Summary
Cellular traction forces influence epithelial behavior, including wound healing and cell extrusion. Here, we describe a simple in vitro traction force microscopy (TFM) protocol using ECM protein-coated polydimethylsiloxane substrate and widefield fluorescence microscopy. We include detailed steps for analysis so readers can obtain traction forces to study the mechanobiology of epithelial cells. We also provide guidelines on when to adopt another common class of TFM protocols based on polyacrylamide hydrogels.
For complete details on the use and execution of this protocol, please refer to Saw et al. (2017) and Teo et al. (2020).
Graphical Abstract

Highlights
-
•
A 2D, PDMS-based TFM experimental protocol that is easy to adopt
-
•
Detailed steps of analysis to obtain traction forces
-
•
A brief note and guidelines on other materials that can be used for TFM
Cellular traction forces influence epithelial behavior, including wound healing and cell extrusion. Here, we describe a simple in vitro traction force microscopy (TFM) protocol using ECM protein-coated polydimethylsiloxane substrate and widefield fluorescence microscopy. We include detailed steps for analysis so readers can obtain traction forces to study the mechanobiology of epithelial cells. We also provide guidelines on when to adopt another common class of TFM protocols based on polyacrylamide hydrogels.
Before You Begin
Traction Force Microscopy: A Brief Note
Traction force microscopy (TFM) is a technique that measures forces, also termed as tractions, that cells exert on a substrate. These tractions are in the form of vectoral quantities and consist of both in-plane (Tx and Ty) and out-of-plane components (Tz). It is important to clarify that although these quantities are termed forces (units of Newton, N), the usual output by TFM is force per unit area, i.e., mechanical stress (units of Pascal, Pa). This also holds true for the software we have recommended here. Tz, which cannot be neglected in general (Hur et al., 2012), is computationally and experimentally challenging to derive compared to Tx and Ty.
In this introductory protocol and guide, we focus on 2D-TFM (i.e., Tx and Ty components) which still provides important mechanical information on epithelial monolayers. For example, traction magnitude which tells us the average strength of this force, could be indirectly associated with acto-myosin and stress fiber contractile strength (Rape et al., 2011). Also, the direction of local traction vectors could be correlated with distinct modes of cell migration into cell-free regions (Kim et al., 2013, Ravasio et al., 2015, Vedula et al., 2012) and cell extrusion (Kocgozlu et al., 2016). Furthermore, global spatio-temporal patterns of traction forces could be used to understand long-range collective migration of epithelial monolayers migrating into free space (Trepat et al., 2009, Vishwakarma et al., 2018).
Key Resources Table
Materials and Equipment
| Reagent | Final Concentration (mM or μM) | Volume (μL or mL) |
|---|---|---|
| Tris solution | 100 mM (w/v) Note: Leftover Tris solution can be kept at room temperature (~22°C–25°C) for future use |
200 mL |
| Cy-A solution | 100% Note: Excess Cy-A and Cy-B can be kept at room temperature (~22°C–25°C) for future use. Note: This two-part PDMS material is different from the more commonly used Sylgard-184, as the viscosity of the solidified PDMS varies less while its stiffness is varied (Kenry et al., 2015) |
50 mL |
| Cy-B solution | 100% Note: Excess Cy-A and Cy-B can be kept at room temperature (~22°C–25°C) for future use. Note: This two-part PDMS material is different from the more commonly used Sylgard-184, as the viscosity of the solidified PDMS varies less while its stiffness is varied (Kenry et al., 2015) |
50 mL |
| 100–200 nm carboxylated nanobeads, with fluorescence of your choice | 0.05% (v/v) | 5 mL |
| Fibronectin solution | 1mg/mL (w/v) | 1 mL |
| Pluronic | 1% (w/v) Note: Filter sterilize pluronic solution and store at room temperature. |
50 mL |
| Sodium Dodecyl Sulfate (SDS) | 10% (w/v) Note: Excess 10% SDS can be kept at room temperature (~22°C–25°C) for future use. |
50 mL |
CRITICAL: Mix fibronectin well by gently pipetting up and down, using wide tips. Vigorous pipetting can induce the formation of fibronectin fibers (Nguyen et al., 2013), which impedes uniform coating on PDMS substrate. One can visualize whether there is fiber formation by using fibronectin conjugated with fluorescent proteins (https://www.sigmaaldrich.com/technical-documents/articles/biofiles/atto-dyes-and-tracy-dyes-for-fluorescent-protein-labeling.html)
Alternatives: Apart from fibronectin, other proteins of choice could be used as well as this protocol depends on non-specific binding to the PDMS surface (see step 20).
Step-By-Step Method Details
Polydimethylsiloxane Substrate Preparation
Timing: 2–2.5 h
Prepare polydimethylsiloxane (PDMS) mixture and coat glass bottom dishes
-
1.
Pipette equal weights of Cy-A and Cy-B using a P1000 cut tip into a weigh tray. For example, 0.5 g of Cy-A and 0.5 g of Cy-B.
Note: This gives rise to a substrate stiffness of approximately 10–20 kPa, measured by Atomic Force Microscopy (AFM), (Kenry et al., 2015). To generate different substrate stiffness, other mixing ratios can be adopted. Cy-A:Cy-B – 1.2:1 for 2–3 kPa, and Cy-A:Cy-B – 1:1.2 for 30–40 kPa.
-
2.
Stir PDMS mixture well with a P200 cut tip. Mixing by pipetting may be less efficient due to the viscosity of PDMS.
CRITICAL: The mixed PDMS thickens within 20 min of mixing the two-part PDMS and it is advisable to proceed to steps 3) and 4) in a similar duration. This is particularly important when preparing multiple samples at the same time. Check section on Troubleshooting.
-
3.
Using a P200 cut tip, add 0.08 g (approximately 80 μL) of PDMS mixture onto a glass bottom dish.
Note: Pipette amount by weight and not volume as PDMS is viscous. This weight does not need to be changed if dishes of similar size or bigger are used. Ensure that the mixture is dropped at the center of the dish to generate an even layer of substrate coating. The drop would have a diameter of approximately 0.5–1 cm before spin coating.
-
4.
Spin coat at 500 rpm for 30 secs to generate a substrate thickness of >100 μm (Figure 1) (Yoshie et al., 2019).
Note: As the software assumes cells exert forces on an infinite half-plane, this thickness was chosen such that cells are less likely to sense the stiff glass surface at the bottom, allowing this assumption to be better met (Buxboim et al., 2010, Tusan et al., 2018). At the same time, this allows relatively flat PDMS to be fabricated.
-
5.
Cure PDMS substrate for 2 h at 80°C.
CRITICAL: Be sure there are no obvious bubbles in the PDMS prior to curing. Check section on Troubleshooting.
Pause Point: Once PDMS is cured, proceed to the next steps, or seal the dishes with parafilm and store at room temperature (∼22°C–25°C) for future use. The solidified PDMS in the dishes can be kept for 1–2 months without significant deterioration.
Figure 1.
Schematic to Illustrate the Process of Spin-Coating a PDMS Substrate Droplet onto a Dish with a Glass Coverslip to Achieve a Flattened Layer with Certain Height
Be sure to align the centers of the spin-coater, the PDNS drop, and the Petri dish.
Prepare PDMS Surface for Bead Incubation
Timing: 40 min–1 h
Silanize PDMS Surface
-
6.
Prepare 5% (v/v) APTES solution in 100% ethanol.
Note: APTES is sensitive to moisture and should be stored by filling the pocket of gas in the bottle with inert gas such as nitrogen. The solution should be prepared fresh. Do not keep any unused solution.
-
7.
Add 2 mL of 5% APTES solution on cured PDMS dish for 10 min.
-
8.
Remove 5% APTES solution and wash PDMS dish 3 times with 3 mL of 100% ethanol.
Note: The surface is soft and slightly sticky, be careful not to pipette vigorously close to the surface nor touch it, in order to not generate unwanted plastic deformations.
-
9.
Dry PDMS dish at 80°C for 30 min with the lid ajar.
Prepare Nanobead Solution
Timing: 30 min
-
10.
Prepare 5 mL of 0.05% (v/v) beads in distilled water.
-
11.
Sonicate bead solution at around 50–60 Hz for 10 min.
Note: Check section on Troubleshooting.
-
12.
Filter bead solution through a 0.45 μm filter.
Functionalize PDMS with Nanobeads
Timing: 45 min–1 h
-
13.
Incubate PDMS dish with bead solution for 5 min at room temperature (∼22°C–25°C).
-
14.
Remove bead solution and wash PDMS dish 3 times with 3 mL of distilled water.
Note: During washing, care should be taken to not have any parts of the PDMS dry at any time, which can generate streaks and non-uniform bead distribution.
-
15.
Dry dish at 80°C for 15 min with the lid ajar.
-
16.
Incubate dish with 3 mL of 100 mM Tris solution for 10 min at room temperature (∼22°C–25°C). This is to inactivate the bead surfaces.
-
17.
Remove Tris solution and wash PDMS dish 3 times with 3 mL of distilled water.
-
18.
Dry dish at 80°C for 15 min with the lid ajar.
Note: PDMS dish coated with beads should be protected from light from here on. For example, keep dishes in an opaque box or a container wrapped with aluminum foil.
Coat PDMS Dish with Fibronectin and Seed Cells
Timing: 1–2 h
-
19.
Prepare 50 μg/mL of fibronectin solution in distilled water or 1× PBS.
-
20.
Add 2 mL of fibronectin solution to PDMS dish. Incubate PDMS dish at room temperature (∼22°C–25°C) for 1 h.
Note: Ensure that the dish is fully covered with fibronectin solution. Fibronectin adheres to the PDMS surface through non-specific binding, it is thus expected that other ECM proteins can also adhere to the surface with a similar protocol.
-
21.
Remove fibronectin solution and wash PDMS dish 3 times with 3 mL of PBS.
-
22.
Add 1 mL of 1% pluronic to PDMS dish for sterilization. Incubate at room temperature (∼22°C–25°C) for 30 min.
-
23.
Remove pluronic and wash PDMS dish 3 times with 3 mL of PBS.
-
24.
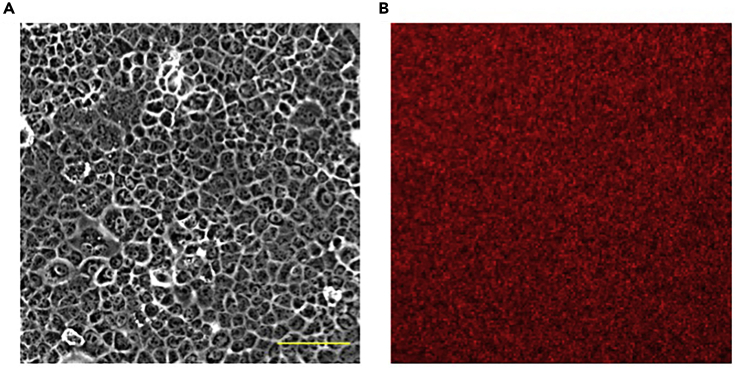
Seed cells onto PDMS dish so that a confluent monolayer is established in 24 h (Figure 2).
Note: This protocol can be adopted to measure traction forces from single cells as well.
Figure 2.
Confluent Monolayer of AML12 Cells on PDMS Substrate
Representative phase contrast image of AML12 (A) seeded on PDMS substrate functionalized with fluorescent beads (B). Scale bar, 100 μm.
Imaging
Timing: 26 h
-
25.
Transfer dish to a widefield fluorescence microscope. For instance, we used the Nikon Ti-E Inverted Microscope equipped with a Hamamatsu Flash 4.0 sCMOS camera, Lumencor 7 line LED light source, motorized X-Y-Z stage, piezo Z-drive and a CO2 and temperature incubator box.
-
26.
Using a 10× (or 20×) dry objective, acquire images of beads with a large field of view (e.g., 1,000 × 1,000 μm depending on the camera and objective).
Note: It is advised to set up a single z-plane each for imaging channels of beads and cells as they are usually not in the same focal-plane. In addition, ensure that images are captured and saved as tiff files.
-
27.
Determine the duration and time interval for capturing the movie. For instance, capturing an image every 15 min for 24 h.
Note: The time interval is not important for traction force calculations, but is important to correlate cell motion with the traction forces. For cells moving at ∼30 μm/h, a 10 min interval is good. For cells moving at few μm/h, ∼60 min interval is enough.
-
28.
At the end of a 24-h run, remove lid of the dish carefully and add 10% SDS.
Note 1: This detaches the cells and obtains the reference bead images when cells are no longer exerting forces on the substrate (Style et al., 2014). Alternatives such as Trypsin and Triton X-100 could also be used to detach cells for the same purpose, but SDS has the advantage of detaching cells within minutes. Do not remove the dish from the microscope to prevent the loss of imaging positions. Generally, movement of the imaging position less than ∼20% of the whole image allows the alignment software to work.
Note 2: Another way to obtain reference states is to make TFM samples on dishes pre-printed with gridlines at the bottom, and image the reference state before cell seeding. The gridlines will allow one to find the corresponding cell region for imaging after cells are seeded. This second method eliminates the need to remove cells at the end of the experiment and allows for cell fixing and staining of antibodies.
-
29.
After ∼1 h, when substrate deformation is fully reversed, capture reference bead images.
Expected Outcomes
A successful run should have cells adhered to the substrate at the end of the run prior to SDS treatment (Figure 3). In addition, cell proliferation should be comparable to cells seeded on a dish without PDMS during the imaging process (unless anti-proliferative drugs are added). This serves as an indication that the cells are healthy and force measurements taken are not a result of cellular toxicity.
Figure 3.
Pre- and Post-24 h Imaging of AML12 Cells Seeded on PDMS Substrate Functionalized with Fluorescent Beads
10% SDS was added to cells at the end of 24 h to remove the cells and to obtain the reference PDMS state. Scale bar, 100 μm.
Bead displacements can be converted to traction forces using an existing Matlab software, PIVlab (Thielicke and Stamhuis, 2014) and ImageJ plugin: Fourier transform traction cytometry (FTTC, (Martiel et al., 2015, Tseng, 2011)). PIVlab measures the deformation map at each time frame. The FTTC plugin further converts the deformation map to traction forces. We detail the parameters and steps we use for analysis in the section below, but users are recommended to read the documentation related to those software packages.
Quantification and Statistical Analysis
-
1.
Install and Open ImageJ.
-
2.
Create a folder on desktop and rename the folder (e.g., “Folder 1”).
-
3.
Repeat step 2 with the reference bead channel without cells (reference beads).
-
4.
Concatenate maximum-projected beads channel with cells and reference beads channel.
-
a.
ImageJ -> Stacks -> Tools -> Concatenate (Ensure that reference beads channel is Image1 and beads channel with cells is Image2).
-
5.
Save concatenated image stack in Folder 1.
-
6.
Run ForBeads-CorrectDriftNGlaring-Align.ijm macro to align beads image and generate image stack for PIV analysis.
-
a.
Duplicate and rename the first frame as “Ref.”
-
b.
Duplicate the remaining stack without the first frame (e.g., Range 2-XX) and rename as “WithCells.”
-
c.
Run macro and save Transformation Matrix.
-
d.
Save the new stack in 8-bit format as Image Sequence in Folder 1.
-
i.
E.g., ForPIV001, ForPIV002, ForPIV_003, etc.
-
7.
Run the PIVlab algorithm version 1.43 (Thielicke and Stamhuis, 2014) in Matlab
-
a.
Open PIVlab and select Load images to import images.
-
b.
In the Analyses setting tab, PIV settings used are as follows:
-
i.
Interrogation windows with three pass: 128(64), 64(32), 32(16). The window size in the final pass should have at least ∼5 beads for the correlation to proceed well (Martiel et al., 2015).
Note: We encourage new users to refer to the PIVlab video tutorial for more information on how to navigate the software and learn best practices.
-
c.
Click Analysis-> Analyze!
-
d.
After analysis, under the post processing tab, click vector validation (Thielicke and Stamhuis, 2014)
-
i.
Select “display all frames in…” and click on Refine velocity limits to remove outlier velocity limits if necessary
-
ii.
Select stdev filter 7, Local median filter threshold 5, epsilon 0.1.
-
iii.
After selecting these parameters, Select Apply to all frames.
-
e.
Save PIVlab session in Folder 1.
-
i.
File -> Save -> PIVlab Session.
-
f.
Export results to Matlab workspace.
-
i.
File -> Save -> All results to Matlab workspace.
-
8.
Convert PIVlab output files into .txt files using the code OutputTxtFilesForFTTC2.m.
Note: A supplemental dataset (Supplemental Data 1) is provided for users to perform a test run.
-
9.
Using PIV.txt files, obtain traction forces by running the FTTC plugin in ImageJ (Tseng, 2011) with a Poisson ratio of 0.5 (Martiel et al., 2015). Choose the stiffness depending on the mixing ratio that the user has used to fabricate the PDMS (see step 1). Although a stiffness range was given for each mixing ratio, users can take the midpoint of each range without loss of generality but keeping in mind to consistently use the same value. A regularization parameter can be tuned to remove highly fluctuating signals due to noise (Martiel et al., 2015). Note that traction force outputs are in the units of Pascal (Pa).
Note: The higher the value of regularization, the more the traction forces output are smoothed. The values suggested by Martiel et al. 2015 are ∼8e−11, but this is not a strict guideline. An important rule of thumb is to choose a value which gives traction forces that make sense, and use the same values when comparing between experimental conditions. This mean that high spatial frequency components representing noise are not present. For example, if one observes spatial traction values fluctuating much more rapidly than the average distance between focal adhesions, it is advisable to increase the regularization value.
Limitations
Considering that polyacrylamide (PA) hydrogels and PDMS are the two most widely used classes of materials for performing TFM, we provide a brief summary of their differences as well as recommendation of use.
We suggest anyone new to the methodology of TFM should consider using PDMS as a substrate instead of PA hydrogels. Importantly, PDMS is easy to fabricate and functionalize with extracellular matrix proteins (e.g., Fibronectin) compared to PA gels. Like PA gels, PDMS also allows for micro-contact printing and patterning of these proteins in extended-variants of this protocol (Vedula et al., 2014). Further, PDMS does not exhibit poroelasticity as seen in PA hydrogels. This avoids complex material deformation due to fluid flow, occurring at time-scales on the order of 10 min or more, which can complicate the interpretation of traction force measurements (Casares et al., 2015).
However, we do recommend the use of PA hydrogels when low substrate stiffness (<1 kPa) and/or high-resolution force maps are required (down to single Focal Adhesion resolution (Plotnikov et al., 2014)). PA hydrogels are able to exhibit low substrate stiffness (on the order of 100 Pa) without drastically affecting the solidity of the material at this stiffness range. In addition, with a largely uniform substrate stiffness and a refractive index closer to water, more accurate inference of forces at the local scale and higher optical resolution can be achieved. It is noteworthy that long-term culture (more than a few days) of epithelial cells that are prone to pumping ions toward the substrate (e.g., MDCK cells) can affect the flat epithelial morphology and induce three-dimensional, fluid-filled structures on non-permeable PDMS (Latorre et al., 2018). This can be partially circumvented by culturing cells on PA hydrogels that have sufficient gel thickness, as these gels are permeable to liquid and allows the buffering of ion accumulation.
Table 1 below compares several important properties of PDMS and PA hydrogels that one should take into consideration prior to performing TFM.
Table 1.
Key Material Properties of PDMS and PA
| PDMS | PA Hydrogels | |
|---|---|---|
| Minimum Stiffness | Approximately 2 kPa | On the order of 100 Pa |
| Permeability to medium and ions | No | Yes |
| Refractive index | > 1.4 | Approximately 1.33 (that of water) |
| Uniformity of stiffness | Treatment with oxygen plasma can lead to a stiffer surface compared to the bulk PDMS. | More uniform stiffness throughout the material |
| Mechanical property | Viscoelastic. The type of PDMS recommended here has consistent viscosity when varying stiffness down to approximately 2 kPa, allowing for better characterization of cell behavior as a function of substrate stiffness (Kenry et al., 2015) | Poroelastic (Casares et al., 2015) |
| Surface functionalization with proteins | Simple, non-specific binding | Use of chemicals such as Sulfo-Sanpah, but tricky in general due to hydrophilicity of hydrogels |
Troubleshooting
Problem 1
Beads in different areas are not in focus.
Potential Solution
This tends to happen when the PDMS substrate is not evenly spread. Make sure that the PDMS mixture is dropped right in the center of the dish before spin coating. Ensure that the dish is well-centered on the Spin coater before spinning. After spinning, perform a visual check to see if a uniform layer of the PDMS mixture is formed. There should be no visible accumulation of the PDMS mixture in the dish.
Problem 2
Visible bubbles seen in cured PDMS substrate.
Potential Solution
Prior to spin coating, perform a visual check to ensure that there are no bubbles in the PDMS mixture. If bubbles are present, use a pipette tip to pop the bubbles. Perform a second visual check after spin coating to ensure that bubbles are absent before curing.
Problem 3
Imaging positions for reference beads images are not maintained after adding 10% SDS.
Potential Solution
If the microscope is equipped with a cage incubator, consider imaging the beads and (or) cells in the dish without the lid on. To minimize evaporation of media during the imaging process, place Petri dishes containing water near the sample to humidify the environment. Also, adding mineral oil (which has a lower density than water) gently to the top of the media can help.
Problem 4
Beads are clumped up.
Potential Solution
Ensure that the bead solution is well sonicated. When using an ultrasonic bath, check that the bead solution is well immersed in the water bath throughout the sonication process. In addition, vortex the bead solution just before applying it onto PDMS substrate. In the event that beads are still clumping, consider testing a range of sonication frequencies. A good sonication frequency to adopt would be one that has 1) minimal beads lost, 2) fluorescence intensity similar to beads that have not been sonicated and 3) homogenous beads mixture with minimal clumps.
Problem 5
Cells are dying.
Potential Solution
Check that the imaging set up is not causing phototoxicity. Perform a trial experiment to optimize fluorescence lamp intensity and exposure time. If cells are dying prior to imaging, make sure that fibronectin has attached to the substrate before cells are seeded by performing an antibody staining for fibronectin. If fibronectin is not detected, increase the duration of fibronectin incubation and be gentle during washes to ensure that fibronectin is not being washed off.
Resource Availability
Lead Contact
Further information and requests for resources should be directed to the Lead Contact, Thuan Beng Saw (biestb@nus.edu.sg).
Materials Availability
No unique reagents were generated.
Data and Code Availability
No large dataset or new softwares were developed.
Acknowledgments
T.B.S. acknowledges the CT Lim and Ladoux lab members at the Mechanobiology Institute for the continual development and optimization of this protocol. This work was supported by the National Health and Medical Research Council of Australia (grants 1140090 to A.S.Y. and fellowship APP1139592 to A.S.Y.). C.T.L. was supported by the National Research Foundation, Singapore, under the Mechanobiology Institute at the National University of Singapore and the Human Frontier Science Program (grant LIP000635/2018). J.L.T. was the recipient of an Equity Trustees PhD Scholarship in Medical Research; T.B.S. is supported by the Lee Kuan Yew Postdoctoral fellowship and Singapore Ministry of Education Tier 1 Academic Research Fund (R-397-000-320-114). Optical microscopy was performed at the ACRF/IMB Cancer Biology Imaging Facility, established with the generous support of the Australian Cancer Research Foundation.
Author Contributions
Investigation, J.L.T and T.B.S.; Visualization, J.L.T. and T.B.S.; Writing, J.L.T. and T.B.S.; Resources and Supervision, C.T.L. and A.S.Y.
Declaration of Interests
The authors declare no competing interests.
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.xpro.2020.100098.
Contributor Information
Jessica L. Teo, Email: j.teo@imb.uq.edu.au.
Thuan Beng Saw, Email: biestb@nus.edu.sg.
Supplemental Information
References
- Buxboim A., Rajagopal K., Brown A.E., Discher D.E. How deeply cells feel: methods for thin gels. J Phys Condens Matter. 2010;22:194116. doi: 10.1088/0953-8984/22/19/194116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casares L., Vincent R., Zalvidea D., Campillo N., Navajas D., Arroyo M., Trepat X. Hydraulic fracture during epithelial stretching. Nat Mater. 2015;14:343–351. doi: 10.1038/nmat4206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hur S.S., Del Alamo J.C., Park J.S., Li Y.S., Nguyen H.A., Teng D., Wang K.C., Flores L., Alonso-Latorre B., Lasheras J.C., Chien S. Roles of cell confluency and fluid shear in 3-dimensional intracellular forces in endothelial cells. Proc Natl Acad Sci U S A. 2012;109:11110–11115. doi: 10.1073/pnas.1207326109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenry, Leong M.C., Nai M.H., Cheong F.C., Lim C.T. Viscoelastic effects of silicone gels at the micro- and nanoscale. Procedia IUTAM. 2015;12:20–30. [Google Scholar]
- Kim J.H., Serra-Picamal X., Tambe D.T., Zhou E.H., Park C.Y., Sadati M., Park J.A., Krishnan R., Gweon B., Millet E., Butler J.P., Trepat X., Fredberg J.J. Propulsion and navigation within the advancing monolayer sheet. Nat Mater. 2013;12:856–863. doi: 10.1038/nmat3689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocgozlu L., Saw T.B., Le A.P., Yow I., Shagirov M., Wong E., Mege R.M., Lim C.T., Toyama Y., Ladoux B. Epithelial cell packing induces distinct modes of cell extrusions. Curr Biol. 2016;26:2942–2950. doi: 10.1016/j.cub.2016.08.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latorre E., Kale S., Casares L., Gomez-Gonzalez M., Uroz M., Valon L., Nair R.V., Garreta E., Montserrat N., Del Campo A., Ladoux B., Arroyo M., Trepat X. Active superelasticity in three-dimensional epithelia of controlled shape. Nature. 2018;563:203–208. doi: 10.1038/s41586-018-0671-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martiel J.L., Leal A., Kurzawa L., Balland M., Wang I., Vignaud T., Tseng Q., Thery M. Measurement of cell traction forces with ImageJ. Methods Cell Biol. 2015;125:269–287. doi: 10.1016/bs.mcb.2014.10.008. [DOI] [PubMed] [Google Scholar]
- Nguyen H.T., Huynh K.C., Scharf R.E., Stoldt V.R. Shear-related fibrillogenesis of fibronectin. Biol Chem. 2013;394:1495–1503. doi: 10.1515/hsz-2013-0183. [DOI] [PubMed] [Google Scholar]
- Plotnikov S.V., Sabass B., Schwarz U.S., Waterman C.M. High-resolution traction force microscopy. Methods Cell Biol. 2014;123:367–394. doi: 10.1016/B978-0-12-420138-5.00020-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rape A., Guo W.H., Wang Y.L. Microtubule depolymerization induces traction force increase through two distinct pathways. J Cell Sci. 2011;124:4233–4240. doi: 10.1242/jcs.090563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravasio A., Cheddadi I., Chen T., Pereira T., Ong H.T., Bertocchi C., Brugues A., Jacinto A., Kabla A.J., Toyama Y. Gap geometry dictates epithelial closure efficiency. Nat Commun. 2015;6:7683. doi: 10.1038/ncomms8683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saw T.B., Doostmohammadi A., Nier V., Kocgozlu L., Thampi S., Toyama Y., Marcq P., Lim C.T., Yeomans J.M., Ladoux B. Topological defects in epithelia govern cell death and extrusion. Nature. 2017;544:212–216. doi: 10.1038/nature21718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Style R.W., Boltyanskiy R., German G.K., Hyland C., Macminn C.W., Mertz A.F., Wilen L.A., Xu Y., Dufresne E.R. Traction force microscopy in physics and biology. Soft Matter. 2014;10:4047–4055. doi: 10.1039/c4sm00264d. [DOI] [PubMed] [Google Scholar]
- Teo J.L., Gomez G.A., Weeratunga S., Davies E.M., Noordstra I., Budnar S., Katsuno-Kambe H., Mcgrath M.J., Verma S., Tomatis V. Caveolae control contractile tension for epithelia to eliminate tumor cells. Dev Cell. 2020;54:75–91.e7. doi: 10.1016/j.devcel.2020.05.002. [DOI] [PubMed] [Google Scholar]
- Thielicke W., Stamhuis E.J. PIVlab – towards user-friendly, affordable and accurate digital particle image velocimetry in MATLAB. Journal of Open Research Software. 2014;2:e30. [Google Scholar]
- Trepat X., Wasserman M.R., Angelini T.E., Millet E., Weitz D.A., Butler J.P., Fredberg J.J. Physical forces during collective cell migration. Nature Physics. 2009;5:426–430. [Google Scholar]
- Tseng Q. Traction force microscopy. 2011. https://sites.google.com/site/qingzongtseng/tfm
- Tusan C.G., Man Y.H., Zarkoob H., Johnston D.A., Andriotis O.G., Thurner P.J., Yang S., Sander E.A., Gentleman E., Sengers B.G., Evans N.D. Collective cell behavior in mechanosensing of substrate thickness. Biophys J. 2018;114:2743–2755. doi: 10.1016/j.bpj.2018.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vedula S.R., Hirata H., Nai M.H., Brugues A., Toyama Y., Trepat X., Lim C.T., Ladoux B. Epithelial bridges maintain tissue integrity during collective cell migration. Nat Mater. 2014;13:87–96. doi: 10.1038/nmat3814. [DOI] [PubMed] [Google Scholar]
- Vedula S.R., Leong M.C., Lai T.L., Hersen P., Kabla A.J., Lim C.T., Ladoux B. Emerging modes ofs collective cell migration induced by geometrical constraints. Proc Natl Acad Sci U S A. 2012;109:12974–12979. doi: 10.1073/pnas.1119313109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vishwakarma M., Di Russo J., Probst D., Schwarz U.S., Das T., Spatz J.P. Mechanical interactions among followers determine the emergence of leaders in migrating epithelial cell collectives. Nat Commun. 2018;9:3469. doi: 10.1038/s41467-018-05927-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshie H., Koushki N., Molter C., Siegel P.M., Krishnan R., Ehrlicher A.J. High throughput traction force microscopy using PDMS reveals dose-dependent effects of transforming growth factor-beta on the epithelial-to-mesenchymal transition. J Vis Exp. 2019 doi: 10.3791/59364. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No large dataset or new softwares were developed.