Summary
Zinc (Zn2+) plays a vital role in the functioning of the cell. Cells have influx and efflux zinc transporters to regulate the levels of Zn2+ in the cytoplasm and organellar compartments to maintain homeostasis. We present a protocol to measure changes in cellular zinc concentrations using either a low-affinity membrane permeable or a high-affinity membrane impermeable fluorescent dye. Overall, zinc-specific fluorescent indicators using the assay can reliably detect the Zn2+ flux into or out of cultured cells.
For complete details on the use and execution of this protocol, please refer to Sanchez et al. (2019).
Graphical Abstract

Highlights
-
•
Reliable measure of zinc flux into or out of cultured adherent cells
-
•
Amenable for use with fluorescent dyes or genetically encoded zinc indicators
-
•
Data and statistical analyses can be done using a standard spreadsheet
-
•
Adaptable assay for other metal ions that bind specific fluorescent dyes
Zinc (Zn2+) plays a vital role in the functioning of the cell. Cells have influx and efflux zinc transporters to regulate the levels of Zn2+ in the cytoplasm and organellar compartments to maintain homeostasis. We present a protocol to measure changes in cellular zinc concentrations using either a low-affinity membrane permeable or a high-affinity membrane impermeable fluorescent dye. Overall, zinc-specific fluorescent indicators using the assay can reliably detect the Zn2+ flux into or out of cultured cells.
Before You Begin
Cell Line and Fluorophore Selection
Note: The current zinc flux assay uses two zinc-specific fluorescent indicators that are sensitive enough to detect the activity of endogenous zinc transporters, namely Newport Green (cell membrane permeant) and Fluozin-3 (cell membrane impermeant). Many human cell lines express endogenous zinc influx (SLC39/ZIP) and efflux (SLC30/ZNT) protein transporters (www.proteinatlas.org).
CRITICAL: In our experience, you must use an adherent cell line that expresses the protein-of-interest (e.g. influx or efflux type; plasma membrane or compartment localization) or use an adherent cell line for heterologous over-expression of the protein to be studied.
Note: Users must also decide the appropriate fluorescent zinc indicator (e.g. membrane permeant or impermeant; high affinity or low affinity) to achieve their research goals. Other more sensitive fluorescent zinc indicators and genetically encoded probes have been developed (Dittmer et al., 2009, Minckley et al., 2019); these may be utilized for the current assay by modifying the protocol according to the users’ project aims.
Coat 96-Well Plate with Poly-D-Lysine
Timing: 2.5 h
Note: This assay requires flat, clear bottom black 96-well cell culture plate that is used for fluorescent applications.
Note: Using black plates will reduce background light and crosstalk between wells, because the black hue tends to absorb light.
-
1.
Coat the 96-well plate with Poly-D-Lysine (PDL).
-
2.
The PDL is reconstituted in sterile water at a concentration of 0.1 mg/mL.
-
3.
The wells are incubated with PDL for 2 h, and then washed with sterile water three times.
-
4.
Let it dry. Wrap the plates in parafilm and store at 4°C.
-
5.
These plates can be stored in this manner for up to a year.
Key Resources Table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Chemicals, Peptides, and Recombinant Proteins | ||
| Pluronic F-68 Polyol (10% solution) | Cellgro | Cat#13-901-CI |
| 2-Mercaptopyridine N-oxide | Alfa Aesar | Cat#CAS1121-31-9 |
| Newport Green DCF Diacetate, cell permeable | Invitrogen | Cat#CATN7991 |
| FluoZin-3, Tetrapotassium Salt, cell impermeable | Invitrogen | Cat#F24194 |
| Poly-D-Lysine hydrobromide | Sigma-Aldrich | Cat#P6407-5MG |
| Zinc Chloride (ZnCl2) | Acros Organics | Cat#196840010 |
| Hydochloric acid 1N solution | Fisher | Cat#SA54-1 |
| Dimethylsulfoxide | Sigma-Aldrich | Cat#276855 |
| GlutaMAX-1 (100×) | Gibco | Cat#35050-061 |
| Sodium Pyruvate (100 mM) 100× | Gibco | Cat#11360-070 |
| Sodium Chloride (NaCl) | Fisher | Cat#S671-3 |
| Potassium Chloride (KCl) | Fisher | Cat#BP366-500 |
| Calcium Chloride dihydrate (CaCl2·2H2O) | Sigma-Aldrich | Cat#C3306 |
| HEPES, 1 M buffer soln., pH 8.0 | Alfa Aesar | Cat#J64578 |
| D-Glucose | MPI | Cat#194672 |
| Magnesium Chloride anhydrous (MgCl2) | Sigma-Aldrich | Cat#M8266-100G |
| Sodium Phosphate dibasic anhydrous (Na2HPO4) | Fisher | Cat#S374-500 |
| Sodium Phosphate monobasic monohydrate (NaH2PO4·H2O) | Fisher | Cat#S369-500 |
| Sodium bicarbonate (NaHCO3) | Sigma-Aldrich | Cat#S-8875 |
| Experimental Models: Cell Lines | ||
| HeLa Cell line | ATCC | Cat#CCL-2 |
| Recombinant DNA | ||
| pmCherry-N1 vector | Takara Bio USA | Cat#632523 |
| TMEM163-mCherry expression vector | Sanchez et al. 2019 | N/A |
| ZNT4-mCherry expression vector | Cuajungco Laboratory | N/A |
| Software and Algorithms | ||
| Gen5 software | BioTek | Cat#GEN5IPLUS |
| Excel software | Microsoft Office 365 | N/A |
| Graphpad Prism 8.0 | GraphPad | N/A |
| Other | ||
| Multi-channel pipet (8-channel, Eppendorf 2231302079) | Fisher | Cat#05412540 |
| Reagent reservoirs (disposable, sterile, Corning 4872) | Fisher | Cat#07200129 |
| 96-well culture-treated plate (Black, flat-bottom, Corning 3904) | Fisher | Cat#07200588 |
| Nalgene Filter unit PES membrane 500 mL (disposable, Thermo Scientific 1660045) | Fisher | Cat#0974063B |
| BioTek 405 TS plate washer | BioTek | Cat#405TSUS |
| BioTek Cytation 5 plate reader | BioTek | Cat#CYT5MV |
Resource Availability
Lead Contact
Math P. Cuajungco.
Materials Availability
The human TMEM163 (ZNT11)-mCherry and ZNT4-mCherry expression constructs are available upon request by contacting the lead contact.
Data and Code Availability
Not applicable.
Materials and Equipment
For Newport Green Assay: Standard Buffer (pH 7.4)
| Reagent/Chemical | Final Concentration (mM) |
|---|---|
| NaCl | 135 |
| KCl | 5 |
| CaCl2 | 1 |
| HEPES | 10 |
| D-Glucose | 5 |
For Fluozin-3 Assay: Kreb’s Ringer Bicarbonate Buffer (pH 7.4)
| Reagent/Chemica | Final Concentration (mM) |
|---|---|
| NaCl | 119.8 |
| KCl | 4.6 |
| MgCl2 | 0.5 |
| Na2HPO4 | 0.7 |
| NaH2PO4 | 1.3 |
| NaHCO3 | 15 |
| D-Glucose | 1 |
CRITICAL: To get rid of particulate contaminants in solution, all buffers are filter-sterilized using a Nalgene Rapid Flow filter unit (45 μm, PES membrane).
-
•
Make 10 mM stock solution of 2-Mercaptopyridine N-oxide (Pyrithione) reconstituted in water. This light sensitive chemical can be stored at 20°C to 25°C (room temperature) for 6 months.
CRITICAL: Pyrithione is very toxic and it is an irritant. It should be handled with personal protective equipment (PPE) such as gloves, eye protection goggles, and dust mask type N95.
-
•
Make a stock solution of 100 mM Zinc chloride (ZnCl2) in 0.02 N Hydrochloric acid (HCl).
CRITICAL: HCl is corrosive and concentrated HCl can release acidic vapors that are also dangerous. It should be handled in a chemical fume hood while wearing proper PPE.
CRITICAL: The Zinc-Pyrithione chelate solution (working concentration of 200 μM ZnCl2 and 20 μM 2-Mercaptopyridine N-oxide) must be made fresh on the day of the experiment.
For the Newport Green Assay
-
•
Make a 1 mM Newport Green DCF diacetate stock solution by dissolving the dye in Dimethylsulfoxide (DMSO). Newport Green is a low-affinity zinc dye (Kd ≈ 1 μM; excitation = 505 nm, emission = 535 nm; Johnson, 2010). The diacetate moiety makes Newport Green cell membrane permeable.
CRITICAL: Newport Green fluorescent dye is a light- and temperature-sensitive compound. Repeated freezing and thawing will degrade the dye resulting in reduced fluorescence. Make aliquots of the stock solution and store at –20°C for up to 6 months.
-
•
Make 5 μM Newport Green DCF working solution: 1 mM Newport Green stock solution is diluted in Standard Buffer with nonionic detergent 0.02% Pluronic F-68 Polyol. The detergent helps to disperse the non-polar ester in the buffer.
-
•
Supplement the Standard Buffer with 2 mM Glutamax (100×) and 1 mM Sodium pyruvate (100×) and keep at 20°C to 25°C until used. We found that these supplements help cell viability during the assay.
-
•
1 h before the assay, make a working solution of 200 μM ZnCl2 with 20 μM 2-Mercaptopyridine N-oxide (i.e. Zinc-Pyrithione complex) by using 6 mL Standard Buffer + 12 μL of 10 mM 2-Mercaptopyridine N-oxide + 12 μL 100 mM ZnCl2.
CRITICAL: The 200 μM ZnCl2 and 20 μM 2-Mercaptopyridine N-oxide solutions should be made fresh on the day of the experiment to avoid variations between trials. The complex is stable for at least 2 h at 20°C to 25°C.
For the Fluozin-3 Assay
-
•
Make a 1 mM Fluozin-3 tetrapotassium stock solution by reconstituting the dye in water. Fluozin-3 is a high affinity zinc dye (Kd = 15 nM; excitation = 494 nm, emission = 516 nm; Johnson, 2010). The tetrapotassium salt moiety makes Fluozin-3 cell membrane impermeable.
CRITICAL: Similar to Newport Green fluorescent dye, the Fluozin-3 dye is also light- and temperature sensitive. Make aliquots of the stock and store at –20°C.
-
•
Make a working solution of 2 μM Fluozin-3 using a 1 mM Fluozin-3 stock solution diluted in Kreb’s Ringer Bicarbonate buffer.
-
•
Supplement the Kreb’s Ringer Bicarbonate buffer with 2 mM Glutamax (100×) and 1 mM Sodium pyruvate (100×) to enhance cell viability during the assay and keep the solution at 20°C to 25°C until used.
Equipment Notes
-
•
Plate washer: BioTek 405 TS: The availability of the automated washer (Figure 1) allows us to wash the plate more quickly. The aspiration and washing program settings are shown in Figures 2 and 3, respectively.
Figure 1.

BioTek 405 TS Microplate Washer
The instrument was programmed to aspirate and wash the wells of the 96-well plate.
Figure 2.
Aspiration Tube Position
The touch screen image shows the exact position of the manifold tubes in order to wash the plate. Defining the position correctly will minimize or prevent cell disruption on the plate surface.
Figure 3.
The Washer Dispensing Tube Position
The touch screen image illustrates the required position of the manifold tubes to gently dispense the buffer solution.
Alternatives: A multi-channel pipet can be used to manually wash the 96-well plate. A gentle approach is necessary to avoid dislodging the cells.
-
•
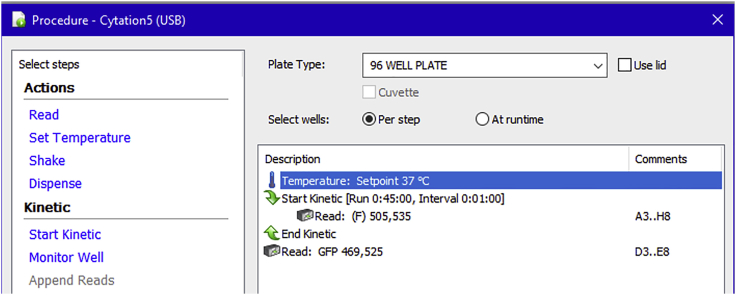
Plate reader: BioTek Cytation 5: The Cytation 5 plate reader is a multi-mode reader that is capable of fluorescence imaging (Figure 4). The instrument is controlled by the Gen5 software (Figures 5 and 6) that allows us to export the raw relative fluorescent unit (RFU) data to an Excel file. Background subtraction (of control RFUs) is then performed using the Excel software during data analysis.
Figure 4.

BioTek Cytation 5 Plate Reader
The instrument is a multi-mode spectrofluorometer with imaging capability. The plate reader includes an automated injector situated on the top of the instrument. Kinetic reading of plate wells was pre-programmed for each zinc flux assay using the Gen5 software that controls the instrument.
Figure 5.
A Sample Gen5 Program Setting for the Newport Green Assay
The fluorescence (Excitation 505 nm/Emission 535 nm) is read every minute for 45 min. An image of the cells (GFP channel) is taken at the end of the reading to ensure that the wells treated with ZnCl2 and 2-Mercaptopyridine N-oxide are loaded with zinc. The average fluorescence values from zinc-absent treatment wells are considered background and subtracted from treatment values during analysis.
Figure 6.
A Sample Gen5 Program Setting for the Fluozin-3 Assay
The background fluorescence is measured prior to the addition of Fluozin-3 dye and is subtracted out during data analysis. The dispenser injects 100 μL of the Fluozin-3 dye and the fluorescence (Excitation 495 nm/Emission 536 nm) is read every minute for 30 min.
Alternatives: Any microplate reader that is capable of reading fluorescence will suffice (e.g. Molecular Devices, Promega, ThermoScientific). A plate reader that is able to perform a kinetic read is used in the current protocol; however, a plate reader that only performs end-point reading can also be used. A plate reader that takes fluorescence imaging is used in the current protocol, but users who have an instrument that does not have this capability may use a standard inverted fluorescence microscope to image zinc loading in cells.
The plate aspiration settings must be defined before using the instrument (Figure 2). The wells are aspirated at a travel rate of 6 cell wash (6 CW).
The plate wash settings must also be specified before performing the wash (Figure 3). The wells are washed with 290 μL/well at a flow rate of 1 CW and aspirated at a travel rate of 6 CW. At the end of the wash, 100 μL/well of Standard Buffer is dispensed at a flow rate of 1 CW.
CRITICAL: Setting the correct tube position for the washer is crucial because it is important to dispense the solution without disrupting the cells.
Step-By-Step Method Details
Day 1: Seeding Cells for the Assay
Timing: 1 h
This step describes how to prepare cells on coated plates.
Note: Here, we describe the use of HeLa cells that stably express human TMEM163 (ZNT11) and mutant variants of the protein (Sanchez et al., 2019). We have also had success using HeLa cells with transiently expressed proteins, human primary fibroblast cells, and human embryonic kidney (HEK)-293 cells with RNA interference treatment to quantify cellular zinc flux (Eichelsdoerfer et al., 2010, Cuajungco et al., 2014).
-
1.
Warm PDL-treated plates to ambient temperature.
-
2.
Before use, inspect the coating on the PDL-treated plate to make sure that there are no cracks.
-
3.
Seed 25,000 stably transfected HeLa cells per well in the PDL-treated 96-well plate.
CRITICAL: Seeding 25,000 stably transfected cells resulted in about 90% confluency on Day 2. Seeding less than the recommended cell number will produce low confluency on Day 2, which could result in a very low fluorescence measurement.
Note: For zinc flux assays, it is ideal to create stable cell lines that express a specific zinc transporter protein to reduce variability caused by issues in transfection efficiency.
Note: Due to several wash steps in the protocol, we highly recommend using adherent monolayer cell lines that attach strongly on well surface.
-
4.
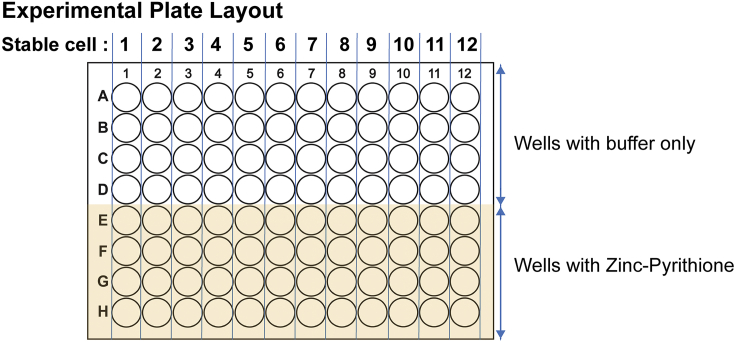
Plate stable cell lines expressing different protein variants in columns (see Figure 7, plate layout) so that there are eight experimental replicates per protein variants.
Figure 7.
Sample Plate Layout for Cell Seeding
Each column (1–12) represents specific stable cells that over-express the wild-type and protein variants of TMEM163 (ZNT11) zinc efflux transporter.
Alternatives: We were able to assay zinc flux in HeLa and human embryonic kidney (HEK)-293 cells using transient transfection (Cuajungco et al., 2014, Sanchez et al., 2019). See Quantification and Statistical Analysis section for sample data from transfected cells.
Alternatives: If you are going to transiently transfect the cells, then plate 10,000 Hela cells per well on Day 1. Transfect the cells on Day 2 using the appropriate expression vectors and transfection reagent. Perform the zinc flux assay on Day 3.
CRITICAL: The transfection approach may produce cytotoxicity depending on the cell lines, the transfection reagent or method, and the recombinant protein used in the study. The users are advised to optimize the process so that the cells can reach around 90% confluency on Day 3 of the zinc flux assay.
Day 2: Newport Green Assay
Timing: 2–3 h
This step describes the quantification of intracellular zinc flux.
-
5.
Aspirate the plate using the automated plate washer. Add 100 μL of 5 μM Newport Green working solution per well. The Newport Green dye is light sensitive, so cover the plate with foil. Place in an incubator for 20 min at 37°C.
-
6.
Wash twice with Standard Buffer using the plate washer. Bring the volume to 100 μL.
Alternatives: If an automated plate washer is not available, manual washing is acceptable, but it must be done carefully to safeguard the cells from lifting off the plate surface.
-
7.
Leave the plate for 30 min at 20°C to 25°C on the bench to allow for ester cleavage of the dye.
-
8.
Two different treatment groups are mapped on the plate (Figure 8).
Figure 8.
Sample Experimental Plate Layout
The first four rows (A-D) represent stable cells exposed to Standard Buffer, while the remaining lower rows (E-H, shaded area) represent stable cells treated with Zinc-Pyrithione complex.
-
a.
No Zinc: Add 100 μL Standard Buffer to the wells.
-
b.
Zinc treatment: Add 100 μL of Standard Buffer containing 200 μM ZnCl2 and 20 μM 2-Mercaptopyridine N-oxide.
-
9.
Place the plate in the 37°C incubator for 20 min.
-
10.
Wash the cells five times with Standard Buffer using the automated plate washer. After aspiration, bring the volume up to 100 μL with the Standard Buffer.
Alternatives: Manual washing may be done if an automated plate washer is unavailable. Be careful washing the plate to avoid lifting the cells off the surface.
-
11.
Place the plate in the spectrofluorometer instrument. Take the kinetic readings every 1 min for 45 min.
Alternatives: Depending on the research goals, the users have the option to measure zinc levels using an end-point reading at a specific time point (e.g. read at 20 min post-zinc exposure).
Day 2 Alternative: Fluozin-3 Assay
Timing: 1 h
This step describes quantification of extracellular zinc flux.
-
12.
Aspirate the plate using the automated plate washer and replace the wells with treatment and control buffer.
Alternatives: The plate may be manually washed if an automated plate washer is not available, but exercise care to ensure that the cells do not detach.
-
13.
There are two different treatments for the wells.
-
a.
No Zinc: Add 100 μL Kreb’s Ringer Bicarbonate buffer to the wells.
-
b.
Zinc treatment: Add 100 μL of a solution of Kreb’s Ringer Bicarbonate buffer containing 200 μM ZnCl2 and 20 μM 2-Mercaptopyridine N-oxide solution.
-
14.
Incubate the plate in the 37°C incubator for 10 min.
-
15.
Wash the plate five times with Standard Buffer. After aspiration, bring the volume up to 100 μL using the Standard Buffer. The plate may be washed by hand if an automated washer is not available.
-
16.
Place the cells in the plate reader. Perform an initial baseline reading – this reading will be used for background fluorescence normalization. Use the automated injector in the plate reader to add 2 μM cell impermeant Fluozin-3 to get a final concentration of 1 μM.
Alternatives: Use a multi- channel pipet to add the 2 μM cell impermeant Fluozin-3 to get a final concentration of 1 μM if an injector is not available.
-
17.
Take the readings every 1 min for 30 min. Similar to the Newport Green assay, the users may perform an end-point reading at 20 min post-exposure to Fluozin-3 dye.
Expected Outcomes
Newport Green Assay
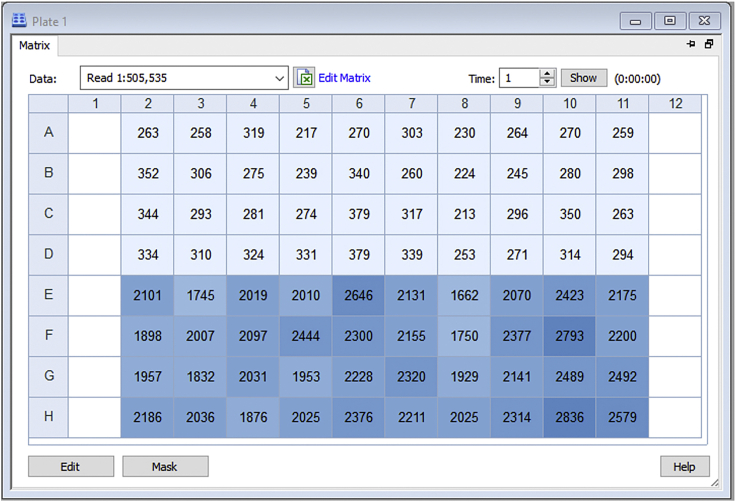
The fluorescence is read every minute for 45 min. Only columns 2–11 were used in the specific experiment shown below. Cells within rows A-D were not exposed to zinc, while rows E-H were pre-incubated with zinc (Figure 9). Each treatment group displayed fluorescence values that correspond to the absence or presence of zinc.
Figure 9.
Newport Green Fluorescence Values of Stable Cells in the Absence (Rows A-D) or Presence (Rows E-H) of Zinc
The fluorescence is detected inside the cells.
The protocol is setup to take an image of the cells using the green fluorescent protein (GFP) channel after the kinetic reading step (Figure 10). This confirms that the cells treated with zinc were indeed loaded with zinc. A representative image below shows that the cells are loaded with zinc as evidenced by the Newport Green fluorescence.
Alternatives: For users that do not have a combined plate reader and imager such as the Cytation 5, using a standard inverted fluorescent microscope is sufficient to image and confirm proper zinc loading of cells prior to and after performing the zinc flux assay.
Figure 10.
Newport Green Fluorescence Image of HeLa Cells Exposed to Zinc
Scale bar, 100 μm.
Fluozin-3 Assay
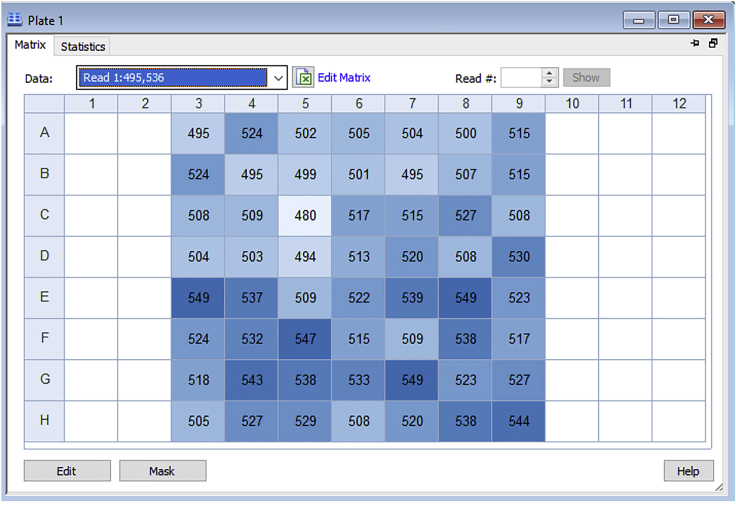
The plate is first read before the addition of the Fluozin-3 dye solution. The fluorescence values of the initial reading represent the background RFUs. The values obtained should be low as demonstrated in columns 3–9 of a particular experiment shown below (Figure 11).
Figure 11.
Background Fluorescence Values of Stable Cells in the Absence of Fluozin-3
The values obtained likely represent fluorescent intracellular materials such as lipofuscin.
After the addition of Fluozin-3 dye indicator, the fluorescence readings were taken every minute for 30 min. The image below shows fluorescence values at 21 min. This particular experiment contained cells within columns 3–9 where rows E-H were exposed to zinc, while rows A-D were not treated with zinc. The fluorescence values are higher in cells that were incubated with Zinc-Pyrithione complex (Figure 12).
Figure 12.
Fluozin-3 Fluorescence Values
The values are detected from extracellular zinc flux in the absence (rows A–D) or presence (rows E–H) of exogenously added zinc.
Quantification and Statistical Analysis
The BioTek Cytation5 plate reader uses the Gen5 software, which allows for a quick export of data into Excel. As mentioned previously, the normalization (background subtraction) and analysis are done manually in Excel using the exported raw data. Thus, the users do not need the Gen5 software to perform data analysis. Users may perform statistical analysis using a software such as Excel or GraphPad Prism to determine significant values between treatment and control data.
Alternatives: The users may perform an end-point analysis to detect changes in intracellular or extracellular zinc concentrations. Indeed, we previously reported data taken from end-point assays to show zinc flux in cultured cells (Eichelsdoerfer et al., 2010, Cuajungco et al., 2014).
For this protocol, we used kinetic reading because we were interested in showing how the over-expression of TMEM163 (ZNT11) affects zinc flux in the presence of endogenous zinc influx and efflux transporters in HeLa cells. It has been determined that HeLa cells express several zinc influx (SLC39/ZIP) and efflux (SLC30/ZNT) proteins (www.proteinatlas.org). Despite the presence of endogenous zinc transporters, we were able to show that TMEM163 (ZNT11) effluxes zinc by reducing intracellular or increasing extracellular zinc levels upon exogenous zinc incubation (Sanchez et al., 2019). The kinetic data analysis is explained below.
At each time point (Time X), the average fluorescence is calculated for each column containing stable cell expressing protein variants either treated with zinc or without zinc (buffer only). The average fluorescence values from the buffer-treated cells (background fluorescence) are subtracted from the average fluorescence values of zinc-treated cells.
| Ave. Fluorescence Time X = Ave. Fluorescence Zinc – Ave. Fluorescence Buffer |
The background-subtracted average fluorescence value at Time 0 is taken as 100%. The average fluorescence values at subsequent time points (Time X) are compared to this value. The change in RFUs will show how zinc is moving into or out of the cells (Newport Green assay) or indicate the changing relative extracellular zinc concentration (Fluozin-3 assay) over the elapsed time (indicated as Time X).
| % Fluorescence Time X = (Ave. Fluorescence Time X ÷ Ave. Fluorescence Time 0) × 100 |
The % fluorescence as a function of time is then plotted. Figure 13 illustrates graphical data from a Newport Green assay using stably expressing HeLa cells (Figure 13A) and a Fluozin-3 assay using transiently transfected HeLa cells (Figure 13B). As mentioned earlier, end-point data analysis may be performed to represent the overall change in intracellular or extracellular zinc flux. In addition, the kinetic data obtained from the Fluozin-3 assay shown in Figure 13B may be represented by way of RFU values as a function of time. Finally, the slope of the curve obtained from the kinetic data analysis may also be used to show changes in intracellular or extracellular zinc flux over time.
Figure 13.
Graphical Representation of Zinc Flux
(A) Newport Green assay using stable cell lines expressing the pmCherry vector and pmCherry-TMEM163 (ZNT11). Normal HeLa cells was used as negative control.
(B) Fluozin-3 assay using transiently transfected HeLa cells expressing pmCherry vector, pmCherry-ZNT4, and pmCherry-TMEM163 (ZNT11) constructs. Representing the Fluozin-3 data as RFU values as a function of time will show a similar trend of increasing extracellular zinc fluorescence upon complexation of the Fluozin-3 dye. The data were calculated and graphed using an Excel software.
Limitations
This assay works optimally for adherent cells in monolayers. The cells have to adhere strongly to the bottom of the wells, since there are many cycles of washes that could lift the cells off the plate surface. Thus, the use of cells in suspension is not advised. While we have used HEK-293 cells with gentle hand washing or with an automated plate washer, if any cells are dislodged from the well surface, the experiment will need to be repeated.
Cell lines used in this assay cannot express recombinant proteins with a GFP or YFP tag, because the fluorescent protein will interfere with the fluorescence reading emitted by Fluozin-3 or Newport Green.
Troubleshooting
Potential Problem
Independent trials may give inconsistent results.
Possible Solutions
-
•
Check that the number of cells seeded was consistent across each well by inspecting the cells under a microscope. See Step-By-Step Method Details section (Day 1: Critical step on seeding cells).
-
•
Mixing the cells by gentle agitation (swirling in a figure eight motion) during plating will give evenly distributed cells in each well.
-
•
Make sure the cells are healthy and low in passage numbers.
-
•
Ensure the buffers are at room temperature when used during the assay. See Materials and Equipment section.
-
•
If transfecting the cells, ensure that the plasmid or the lipid reagent does not contribute to cytotoxicity. Optimization is necessary for transient transfection experiments.
Potential Problem
The fluorescence readings are very low after the addition of the fluorescent zinc indicator.
Possible Solutions
-
•
Ensure the seeding was consistent across each well and the confluency on Day 2 shows about 90%. See Step-By-Step Method Details section (Day 1: Critical step on seeding cells).
-
•
Make sure the cells are completely loaded with zinc. See Expected Outcomes section (Figure 10)
-
•
The Zinc-Pyrithione solution must be made on the day of the experiment and ensure that the working solution is used within 2 h. The Pyrithione solution must be less than 6 months old. See Materials and Equipment section.
-
•
Fluozin-3 and Newport Green fluorescent dyes are both light- and temperature-sensitive compounds. Repeated freezing and thawing will degrade the fluorescent dye resulting in reduced fluorescence. Make aliquots of the stock solution and store at –20°C for up to 6 months. See Materials and Equipment section.
Acknowledgments
This work was supported by funds from the National Institutes of Health (NIH) National Institute of Neurological Disorders and Stroke AREA 2R15 NS101594 grant to M.P.C. The content of this paper is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Author Contributions
S.A. developed and optimized the zinc flux assays and wrote the manuscript. M.P.C. supervised the project and wrote the manuscript. All authors read and approved the final version of the manuscript.
Declaration of Interests
The authors declare no conflicts of interest.
Contributor Information
Saima Ali, Email: saimaali@fullerton.edu.
Math P. Cuajungco, Email: mcuajungco@fullerton.edu.
References
- Cuajungco M.P., Basilio L.C., Silva J., Hart T., Tringali J., Chen C.C., Biel M., Grimm C. Cellular zinc levels are modulated by TRPML1-TMEM163 interaction. Traffic. 2014;15:1247–1265. doi: 10.1111/tra.12205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dittmer P.J., Miranda J.G., Gorski J.A., Palmer A.E. Genetically encoded sensors to elucidate spatial distribution of cellular zinc. J Biol. Chem. 2009;284:16289–16297. doi: 10.1074/jbc.M900501200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichelsdoerfer J.L., Evans J.A., Slaugenhaupt S.A., Cuajungco M.P. Zinc dyshomeostasis is linked with the loss of mucolipidosis IV-associated TRPML1 ion channel. J. Biol. Chem. 2010;285:34304–34308. doi: 10.1074/jbc.C110.165480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson I.D. Fluorescent indicators for zinc and other metal ions. In: Johnson I.D., Spence M.T.Z., editors. Molecular Probes Handbook. Eleventh Edition. Life Technologies Corporation; 2010. pp. 869–870. Chapter 19.7. [Google Scholar]
- Minckley T.F., Zhang C., Fudge D.H., Dischler A.M., LeJeune K.D., Xu H., Qin Y. Sub-nanomolar sensitive GZnP3 reveals TRPML1-mediated neuronal Zn(2+) signals. Nat. Commun. 2019;10:4806. doi: 10.1038/s41467-019-12761-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez V.B., Ali S., Escobar A., Cuajungco M.P. Transmembrane 163 (TMEM163) protein effluxes zinc. Arch. Biochem. Biophys. 2019;677:108166. doi: 10.1016/j.abb.2019.108166. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.