Summary
Many studies in systems neuroscience use head-fixation preparations for in vivo experimentation. While head-fixation confers several advantages, one major limitation is the lack of behavioral measures that quantify whole-body movements. Here, we detail a step-by-step protocol for using a novel head-fixation device that measures the forces exerted by head-fixed mice in multiple dimensions. We further detail how this system can be used in conjunction with in vivo electrophysiology and optogenetics to study dopamine neurons in the ventral tegmental area.
For complete details on the use and execution of this protocol, please refer to Hughes et al. (2020a, 2020b)
Graphical Abstract

Highlights
-
•
Protocol for using a novel head-fixation device to measure head and body forces in mice
-
•
Protocol allows recording from optogenetically tagged dopamine neurons
-
•
Device can identify start and end of movements for correlation with neuron activity
Many studies in systems neuroscience use head-fixation preparations for in vivo experimentation. While head fixation confers several advantages, one major limitation is the lack of behavioral measures that quantify whole-body movements. Here, we detail a step-by-step protocol for using a novel head-fixation device that measures the forces exerted by head-fixed mice in multiple dimensions. We further detail how this system can be used in conjunction with in vivo electrophysiology and optogenetics to study dopamine neurons in the ventral tegmental area.
Before You Begin
To monitor head and body movements during head-fixed experiments, we built a head-fixation device that incorporates five high-precision force sensors (load cells). When load cells are deformed due to an external force, they produce continuous analog signals that are proportional to the force applied. Three load cells are located above the head. These are directly attached to the part of the apparatus that clamps onto the rigid head-restraint bar attached to the skull. These load cells are arranged so that the animals’ movements can be detected in three different directions: up and down, side-to-side, and front-to-back. Two additional sensors are also placed below the feet to measure the movement of the limbs (Figure 1). The device must be 3D-printed. The load cells can be purchased from a hobby parts retailer, making the device very inexpensive to build (∼$120; see Key Resources Table for further information). Once assembled and calibrated, the system requires little maintenance. The load cell signals can be recorded in parallel with neural data or optogenetic stimulation timestamps to better understand the relationship between neural activity and behavior. This protocol first details the assembly and use of the head-fixation system, and then further describes how it can be used in conjunction with electrophysiology and optogenetics to record from dopamine (DA) neurons in the ventral tegmental area (VTA).
Figure 1.
Illustration of the Five Force Sensors Incorporated into the Head-Fixation Device
This device precisely quantifies whole-body and head movements while mice are head-restrained. Three load cells detect movements of the head along three different axes, and two load cells resting beneath the animal detect movements of the left and right feet.
Assembly of the Head-Fixation Device
Timing: 2 days
The individual components of the calibration and head-fixation devices must be 3D printed by the user. Printing files are available by request. Additional step-by-step procedures, graphical descriptions, and videos of the assembly of the system can be found in a previous publication (Hughes et al., 2020a).
-
1.
Print out the components listed in Materials and Equipment.
-
2.
Mount the base supports using ¼″ (×8) screws onto the breadboard so that the inner bottom edges of the base supports are 7 inches apart.
-
3.Mount the perch main body onto the breadboard using ¼″ (×2) screws so that it is squarely between the base supports.
-
a.Leave the screws loose until assembly of the entire system is complete.
-
a.
-
4.
Connect the curved base connectors to the base supports using M6 (×8) bolts and nuts.
-
5.
Link the curved base connectors together with the keystone beam using M6 (×4) blots and nuts.
-
6.
Attach the unwired end of the Left/Right load cell to the keystone beam using M2.5 (×2) screws. The bolt holes are threaded, so no nuts are required. Make sure the head of the screw rests on the plastic. The end of the load cell with the wires will point upward.
-
7.
Attach the top load cell connector to the wired end of the Left/Right load cell using M2.5 (×2) screws. Make sure the wires are not being pinched in any way.
-
8.
Attach the wired end of the Front/Back load cell to the top load cell connector using M2.5 (×2) screws. Ensure that the wires are not being pinched.
-
9.
Attach the bottom load cell connector to the unwired end of the Front/Back load cell using M2.5 (×2) screws.
-
10.
Attach the wired end of the Up/Down load- ell to the bottom load cell connector using M2.5 screws (×2). Ensure that the wires are not being pinched.
-
11.
Attach the unwired end of the Up/Down load cell to the Main load cell connector using M2.5 (×2) screws.
-
12.Assemble the perch.
-
a.Attach wired ends of the left and right foot load cells to the curved cover piece using M2.5 (×4) screws.
-
b.Attach the foot platforms to the unwired ends of the load cells using M2.5 (×4) screws.
-
c.Super glue the perch to the main body.
-
a.
Optional: We use our system in sound-attenuating boxes with doors that are closed during testing. The mice will often make large movements (which can be seen in the load cell signals) if they are disturbed during the performance of a task, so minimizing such disturbances is helpful. We also play white noise in the boxes to prevent them from being distracted by noises in the environment.
CRITICAL: For 3D printing, any filament can be used for the head-fixation apparatus. PLA is very rigid but also notorious for wearing down over time. ABS is tougher, but it is harder to print because of higher temperature requirements. Alloy 910 and NylonX are both extremely tough and resistant to wear, but they require special care in storage, printer nozzle type , and print settings.
CRITICAL: The frame that attaches to the mice’s head-restraint bar must be customized to accommodate different experimental set ups. Make sure your lab-specific design provides enough room for recording head stages and optical fiber cables. Additionally, make sure that the restraining hardware can be adjusted up and down and forward and back to accommodate as many different applications as possible.
Load Cell Calibration Device Assembly
Timing: 1 day
The goal of calibration is to determine the relationship between the applied force and the voltage signal. The key is to fix the load cell rigidly in the horizontal plane by one end and apply a known mass to the other end while recording the voltage output. The load cell should be calibrated in both deflection directions (Figure 2) to be able to reliably calculate the forces exerted in opposite directions by the animal.
-
13.
3D-print two copies of part A, one copy of part B, and one weighing platform (Figure 2).
-
14.
Fasten the two copies of A together using part B using M4 screws.
-
15.
Using two M2.5 screws, attach the wired end of the load cell to part B. This acts as the rigid anchor and holds the load cell in place for calibration (Figure 2).
-
16.
Make a note of the mass of the weighing platform. This value will be added to the known masses that will be applied to improve calibration accuracy.
-
17.
Attach the weighing platform to the unwired end of the load cell with two more M2.5 screws (Figure 2).
Figure 2.
Schematic of Calibration Apparatus Designed to Bi-directionally Calibrate Load Cells
This calibration device allows you to change the direction that the load cell is facing. This makes it easy to calibrate bidirectional movements of the load cell. It is important to measure the mass of the weight platform and incorporate this value into the force calculations.
Notes on Amplification Circuit Assembly
The INA125 amplifiers are robust and should have a long life if the soldering and wire connections are done well. The circuit design described in (Hughes et al., 2020a) is recommended because it has been adapted to allow for bidirectional forces (e.g., forward/backward, left/right, and up/down). The most common source of circuit malfunctions is from faulty connections. While it is possible to assemble the wiring by hand on a prototyping board, we recommend getting a circuit board professionally printed (see below, Figure 6).
Figure 6.
Optional Printed Circuit Board Containing Five Circuits with Surface Mount Components for Each Load Cell
The printed circuit board is an alternative solution to wiring separate circuits by hand for each load cell. Inset shows zoomed in view of one circuit module. The circuit components are soldered by hand. Note that the amplifying chip used here is the surface mount version (INA125UA), as is the potentiometer.
Optogenetically Tagging Dopamine Neurons
DAneurons have diverse action potential waveforms that make them difficult to identify during in vivo electrophysiology experimentsBarter, 2015. Although some have argued that DA neurons can be distinguished from other neuronal subtypes based on their firing rate and waveform shape, this approach may result in some false positive identifications, especially in the VTA(Margolis et al., 2010, Ungless and Grace, 2012). Thus, many studies investigating DA neurons now use optogenetic tagging (optotagging) to confirm neurons are dopaminergic. This approach combines extracellular electrophysiological recording with the expression of Cre-dependent channelrhodopsin (ChR2) in neurons that express Cre-recombinase under the control of the dopamine transporter (DAT) promoter. ChR2 is a light sensitive protein that allows for selective excitation of neurons using light. By delivering light immediately adjacent to the recording sites, recorded ChR2-expressing neurons will depolarize.. This confirms they are dopaminergic.
-
18.
To build an optrode (electrode plus optic fiber), it is common to attach an optic fiber to a commercially available electrode array. Various devices that combine recording and light delivery are also available commercially (http://www.inphysiology.com/optogenetic-applications/).
-
19.
To express ChR2 in DA neurons, one can inject a viral vector containing Cre-dependent ChR2 into the VTA of DAT-Cre mice (Cohen et al., 2012), or by cross-breeding DAT-Cre mice with mice genetically modified to express ChR2 in any neuron also expressing Cre (Hughes et al., 2020b, Madisen et al., 2012). The latter approach ensures more uniform expression of ChR2 in DA neurons, as it eliminates variability due to virus injection.
-
20.
Adjust the laser output at the tip of the connecting optic fiber. For DA neurons, we typically use between 2 and 8 mW of power.
Key Resources Table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Bacterial and Virus Strains | ||
| AAV5-EF1a-DIO-hChR2(E123T/T159C)-EYFP | UNC Vector Core | N/A |
| Experimental Models: Organisms/Strains | ||
| Mouse: DAT-IRES-Cre | The Jackson Laboratory | 006660 |
| Mouse: Ai32(RCL – ChR2(H134R)/EYFP) | The Jackson Laboratory | 024109 |
| Software and Algorithms | ||
| MATLAB | MathWorks | https://www.mathworks.com/downloads/web_downloads/download_release?release=R2020a |
| NeuroExplorer | Nex Technologies | http://www.neuroexplorer.com/downloadspage/ |
| Other | ||
| PLA Plastic | Gizmo Dorks | PLA Plus 3D Filament Black, ABS, Alloy 910 or NylonX |
| 12″ × 12″ aluminum breadboard | Thorlabs, Inc. | Part # MB12 |
| ¼″-20 bolts | Thorlabs, Inc. | Part # SH25S100 |
| M6 bolts and nuts | Newlng | Part # N0LG063 |
| 100 g load cell (×5) | RobotShop | Product Code: RB-Phi-203 |
| INA125 Instrumentation amplifier: Through-pin (×5) |
Digikey Electronics | Part # INA125P-ND |
| INA125 Instrumentation Amplifier (×5) Surface mount (×5) |
Digikey Electronics | Part # INA125UA-ND |
| Multi-turn Potentiometer (×5) through-pin | Digikey Electronics | Part # 3006P-102LF-ND |
| Trimmer potentiometer (×5) Surface mount |
Digikey Electronics | Part # ST4ETB101CT-ND |
| Prototyping breadboard | Digikey Electronics | Part # 1988-1001-ND |
| Printed circuit board for amplification circuit | Pcbway | Custom order |
| 1 × M2.5 stainless steel machine screw kit | HVAZI | HVAZI M2.5 Stainless Steel Phillips Flat Head Machine Screws Assortment Kit. Part # 43239-160489 |
| Recording hardware | Various (e.g., Blackrock, National Instruments) | N/A |
| Super glue | Loctite | Part # 1363589 |
| 473 nm laser | Lasermate Group, Inc. | Part # DPF473-200FMA21-H |
| Stereotaxic framea | David Kopf Instruments | Model 963 |
| Head shavera | Wahl | Wahl Pro Clipper #9307-100 |
| Isoflurane delivery devicea | Kent Scientific Corporation | VetFlo Vaporizor |
| Thermoregulating heating pada | Kent Scientific Corporation | RightTemp Jr. |
| Tweezersa | Kent Scientific Corporation | #5, 0.06 mm tips |
| Surgical scissorsa | Kent Scientific Corporation | Dissecting scissors, straight |
| Drilla | Makartt | 30000RPM Nail Drill Machine JD700 |
| Electrodesa | Innovative Neurophysiology | 16 channel fixed & 16 channel movable |
| Microinjectora | Drummond Scientific Company | Nanoject III |
| Iodine wipesa | VWR International, LLC | 1108 |
| Isopropyl alcohol wipesa | VWR International, LLC | First aid alcohol wipes |
| Gelfoam spongea | Pfizer | 9031408 |
| Miniature screwsa | SE | SE Glasses/Watch Repair Screws Set (900 PC.) - JT69900 |
| Dental Cementa | The Vollrath Company, LLC | Item # 51458 |
| Head-restraint barsa | The H.E. Parmer Company | Custom machining order |
| Chemicals, Peptides, and Recombinant Proteins | ||
| Isofluranea | Patterson Veterinary | 14043-704-06 |
| 0.9% salinea | Pfizer | 0409-4887-10 |
| Meloxicama | Boehringer Ingelheim | Megacam 5 mg/mL |
| Bupivacainea | Hospira, Inc. | 0.25% (2.5 mg/mL) |
| Ophthalmic ointmenta | Dechra Veterinary Products | Puralube |
| Hydrogen peroxidea | VWR International, LLC | BDH7540-2 |
For use in surgery
Materials and Equipment
This table details the 3d printed parts required for making the head-fixation and load cell calibration devices.
| Number | Purpose | |
|---|---|---|
| Head-Fixation device | ||
| Base supports | 2× | Supports the entire head load cell array |
| Curved base connectors | 2× | Supports the load cell array above the animals’ head |
| Keystone beam | 1× | Connects the left and right supports as well as attaches the Left/Right load cell |
| Top load cell connector | 1× | Connects the Left/Right load cell to the Forward/Backward load cell |
| Bottom load cell connector | 1× | Connects the Forward/Backward load- cell to the Up/Down load cell |
| Main load cell connector | 1× | Connects the Up/Down load cell to the part of the head-fixation device that clamps the animal in |
| Perch main body | 1× | Supports the animal and the perch which houses the foot loadcells |
| Perch feet platforms | 2× | Provides a platform between the animal’s feet and the feet load cells |
| Perch curved cover | 1× | Provides a covered housing for the animal |
| Load Cell Calibration Device | ||
| Part A | 2× | Provides support and facilitates bidirectional calibration |
| Part B | 1× | Joins the twin A Parts and attaches the load cell for calibration |
| Weighing platform | 1× | Attaches to the free end of the load cell and supports calibration weights |
Step-By-Step Method Details
Load Cell Calibration
Timing: 1 h
Load cells are calibrated by installing them into our custom-designed calibration device and then applying increasing forces on the load cell using known masses. We use increasing amounts of sucrose or water in a lightweight container. The amplified analog force signal from the load cells is recorded as a voltage by the acquisition hardware. The corresponding voltage signals from each known mass can then be plotted to determine the force-to-voltage relationship. The calibration device is designed to determine the force-to-voltage relationship for forces applied bidirectionally to the load cells.
CRITICAL: The load cells only need to be calibrated once. This needs to be done before the installation of the load cell into the head-fixation system.
-
1.
Install the load cell into the calibration device. See above for assembly instructions.
-
2.
Connect the circuit output to an analog recording device. Our lab uses Blackrock data acquisition systems, but we have also used National Instruments data acquisition cards. For calibration, a high-resolution voltage meter is also sufficient (Figure 3).
-
3.
Set the force-to-voltage slope for each circuit.
-
a.
The INA125 amplification chip is connected to a multi-turn trimmer potentiometer between pins 8 and 9. The potentiometer should be adjusted while monitoring the signal in real time, as the neutral set point voltage of the load cell will change.
-
b.
Turning the potentiometer changes the slope of the relationship between the applied force and the corresponding voltage. Counter clock-wise rotation (reducing resistance) will increase the slope and clock-wise rotation (increasing resistance) will reduce the slope (Hughes et al., 2020a). The resistance should be adjusted so the mice cannot max out the voltage signal in either direction. At the same time, the range should also be calibrated for maximum sensitivity. Thus, a good balance of sensitivity and resolution needs to be found through trial-and-error. The range of the signal can be tested by gently moving the calibration platform up and down, producing a sinusoidal signal.
-
c.
Full-grown mice readily produce instantaneous force amplitudes exceeding 1 N. Making sure that a 100 g mass placed on the calibration platform does not max out the signal can help determine the optimal potentiometer position.
-
d.
We have found that the potentiometer resistance value should be around 100 ohms for a good signal range. With lower resistance values, the conversion function can become non-linear with more extreme forces.
-
4.
Calibrate each load cell circuit.
-
a.
Apply increasing masses (e.g., 25 g, 50 g, 100 g) one at a time to the platform of the calibration device and record the voltage signal that results at the circuit output. Keep using greater masses until the signal has reached the end of its range.
CRITICAL: The actual applied mass is equal to the mass of the applied object plus the mass of the weighing platform itself.
-
b.
Flip the device over and repeat with the other side.
-
c.
If the load cell’s signal saturates with a mass lower than 100g, adjust the potentiometer, and then repeat step 4.
-
5.Calculate the voltage-to-force linear function.
-
a.The applied force from each mass can be calculated using Newton’s second law (force = mass x acceleration). We assume a constant acceleration due to gravity (9.8 m/s2).
-
b.Determine the linear function for converting the recorded voltage from the load cell to force. It is useful to first plot the voltage vs. applied force data to confirm a linear relationship. Next, invert the relationship, plotting force vs. voltage and fit a line to the points to determine the slope and the y-axis intercept (see below for further clarification, Figure 14). Because the load cell’s signal should be linear, applying the function to any voltage value results in an accurate force estimate.
-
c.Repeat steps 4 & 5 for each load cell (x5).
-
a.
-
6.Install the load cells into the head-fixation device (see previous section on head-fixation device assembly).
-
a.Make sure that the load cells are oriented in the device in such a way that the directions of the signals are known and intuitive. For example, it is helpful for analysis and interpretation that forward movements increase the voltage signal and backward movements decrease the signal.
-
b.It is helpful for troubleshooting to label the individual wires that connect the circuit board to each load cell as well as to the data acquisition system. This allows you to easily find the appropriate connections.
-
c.Keep track of the analog input channel that each load cell signal is being routed to in the recording system. Labeled wires make this simple and clear.
-
a.
CRITICAL: The load cell should not be used if either non-linearities or slope differences between the two load directions are encountered. These make any simple linear force-voltage transformation more difficult, if not impossible.
CRITICAL: The conversion function should remain stable indefinitely if the trim potentiometer setting does not change and if the load cell does not become permanently deformed from excessive force. If this is done properly, the calibration step only needs to be performed once prior to final head-fixation device assembly.
Figure 3.
Equipment Needed to Calibrate Load Cells
Any voltage meter can be used that detects the voltage of the circuit output. For example, Blackrock systems can also be used. Note the calibration device currently has a load applied to the load cell, which is registering a voltage of 3.9 V.
Figure 14.
Demonstration of the Conversion between Voltage and Force Using the Linear Conversion Function
Load cells produce a linear relationship between force applied to the load cell during calibration and voltage. Inverting this relationship yields a linear function that is used to convert voltage into force.
Solutions for Long-Term Stability and Protection of Wiring
Timing: 1 h
The final assembly of the head-fixation system results in many delicate wires arranged around the mouse. Proper planning and organization of the wiring protects the system from accidental snagging and jostling. Damage can naturally result from installing and removing the animals as well as from their natural movements during experimentation. Here we provide some suggestions for ways to protect the wiring and circuits.
-
7.
The amplifier and trim potentiometer can be protected by soldering them together on a small circuit prototyping breadboard. (Figure 4; see (Hughes et al., 2020a) for wiring diagram).
Figure 4.
Custom-Built Circuit with Trimmer Potentiometer
This circuit is made using the through-pin version of the amplifying chip (INA125P). See (Hughes et al., 2020a) for a full circuit diagram.
-
8.
The circuit boards should be kept some distance away from the head-fixation device to prevent them from being bumped, jostled, or soiled. We have found that it is helpful to place the circuit boards in small modified plastic containers that you can put in the corner of the operant box (Figure 5). Used pipette tip boxes work well.
Figure 5.
Enclosure to Protect Circuit Wiring
Not shown are holes cut in the side of the enclosure to allow for passage of wiring.
-
9.
We have made custom printed circuit boards that work with surface mount variants of the amplifier and trimmer potentiometers. The boards are meant to replace the hand- assembled circuits to reduce assembly error. In our design, all five circuits can be conveniently arranged on one board (Figure 6).
Note: One limitation with some surface mount potentiometers is that they are not multi-turn, meaning that it may be difficult to dial in the right resistance for the optimal signal sensitivity. We have not experimented with multi-turn surface mount potentiometers.
-
10.
The load cells have very delicate wires. We recommend permanently attaching the wires to the plastic components of the head-fixation device. We also have begun to use male and female pin connectors that are glued to the 3D-printed structure components (Figure 7). This approach also makes for easy disconnection if the system must be removed for repair or cleaning.
Figure 7.
Load Cells Connected to Circuit with Male-to-female Connector Pins for Easy Disassembly
We recommend attaching these connectors with super glue to the base support and curved support pieces of the head-fixation system.
-
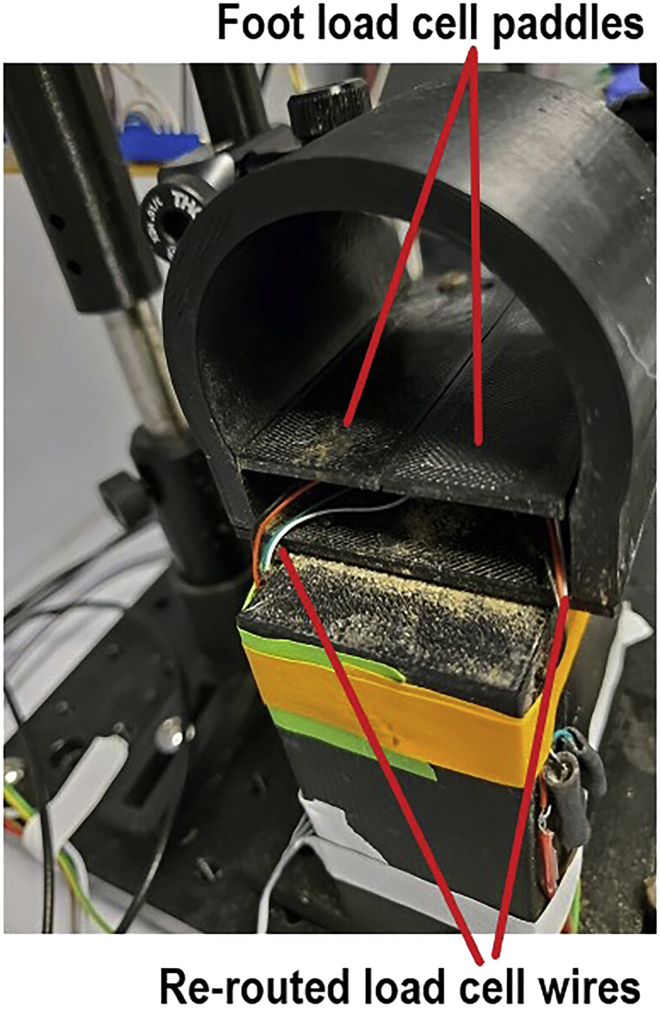
11.
The wiring of the loadcells installed under the animals’ feet exit out the back of the perch (Figure 8). As a result, these wires can be easily damaged by movements that occur during the task, as well as when you install the mice into the perch. They should be routed down and to the sides of perch stand to keep them protected and immobile.
Figure 8.
Rear-View of Behavioral Perch Showing the Routing and Protection of the Load Cell Wiring from the Foot Force Sensors
Wires in the back of the perch are the most prone to damage from normal movements. Route these wires around the sides of the perch.
Implanting and Preparing Mice with Optogenetic and Electrophysiological Equipment in the VTA
Timing: 4 h
Recording from VTA DA neurons can be a difficult and tedious process. However, there are some useful tips that can help you increase the yield.
CRITICAL: Even with good surgical technique, DA neurons often will not be immediately detectable when using chronic electrode implants. In these cases, being patient and simply waiting long enough after surgery often results in obtaining good DA neurons.
-
12.Prepare mice for surgery.
-
a.Additional surgical preparation procedures can be found in (Geiger et al., 2008).
-
b.Sterilize surgical instruments using an autoclave or dry bead sterilizer. Animals are initially anesthetized with isoflurane (2%–3%) mixed with oxygen flowing at 0.5 L/min.
-
c.Once installed in the stereotaxic device, animals are maintained during surgery using 0.8% to 1.5% isoflurane in 0.5 L/min oxygen.
-
d.Maintain animal body temperature using a thermoregulating heating pad.
-
e.Shave hair over the entire scalp using electric clippers and clean the shaved areas with iodine and 70% isopropyl alcohol.
-
f.Cover the eyes with ophthalmic ointment for protection.
-
g.Deliver a subcutaneous injection of meloxicam (2 mg/kg) for preemptive analgesia.
-
a.
-
13.Level the head and determine the location of the craniotomy.
-
a.Tent the skin over the scalp by pulling it gently using sterile forceps. Cut the skin with sterile surgical scissors so that the scalp is exposed. The resulting incision should be about 10 mm in diameter.
-
b.Clean the bone surface with hydrogen peroxide to remove periosteal tissue, then rinse with sterile 0.9% saline and dry it with Gelfoam.
-
c.Fix a sturdy vertical coordinate probe (such as a syringe tip or a spare optical fiber implant) to the stereotaxic arm and ensure it travels consistently along the entire exposed sagittal suture of the skull in the anterior-posterior axis.
-
i.This ensures that the skull is centered in the apparatus.
-
ii.If the probe and suture deviate, adjust the ear bar positions to correct the deviation.
-
i.
-
d.Locate lambda and bregma (See methods from Paxinos and Franklin, 2004 for useful suggestions on determining these landmarks).
-
e.Ensure that lambda and bregma are level by moving the probe to both positions and making note of the dorsal-ventral coordinate at each landmark. We aim for a difference of less than 100 μm.
-
a.
-
14.Perform a small craniotomy over the VTA and four additional craniotomies for skull screws.
-
a.We use the following coordinates to target the VTA relative to bregma: AP: −3.3 mm, ML: 0.5 mm for the center of the craniotomy (Figure 9 left).
-
a.
Figure 9.
Craniotomy Targeting for the VTA Electrode Implantation and Locations of Bone Screws around Perimeter of Exposed Skull
Left: Coordinate probe is used to level the head and determine the location of the craniotomy relative to bregma. Right: Screws are implanted around the edges of the exposed skull where it is thickest. This electrode does not have an optical fiber attachment. Reference wire is wrapped around one screw.
-
b.
The size and location of the craniotomy will depend on the size of the electrode array.
-
c.
Drivable electrodes that we use have recording wires bundled in a cannula that is 200 μm in diameter and require the smallest craniotomy.
CRITICAL: Take caution when using larger electrode arrays. Avoid implanting them too close to the midline. Mice have a higher mortality rate when this is done due to the increased risk of rupturing the sagittal sinus.
-
d.
Implant several miniature screws (4 are recommended) along the lateral portions of the front and back of the skull, forming roughly a square (Figure 9, right). The bone is thicker further from the midline as well as near the cerebellum. The headcap can stay fixed to the skull for over a year even with regular head-fixation experiments.
-
e.
Turn the screws 2–3 times, so that the first or second complete thread has cut into the bone. Inserting the screw beyond this point only damages the brain and does not improve its stability in the skull.
-
i.
If you are unsure of how well the screw is fastening to the skull, take some tweezers and try to lightly wiggle the screw. If it does not move, it is well fastened. However, if it does, unscrew it from the skull and make a new craniotomy.
-
15.Position the electrode just above the craniotomy. Ground the electrode by wrapping the refence wire around only one dental screw.
-
a.Wrap the reference wire around the screw 4–5 times, then turn the screw by ½ a turn so the ground wire becomes tightly wrapped around the screw.
-
i.Good grounding is important for optimal neural activity with a good signal-to- noise ratio.
-
ii.Excessive bleeding under the screw may form a clot between the metal and the brain surface, which will worsen grounding. It is advised to use a flat-bottomed screw for the reference to minimize clotting.
-
iii.Remove any additional wiring by cutting the remaining wire coming off the skull screw.
-
i.
-
a.
CRITICAL: Be sure to wrap the reference wire around the screw before implanting the electrode. Otherwise, wrapping the wire around the screw while it is in the brain will move the electrode, which can cause significant damage to brain tissue.
-
16.Slowly lower the electrode array into the VTA.
-
a.Initially lower the electrode in 500 μm steps. Between each advance of the electrode, wait 2–3 min, then lower another 500 μm.
-
b.Approximately 1 mm above the VTA (2.8–3.0 mm from brain surface), begin to lower the electrode in 100 μm steps. After each increment, stop and wait 2–3 min. Lowering the electrode array too rapidly will affect your yield of DA neurons.
-
c.Stop anywhere between (dorsal-ventral) 3.9 - 4.5 mm from brain surface.
-
i.If using a drivable electrode, we recommend stopping the cannulae just above the VTA around 3.8 mm.
-
ii.See also (Coddington and Dudman, 2018). They were able to optically tag DA neurons as shallow as 3.6 mm and as deep as 4.9 mm. We have not attempted to record from these more extended coordinates.
-
i.
-
a.
CRITICAL: Lowering the electrode slowly, especially for the final millimeter, is essential for high-quality recordings with a good signal-to-noise ratio.
-
17.
Position the head-restraint implant around the electrode and apply dental cement around both to secure them in place for chronic recording (Figure 10).
Figure 10.
Release of a Drivable Optical Electrode after Application of Dental Cement
Left: Release of a cemented drivable optrode targeting the VTA. Right: View from the front along with the head bar embedded into the cement.
Note: Different head-restraint implants are often used in individual labs to accommodate for different recording hardware needs. Our restraint bars are custom made by a machine shop.
-
18.
Remove the animal from the stereotaxic device and place them in a clean cage.
-
19.
Place the cage on a heating pad for recovery.
CRITICAL: After an implantation of an electrode array, we often will wait up to several months before viable DA neurons can be observed. If no DA neurons appear at first, e.g., if the electrode contains neurons that cannot be optogenetically tagged or do not have a characteristic DA waveform, wait and recheck neural activity after several weeks.
Recording Force and Neural Signals from Behaving Animals
Timing: 1 h
The device requires an acquisition system that can record a minimum of five analog voltage signals in parallel. Our lab uses Blackrock Cerebus systems. These systems can record neural data in parallel with analog behavioral data and digital time stamps such as laser events or licking time stamps.
Note: We use two computers for our experimental system. One computer controls the behavioral tasks using MATLAB via a small data acquisition (DAQ) system (National Instruments). The DAQ outputs cue, reward, and laser stimulation commands that are copied to the digital inputs of the Blackrock Cerebus recording system. The second computer records all the neural, analog, and digital timestamp data via the Blackrock system. In this design, all experimental manipulations, as well as the neural and behavioral data from the animal, are saved onto one system for ease of analysis.
CRITICAL: We recommend using an all-in-one system for recording neural and force data. However, many neural recording acquisition systems are unable to accommodate many analog inputs. It is possible to use other systems for recording the analog signals, such as acquisition cards from National Instruments. If recording neural data and load cell force data on separate devices, it is essential to be able to synchronize the two systems in some manner.
-
20.Set up the recording system and software.
-
a.Ensure cues and rewards are being delivered correctly.
-
b.When running optogenetic experiments, check the power output from the laser and confirm that the behavioral program is correctly outputting the proper frequency of stimulation.
-
a.
-
21.Prepare data recording hardware.
-
a.Enter file name for saving.
-
b.Also prepare head stages and optical stimulation hardware.
-
a.
-
22.Double check force sensors are working properly.
-
a.Gently push on the pivot point of the head-fixation system where the head force sensors are located.
-
i.Blackrock systems make this step easy, as it is possible to observe all of the analog signals together using the “raster plot” window found on the control panel of the Blackrock recording software suite (Central) and selecting the appropriate analog channels. This will provide a real-time read out of the voltage signals from the load cells.
-
b.Gently push on the foot platforms to check the feet force sensors.
-
i.
-
a.
-
23.Install mice in the head-fixation system.
-
a.The perch that the mice sit in has a cover. Mice will learn to walk forward under the cover to the front side with the head-restraint attachments. We deliver a small reward they can collect once they walk through. Do not try to force them through the perch. They will naturally approach the front. This is also much easier than trying to back the mice into the system.
-
i.Optical fibers and recording head stages should only be attached after the mice are head-restrained.
-
i.
-
b.Animals should be positioned so that they are comfortably resting on the floor of the mouse perch. The head should not be too high or too low relative to the floor. The mice will often use their forepaws to pull against the edges of the perch (Figures 11 and 12).
-
a.
-
c.
When first putting the mouse on the platform, perform a quick visual check that the loadcell signal baselines are at neutral values and that it is possible to obtain equal measures of movement in both directions for each load cell. It is possible that adding or removing the animal can shift the load cells slightly, cause an offset from a neutral position, or damage wiring.
-
24.
Complete experiment and stop recording signals.
-
25.
Remove fiber optic cables or head stages.
-
26.
Loosen the head-restraint clamps and simply allow the mouse to walk onto one’s hand.
Optional: To keep the device in good working condition, clean up the perch between experiments. However, we have not noticed any impact on behavior by training animals consecutively in the device without cleaning. We clean at the end of the day by vacuuming droppings.
Figure 11.
Wide View of a Mouse Installed in the Head-Fixation System
Note mouse positioning and the generous space afforded to accommodate various recording hardware.
Figure 12.
Close-Up View of Optimal Mouse Positioning
Note the forepaws are able to reach the edge of the perch.
Optically Tagging DA Neurons
Timing: 10 min
DA neurons can be optically tagged if the DA neurons express ChR2. Light can then be delivered to the recording site immediately adjacent to the tips of the electrode. Ensure that the laser is connected to the optic fiber implant before beginning.
-
27.
We normally attempt to tag DA neurons at the end of the experiment.
-
28.
Stop the behavioral programs, but do not stop recording neural signals.
-
29.Activate the laser and initiate the stimulation.
-
a.We use 10-20 5 ms pulses at 10–20 Hz delivered for 1 second. Each pulse train istriggered every 5–15 seconds for 20 total trials. Use a random inter-trial-interval to keep animals from anticipating the stimulation.
-
a.
-
30.
Once laser stimulation is completed, stop recording the neural signals and remove the mouse.
-
31.
See (Hughes et al., 2020b) for examples of optically tagged neurons.
CRITICAL: Save any spiking evoked by optical tagging in the same file that contains the neural activity recorded during the behavioral session. This allows spontaneously evoked and stimulation- evoked spikes to be sorted together, and will greatly aid in both identifying DA neurons and connecting them to activity recorded during the experiment.
Expected Outcomes
Properly setting up and configuring the system should yield many hours of productive recording without the need for much adjustment or repair. Proper calibration of the load cells results in a linear calibration function with very little residual error. Careful adjustment of the trimmer potentiometer should result in high-resolution voltage signals from the load cells. The force sensor signals should be highly sensitive to the mouse’s movements. Both high- and low-frequency oscillations, as well as large and small amplitude movements,should be visible in the sensors (Figure 15). The relationships between neural activity and the continuous forces produced by miceare still poorly understood. We have recently demonstrated that DA neurons in the ventral tegmental area (VTA) are responsible for controlling the impulse vector (force exerted in a certain direction over time) in a fixed-time task (Hughes et al., 2020b). It is therefore predicted that neural activity in regions innervated by DA neurons are also related to forces exerted by the head and body. We have also recently demonstrated that putative VTA gamma-aminobutyric acid (GABA) neurons co-vary with force during a standard Pavlovian conditioning task (Hughes et al., 2020a). These movements were unique on a trial-by-trial basis, even though the animals experienced identical conditioned (CS) and unconditioned stimulus (US) presentations. This revealed that averaging across trials can obfuscate the relationship between behavior and neural activity. Furthermore, we showed that when the neural activity is aligned to experimenter-defined events such as cues, but sorted according to the continuous force measures as shown below, the neural activity more accurately reflects the continuous behavior. Thus, our systems reveals the importance of measuring behavior as a continuous process while head-fixed. If neural activity in other regions besides those we have found are related to the time-varyingforces generated while head-fixed, then the successful use of our system by other groups may reveal previously overlooked functions of the brain.
Figure 15.
Procedure to Detect Resting Periods between Movement Events
Top: One minute of continuously sampled raw data collected from one animal. Middle: The same signal as above has been converted into force in the forward and backward directions. Bottom: Taking the time derivative of force reveals the periods of immobility (shaded bars) that are necessary for subsequent movement time point estimation.
Quantification and Statistical Analysis
Sorting Neural Activity
Once the neural activity has been recorded, it can be sorted offline to isolate single units for classification and analysis. Offline sorting, though time-consuming, is recommended to obtain high-quality single unit data.
-
1.
Open recorded neural activity in an offline sorting program.
-
a.
We use Plexon Offline Sorter.
-
2.
Sort neural activity by going through each individual channel and adding units.
-
a.
Offline Sorter provides an automated principal component analysis that clusters the waveforms. Clusters should be well-isolated and easy to differentiate (Figure 13).
Figure 13.
Sorted Dopamine Neurons Recorded on a Single Channel Using Plexon Offline Sorter
Left: Action potential waveforms of three sorted VTA DA neurons from a single electrode channel. Right: Clusters formed by the distinct action potential waveforms plotted in principal component space.
-
b.
Offline Sorter also offers the option to “find units” that automatically adds units. However, their algorithm can make mistakes, so it is important to confirm their classified units manually.
-
3.
Save the sorted data. If using Blackrock, the sorted data file will not contain the raw continuous voltage data anymore. It is necessary to open the original file and export it to the sorted file in Neuroexplorer.
Note: See Plexon Offline Sorter manual for further instructions on sorting (https://plexon.com/wp-content/uploads/2018/02/Offline-Sorter-v4.4-User-Guide-a.pdf)
Conversion of Voltage to Force
The load cell voltage is linearly proportional to the force applied to the sensor. Calibration yields a linear formula that can be applied to voltage signals to obtain the force exerted by the mouse.
-
4.
Open the recording data in NeuroExplorer.
-
a.
We use NeuroExplorer because it contains many inbuilt functions for aligning data to time stamps.
-
b.
Data organization can also be done using MATLAB or other programming software, but we recommend NeuroExplorer. It has a scripting language that allows for efficient renaming and conversion of the channels and the data for each experiment performed.
-
5.
Relabel the analog input channels to reflect the load cell that the signal was recorded from.
-
a.
NeuroExplorer will use generic labels for each channel (e.g., ainp1, ainp2, etc.).
-
6.
Export the voltage signals into MATLAB from NeuroExplorer.
-
a.
Select the continuous voltage variables in NeuroExplorer.
-
b.
Under the “Matlab” tab in NeuroExplorer, select “Send Selected Variables to Matlab”
-
7.
Convert the voltage to force using the conversion functions that were determined during calibration (Figure 14).
Movement Detection and Sorting Trials
Movements are detected in the continuous load cell data, which provides information on the magnitude, duration, and direction of movement. Detecting and sorting these signals is an important step in understanding the relationships between movement and neural activity. In Figure 15, example traces of individual movement events produced by a mouse in our system are shown. Each movement is unique and shows distinct dynamics.
-
8.
Detect resting periods
-
a.
Animals will periodically rest between movement bouts. These periods are detected by taking the time derivative of force (Figure 15).
-
b.
Determine a threshold to identify the rest periods. We have found that it is sufficient to identify times when the change in force is below the 50th percentile of the absolute value of all force derivative measures.
-
9.
Determine the start and end times of each movement event.
-
a.
Movements begin when the rest periods end and are completed when rest periods begin. Each resting period provides a baseline rest voltage for the following movement.
-
b.
For each rest period, identify the subsequent forward movement periods as those that contain forces greater than the resting baseline force (Figure 16).
Figure 16.
Example Forward and Backward Movement Detection Strategy
Three example movement events detected on the Front/Back load cell. Blue shading reflects the period of rest that occurs in between movements. Green points indicate the initiation times of movement and red points indicate movement end times. Forward and backward movements are determined by identifying periods of positive and negative force.
-
c.
Identify the subsequent backward movements as those that are less than the resting baseline voltage (Figure 16).
Note: We created a MATLAB script for this step. It is available upon request.
-
10.
Send converted force data back to NeuroExplorer
-
a.
Under the “Matlab” tab in NeuroExplorer, select “Get Data from Matlab” and choose “Get Continuous Variables with Timestamps” to import data from Matlab.
-
11.
Send time stamps of movement start and end times back to NeuroExplorer
-
12.
Sort trials according to movement duration
-
a.
This is easily accomplished in NeuroExplorer by using the Sort Trials function. Use the movement start-times as the peri-event histogram reference and the movement end times as the Sorting Reference.
-
b.
Select “First after reference” as the Sorting Event (Figure 17).
Figure 17.
All Detected Movement Events from One Recording Session
The forces produced by the movements are sorted according to the movement duration. Dotted line reflects the end times of each movement.
-
c.
Compare the corresponding neural activity that will also be sorted according to the movement durations.
Limitations
Our system is compatible with chronically implanted electrodes and one-photon calcium imaging miniscopes that are attached directly to the head with a baseplate (www.miniscope.org). However, our head-fixation system is likely too flexible to be used in tandem with optical or electrophysiological approaches that require a completely rigid and immobilized preparation, such as two-photon microscopy or acutely implanted silicon electrodes. We have not tested the system for either technique. Alternative force sensors used for behavior are more rigid and could potentially be used. However, they are rated for much higher loads (> 1 kg). As a result, their ability to detect small movements made by mice may be more limited.
Troubleshooting
Problem 1: Loss of Load Cell Signals
Signal loss normally occurs when the system is first being set up, as this is the time that loose connections reveal themselves.
Potential Solution
The wiring will need to be carefully examined. It is very rare that the amplifier or potentiometer are the cause of this problem. Double check the connections as compared to the circuit diagram. Check each connection that was soldered manually. Often the circuit board will have shorted connections because the pins and connections are close to the the amplifier. This is potentially even more of a risk with the printed circuit board and the smaller size of the surface mount components. The delicate wires coming out of the load cells can also become disconnected and this can be difficult to notice - check these wires first.
Load-cells that are placed under the mice's feet can sometimes lose signal because of a mechanical problem – the paddles that they stand on can get stuck either to one another or to the side of the perch. Check that these paddles are not hindered in their normal movement before investigating the electrical components.
Urine from the mice can easily get under the foot pads of the perch design. However, it is difficult to clean under the foot pads without fully disassembling the perch. If left and right feet force sensors stop working, it is possible that urine has collected beneath the foot pads and onto the force sensors. We have not had any issues with this as we use water-deprivation in our experiments, and they do not frequently urinate in the perch. If the paddles are moving smoothly and the electrical circuit is working, full disassembly of the perch may be required to clean under the paddles. While the sensitive components of the load-cell are covered in a protective material, liquid may still potentially damage them.
CRITICAL: We recommend keeping a known working circuit and load cell available to troubleshoot with. It would be beneficial to design the wiring components in such a way that disconnection and testing with known components is effortless and does not require full disassembly of the system (see Figures 7 and 8 for a suggestion as to how to accomplish this).
Problem 2: Noise in the Load Cell Signals
Load cell signals “flicker” in and out or are noisy.
Potential Solution
There are several causes of this. Noise in a subset of load cells, sometimes accompanied by intermittent loss and reappearance of signal, indicates a faulty connection. Inspect the connections from the noisy load cell and ensure they are properly soldered and intact. Noise across all load cells, however, could indicate electrical interference from other devices such as the laser power supply. We have not found a single solution to this problem. There are several things however that can be done to reduce it. One important solution is to use wiring between the load cell and the amplifier that is shielded. Noise can also be reduced using a common ground and power supply for all load cells, which is isolated from other circuitry.
Problem 3: Noise in the Neural Recordings
Neural recordings can display high amounts of electrical noise and a low signal-to-noise ratio
Potential Solution
There are several potential sources for both electrical noise and a low signal-to-noise ratio. Make sure the neural recording system is grounded and separated from other electrical devices. In addition, make sure that the electrode is well grounded on the skull screw during surgery. Tighten the wire around the screw and make sure it is firm during surgery. This will prevent any potential movement of the wire on the screw while the mice are moving. Lowering the electrode very slowly in the last millimeter is also important for obtaining high-quality recordings.
Problem 4: Wearing Out of Plastic Pieces
Extensive use of the system will eventually wear down the printed components, particularly if they are printed with a low infill percentage PLA. The problem is usually one of rotation, whereby torque is continually applied to the plastic components at the screw holes. This gradually widens the holes and results in the load cell rotating orthogonally to its intended sensing direction. As a result, it distorts the registered force signal.
Potential Solution
The best solution we have found for this issue is to print with a more durable material (e.g., Alloy910 or NylonX) and to modify the components to include a small anti-rotation tab during printing that helps stabilize the load cell in the assembly.
Resource Availability
Lead Contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Henry H. Yin (hy43@duke.edu).
Materials Availability
Printing files for the calibration and the head-fixation devices are available upon request.
Data and Code Availability
Data and code are available upon request.
Acknowledgments
This work was supported by NIH grants NS904754, DA040701, and MH 112883 to H.H.Y.
Author Contributions
R.N.H., J.W.B., and H.H.Y. conceptualized the method. R.N.H., J.W.B., J.Z., and K.I.B. designed and tested the head-fixation apparatus. K.I.B. and R.N.H. drafted the manuscript. All authors read and approved the manuscript.
Declaration of Interests
The authors declare no competing interests.
References
- Barter Joseph. Beyond reward prediction errors: the role of dopamine in movement kinematics. Frontiers in integrative neuroscience. 2015 doi: 10.3389/fnint.2015.00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coddington L.T., Dudman J.T. The timing of action determines reward prediction signals in identified midbrain dopamine neurons. Nat. Neurosci. 2018;21:1563–1573. doi: 10.1038/s41593-018-0245-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J.Y., Haesler S., Vong L., Lowell B.B., Uchida N. Neuron-type-specific signals for reward and punishment in the ventral tegmental area. Nature. 2012;482:85–88. doi: 10.1038/nature10754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger B.M., Frank L.E., Caldera-Siu A.D., Pothos E.N. Survivable stereotaxic surgery in rodents. J. Vis. Exp. 2008;20 doi: 10.3791/880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes R.N., Bakhurin K.I., Barter J.W., Zhang J., Yin H.H. A head-fixation system for continuous monitoring of force generated during behavior. Front. Integr. Neurosci. 2020;14:11. doi: 10.3389/fnint.2020.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes R.N., Bakhurin K.I., Petter E.A., Watson G.D.R., Kim N., Friedman A.D., Yin H.H. Ventral tegmental dopamine neurons control the impulse vector during motivated behavior. Curr. Biol. 2020;30:2681–2694.e5. doi: 10.1016/j.cub.2020.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madisen L., Mao T., Koch H., Zhuo J.M., Berenyi A., Fujisawa S., Hsu Y.W., Garcia A.J., 3rd, Gu X., Zanella S., Kidney J., Gu H., Mao Y., Hooks B.M., Boyden E.S., Buzsaki G., Ramirez J.M., Jones A.R., Svoboda K., Han X., Turner E.E., Zeng H. A toolbox of Cre-dependent optogenetic transgenic mice for light-induced activation and silencing. Nat. Neurosci. 2012;15:793–802. doi: 10.1038/nn.3078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis E.B., Coker A.R., Driscoll J.R., Lemaitre A.I., Fields H.L. Reliability in the identification of midbrain dopamine neurons. PLoS One. 2010;5:e15222. doi: 10.1371/journal.pone.0015222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G., Franklin K.B.J. Elsevier Academic Press; 2004. The Mouse Brain in Stereotaxic Coordinates. [Google Scholar]
- Ungless M.A., Grace A.A. Are you or aren't you? Challenges associated with physiologically identifying dopamine neurons. Trends Neurosci. 2012;35:422–430. doi: 10.1016/j.tins.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data and code are available upon request.