 mm Hg PO2 70 mm Hg HCO3– 25 mmol/l |
pH 7.45 PCO2 59 mm Hg PO2 66.7 mm Hg HCO3– 38 mmol/l |
| PaO2/FiO2 after HFNC | 359 | 360 | 343 | 335 | 315 |
| ROX index 2 h-HFNC |
8.8 | 7.5 | 8.5 | 6.8 | 5.5 |
| ROX index 12 h-HFNC |
10.5 | 9.6 | 10.2 | 9.5 | 6.7 |
ABG, arterial blood gas; COVID-19, coronavirus disease 2019; CPAP, continuous positive airway pressure; HFNC, high flow nasal cannula; NIV, non-invasive mechanical ventilation.
Two patients had a PaO2/FiO2 < 200 mmHg at hospital admission, and immediately required ventilatory support with CPAP or NIV. The remaining three subjects were characterised by a PaO2/FiO2 ratio ranging from 200 to 300 mmHg, and were initially treated with COT. However, these patients developed deterioration of respiratory function after 3.5 ± 2.5 days, and required ventilatory support. A first course of CPAP or NIV treatment was tried in all five patients but, despite application of different interfaces and pressure levels, these procedures were not well tolerated, thereby generating feelings of claustrophobia and anxiety, which in some cases required sedation (Patients 2 and 5).
All five patients were not responsive to CPAP or NIV, and they were shifted to HFNC after a rapid decline of respiratory function.
Blood gas analysis before HFNC treatment showed hypoxemic/hypocapnic lung failure and respiratory alkalosis in four subjects (Patients 1, 2, 3 and 4); hypoxemia and hypercapnia, associated with respiratory acidosis, were present only in the patient suffering from idiopathic pulmonary fibrosis (Patient 5) (Table 3).
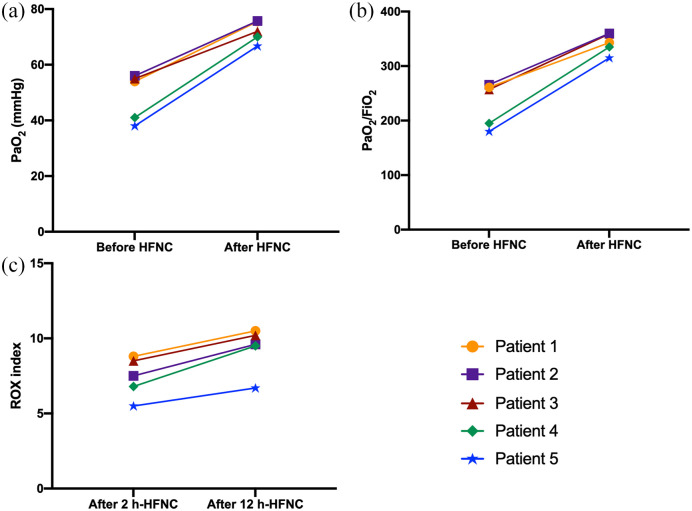
After HFNC treatment, all five patients experienced a trend towards a PaO2 increase that almost reached the threshold of statistical significance (48.80 ± 8.585 mm Hg versus 71.98 ± 3.809 mm Hg; p = 0.06) (Figure 1A). Moreover, after HFNC therapy PaO2/FiO2 ratio enhanced from 231.8 ± 40.91 mm Hg to 342.4 ± 18.65 mm Hg; this increase also almost accomplished the task of satisfying the criteria of statistical significance (p = 0.06) (Figure 1B). After 2 h of treatment with HFNC, mean ROX index was 7.420 ± 1.337 and, after 12 h, it increased to 9.300 ± 1.512 (p = 0.06), thus indicating that HFNC therapy was quite successful (Figure 1C). After 24 h, SpO2 persisted within values ranging from 94% to 99%. During treatment, SpO2 increased gradually; patients were switched to COT after a mean of 5.38 ± 2.07 days, when their conditions definitely improved.21 All five patients showed high tolerance and good compliance to HFNC. Blood gas analysis parameters reached normal values (Table 3). Subsequently, four patients further improved and reached a complete clinical and microbiological cure. Only the patient with terminal pulmonary fibrosis, Parkinson’s disease and diabetes died, but because of non-respiratory complications (internal bleeding), as described elsewhere.22
Figure 1.
PaO2, PaO2/FiO2 and ROX index variations.
FiO2, fractional inspired oxygen; HFNC, high-flow nasal cannula; PaO2, arterial oxygen partial pressure; ROX, rate of oxygenation.
Discussion
There are currently no specific recommendations indicating when it could be appropriate to choose either HFNC, CPAP or NIV to treat severe respiratory failure caused by Sars-CoV-2. The use of HFNC for the management of COVID-19 pneumonia is still controversial. Some guidelines discourage the routine use of HFNC or any noninvasive, potentially aerosol-generating approach such as CPAP or NIV.23
On the other hand, the Surviving Sepsis/Society of Critical Care Medicine guidelines recommend HFNC as a first-line approach.11 Other hospital centres favour early intubation, and strongly discourage the use of other non-invasive therapies approaches. The rationale of this approach is that failure rate of noninvasive ventilatory techniques in COVID-19 patients is high, and these AGP place caregivers at increased risk of contracting COVID-19 infection.24
It is well known that, among other advantages, HFNC is often well tolerated. Furthermore, HFNC can improve clinical prognosis in patients with acute respiratory failure,7,8 and decrease the probability of unnecessary intubation, thus preserving much-needed critical care ventilators, which have been in short supply in some areas.
Therefore, we decided to use HFNC in five COVID-19 patients with respiratory failure, not responsive to CPAP. During treatment of these patients, we observed that HFNC was able to satisfy their oxygen requirements. Despite current guidelines recommend CPAP when ARDS or hypoxemia cannot be corrected by means of standard oxygen therapy, in our real-life practice HFNC was more advantageous than CPAP. Indeed, our limited experience, mostly referring to elderly and uncooperative patients, characterised by severe respiratory failure associated with multiple comorbidities, suggests that such subjects are often quite anxious, claustrophobic and not compliant with CPAP, which instead requires marked man–device cooperation. The latter condition can thereby be hardly achievable in elderly and poorly collaborative patients.13 However, a low degree of tolerance to CPAP can occur even in middle-aged patients, thus possibly also impairing the effectiveness of respiratory failure therapy.25 Notably, in comparison with CPAP, HFNC therapy appears to be more manageable and easier to be utilised by a wide range of physicians, not just pulmonologists and intensive care specialists. However, healthcare professionals should still pay close attention to changes in patients’ oxygenation rates and respiratory frequency,15 thus strictly monitoring the progression from mild/moderate to severe ARDS.
Our results are in agreement with what was previously reported by Geng et al.,13 who pointed out how the use of HFNC in eight patients with severe disease was beneficial. None of the patients of this study with critical disease required NIV; only one patient was intubated.13 Moreover, a recent small trial from China indicated HFNC as the most common ventilation support for COVID-19 patients with pneumonia, and showed that 7 out 17 patients treated with HNFC experienced treatment failure. Among patients with PaO2/FiO2 ratio <200, failure rate reached 63%. Hence, patients characterised by lower PaO2/FiO2 ratio were more likely to experience HFNC failure.7 Another case series of four patients has shown that HFNC could prevent intubation in some patients, also avoiding complications such as ventilator-associated pneumonia and deep-vein thrombosis. This treatment reduced workloads for healthcare professionals, had good tolerability for patients, and might not significantly increase the risk of infection for healthcare professionals. Two patients survived after treatment, while the other two died because of ARDS and heart failure, respectively.15 Lastly, a case report of a 44-year-old COVID-19 positive male patient, suffering from hypoxemic respiratory failure, has been published recently. This subject was treated successfully with HFNC therapy in a negative pressure intensive care room, suggesting that this non-invasive modality can be an alternative respiratory support in selected patients with respiratory failure.16
Using HFNC, a balance between benefits and risks of droplet dispersion must be evaluated.26 Although several authors have hypothesised a higher risk of droplet scattering and contamination for healthcare professionals facing patients undergoing HFNC, with respect to CPAP, this topic is still highly debated.27–31 Some studies showed that the risk of pathogen dispersal during HFNC therapy was limited to the proximal area of face and nasal cannula, thus suggesting that this therapeutic approach does not increase the risk of droplet production and contact infection. Furthermore, Li et al. reported that the risk of bio-aerosol dispersion through HFNC was similar to that referring to standard oxygen masks.31,32 Indeed, by correctly positioning the surgical mask on the patient’s face, hypoxemic COVID-19 subjects might benefit from HFNC, without the adjunctive risks of contamination for medical staff.26 Hence, such risks can be minimised by observing the well-known behavioural rules that are mandatory when managing COVID-19 patients.
It has been described that aerosol-mitigating interventions, such as the use of high-energy particulate accumulator (HEPA) filters, negative–pressure rooms and full PPE, are sufficient to protect medical and nursing staff.24 Several studies have examined aerosol dispersion during use of various AGP, and the results regarding HFNC have been reassuring. When compared with other AGP, HFNC are characterised by a shorter air dispersion distance.27,30,33 Recently, Gaeckle et al. measured particle and droplet generation from the respiratory tract of 10 healthy individuals receiving oxygen with various modes of delivery.34 They observed that, when compared with breathing room air or non-humidified oxygen modalities, there was no evidence of increase in the concentration of aerosol generated with the use of HFNC or NIV.34 Iwashyna et al. enrolled four healthy volunteers and observed that, in a simulated hospital room, there was no evidence of increased aerosolisation above room levels with nasal cannula, non-rebreather mask or HFNC, up to maximal flow rates of 60 l/min.35 In any case, for maximal safety of staff, patients receiving HFNC should be placed in a negative pressure room and closely monitored in a setting where intubation can be immediately performed in case of clinical deterioration.36 If negative-pressure rooms are not available, as in our case, rooms with natural ventilation characterised by airflow of at least 160 l/s per patient are recommended.24
Conclusion
Based on our experience and after a review of the literature, we consider HFNC a better treatment option than CPAP for some fragile COVID-19 patients with respiratory failure. In this regard, it is important to point out that none of the critically ill COVID-19 patients admitted to our hospital ward required intubation. In conclusion, the main limitations of this observational clinical investigation regard the small number of enrolled patients and, like most real-life studies, the lack of a suitable control group. However, our results convincingly suggest that, in the management of COVID-19-associated ARDS, HFNC can be usefully utilised, especially for elderly and/or uncooperative patients.
Supplemental Material
Supplemental material, Author_Response for Oxygen therapy via high flow nasal cannula in severe respiratory failure caused by Sars-Cov-2 infection: a real-life observational study by Giada Procopio, Anna Cancelliere, Enrico Maria Trecarichi, Maria Mazzitelli, Eugenio Arrighi, Graziella Perri, Francesca Serapide, Corrado Pelaia, Elena Lio, Maria Teresa Busceti, Maria Chiara Pelle, Marco Ricchio, Vincenzo Scaglione, Chiara Davoli, Paolo Fusco, Valentina La Gamba, Carlo Torti and Girolamo Pelaia in Therapeutic Advances in Respiratory Disease
Supplemental material, Reviewer_1_v.1 for Oxygen therapy via high flow nasal cannula in severe respiratory failure caused by Sars-Cov-2 infection: a real-life observational study by Giada Procopio, Anna Cancelliere, Enrico Maria Trecarichi, Maria Mazzitelli, Eugenio Arrighi, Graziella Perri, Francesca Serapide, Corrado Pelaia, Elena Lio, Maria Teresa Busceti, Maria Chiara Pelle, Marco Ricchio, Vincenzo Scaglione, Chiara Davoli, Paolo Fusco, Valentina La Gamba, Carlo Torti and Girolamo Pelaia in Therapeutic Advances in Respiratory Disease
Supplemental material, Reviewer_2_v.1 for Oxygen therapy via high flow nasal cannula in severe respiratory failure caused by Sars-Cov-2 infection: a real-life observational study by Giada Procopio, Anna Cancelliere, Enrico Maria Trecarichi, Maria Mazzitelli, Eugenio Arrighi, Graziella Perri, Francesca Serapide, Corrado Pelaia, Elena Lio, Maria Teresa Busceti, Maria Chiara Pelle, Marco Ricchio, Vincenzo Scaglione, Chiara Davoli, Paolo Fusco, Valentina La Gamba, Carlo Torti and Girolamo Pelaia in Therapeutic Advances in Respiratory Disease
Acknowledgments
We want to thank all our patients and our nurses. We also thank the Infectious Diseases and Tropical Medicine (IDTM) of the University ‘Magna Graecia’ (UMG) COVID-19 Group, which is composed, besides the main authors, by the following people: Domenico Laganà, Maria Petullà, Bernardo Bertucci, Angela Quirino, Giorgio Settimo Barreca, Aida Giancotti, Luigia Gallo, Angelo Lamberti, Maria Carla Liberto, Nadia Marascio, Adele Emanuela De Francesco.
Footnotes
Author contribution(s): Giada Procopio: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Project administration; Supervision; Visualization; Writing-original draft; Writing-review & editing.
Anna Cancelliere: Conceptualization; Formal analysis; Methodology; Writing-original draft.
Enrico Maria Trecarichi: Methodology; Writing-review & editing.
Maria Mazzitelli: Investigation; Writing-review & editing.
Eugenio Arrighi: Investigation; Writing-review & editing.
Graziella Perri: Methodology; Writing-review & editing.
Francesca Serapide: Conceptualization; Writing-review & editing.
Corrado Pelaia: Conceptualization; Data curation; Formal analysis; Writing-original draft; Writing-review & editing.
Elena Lio: Conceptualization; Writing-review & editing.
Maria Teresa Busceti: Formal analysis; Investigation; Writing-review & editing.
Maria Chiara Pelle: Formal analysis; Writing-review & editing.
Marco Ricchio: Methodology; writing-review & editing.
Vincenzo Scaglione: Conceptualization; Writing-review & editing.
Chiara Davoli: Investigation; Writing-review & editing.
Paolo Fusco: Conceptualization; Writing-review & editing.
Valentina La Gamba: Investigation; Writing-review & editing.
Carlo Torti: Formal analysis; Methodology; Writing-review & editing.
Girolamo Pelaia: Conceptualization; Data curation; Methodology; Writing-review & editing.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Giada Procopio  https://orcid.org/0000-0001-5148-3610
https://orcid.org/0000-0001-5148-3610
Corrado Pelaia  https://orcid.org/0000-0002-4236-7367
https://orcid.org/0000-0002-4236-7367
Girolamo Pelaia  https://orcid.org/0000-0001-9288-8913
https://orcid.org/0000-0001-9288-8913
Supplemental material: The reviews of this paper are available via the supplemental material section.
Contributor Information
Giada Procopio, Infectious and Tropical Disease Unit, ‘Magna Graecia’ University, Viale Europa, Catanzaro, 88100, Italy.
Anna Cancelliere, Infectious and Tropical Disease Unit, Department of Medical and Surgical Sciences, ‘Magna Graecia’ University of Catanzaro, Catanzaro, Italy.
Enrico Maria Trecarichi, Infectious and Tropical Disease Unit, Department of Medical and Surgical Sciences, ‘Magna Graecia’ University of Catanzaro, Catanzaro, Italy.
Maria Mazzitelli, Infectious and Tropical Disease Unit, Department of Medical and Surgical Sciences, ‘Magna Graecia’ University of Catanzaro, Catanzaro, Italy.
Eugenio Arrighi, Infectious and Tropical Disease Unit, Department of Medical and Surgical Sciences, ‘Magna Graecia’ University of Catanzaro, Catanzaro, Italy.
Graziella Perri, Infectious and Tropical Disease Unit, Department of Medical and Surgical Sciences, ‘Magna Graecia’ University of Catanzaro, Catanzaro, Italy.
Francesca Serapide, Infectious and Tropical Disease Unit, Department of Medical and Surgical Sciences, ‘Magna Graecia’ University of Catanzaro, Catanzaro, Italy.
Corrado Pelaia, Respiratory Disease Unit, ‘Magna Graecia’ University Hospital of Catanzaro, Catanzaro, Italy.
Elena Lio, Infectious and Tropical Disease Unit, Department of Medical and Surgical Sciences, ‘Magna Graecia’ University of Catanzaro, Catanzaro, Italy.
Maria Teresa Busceti, Respiratory Disease Unit, ‘Magna Graecia’ University Hospital of Catanzaro, Catanzaro, Italy.
Maria Chiara Pelle, Infectious and Tropical Disease Unit, Department of Medical and Surgical Sciences, ‘Magna Graecia’ University of Catanzaro, Catanzaro, Italy.
Marco Ricchio, Infectious and Tropical Disease Unit, Department of Medical and Surgical Sciences, ‘Magna Graecia’ University of Catanzaro, Catanzaro, Italy.
Vincenzo Scaglione, Infectious and Tropical Disease Unit, Department of Medical and Surgical Sciences, ‘Magna Graecia’ University of Catanzaro, Catanzaro, Italy.
Chiara Davoli, Infectious and Tropical Disease Unit, Department of Medical and Surgical Sciences, ‘Magna Graecia’ University of Catanzaro, Catanzaro, Italy.
Paolo Fusco, Infectious and Tropical Disease Unit, Department of Medical and Surgical Sciences, ‘Magna Graecia’ University of Catanzaro, Catanzaro, Italy.
Valentina La Gamba, Infectious and Tropical Disease Unit, Department of Medical and Surgical Sciences, ‘Magna Graecia’ University of Catanzaro, Catanzaro, Italy.
Carlo Torti, Infectious and Tropical Disease Unit, Department of Medical and Surgical Sciences, ‘Magna Graecia’ University of Catanzaro, Catanzaro, Italy.
Girolamo Pelaia, Department of Health Sciences, ‘Magna Graecia’ University of Catanzaro, Catanzaro, Italy.
References
- 1. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. Epub ahead of print 7 February 2020. DOI: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Renda T, Corrado A, Iskandar G, et al. High-flow nasal oxygen therapy in intensive care and anaesthesia. Br J Anaesth 2018; 120: 18–27. [DOI] [PubMed] [Google Scholar]
- 3. Mauri T, Turrini C, Eronia N, et al. Physiologic effects of high-flow nasal cannula in acute hypoxemic respiratory failure. Am J Respir Crit Care Med 2017; 195: 1207–1215. [DOI] [PubMed] [Google Scholar]
- 4. Chanques G, Riboulet F, Molinari N, et al. Comparison of three high flow oxygen therapy delivery devices: a clinical physiological cross-over study. Minerva Anestesiol 2013; 79: 1344–1355. [PubMed] [Google Scholar]
- 5. Dysart K, Miller TL, Wolfson MR, et al. Research in high flow therapy: mechanisms of action. Respir Med 2009; 103: 1400–1405. [DOI] [PubMed] [Google Scholar]
- 6. Spoletini G, Alotaibi M, Blasi F, et al. Heated humidified high-flow nasal oxygen in adults: mechanisms of action and clinical implications. Chest 2015; 148: 253–261. [DOI] [PubMed] [Google Scholar]
- 7. Wang K, Zhao W, Li J, et al. The experience of high-flow nasal cannula in hospitalized patients with 2019 novel coronavirus-infected pneumonia in two hospitals of Chongqing, China. Ann Intensive Care 2020; 10: 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Slessarev M, Cheng J, Ondrejicka M, et al. ; Critical Care Western Research Group. Patient self-proning with high-flow nasal cannula improves oxygenation in COVID-19 pneumonia. Can J Anaesth. Epub ahead of print 21 April 2020. DOI: 10.1007/s12630-020-01661-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Delclaux C, L’Her E, Alberti C, et al. Treatment of acute hypoxemic nonhypercapnic respiratory insufficiency with continuous positive airway pressure delivered by a face mask: a randomized controlled trial. JAMA 2000; 284: 2352–2360. [DOI] [PubMed] [Google Scholar]
- 10. Rochwerg B, Brochard L, Elliott MW, et al. Official ERS/ATS clinical practice guidelines: noninvasive ventilation for acute respiratory failure. Eur Respir J 2017; 50: 1602426. [DOI] [PubMed] [Google Scholar]
- 11. Alhazzani W, Møller MH, Arabi YM, et al. Surviving sepsis campaign: guidelines on the management of critically ill adults with coronavirus disease 2019 (COVID-19). Intensive Care Med 2020; 46: 854–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Winck JC, Ambrosino N. COVID-19 pandemic and non invasive respiratory management: every Goliath needs a David. An evidence based evaluation of problems. Pulmonology. Epub ahead of print 27 April 2020. DOI: 10.1016/j.pulmoe.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Geng S, Mei Q, Zhu C, et al. High flow nasal cannula is a good treatment option for COVID-19. Heart Lung J Crit Care. Epub ahead of print 11 April 2020. DOI: 10.1016/j.hrtlng.2020.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. He G, Han Y, Fang Q, et al. [Clinical experience of high-flow nasal cannula oxygen therapy in severe corona virus disease 2019 (COVID-19) patients]. Zhejiang Da Xue Xue Bao Yi Xue Ban 2020; 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lu X, Xu S. Therapeutic effect of high-flow nasal cannula on severe COVID-19 patients in a makeshift intensive-care unit: a case report. Medicine (Baltimore) 2020; 99: e20393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Karamouzos V, Fligou F, Gogos C, et al. High flow nasal cannula oxygen therapy in adults with COVID-19 respiratory failure. A case report. Monaldi Arch Chest Dis 2020; 90. [DOI] [PubMed] [Google Scholar]
- 17. Borghesi A, Maroldi R. COVID-19 outbreak in Italy: experimental chest X-ray scoring system for quantifying and monitoring disease progression. Radiol Med (Torino) 2020; 125: 509–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Percorso assistenziale per il paziente affetto da COVID-19. Sezione 2 - Raccomandazioni per la gestione locale del paziente critico - versione 02. SIAARTI, http://www.siaarti.it/SiteAssets/News/COVID19%20-%20documenti%20SIAARTI/Percorso%20COVID-19%20-%20Sezione%201%20-%20Procedura%20Area%20Critica%20-%20Rev%202.0.pdf
- 19. Roca O, Caralt B, Messika J, et al. An index combining respiratory rate and oxygenation to predict outcome of nasal high-flow therapy. Am J Respir Crit Care Med 2019; 199: 1368–1376. [DOI] [PubMed] [Google Scholar]
- 20. Gautret P, Lagier J-C, Parola P, et al. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int J Antimicrob Agents. Epub ahead of print 20 March 2020. DOI: 10.1016/j.ijantimicag.2020.105949. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 21. Mazzitelli M, Arrighi E, Serapide F, et al. Use of subcutaneous tocilizumab in patients with COVID-19 pneumonia. J Med Virol. Epub ahead of print 15 May 2020. DOI: 10.1002/jmv.26016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mazzitelli M, Serapide F, Tassone B, et al. Spontaneous and severe haematomas in patients with COVID-19 on low-molecular-weight heparin for paroxysmal atrial fibrillation: Mediterr J Hematol Infect Dis 2020; 12: e2020054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. World Health Organization. Clinical management of severe acute respiratory infection when novel coronavirus (2019-nCoV) infection is suspected: interim guidance, https://apps.who.int/iris/handle/10665/330893 (2020, accessed 28 January 2020).
- 24. Raoof S, Nava S, Carpati C, et al. High-flow, noninvasive ventilation and awake (nonintubation) proning in patients with COVID-2019 with respiratory failure. Chest. Epub ahead of print 15 July 2020. DOI: 10.1016/j.chest.2020.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cortegiani A, Crimi C, Noto A, et al. Effect of high-flow nasal therapy on dyspnea, comfort, and respiratory rate. Crit Care 2019; 23: 201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Agarwal A, Basmaji J, Muttalib F, et al. High-flow nasal cannula for acute hypoxemic respiratory failure in patients with COVID-19: systematic reviews of effectiveness and its risks of aerosolization, dispersion, and infection transmission. Can J Anaesth. Epub ahead of print 15 June 2020. DOI: 10.1007/s12630-020-01740-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hui DS, Chow BK, Lo T, et al. Exhaled air dispersion during high-flow nasal cannula therapy versus CPAP via different masks. Eur Respir J 2019; 53: 1802339. [DOI] [PubMed] [Google Scholar]
- 28. Remy KE, Lin JC, Verhoef PA. High-flow nasal cannula may be no safer than non-invasive positive pressure ventilation for COVID-19 patients. Crit Care 2020; 24: 169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Leung CCH, Joynt GM, Gomersall CD, et al. Comparison of high-flow nasal cannula versus oxygen face mask for environmental bacterial contamination in critically ill pneumonia patients: a randomized controlled crossover trial. J Hosp Infect 2019; 101: 84–87. [DOI] [PubMed] [Google Scholar]
- 30. Hui DS, Hall SD, Chan MTV, et al. Exhaled air dispersion during oxygen delivery via a simple oxygen mask. Chest 2007; 132: 540–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Li J, Fink JB, Ehrmann S. High-flow nasal cannula for COVID-19 patients: low risk of bio-aerosol dispersion. Eur Respir J 2020; 55: 2000892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kotoda M, Hishiyama S, Mitsui K, et al. Assessment of the potential for pathogen dispersal during high-flow nasal therapy. J Hosp Infect 2020; 104: 534–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hui DS, Hall SD, Chan MTV, et al. Noninvasive positive-pressure ventilation: an experimental model to assess air and particle dispersion. Chest 2006; 130: 730–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gaeckle NT, Lee J, Park Y, et al. Aerosol generation from the respiratory tract with various modes of oxygen delivery. Am J Respir Crit Care Med. Epub ahead of print 21 August 2020. DOI: 10.1164/rccm.202006-2309OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Iwashyna TJ, Boehman A, Capelcelatro J, et al. Variation in aerosol production across oxygen delivery devices in spontaneously breathing human subjects. medRxiv 2020.04.15.20066688. [Google Scholar]
- 36. Fowler RA, Guest CB, Lapinsky SE, et al. Transmission of severe acute respiratory syndrome during intubation and mechanical ventilation. Am J Respir Crit Care Med 2004; 169: 1198–1202. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Author_Response for Oxygen therapy via high flow nasal cannula in severe respiratory failure caused by Sars-Cov-2 infection: a real-life observational study by Giada Procopio, Anna Cancelliere, Enrico Maria Trecarichi, Maria Mazzitelli, Eugenio Arrighi, Graziella Perri, Francesca Serapide, Corrado Pelaia, Elena Lio, Maria Teresa Busceti, Maria Chiara Pelle, Marco Ricchio, Vincenzo Scaglione, Chiara Davoli, Paolo Fusco, Valentina La Gamba, Carlo Torti and Girolamo Pelaia in Therapeutic Advances in Respiratory Disease
Supplemental material, Reviewer_1_v.1 for Oxygen therapy via high flow nasal cannula in severe respiratory failure caused by Sars-Cov-2 infection: a real-life observational study by Giada Procopio, Anna Cancelliere, Enrico Maria Trecarichi, Maria Mazzitelli, Eugenio Arrighi, Graziella Perri, Francesca Serapide, Corrado Pelaia, Elena Lio, Maria Teresa Busceti, Maria Chiara Pelle, Marco Ricchio, Vincenzo Scaglione, Chiara Davoli, Paolo Fusco, Valentina La Gamba, Carlo Torti and Girolamo Pelaia in Therapeutic Advances in Respiratory Disease
Supplemental material, Reviewer_2_v.1 for Oxygen therapy via high flow nasal cannula in severe respiratory failure caused by Sars-Cov-2 infection: a real-life observational study by Giada Procopio, Anna Cancelliere, Enrico Maria Trecarichi, Maria Mazzitelli, Eugenio Arrighi, Graziella Perri, Francesca Serapide, Corrado Pelaia, Elena Lio, Maria Teresa Busceti, Maria Chiara Pelle, Marco Ricchio, Vincenzo Scaglione, Chiara Davoli, Paolo Fusco, Valentina La Gamba, Carlo Torti and Girolamo Pelaia in Therapeutic Advances in Respiratory Disease