Abstract
Because chiral dialkyl carbinols, as well as their derived esters, are significant as intermediates and endpoints in fields such as organic, pharmaceutical, and biological chemistry, the development of efficient approaches to their asymmetric synthesis is an important endeavor. In this report, we describe a method for the direct catalytic enantioselective synthesis of such esters, beginning with an alkyl halide (derived from an aldehyde and an acyl bromide), an olefin, and a hydrosilane, catalyzed by nickel, an earth-abundant metal. The method is versatile, tolerating substituents that vary in size and that bear a range of functional groups. We further describe a four-component variant of this process, wherein the alkyl halide is generated in situ, thus obviating the need to isolate either an alkyl electrophile or an alkylmetal, while still effecting an alkyl–alkyl coupling. Finally, we apply our convergent method to the efficient catalytic enantioselective synthesis of three esters that are bioactive themself or that have been utilized in the synthesis of bioactive compounds.
Graphical Abstract
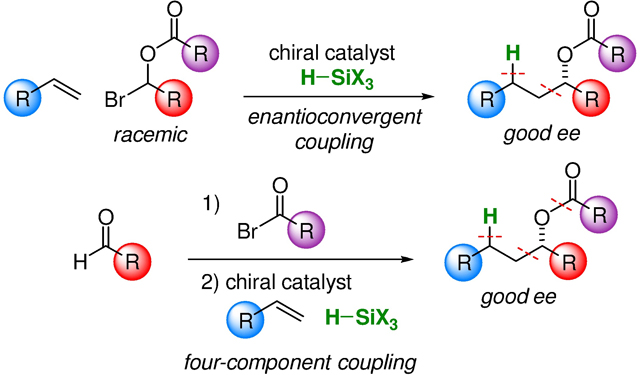
INTRODUCTION
Esters of enantioenriched dialkyl carbinols, as well as the dialkyl carbinols themselves, are frequently encountered in organic chemistry, pharmaceutical science, biochemistry, and related fields (e.g., Figure 1A). With regard to catalytic asymmetric approaches to the generation of such esters, the dynamic kinetic resolution of racemic alcohols is one attractive strategy, although the scope of suitable partners is somewhat limited.1 Another straightforward retrosynthesis involves the acylation of an enantioenriched alcohol, which might in turn be synthesized via the reduction of a ketone or via the addition of an organometallic nucleophile to an aldehyde. However, the asymmetric reduction of dialkyl ketones that bear two alkyl groups of similar size typically proceeds with poor enantioselectivity,2 and studies of the asymmetric addition of alkylmetals to aliphatic aldehydes have largely focused on relatively simple nucleophiles.3 Consequently, alternative approaches to the catalytic asymmetric synthesis of enantioenriched dialkyl carbinols and/or their ester derivatives have been pursued.4
Figure 1.
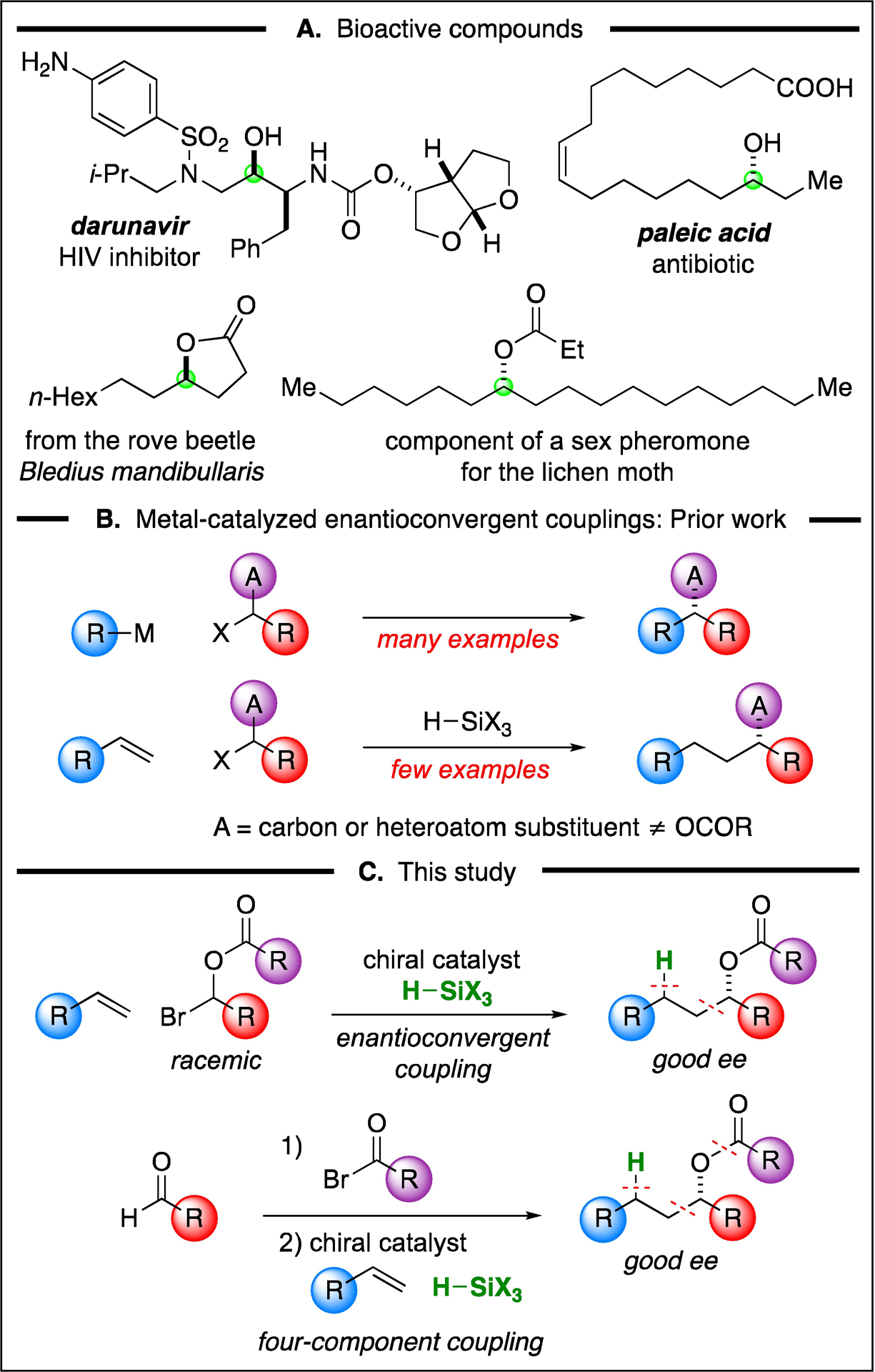
(A) Examples of bioactive compounds that include a chiral dialkyl carbinol, free or acylated. (B) Approaches to metal-catalyzed enantioconvergent alkyl–alkyl couplings of racemic electrophiles. (C) This study.
The metal-catalyzed enantioconvergent coupling of racemic alkyl electrophiles with alkyl nucleophiles has begun to emerge as a powerful strategy in asymmetric synthesis (top of Figure 1B).5 Recently, it has been established that, in certain instances, an olefin (in combination with a hydrosilane) can be used in place of an alkyl nucleophile as the coupling partner (bottom of Figure 1B), thereby obviating the need to synthesize a discrete alkylmetal reagent,6,7 which can afford significant practical advantages.
We have begun to explore whether the metal-catalyzed enantioconvergent coupling of racemic alkyl electrophiles with olefins can be applied to interesting new families of electrophiles to generate useful new classes of products. For example, we envisioned that it might be possible to accomplish a direct catalytic asymmetric synthesis of esters derived from enantioenriched dialkyl carbinols (top of Figure 1C). In this report, we describe the achievement of this objective, as well as the development of a variant wherein the same products are generated in a four-component coupling of readily available reaction partners (aldehyde, acyl bromide, olefin, and hydrosilane; bottom of Figure 1C).8
RESULTS AND DISCUSSION
In an initial study, we examined the coupling of racemic 1-bromopropyl benzoate with vinyl cyclohexane (Figure 2A), and we determined that NiBr2·diglyme and chiral bis(oxazoline) ligand L* can accomplish the desired enantioconvergent reductive coupling in good yield and ee (78% yield, 92% ee; entry 1). In the absence of NiBr2·diglyme or of ligand L*, essentially no carbon–carbon bond formation is observed (entries 2 and 3). An array of other ligands are less effective than is ligand L* (entries 4–8), whereas the use of i-Pr2O in place of MTBE leads to a similar outcome (entry 9). If the coupling is conducted with less catalyst, less olefin, less hydrosilane/base, or for less time, then a lower yield and/or ee are obtained (entries 10–13). The presence of a small amount of water (0.1 equiv) has virtually no effect on the coupling (entry 14), and a reaction run under air in a closed vial proceeds relatively smoothly (entry 15).
Figure 2.
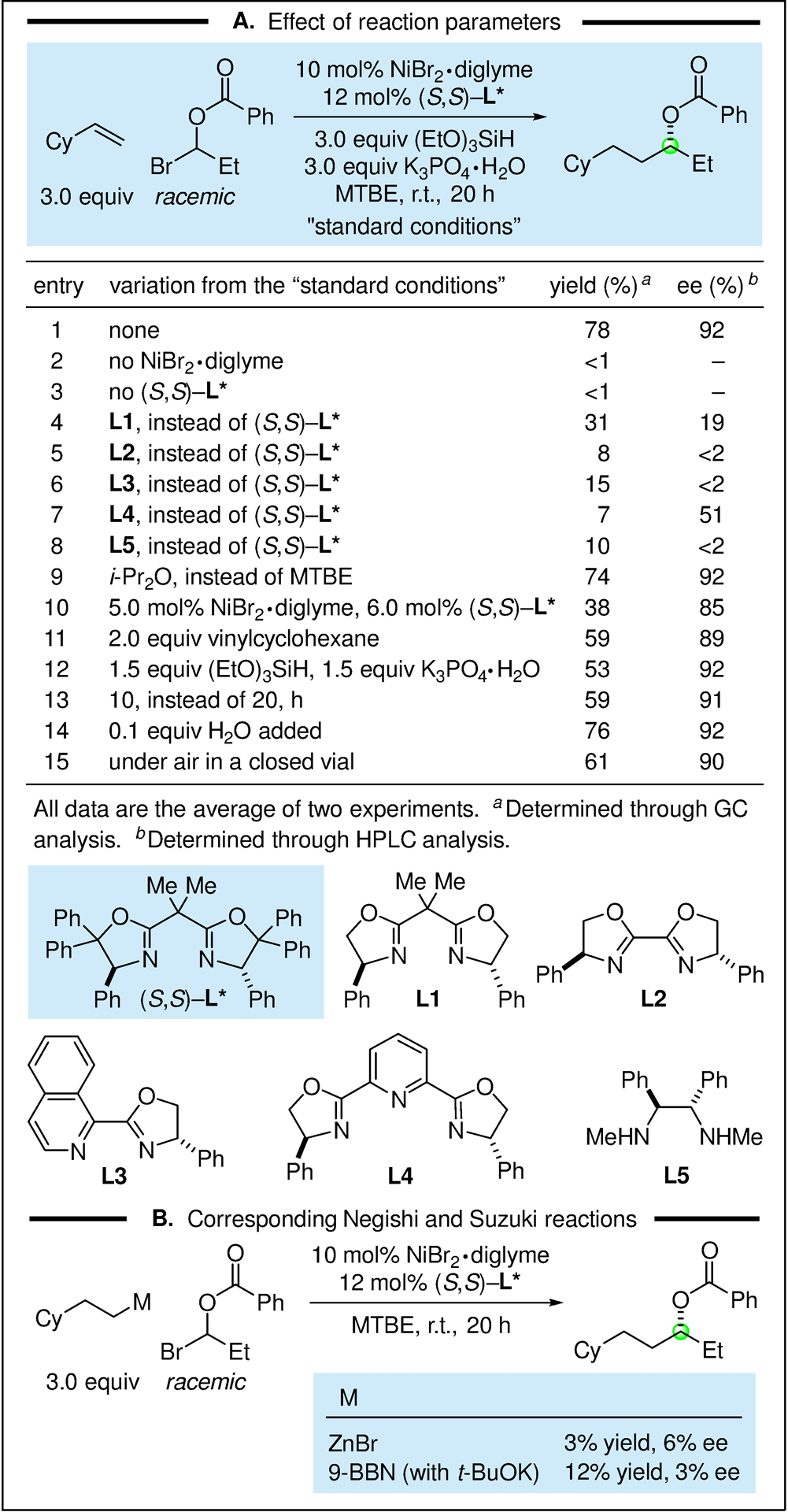
Catalytic asymmetric synthesis of an ester of a chiral dialkyl carbinol. (A) Effect of reaction parameters. (B) Comparison with the corresponding Negishi and Suzuki reactions.
It is interesting to note that, if the olefin/hydrosilane are replaced with an alkylmetal reagent (an alkylzinc or an alkylborane), carbon–carbon bond formation proceeds with poor yield and enantioselectivity (Figure 2B). Thus, in addition to the practical advantage of not having to generate a stoichiometric quantity of an alkylmetal reagent, the use of an olefin/hydrosilane may enable couplings that are not readily achieved via the traditional nucleophile–electrophile approach.
The scope of this method for the catalytic enantioconvergent synthesis of acylated dialkyl carbinols is fairly broad with respect to the various substituents (Figure 3). For example, the alkyl substituent α to bromine can vary in size from methyl to neopentyl to isopropyl, and consistently good yields and ee’s are observed (entries 1–8). A variety of functional groups, including an ether and an unactivated primary alkyl chloride and bromide, are compatible with the method (entries 9–12). In the case of an electrophile that bears a nearby stereocenter, the stereochemistry of the catalyst, rather than that of the substrate, predominantly controls the stereochemistry of the product (entries 13–14). On a gram-scale (1.21 g of product), the coupling illustrated in entry 6 proceeds in similar yield and ee as for a reaction conducted on a 0.8-mmol scale.
Figure 3.
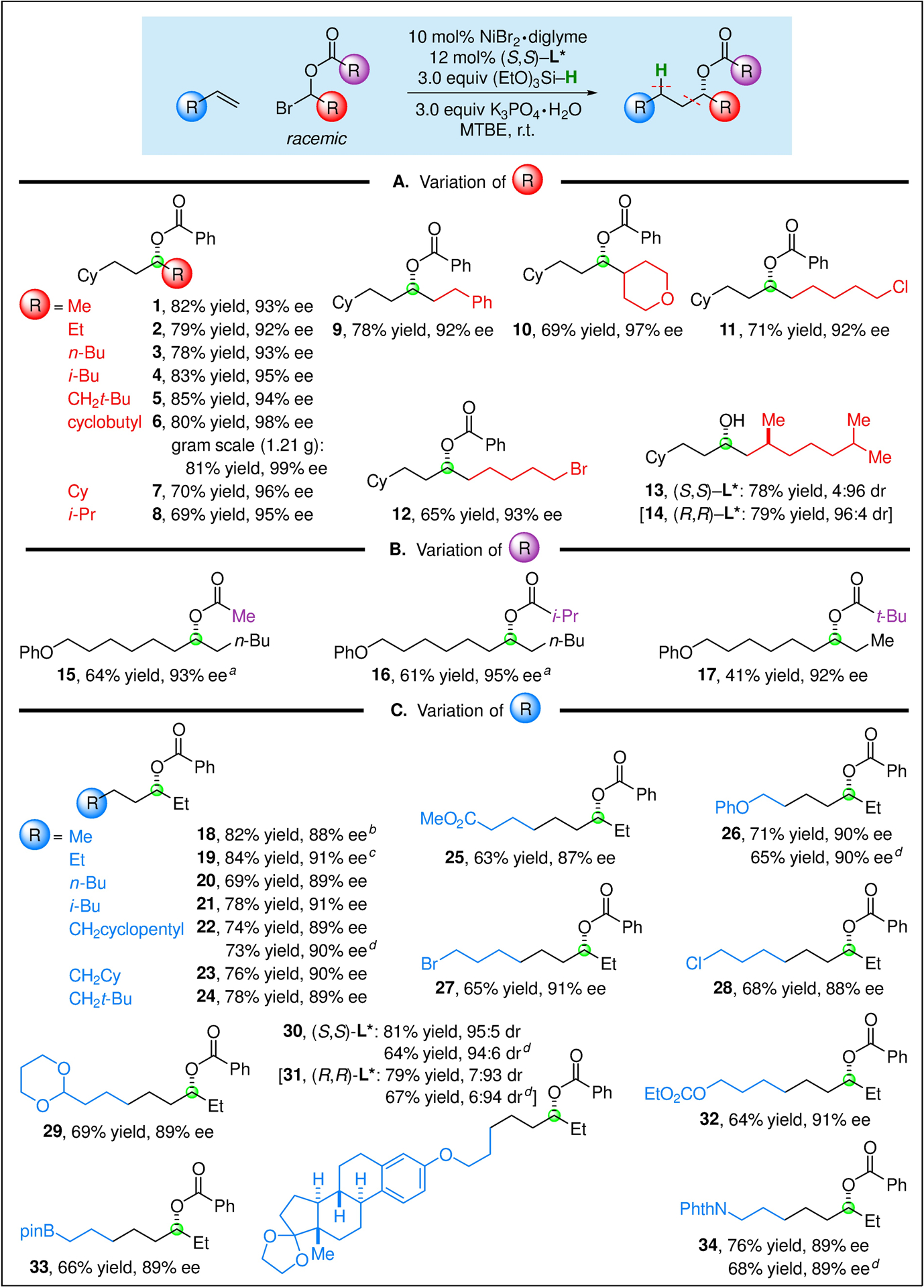
Catalytic asymmetric synthesis of esters of chiral dialkyl carbinols: Scope. All data are the average of two experiments. Unless otherwise noted, couplings were conducted on a 0.8-mmol scale. a In place of the standard conditions, 2.0 equivalents of (EtO)3SiH and 2.0 equivalents of K3PO4•H2O were used, with i-Pr2O as the solvent. b In place of the standard conditions, a balloonful of propylene was used. c In place of the standard conditions, 5.0 equiv of 1-butene (b.p. 21 °C) was used. d In place of the standard conditions, 2.0 equiv of the olefin was used.
With regard to the acyl substituent of the electrophile, not only an aryl group (Figure 3, entries 1–14), but also an alkyl group (entries 15–17), can be present. For these electrophiles, less (EtO)3SiH and K3PO4•H2O may be used (2.0 equiv), and a small enhancement in yield is observed when the coupling is conducted in i-Pr2O. However, in the case of a t-butyl substituent, a modest yield (but good enantioselectivity) is observed under either conditions (entry 17).
The scope of the enantioconvergent coupling is broad with respect to the olefin, leading to an array of products with ~90% ee. For example, the substituent can range in size from methyl to neopentyl (Figure 3, entries 18–24; for Cy, see entries 1–14), and a variety of functional groups can be present, including an acetal, carbonate, boronate ester, and imide (entries 25–34). Additive studies establish that an aldehyde, aniline, aryl bromide, aryl chloride, aryl tosylate, aryl triflate, benzofuran, benzothiophene, epoxide, ketone, N-methylindole, thioether, and unactivated secondary alkyl bromide are fully compatible with the reaction conditions (see the Supporting Information). Consistent with the data provided in entry 11 of Figure 2A, the use of 2.0, rather than 3.0, equivalents of the olefin generally leads to a modest loss in yield, but little change in enantioselectivity (Figure 3, entries 22, 26, 30, 31, and 34; drop in yield: 1–17%).
These three-component reactions can also be conducted as four-component reactions (aldehyde, acyl bromide, olefin, and hydrosilane) by preparing the alkyl bromide in situ. As illustrated in Figure 4A, the four-component reactions proceed in good yield, leading to the formation of a C–H, a C–C, and a C–O bond, as well as the creation of a stereocenter with good enantioselectivity.
Figure 4.
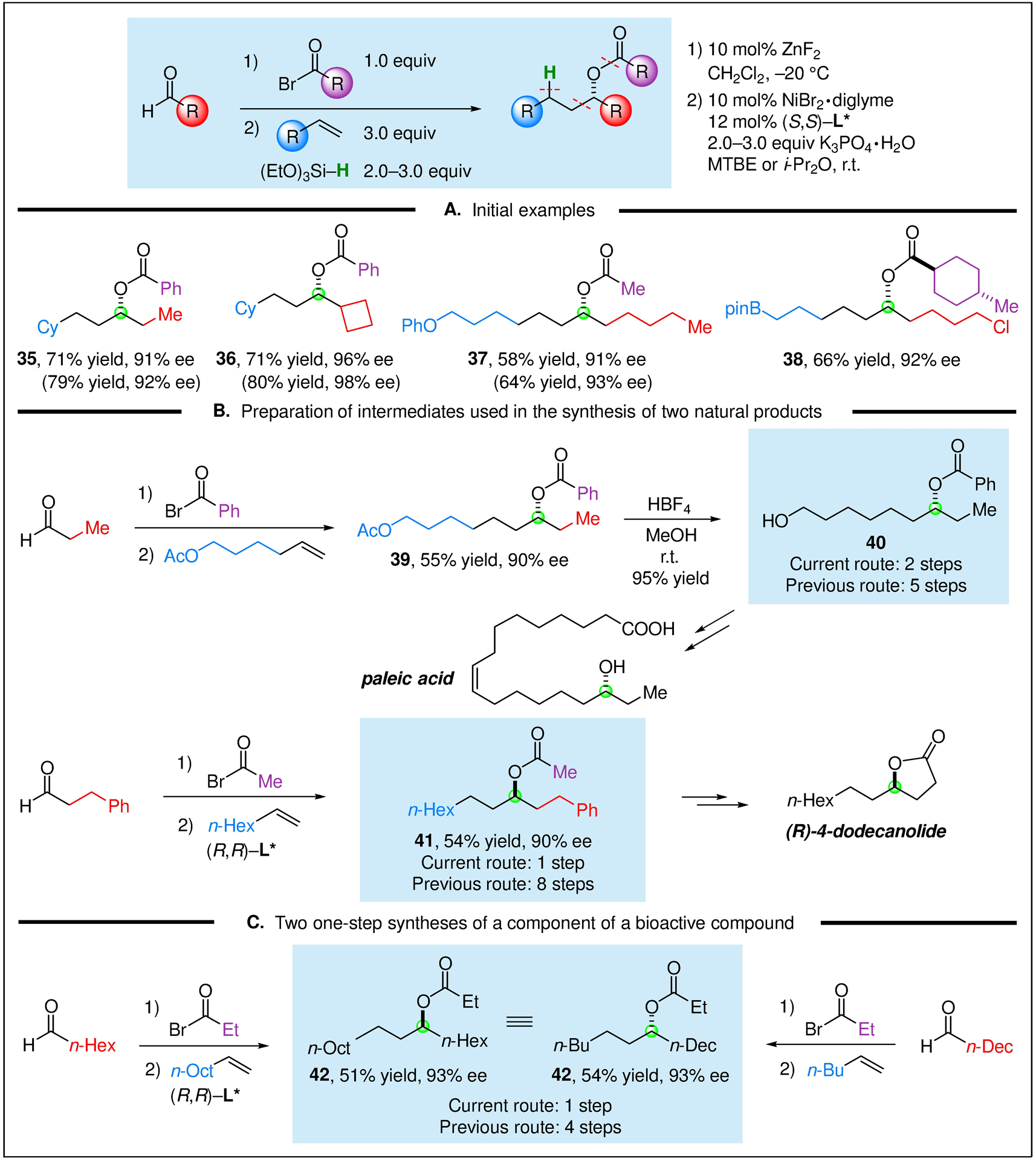
Catalytic asymmetric four-component couplings. (A) Initial examples. (B) Preparation of intermediates used in the synthesis of paleic acid and (R)-dodecanolide. (C) One-step syntheses of (S)-heptadecan-7-yl propionate, a component of a sex pheromone for the lichen moth. All data are the average of two experiments.
This nickel-catalyzed four-component coupling provides efficient access to enantioenriched compounds that have been applied by others in the synthesis of natural products (Figure 4B). Thus, benzoate ester 40, an intermediate in the synthesis of paleic acid, an antimicrobial agent that is effective against Mannheimia and Pasteurella, can be generated from commercially available compounds in two steps and 90% ee via our catalytic asymmetric coupling, whereas it had previously been synthesized in five steps.9
Enantioenriched ester 41 (Figure 4B), generated earlier in eight steps via a lipase-catalyzed kinetic resolution of a racemic alcohol, has served as an intermediate in a synthesis of (R)-4-dodecanolide, which is part of a defensive secretion of rove beetle Bledius mandibullaris.10 Using our catalytic enantioselective four-component coupling, we can produce ester 41 in one step and 90% ee from commercially available building blocks.
Finally, we have applied our method to the catalytic asymmetric synthesis of (S)-heptadecan-7-yl propionate (Figure 4C; 42), which is a component of a sex pheromone for lichen moths.11 Ester 42 has previously been synthesized in four steps via a Jacobsen kinetic resolution of a racemic epoxide; using our method, we have produced ester 42 in one step by two different disconnections from commercially available compounds.
CONCLUSIONS
We have developed an efficient and versatile method for the direct synthesis of esters of enantioenriched dialkyl carbinols via a nickel-catalyzed enantioconvergent coupling of a racemic alkyl halide with an olefin in the presence of a hydrosilane. The method tolerates substituents of varying size on the electrophile and on the olefin, and it displays good functional-group tolerance.
Furthermore, we have demonstrated the viability of a four-component variant of this method wherein the alkyl halide is generated in situ, and we have applied this convergent approach to the efficient synthesis of enantioenriched esters that are bioactive themself or that have been employed as intermediates in previous syntheses of bioactive natural products. In contrast to traditional alkyl–alkyl couplings of electrophiles and nucleophiles, in this approach the alkyl electrophile is generated in situ and the need to synthesize a stoichiometric alkylmetal nucleophile is obviated. Additional efforts to apply earth-abundant metals to useful coupling reactions are underway.
Supplementary Material
ACKNOWLEDGMENTS
Support has been provided by the National Institutes of Health (National Institute of General Medical Sciences; grant R01-GM062871), the Shanghai Institute of Organic Chemistry (fellowship to Z.-P.Y.), and the Dow Next-Generation Educator Fund (grant to Caltech). We thank Dr. Caiyou Chen, Dr. Haohua Huo, Dr. Paul H. Oyala, Dr. David G. VanderVelde, Dr. Scott C. Virgil, and Dr. Zhaobin Wang for assistance and for helpful discussions.
Footnotes
The authors declare no competing financial interest.
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website at DOI:
Procedures, characterization data, and additional references (PDF)
REFERENCES
- (1). For a review, see:; Verho O; Bäckvall J-E Chemoenzymatic Dynamic Kinetic Resolution: A Powerful Tool for the Preparation of Enantiomerically Pure Alcohols and Amines. J. Am. Chem. Soc 2015, 137, 3996–4009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2). For overviews, see:; (a) Arai N; Ohkuma T Reduction of Carbonyl Groups: Hydrogenation Science of Synthesis, Stereoselective Synthesis; De Vries JG; Molander GA; Evans PA, Eds.; Georg Thieme Verlag: Stuttgart, 2011; Vol. 2, pp. 9–57. [Google Scholar]; (b) Zaidlewicz M; Pakulski MM Reduction of Carbonyl Groups: Transfer Hydrogenation, Hydrosilylation, Catalytic Hydroboration, and Reduction with Borohydrides, Aluminum Hydrides, or Boranes Science of Synthesis, Stereoselective Synthesis; De Vries JG; Molander GA; Evans PA, Eds.; Georg Thieme Verlag: Stuttgart, 2011; Vol. 2, pp. 59–131. [Google Scholar]; (c) Stefane B; Pozgan F Advances in Catalyst Systems for the Asymmetric Hydrogenation and Transfer Hydrogenation of Ketones Catal. Rev 2014, 56, 82–174. [Google Scholar]
- (3).Ramon DJ; Yus M Alkylation of Carbonyl and Imino Groups Science of Synthesis, Stereoselective Synthesis; De Vries JG; Molander GA; Evans PA, Eds.; Georg Thieme Verlag: Stuttgart, 2011; Vol. 2, pp. 349–400. [Google Scholar]
- (4). For illustrative examples, see:; (a) León F; González-Liste PJ; García-Garrido SE; Arribas I; Rubio M; Cadierno V; Pizzano A Broad Scope Synthesis of Ester Precursors of Nonfunctionalized Chiral Alcohols Based on the Asymmetric Hydrogenation of α,β-Dialkyl‐, α,β-Diaryl‐, and α‐Alkyl-β-aryl-vinyl Esters. J. Org. Chem 2017, 82, 5852–5867. [DOI] [PubMed] [Google Scholar]; (b) Schmidt J; Choi J; Liu AT; Slusarczyk M; Fu GC A General, Modular Method for the Catalytic Asymmetric Synthesis of Alkylboronate Esters. Science 2016, 354, 1265–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).(a) Fu GC Transition-Metal Catalysis of Nucleophilic Substitution Reactions: A Radical Alternative to SN1 and SN2 Processes. ACS Cent. Sci 2017, 3, 692–700. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Choi J; Fu GC Transition Metal-Catalyzed Alkyl–Alkyl Bond Formation: Another Dimension in Cross-Coupling Chemistry. Science 2017, 356, eaaf7230. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Kaga A; Chiba S Engaging Radicals in Transition Metal-Catalyzed Cross-Coupling with Alkyl Electrophiles: Recent Advances. ACS Catal. 2017, 7, 4697–4706. [Google Scholar]; (d) Iwasaki T; Kambe N Ni-Catalyzed C–C Couplings using Alkyl Electrophiles. Top. Curr. Chem 2016, 374, 66. [DOI] [PubMed] [Google Scholar]; (e) Geist E; Kirschning A; Schmidt T sp3-sp3 Coupling Reactions in the Synthesis of Natural Products and Biologically Active Molecules. Nat. Prod. Rep 2014, 31, 441–448. [DOI] [PubMed] [Google Scholar]
- (6). FFor a pioneering study of a non-asymmetric nickel-catalyzed coupling, see:; Lu X; Xiao B; Zhang Z; Gong T; Su W; Yi J; Fu Y; Liu L Practical Carbon–Carbon Bond Formation from Olefins Through Nickel-Catalysed Reductive Olefin Hydrocarbonation. Nat. Commun 2016, 7, 11129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).(a) Wang Z; Yin H; Fu GC Catalytic Enantioconvergent Coupling of Secondary and Tertiary Electrophiles with Olefins. Nature 2018, 563, 379–383. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) He S-J; Wang J-W; Li Y; Xu Z-Y; Wang X-X; Lu X; Fu Y Nickel-Catalyzed Enantioconvergent Reductive Hydroalkylation of Olefins with α‐Heteroatom Phosphorus or Sulfur Alkyl Electrophiles. J. Am. Chem. Soc 2020, 142, 214–221. [DOI] [PubMed] [Google Scholar]; For a related process, see:; Zhou F; Zhang Y; Xu X; Zhu S NiH-Catalyzed Remote Asymmetric Hydroalkylation of Alkenes with Racemic α-Bromo Amides. Angew. Chem. Int. Ed 2019, 58, 1754–1758. [DOI] [PubMed] [Google Scholar]
- (8). For reductive coupling strategies that introduce allyl and other unsaturated groups to aldehydes and other electrophiles, see:; Holmes M; Schwartz LA; Krische MJ Intermolecular Metal-Catalyzed Reductive Coupling of Dienes, Allenes and Enynes with Carbonyl Compounds and Imines. Chem. Rev 2018, 118, 6026–6052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).(a) Watanabe T; Kurata I; Hayashi C; Igarashi M; Sawa R; Takahashi Y; Akamatsu Y The Synthesis of Paleic Acid, an Antimicrobial Agent Effective Against Mannheimia and Pasteurella, and its Structurally Related Derivatives. Bioorg. Med. Chem. Lett 2010, 20, 5843–5846. [DOI] [PubMed] [Google Scholar]; (b) Jones GB; Huber RS; Chapman BJ Catalytic Enantioselective Synthesis of Macrolides via Asymmetric Alkylation. Tetrahedron: Asymmetry 1997, 8, 1797–1809. [Google Scholar]
- (10).(a) Vlasyuk AL; Voblikova VA; Gamalevich GD; Serebryakov EP The Absolute Configuration of (+)- and (−)-Phenylundec-4-yn-3-ols. Synthesis of (R)-4-Dodecanolide, a Component of the Defensive Secretion of Rove Beetle Bledius Mandibullaris. Russ. Chem. Bull., Int. Ed 2013, 62, 2032–2036. [Google Scholar]; (b) Vlasyuk AL; Gamalevich GD; Ignatenko AV; Serebryakov EP; Struchkova MI Lipase-Mediated Deracemization of Secondary 1-Phenyl-Substituted Propargylic Alcohols of Different Topology. Russ. Chem. Bull., Int. Ed 2004, 53, 693–702. [Google Scholar]
- (11).Fujii T; Yamakawa R; Terashima Y; Imura S; Ishigaki K; Kinjo M; Ando T Propionates and Acetates of Chiral Secondary Alcohols: Novel Sex Pheromone Components Produced by a Lichen Moth Barsine Expressa (Arctiidae: Lithosiinae). J. Chem. Ecol 2013, 39, 28–36. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


