SUMMARY
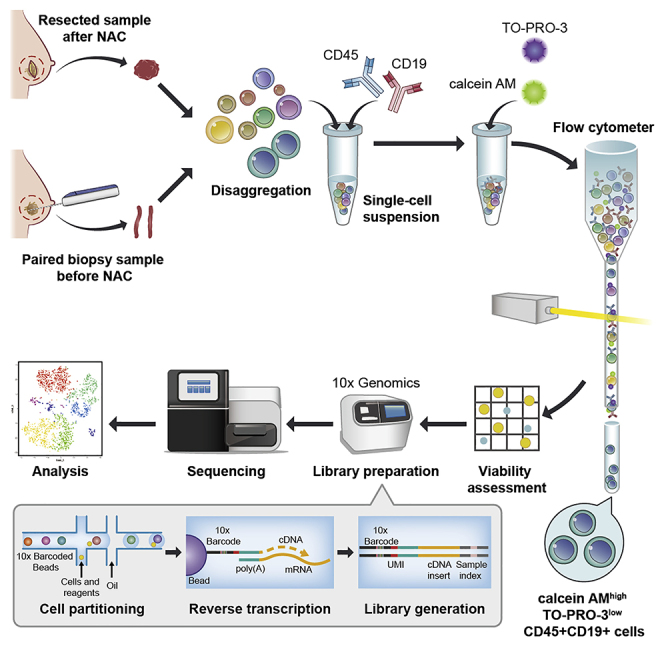
Single-cell analysis of tumor-infiltrating lymphocytes obtained before and after preoperative therapy reflects the dynamic interplay of the tumor and immune system during treatment. Here, we present a protocol to implement single-cell analysis of tumor-infiltrating B cells, which were isolated from paired human breast cancers before and after neo-adjuvant chemotherapy. This protocol also facilitates isolation and single-cell analysis of other tumor-infiltrating lymphocytes.
For complete information on the generation and use of this protocol, please refer to Lu et al. (2020).
Graphical Abstract
Highlights
-
•
Isolation and storage of tumor-infiltrating B cells for single-cell analysis
-
•
Cancer tissues of patients were obtained before and after neo-adjuvant chemotherapy
-
•
The viability and purity are critical for preparing cell suspension
Single-cell analysis of tumor-infiltrating lymphocytes obtained before and after preoperative therapy reflects the dynamic interplay of the tumor and immune system during treatment. Here, we present a protocol to implement single-cell analysis of tumor-infiltrating B cells, which were isolated from paired human breast cancers before and after neo-adjuvant chemotherapy. This protocol also facilitates isolation and single-cell analysis of other tumor-infiltrating lymphocytes.
BEFORE YOU BEGIN
Prepare Biopsy Samples from Patients before Receiving Neo-adjuvant Chemotherapy
Timing: ∼2 h
-
1.
Obtain biopsy tissue when operating ultrasound-guided core needle biopsy for patients with suspicious breast cancer. Biopsy tissues were obtained by EnCor Enspire™ Breast Biopsy System with 7-G EnCor Enspire™ needle probe (BARD GmbH, Karlsruhe, Germany). 1–2 pieces of specimens were collected from each patient.
-
2.
Collect tissue and place it in MACS Tissue Storage Solution (Cat# 130-100-008, Miltenyi Biotec) on ice.
Prepare Paired Resected Samples from the Same Patients after Receiving Neo-adjuvant Chemotherapy
Timing: ∼2 h
-
3.
Obtain paired tumor tissue when resecting breast tumors for the same patients after receiving neo-adjuvant chemotherapy.
-
4.
Collect tissue and place it in MACS Tissue Storage Solution on ice.
Digestion Solution for Tumor Tissue
Timing: 10 min
-
5.
Combine the following reagents in gentle MACS C Tubes.
-
6.
Pre-warm at 37°C for 30 min.
Dulbecco’s modified Eagle medium (DMEM)
5% fetal bovine serum
2 mg/mL collagenase I (Cat# LS004196, Worthington Biochemical)
2 mg/mL collagenase Ⅲ (Cat# LS004182, Worthington Biochemical)
2 mg/mL hyaluronidase (Cat# H3506, Sigma-Aldrich)
CRITICAL: Fresh tumor tissue sample and fast processing is most important for B cells isolation. The role of tumor-infiltrating B cells is still controversial. Previous studies have demonstrated that tumor-infiltrating B cells promote tumor progression in various ways, such as producing IL-10, TGF-β, and IL-35 (Bodogai et al., 2015, Olkhanud et al., 2011, Shalapour et al., 2015, Yuen et al., 2016). In contrast, cumulating studies have reported that B cells could enhance anti-tumor immunity and are positively correlated to better prognosis in patients receiving immunotherapy (Cabrita et al., 2020, Helmink et al., 2020, Petitprez et al., 2020). These findings indicate that tumor-infiltrating B cells have distinct subsets and heterogeneity under different therapies. Thus, we examined tumor-infiltrating B cells from samples before and after neo-adjuvant chemotherapy.
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Pacific Blue™ anti-human CD45 | Biolegend | Cat# 304022; RRID: AB_493655 |
| Pacific Blue™ Mouse IgG1, κ Isotype Ctrl Antibody | Biolegend | Cat# 400151 |
| PE anti-human CD19 | Biolegend | Cat# 392506; RRID: AB_2750097 |
| PE Mouse IgG1, κ Isotype Ctrl Antibody | Biolegend | Cat# 400114 |
| Chemicals, Peptides, and Recombinant Proteins | ||
| MACS Tissue Storage Solution | Miltenyi Biotec | Cat# 130-100-008 |
| Collagenase, typeⅠ | Worthington Biochemical | Cat# LS004196 |
| Collagenase, typeⅢ | Worthington Biochemical | Cat# LS004182 |
| Hyaluronidase | Sigma-Aldrich | Cat# H3506 |
| Calcein AM | ThermoFisher Scientific | Cat# C3100MP |
| TO-PRO-3 iodide | ThermoFisher Scientific | Cat# T3605 |
| Trypan Blue Solution | ThermoFisher Scientific | Cat# 15250061 |
| Critical Commercial Assays | ||
| FcR Blocking Reagent, human | Miltenyi Biotec | Cat# 130-059-901 |
| Chromium Single Cell 3′ Library & Gel Bead Kit v3 | 10× Genomics | Cat# 1000075 |
| Other | ||
| gentleMACS C Tubes | Miltenyi Biotec | Cat# 130-093-237 |
| gentleMACS™ Dissociator | Miltenyi Biotec | Cat# 130-093-235 |
| MACSmix Tube Rotator | Miltenyi Biotec | Cat# 130-090-753 |
Resource Availability
Lead Contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Shicheng Su (sushch@mail.sysu.edu.cn).
Materials Availability
This study did not generate new unique reagents.
Data and Code Availability
This study did not generate/analyze new datasets/code.
STEP-BY-STEP METHOD DETAILS
Generation of Single-Cell Suspension Using Tumor Tissues
Timing: ∼4 h
This step provides a detailed procedure for tissue dissociation and cell suspension preparation.
-
1.
Take out the tissue from the MACS Tissue Storage buffer. Wash tissue samples with excess cold PBS on ice to prevent the contamination of peripheral blood.
-
2.
Cut tissue samples into small pieces (1–2 mm diameter) with autoclaved surgical instruments (Huang et al., 2018, Su et al., 2018a) on ice.
-
3.
Transfer the tissue pieces into MACS C Tube containing pre-warmed (37°C) digestion solution.
-
4.
Attach C Tube upside down onto the sleeve of the gentleMACS Dissociator and choose the program: h_tumor_01.
-
5.
After completion of the program, detach C Tube and incubate sample for 2 h at 37°C under continuous rotation using the MACSmix Tube Rotator. During digestion, vortex the tissue pieces in solution every 15 min.
-
6.
Repeat step 4 and apply the suspension to 100 μm and 70 μm cell strainer placed on a 50 mL tube successively on ice. Wash the cell strainers twice with 20 mL sterile cold PBS.
-
7.
Pre-cool the spin to 4°C. Centrifuge at 350 ×g for 10 minutes at 4°C and discard supernatant. Resuspend cells with cold PBS and place the tube on ice.
Isolation of Tumor-Infiltrating B Cells
Timing: ∼3 h
This step provides a detailed procedure for viable B cell isolation from the cell suspension of tumor tissue.
-
8.
Count cells with hemocytometer.
-
a.
Transfer 10 μL of the cell suspension under the cover slip on the hemocytometer. Wait until capillary action draw the fluid inside and the chamber is fulfilled.
-
b.
Observe the hemocytometer under the microscope. Count the cells in four sets of 16 squares. For cells located on the line, only count ones on the left-hand or top boundary line.
-
c.
No. of cells/mL = (Cell count)/4(number of chambers counted) × 104.
-
9.
Resuspend cells up to 107 cells per 80 μL of cold PBS with 2% FBS and 2 mM EDTA, and add 20 μL of Fc receptor blocking reagent (Cat# 130-059-901, Miltenyi Biotec). Mix the cells and incubate for 10 min at 4°C. Use a small number of cells for single channel control and isotype staining.
-
10.
Add Pacific Blue™ anti-human CD45 (Cat# 304022, Biolegend) and PE anti-human CD19 (Cat# 392506, Biolegend) antibodies to the suspension and incubate at 4°C for 30 min in the dark (Su et al., 2018b).
-
11.
Wash with cold PBS by centrifugation at 350 ×g for 5 min at 4°C.
-
12.
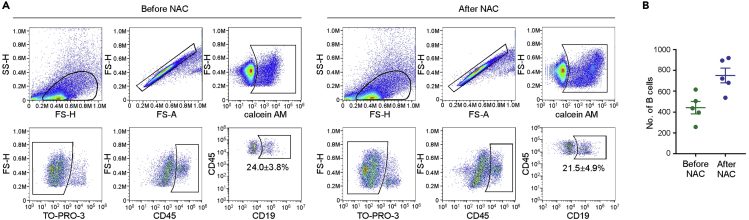
To exclude dead cells, use calcein AM (Cat# C3100MP, ThermoFisher Scientific) to stain live cells, and TO-PRO-3 (Cat# T3605, ThermoFisher Scientific) to stain dead cells. Only cells that are both positive for calcein AM and negative for TO-PRO-3 could be gated as viable (Figure 1A) (Puram et al., 2017). Prepare calcein AM solution by dissolving 50 μg reagent with 50 μL DMSO. Prepare TO-PRO-3 solution by diluting 10 μL reagent with 20 μL DMSO. Restore the solution to 20∼25°C before use.
Figure 1.
B Cell Isolation from Paired Breast Cancer Tissues of Patients before and after Receiving Neo-adjuvant Chemotherapy (NAC)
(A) Gating strategy of tumor-infiltrating B cells in FACS.
(B) Number of B cells isolated from paired breast cancer tissues of five patients.
-
13.
Prepare calcein AM and TO-PRO-3 staining solution by diluting the solution with PBS (1:1000 for both). Incubate the cells in calcein AM and TO-PRO-3 staining solution at 20∼25°C for 15 min in the dark.
-
14.
Wash with cold PBS and centrifuge at 350 ×g for 5 min at 4°C. Resuspend the cells in 500 μL of cold PBS on ice.
-
15.
Perform fluorescence activated cell sorting (FACS) with flow cytometer (BD Biosciences) using 488 nm (calcein AM, 530/30 filter), 640 nm (TO-PRO-3, 670/14 filter), 405 nm (Pacific blue™, 450/50 filter) and 561 nm (PE, 586/15 filter) lasers. Standard forward scatter height versus area criteria were used to discard doublets and capture singlets. Collect the calcein AMhigh TO-PRO-3low CD45+ CD19+ cells with tube containing sterile cold PBS with 10% FBS on ice. The time for cell sorting should not exceed 4–5 h.
-
16.
Pipette the sorted cell suspension and remove 20 μL to a new tube and add 20 μL of 0.4% Trypan Blue (Cat# 15250061, ThermoFisher Scientific). Mix by gentle pipetting and use 10 μL to fill the hemocytometer.
-
17.
Count the number of live (seen as bright cells) and dead cells (stained blue) with microscope. No. of cells/mL = (Cell count)/4(number of chambers counted) × 2 (Dilution with Trypan Blue) × 104.
-
18.
Divide the live cell count by the total cell count to calculate the viability.
Storage of B Cells
Timing: ∼20 min
This step provides a detailed procedure for storage of B cells for single-cell analysis or other application.
-
19.
Pre-cool the spin to 4°C. Centrifuge the isolated viable B cells at 350 ×g for 5 min at 4°C. Discard the supernatant.
-
20.
Resuspend the cells with the 1 mL of freezing medium (90% FBS supplemented with 10% DMSO).
-
21.
Remove the cell suspensions into cryovials.
-
22.
Put the vials in a gradient cooling box and store at -80°C.
-
23.
After 24 h, transfer the vials containing cells to liquid nitrogen.
Thawing and Resuspension of Frozen Cells
Timing: ∼20 min
This step provides a detailed procedure for thawing and resuspension of frozen cells.
-
24.
After confirming the treatment information of patients, pick the paired samples of breast cancers before and after neo-adjuvant chemotherapy.
-
25.
Remove the cryovials from liquid nitrogen and thaw in the water bath at 37°C.
-
26.
Pre-cool the spin to 4°C. Transfer thawed cells to a 50 mL conical tube and dilute cells with 30 mL of DMEM containing 10% FBS. Centrifuge at 350 ×g for 5 min at 4°C.
-
27.
Remove most of the supernatant and leave ∼1 mL.
-
28.
Add 9 mL of cold Dulbecco’s PBS + 0.04% BSA containing 10% FBS.
-
29.
Determine the cell viability and concentration with 0.4% Trypan Blue with Countess II Automated Cell Counter. Take out 20 μL of cell suspension and place in an Eppendorf tube. Add 20 μL of 0.4% Trypan Blue and mix gently.
-
30.
Count cells with Countess II Automated Cell Counter. Load 10 μL of the mixure into a disposable Countess chamber slide. Insert the slide into the instrument and press “Capture” to read the concentration and viability.
-
31.
Centrifuge cell suspension at 350 ×g for 5 min at 4°C.
-
32.
Discard the supernatant and resuspend cells with cold DPBS + 0.04% BSA at concentration of 1 × 105–2 × 105 cells/mL (Savas et al., 2018) on ice.
-
33.
Load the cell suspension to v3 reagent kit (10 × Genomics, Pleasanton, CA, USA) for single-cell RNA sequencing following the 10 × Genomics Chromium single-cell protocol for the v3 reagent kit.
EXPECTED OUTCOMES
About 100–1,000 viable tumor-infiltrating B cells could be isolated from the specimen of each patient.
Before loading the cell suspension to v3 reagent kit, the viability of cells ideally should be at least 90%.
LIMITATIONS
The number of isolated tumor-infiltrating B cells varies among different patients due to the tumor heterogeneity. In addition, tumor-infiltrating B cells from different patients may be pooled together for single-cell analysis due to the limited number of isolated cells.
TROUBLESHOOTING
Problem
Low cell viability.
Potential Solution
The viability for B cells isolated from tumor tissue could be improved by always placing tissue or cells on ice (steps 1–15 in STEP-BY-STEP METHOD DETAILS). In addition, it is critical to exclude dead cells, debris and doublets in FACS (steps 12–15 in STEP-BY-STEP METHOD DETAILS).
Problem
Cell loss in the multiple steps.
Potential Solution
Due to very small number of cells isolated from primary tumor tissue, the cell pellet may not be visible after centrifuge. Cell loss could be reduced by leaving a bit of supernatant in the tube instead of removing all (steps 7, 11, 14, 19, 27, 32 in STEP-BY-STEP METHOD DETAILS).
ACKNOWLEDGMENTS
This work was supported by grants from the National Key Research and Development Program of China (2017YFA0106300), the Natural Science Foundation of China (91942309, 81672614, 81971481, 81672621, 81872155), the Natural Science Foundation of Guangdong Province (2016A030306023, 2017A030313878, 2018A030313769), Pearl River S&T Nova Program of Guangzhou (201710010083, 201710010048), Tip-top Scientific and Technical Innovative Youth Talents of Guangdong special support program (no. 2016TQ03R553), Guangdong Science and Technology Department (2017B030314026).
AUTHOR CONTRIBUTIONS
S.S. designed the experiments and supervised the study. Y.L. performed the experiments and wrote the manuscript. J.-Y.L. performed the experiments. All authors discussed the results and commented on the manuscript.
DECLARATION OF INTERESTS
The authors declare no competing interests.
References
- Bodogai M., Moritoh K., Lee-Chang C., Hollander C.M., Sherman-Baust C.A., Wersto R.P., Araki Y., Miyoshi I., Yang L., Trinchieri G. Immunosuppressive and prometastatic functions of myeloid-derived suppressive cells rely upon education from tumor-associated B cells. Cancer Res. 2015;75:3456–3465. doi: 10.1158/0008-5472.CAN-14-3077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabrita R., Lauss M., Sanna A., Donia M., Skaarup Larsen M., Mitra S., Johansson I., Phung B., Harbst K., Vallon-Christersson J. Tertiary lymphoid structures improve immunotherapy and survival in melanoma. Nature. 2020;577:561–565. doi: 10.1038/s41586-019-1914-8. [DOI] [PubMed] [Google Scholar]
- Helmink B.A., Reddy S.M., Gao J., Zhang S., Basar R., Thakur R., Yizhak K., Sade-Feldman M., Blando J., Han G. B cells and tertiary lymphoid structures promote immunotherapy response. Nature. 2020;577:549–555. doi: 10.1038/s41586-019-1922-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang D., Chen J., Yang L., Ouyang Q., Li J., Lao L., Zhao J., Liu J., Lu Y., Xing Y. NKILA lncRNA promotes tumor immune evasion by sensitizing T cells to activation-induced cell death. Nat. Immunol. 2018;19:1112–1125. doi: 10.1038/s41590-018-0207-y. [DOI] [PubMed] [Google Scholar]
- Lu Y., Zhao Q., Liao J.-Y., Song E., Xia Q., Pan J., Li Y., Li J., Zhou B., Ye Y. Complement signals determine opposite effects of B cells in chemotherapy-induced immunity. Cell. 2020;180:1–17. doi: 10.1016/j.cell.2020.02.015. [DOI] [PubMed] [Google Scholar]
- Olkhanud P.B., Damdinsuren B., Bodogai M., Gress R.E., Sen R., Wejksza K., Malchinkhuu E., Wersto R.P., Biragyn A. Tumor-evoked regulatory B cells promote breast cancer metastasis by converting resting CD4(+) T cells to T-regulatory cells. Cancer Res. 2011;71:3505–3515. doi: 10.1158/0008-5472.CAN-10-4316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petitprez F., de Reynies A., Keung E.Z., Chen T.W., Sun C.M., Calderaro J., Jeng Y.M., Hsiao L.P., Lacroix L., Bougouin A. B cells are associated with survival and immunotherapy response in sarcoma. Nature. 2020;577:556–560. doi: 10.1038/s41586-019-1906-8. [DOI] [PubMed] [Google Scholar]
- Puram S.V., Tirosh I., Parikh A.S., Patel A.P., Yizhak K., Gillespie S., Rodman C., Luo C.L., Mroz E.A., Emerick K.S. Single-cell transcriptomic analysis of primary and metastatic tumor ecosystems in head and neck cancer. Cell. 2017;171:1611–1624.e24. doi: 10.1016/j.cell.2017.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savas P., Virassamy B., Ye C., Salim A., Mintoff C.P., Caramia F., Salgado R., Byrne D.J., Teo Z.L., Dushyanthen S. Single-cell profiling of breast cancer T cells reveals a tissue-resident memory subset associated with improved prognosis. Nat. Med. 2018;24:986–993. doi: 10.1038/s41591-018-0078-7. [DOI] [PubMed] [Google Scholar]
- Shalapour S., Font-Burgada J., Di Caro G., Zhong Z., Sanchez-Lopez E., Dhar D., Willimsky G., Ammirante M., Strasner A., Hansel D.E. Immunosuppressive plasma cells impede T-cell-dependent immunogenic chemotherapy. Nature. 2015;521:94–98. doi: 10.1038/nature14395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su S., Chen J., Yao H., Liu J., Yu S., Lao L., Wang M., Luo M., Xing Y., Chen F. CD10(+)GPR77(+) cancer-associated fibroblasts promote cancer formation and chemoresistance by sustaining cancer stemness. Cell. 2018;172:841–856 e816. doi: 10.1016/j.cell.2018.01.009. [DOI] [PubMed] [Google Scholar]
- Su S., Zhao J., Xing Y., Zhang X., Liu J., Ouyang Q., Chen J., Su F., Liu Q., Song E. Immune checkpoint inhibition overcomes ADCP-induced immunosuppression by macrophages. Cell. 2018;175:442–457.e23. doi: 10.1016/j.cell.2018.09.007. [DOI] [PubMed] [Google Scholar]
- Yuen G.J., Demissie E., Pillai S. B lymphocytes and cancer: a love-hate relationship. Trends Cancer. 2016;2:747–757. doi: 10.1016/j.trecan.2016.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This study did not generate/analyze new datasets/code.