Abstract
Despite major therapeutic advances in the management of patients with breast cancer, central nervous system (CNS) metastases remain an intractable problem, particularly in patients with metastatic HER2 positive and triple-negative breast cancer. As systemic therapies to treat extracranial disease improve, some patients are surviving longer, and the frequency of CNS involvement appears to be increasing. Furthermore, in the early-stage setting, the CNS remains a potential sanctuary site for relapse. This Review highlights advances in the development of biologically relevant preclinical models including the development of brain-tropic cell lines for testing of agents to prevent and treat brain metastases, and summarizes our current understanding of the biology of CNS relapse. From a clinical perspective, a variety of therapeutic approaches are discussed, including methods to improve drug delivery, novel cytotoxic agents, and targeted therapies. Challenges in current trial design and endpoints are reviewed. Finally, we discuss promising new directions, including novel trial designs, correlative imaging techniques, and enhanced translational opportunities.
Introduction
Historically, brain metastases were a relatively uncommon complication of breast cancer, reported to affect 10–15% of patients with metastatic disease (1). In prior decades, median survival after diagnosis for all-comers was estimated at less than 6 months, even with whole brain radiation therapy (WBRT) treatment (2). Patients were often diagnosed in the setting of progressive extracranial disease, and lack of systemic disease control was a major limiting factor in overall survival. It is in this context that research into this “old challenge” traditionally took place, and hence, generally held a lower priority than other important challenges in breast cancer. Furthermore, because of their poor expected survival, patients with brain metastases were often entirely excluded from participation in clinical trials. However, new biological insights, coupled with recognition of a very high rate of CNS metastases in particular breast cancer subtypes, and improved overall survival in some subsets of patients, have elevated the clinical and translational relevance of this old clinical problem.
Differences in Behavior by Breast Cancer Subtype
The patterns of metastatic relapse vary significantly according to tumor subtype (3–6). Furthermore, survival after a brain metastasis diagnosis also varies by breast cancer subtype. Although CNS is an uncommon site of first relapse in patients with HER2-positive breast cancer treated with trastuzumab (for example, 2.2% at 4 years median follow-up in the HERA trial), over time, 30–55% of patients with metastatic HER2-positive breast cancer will develop brain metastases, often in the setting of systemic disease control (Table 1) (3, 7–11). Indeed, among over 400 patients who died during follow up on the adjuvant HERA trial, approximately half were diagnosed with CNS relapse prior to death (7, 12). Among patients with HER2-positive breast cancer, the risk of brain metastases is further elevated in the setting of hormone receptor negativity (13). Of note, CNS events in this patient population appear to be widely distributed over time (7, 10). This could be the case either because of CNS seeding on a continuous basis over the course of a patient’s disease, and/or awakening of dormant tumor cells in the brain, the latter of which have been demonstrated in preclinical imaging studies (14). It is in patients with HER2-positive disease that clinical outcomes have changed the most over recent years, likely as a result of improvements in systemic disease control with HER2-directed agents, as well as improvements in CNS-directed local therapies to salvage patients at the time of CNS progression.(15, 16) Indeed, in a multi-institutional, retrospective study, the subgroup of fit patients with estrogen receptor (ER)-positive, HER2-positive brain metastases were found to have a median survival in the range of 2 years after CNS diagnosis, a four-fold increase compared to historical control estimates.(17)
Table 1.
Incidence of Brain Metastases and Overall Survival Among Patients with Metastatic Breast Cancer, According to Breast Cancer Subtype
| 1st author | All-comers Incidence Median OS | HER2-Positive Incidence Median OS | Triple-negative Incidence Median OS | ER-positive Incidence Median OS |
|---|---|---|---|---|
| Barnholtz-Sloan(1) | 14%NR | NR | NR | NR |
| Bendell 2003(9) | NR | 34%13 months | NR | NR |
| Eichler 2008 (104) | NR8.3 months | NR17.1 months | NR4 months | NR |
| Lin 2008 (19) | NR | NR | 46%4.9 months | |
| Nam 2008 (105) | 17%4.5 months | 25%12.8 months* | 25%3.4 months | 8%7.3 months |
| Anders 2010 (106) | NR7.8 months | NR14.2 months in ER-/HER2+; 15.2 months in ER+/HER2+ | NR2.9 months | NR9.6 months |
| Metro 2011 (58) | NR | NR28 months | NR | NR |
| Brufsky 2011 (8) | NR | 37%13 months | NR | NR |
| Olson 2013 (107) | NR | 55%NR | NR | NR |
| Sperduto 2013 (108) | NR | NR17.9 months in ER-/HER2+; 22.9 months in ER+/HER2+ | NR7.3 months | NR10 months |
Median OS calculated from date of first brain metastasis diagnosis.
In patients who received trastuzumab.
Abbreviations: OS, overall survival; ER-positive refers to patients with estrogen receptor positive and HER2 negative tumors; NR, not reported
Patients with metastatic triple-negative breast cancer are also at high risk of CNS relapse, with estimates ranging from 25–46% in retrospective experiences (Table 1).(3, 18, 19) However, in contrast to patients with HER2-positive breast cancer, CNS involvement in patients with triple-negative breast cancer typically occurs in the setting of simultaneous extracranial disease progression, and tends to cluster relatively early in a patient’s disease trajectory.(6, 19–21) Unfortunately, survival from CNS metastases in patients with triple-negative breast cancer remains dismal (less than 6 months) (19, 21, 22).
In summary, far from being a niche problem, CNS metastases are very common in patients with advanced HER2-positive or triple-negative breast cancer. However, despite similarities in the incidence of CNS involvement, the status of extracranial disease control and overall survival differs sharply by breast cancer subtype. This should be taken into consideration in both clinical management and trial design.
Preclinical Models of Breast Cancer Brain Metastasis
In preclinical models, the spontaneous development of brain metastases is relatively uncommon. Occasional spontaneous brain lesions have been reported from Rag2 −/−; IL2rg−/− double knockout mice and human MCF-10A mammary epithelial cells expressing H-Ras and BM1 (23, 24). The rarity of spontaneous models is perhaps not surprising, given that in humans, brain metastases are not typically the first site of tumor recurrence. Furthermore, particularly in HER2-positive and ER-positive breast cancer, brain metastases tend to occur somewhat later in a patient’s disease course, often in the setting of simultaneous extracranial disease control with systemic therapy (9). Hence, most preclinical models of brain metastases generally fall into one of two categories: the development of cell lines that preferentially metastasize in the brain when injected into the arterial circulation, or the use of stereotactic injections of tumor cells directly into brain. Brain-tropic derivatives have been selected by sequential rounds of injection, development of the occasional brain lesion, sterile harvest and expansion of the tumor cells in tissue culture. With selection, most of the brain-tropic derivatives will produce multiple CNS lesions within 1–2 months of inoculation, providing a reasonable timeframe for drug dosing studies (Figure 1). The advantages of using brain-tropic lines are that they are posited to more closely resemble normal tumor and host biology. In addition, they may be used to facilitate research into agents that can prevent brain metastatic colonization.
Figure 1. Schematic of preclinical experimental brain metastasis experiments.
Using the 231-BR model system, tumor cells were injected into the left cardiac ventricle of immunocompromised mice on day 0. For metastasis prevention experiments, mice were randomized three days post-injection to either placebo or drug, given continuously until the experimental endpoint. For metastasis treatment experiments, placebo or drug was begun after micrometastases and macrometastases had formed, usually between days 14 and 21 post-injection, and continued until the experimental endpoint. At necropsy, step sections were cut through one brain hemisphere, and lesions quantified under a microscope in five H&E stained sections. Lesions were dichotomized into micrometastases and macrometastases based on a 300 micron cutoff in a single dimension, roughly equivalent to a lesion that is detectable in a human brain on MRI.
Triple-negative cell lines selected for brain tropism include MDA-MB-231, 4T1, and CN34 (25, 26). For MDA-MB-231, the 231-BR brain tropic subline has been shown to resemble human craniotomy specimens in terms of proliferation, apoptosis, and a neuro-inflammatory response (27). ER-positive models are sparse and include MA11 and the MCF-7 line transfected with HER2 (28, 29). The initial HER2-positive brain-tropic model system employed a HER2 transfectant of 231-BR which produces a 2.5–3 fold greater likelihood of brain metastasis compared to vector control (30, 31). Recently, BT474 cells were selected for brain-tropism through three rounds of carotid artery injection (BT-474-Br.3) (32); other naturally HER2 overexpressing lines are under derivation. Leptomeningeal lesions form in some of the models, but not in sufficient numbers or with sufficient consistency to permit rigorous preclinical testing of novel treatment approaches.
Biology of CNS Relapse
The mechanisms underlying CNS relapse remain poorly understood. Bos and colleagues used patient-derived ER-negative pleural malignant cells (CN34) as well as the MDA-MB-231 cell line to develop brain metastatic derivatives using in vivo selection in mice (25). Derivative lines showed a significant increase in the ability to lodge into and grow in the brain after arterial inoculation. Using comparative genome-wide expression analysis, the investigators identified 243 candidate genes that were differentially expressed in derivative versus parental lines. Expression of these genes was assessed in a set of 368 clinically annotated breast tumors to assess correlations with brain relapse. The resulting 17-gene signature showed an association with brain relapse in two independent breast cancer data sets, including when the signature was limited only to ER-negative tumors. As part of the analysis, COX2, EGFR ligands, and ST6GALNAC5 were identified as potential mediators of brain metastasis. In particular, ST6GALNAC5 was found to be specifically enhance brain metastatic potential, whereas COX2 and EGFR ligands also conferred lung metastatic potential. Subsequent functional experiments suggested that ST6GALNAC5 facilitates transmigration across an in vitro model of the blood-brain barrier (BBB). Other putative signaling pathways associated with metastasis to the brain include CXCR4/CXCL12, VEGF, PI3K, and Notch, raising the question whether inhibitors of these pathways might reduce the risk of CNS relapse (33–37). However, we are not aware of further publications validating the 17-gene signature in specific breast cancer populations of interest (eg triple-negative, HER2-positive). This is important because other attempts at identifying reproducible, predictive gene signatures for CNS relapse have failed.
Moreover, predictive signatures for CNS relapse must ideally take into account tumor subtype and not simply recapitulate it, given the strong association between ER and HER2 status and the likelihood of developing brain metastases. For example, among patients with HER2-positive breast cancer, is there a gene signature that is predictive of CNS relapse?
Another approach has been to dissect the actual process of metastatic colonization to the brain. Using intravital microscopy, Kienast and colleagues tracked the fate of individual metastasizing cells in vivo and described a sequence of arrest at vascular branch points, early extravasation, vessel cooption (in the case of melanoma), and early angiogenesis (in the case of lung cancer) (34). Once tumor cells have migrated across the BBB, a number of factors may explain their relative refractoriness to therapy. One hypothesis relates to the effects of the brain microenvironment. The brain represents a unique microenvironment, not only because of the presence of the BBB which may limit drug penetration, but because of unique stromal elements (e.g., pericytes, astrocytes, glial cells) which may contribute to both metastatic colonization and therapeutic response (35). Brain metastases appear to elicit a brain inflammatory response with extensive reactive gliosis (27, 38, 39). In vitro co-cultures with glial cells have demonstrated an increase in anchorage-independent growth of 231-BR cells in soft agar. suggesting that glial cells may play a role in promoting brain metastasis colonization and growth (27). In addition, it is notable that one of the postulated physiological roles of activated astrocytes is to protect neurons from the effects of toxic substances, and could theoretically be coopted by tumor cells to as a protective mechanism.
Current Therapeutic Approaches
Despite advances in systemic therapy for the treatment of metastatic breast cancer, in 2013, the standard of care for patients who present with newly diagnosed brain metastases still includes localized approaches such as surgical resection, WBRT, and/or stereotactic radiosurgery (SRS)(40). Surgical resection is generally reserved for patients who present with a single or few brain metastases, patients in whom the histologic diagnosis is in question, patients who present with significant mass effect or symptoms, or patients with lesion size >3–4 cm. With WBRT, patients receive radiation to the entire brain in 10–20 fractions. SRS delivers a high dose of radiation to a lesion over a single fraction. Although radiotherapy and surgical approaches can be associated with neurocognitive and other morbidities, they are efficacious in reducing tumor burden and palliating neurological symptoms, and thus remain the standard-of-care approaches for patients with newly diagnosed breast cancer brain metastases.
Novel Approaches to Therapy
Over the past several years, there has been increasing interest in evaluating novel therapies for the treatment of breast cancer brain metastases, including local and systemic approaches. At the present time, no cytotoxic or targeted agents have gained regulatory approval for the treatment or prevention of breast cancer metastases. However, a number of promising avenues of investigation have emerged, and there is hope that some of these approaches may eventually complement or even take the place of initial radiotherapy- and/or surgical-based approaches (Table 2). We highlight some of the approaches below, with a focus on systemic therapies.
Table 2.
Selected Ongoing Clinical Trials of Systemic Therapy for Breast Cancer Brain Metastases
| Agent | Phase of Trial | Class or Target | Patient Population | ClinicalTrials.gov Identifier |
|---|---|---|---|---|
| 2B3–101 | I | Anthracycline | Solid tumors and malignant glioma | NCT01386580 |
| TPI-287 | II | Taxane | Breast cancer, all subtypes | NCT01480583 |
| Everolimus + trastuzumab + vinorelbine | II | mTOR | Breast cancer, HER2-positive | NCT01305941 |
| BKM120 + trastuzumab | I | PI3K | Breast cancer, HER2-positive | NCT01132664 |
| Lapatinib + WBRT | II | HER2 | Breast cancer, HER2-positive | NCT01622868 |
| Neratinib | II | HER2 | Breast cancer, HER2-positive | NCT01494662 |
| Afatinib | II | HER2 | Breast cancer, HER2-positive | NCT01441596 |
| ARRY-380 + trastuzumab | I | HER2 | Breast cancer, HER2-positive | Not yet assigned |
| Bevacizumab + carboplatin | II | VEGF | Breast cancer, all subtypes | NCT01004172 |
| Bevacizumab + cisplatin + etoposide | II | VEGF | Breast cancer, all subtypes | NCT01281696 |
Disruption of the Blood-Tumor Barrier
Debate continues as to what degree the blood-tumor barrier is breached by brain metastases, and to what extent therapeutic resistance relates to inadequate CNS drug penetration versus intrinsic tumor resistance and/or stromal protective effects. Data from the team of Quentin Smith and Paul Lockman have indicated that, in mouse models, most experimental brain metastases were more permeable than normal brain (26). For paclitaxel, fold uptake of drug over normal brain was determined in 231-BR metastases. Approximately 10% of lesions exhibited free uptake of radiolabeled drug (>50-fold over normal brain), while 14% of lesions had drug uptake statistically indistinct from normal brain. The remainder of the lesions fell in between these extremes. Only the 10% of lesions with the highest permeability exhibited a cytotoxic response to paclitaxel. Permeability was somewhat greater for lapatinib. The level of lapatinib in areas of the brain distant from metastases was only 1.3–2.8% of plasma concentrations, whereas in brain metastasis, the brain metastasis/plasma concentration ratio reached 26% overall. Like paclitaxel, lapatinib penetration was variable between and within metastases, with some lesions demonstrating very high levels of lapatinib (17% freely permeable) and others not statistically distinct from normal brain, and was not well-correlated with lesion size (Figure 2). A presurgical study conducted in breast cancer patients demonstrated similar findings.(41). In that study, CNS tumor capecitabine and 5-fluorouracil concentrations ranged from 3% to 129% and 168% to 1,422% of serum concentrations; CNS tumor lapatinib concentrations ranged from 21% to 700% of serum concentrations.
Figure 2. Variable penetration of paclitaxel into experimental 231-BR brain metastases of breast cancer.
A-C, Mice were inoculated with EGFP tagged 231-BR-HER2 brain-tropic tumor cells, and permitted to develop metastases. Before necropsy, mice were injected with 3kDa Texas Red dextran (B) or 14C-Paclitaxel (C), and free drug was then perfused from the vasculature. A single section of brain was imaged for metastases (EGFP, A), Texas Red Dextran uptake (B) and imaged for 14C-paclitaxel uptake. Variability is noted within and between lesions. D, The fold-uptake of paclitaxel relative to normal brain was determined for 379 experimental metastases. Only 9.8% of lesions demonstrated >50-fold greater drug uptake as compared to normal brain, while 14% of lesions were statistically indistinct from normal brain. In experiments not shown, paclitaxel only caused cytotoxicity in the 9.8% of freely permeable lesions. E, Paclitaxel distribution over time in experimental metastases (purple) versus systemic lesions and normal brain. While drug uptake is higher in experimental metastases than normal brain, it remains logs below systemic lesions. Adapted from Lockman, et al. (26).
Several methodologies have been proposed to increase blood-tumor permeability, including radiation therapy, transport pump inhibitors, molecular inhibitors, and blood-brain barrier disruption using ultrasound or other techniques (42–44). While many of these approaches achieve some added permeability, the three driving questions are 1) is drug penetration the primary reason for resistance, 2) if so, how much additional penetration is enough to drive a therapeutic effect, and 3) what are the side effects?
Brain Permeable Cytotoxic Chemotherapies
One approach has been to develop cytotoxic agents that are more brain permeable, either within an existing drug class, or as a direct modification of an existing drug. Examples of this approach include sagopilone, patupilone, ANG1005 (GRN1005), and 2B3–101 (45–47). CNS responses have been reported with each of these compounds; however, none have reached late stages of clinical testing. In a phase 2 study of 36 patients treated with patupilone, a BBB permeable epothilone, CNS response were observed in 19% of patients and median progression-free survival was 2.8 months (46). Although a similar response rate was reported with sagopilone, the responses were not sustained and overall, the drug was not deemed of interest for further study in breast cancer (45).
ANG1005 is novel derivative of paclitaxel, in which the cytotoxic agent is conjugated to a peptide vector (Angiopep-2) which is thought to facilitate passage across the BBB relative to free paclitaxel (48, 49). Initial work in the phase I solid tumor study suggested clinical activity in CNS, including in patients with breast cancer (50). A phase 2 study in patients with breast cancer brain metastases was therefore launched. Patients were initially treated at a 650 mg/m2 starting dose; however, the protocol was subsequently amended to test a 550 mg/m2 because of toxicities (fatigue, cytopenias, neuropathy) noted at the 650 mg/m2 dose necessitating dose reductions in a high proportion of patients. Results of the planned interim analysis of the first 30 patients were underwhelming at the 550 mg/m2 starting dose (51). However, of note, interim efficacy results at the 650 mg/m2 starting dose were comparable to that seen in the phase I, with CNS responses observed in one-third of patients. It is possible that the apparent dose response might reflect incomplete passage across the BBB at the lower doses, but this is not definitively known at this time. We await results of the final efficacy analysis which will include all patient enrolled on the study. Whether a loading dose and schedule might maintain efficacy while minimizing toxicity remains to be seen. 2B3–101 is glutathione-pegylated doxorubicin, which in preclinical models, appears to cross the BBB to a greater extent than free doxorubicin. The phase I trial is ongoing. For both ANG1005 and 2B3–101, regardless of the ultimate fate of the parent compounds, the technology to facilitate BBB penetration may be generalizable to other existing cytotoxic agents, and may offer additional opportunities for drug development in the future.
HER2-Directed Agents
Historically, it has been thought that trastuzumab crosses neither the BBB nor the blood-tumor barrier, due to its large molecular size. While a relative increase in trastuzumab cerebrospinal fluid (CSF) levels has been reported after radiotherapy in human breast cancer patients, the ratio still remained 76:1 relative to serum levels (52, 53). Recently, 89zirconium-labeled trastuzumab, suitable for in vivo PET imaging, has been developed (54). In a limited number of breast cancer patients, as expected, the relative uptake value for 89Zr-trastuzumab in normal brain was significantly lower than in liver. However, notably, significant relative uptake in brain metastases was noted, comparable to that of simultaneously imaged bone metastases (54) (Figure 3). Because the human data are based on a relatively small number of patients and CNS lesions, it remains a question what proportion of brain metastases are permeable to trastuzumab and to what extent. It is unknown if these represent the ~10% of freely permeable lesions seen in preclinical models. Nonetheless, these and other data raise questions as to the true mechanisms of trastuzumab resistance in the brain, particularly given the common clinical scenario of isolated CNS progression with continued systemic disease control on trastuzumab. The data also suggest that, if even minimal amounts of trastuzumab penetrate into brain metastases, then more potent derivatives such as trastuzumab emtansine (T-DM1) may have a more favorable therapeutic profile.
Figure 3.
89Zr-trastuzumab-PET demonstrating uptake in a brain metastasis in a patient with HER2-positive breast cancer (adapted from Dijkers, et al (54)).
Small molecule inhibitors of HER2, including lapatinib, neratinib, afatinib, and ARRY-380, are also under active investigation. In the clinic, lapatinib has been studied both as a single agent, and in combination with chemotherapy. The response rate to single agent lapatinib in patients with progressive CNS metastases after radiotherapy is only 6%; however, the response rate increases to 18–38% when given in combination with capecitabine (55–60). In HER2-positive patients with previously untreated brain metastases, a provocative study conducted in France recently reported a CNS objective response rate of 66% (61). Median time to progression was 5.5 months and 1-year survival exceeded 70%. The treatment was not without toxicity and did lead to diarrhea, palmar-plantar erythema, and other adverse effects. Neuro-cognitive outcomes were not assessed. Discussions are now under way to consider potential phase III designs comparing lapatinib plus capecitabine versus standard radiotherapy as upfront therapy of patients with newly diagnosed HER2-positive breast cancer brain metastases. Lapatinib has also been studied as a possible radiosensitizer, given concurrently with whole brain radiotherapy in a phase I trial (Lin et al, manuscript submitted). In preclinical models, lapatinib appeared to sensitize both basal-like/EGFR+ SUM149 breast cancer cells and HER2+ SUM225 breast cancer cells to radiotherapy (62) In the HER2+ model, radiosensitization was correlated with Akt inhibition by lapatinib. In the completed phase I trial, the CNS objective response rate was 79% and just under half of patients remained alive and progression-free in both brain and body at 6 months, which compares favorably to a historical response rate of 30% with WBRT alone. Although this was a non-randomized experience and results must be interpreted with caution, they are encouraging relative to the reported historical response to WBRT in breast cancer patients, and a randomized phase 2 trial is ongoing (NCT01622868). An unanswered clinical question with lapatinib is to what extent its activity (or the activity of other HER2-directed agents) could be improved with better and more consistent CNS penetration.
Neratinib is an irreversible inhibitor of EGFR and HER2. Phase 2 data in patients with HER2-positive metastatic breast cancer have been encouraging with respect to single-agent activity, as well as activity in combination with chemotherapy (63–65). A prospective, phase 2 study evaluating neratinib monotherapy enrolling patients with progressing breast cancer brain metastases has recently completed accrual (NCT01494662) and plans are ongoing to further study the effects of neratinib in the brain when given in conjunction with capecitabine (Dr. Rachel Freedman, personal communication, July 2013). Afatinib is also being evaluated in this setting (NCT01441596). In addition, newer anti-HER2 agents are finding their way into the clinic, including ARRY-380, a HER2-selective tyrosine kinase inhibitor which demonstrates improved penetration of the BBB relative to lapatinib in preclinical models, prolongation of survival in intracranial mouse xenograft models, and which has demonstrated single-agent activity in metastatic breast cancer in a phase I clinical trial (66, 67).
Finally, there are ongoing efforts to increase drug concentrations in the CNS, either with BBB disruption techniques as described above, or direct instillation into the CSF. A handful of case reports in the literature have described responses of patients with leptomeningeal metastases to intrathecal (IT) trastuzumab, though this should still be considered off-label use (68–71). Two phase 1 studies in the U.S. and Europe are attempting to characterize the safety, optimal dosing, and efficacy of IT trastuzumab (NCT01373710, NCT01325207).
Anti-Angiogenic Agents
Brain metastasis-associated blood vessels are dilated, tortuous, and leaky, relative to normal vasculature (35, 72). However, the role of neo-angiogenesis and of effects of angiogenesis inhibitors in the setting of brain metastases has been debated (73). In some preclinical models, VEGF has been shown to promote the growth of brain metastases and treatment with anti-angiogenic agents leads to reductions in metastatic outgrowth as well as tumor regression (34, 74). Using an orthotopic xenograft of BT474 cells, investigators from the Jain laboratory have demonstrated reductions in microvascular density, increase in necrosis of brain lesions, and prolonged survival among mice treated with combined anti-HER2 therapy and anti-VEGF receptor-2 antibody, compared to control or anti-HER2-therapy alone (75). On the other hand, in a melanoma model (Mel57-VEGF-A), although treatment with the VEGFR inhibitor vandetanib led to the undetectability of tumors on contrast-enhanced MRI scans, histologic analysis revealed the presence of multiple nonangiogenic lesions and sustained tumor progression via co-option (76).
From a clinical perspective, out of concern for intracranial hemorrhage, patients with brain metastases have historically been excluded from trials of anti-angiogenic agents. In light of reassuring safety data with respect to CNS hemorrhage in lung cancer patients, two prospective phase 2 trials evaluating the role of bevacizumab/chemotherapy combinations for patients with breast cancer brain metastases were launched. Both studies have reported a high rate of durable CNS responses in excess of 60% (77, 78). Because of the potential issues with interpreting the clinical meaning of response in the CNS in the setting of anti-angiogenic therapies (since anti-angiogenic agents may reduce gadolinium leakage out of vessels, and hence, measurements of enhancing tumor), randomized data, ideally with an overall survival and/or symptom control/quality of life endpoint, will be required to fully evaluate whether there is a role for anti-angiogenic agents in the clinic.
Other Molecular Targets
As is true with breast cancer more generally, a wide range of molecular targets have a strong rationale for exploration in setting of brain metastases. We highlight two potential targets below.
Adamo and colleagues reported a high rate of PI3K pathway activation in a collection of 52 breast cancer brain metastasis samples, across all of the clinically relevant breast cancer subtypes (37). Multiple mTOR, PI3K, and Akt inhibitors are in clinical development in breast cancer. Of note, BKM120, a pan-PI3K inhibitor, is known to cross the intact BBB and activity has been seen in preclinical models of HER2-positive breast cancer (23). In the clinic, one patient with triple-negative cancer treated on the phase 1 trial experienced an objective response in her brain (79). Prospective trials to study the role of PI3K inhibitors in patients with breast cancer brain metastases are under consideration. As highlighted by Tabchy and colleagues in this issue of Clinical Cancer Research, increasingly, it may be possible to analyze molecular alterations in blood (ie “liquid biopsy”), including for tumor PIK3CA mutations and other alterations (80). In the setting of brain metastases, these approaches may be particularly valuable given the difficulty in directly accessing CNS tumor tissue.
Apart from direct tumor targeting with pharmacologic agents, immune-based approaches have a potential rationale in the treatment of brain metastases. Ipilimumab, a monoclonal antibody that blocks CTLA4, has been associated with CNS responses in patients with metastatic melanoma (81, 82). Newer immunomodulatory agents, including anti-PD-1therapies have generated rapid and sustained tumor responses in melanoma patients and early trials in solid tumor patients are ongoing (83, 84). Of particular interest in the setting of brain metastases is the potential for radiotherapy to synergize with immunotherapy, possibly by increasing antigen presentation (85, 86). If anti-PD-1 therapies are found to have activity against breast cancer, trials combining anti-PD-1 therapies with stereotactic radiosurgery could be quite compelling. In addition, because immune-based approaches do not necessarily require agents to actually cross the BBB, if these approaches are able to stimulate an immune response, they may be effective not only against macrometastases with a variably leaky BBB, but to have an impact on micrometastases and have a true CNS preventive effect. Readers are referred to the excellent review by Disis and colleagues for a comprehensive overview of the status of immunotherapy-based approaches in breast cancer (87).
Challenges and New Possibilities in Clinical Trial Design
Historically, clinical trials in patients with brain metastases have suffered from heterogeneity in the enrolled patient populations, inconsistent definition and application of clinical endpoints, and lack of a clear paradigm/pathway for drug development. The Response Assessment in Neuro-Oncology metastatic working group has recently published an extensive review of the challenges in assessing response and progression in the CNS, with the goal of providing guidelines to improve the quality and consistency across clinical trials (88). As an example, because of limitations of the existing response criteria (e.g. RECIST, MacDonald), investigators have made a wide variety of adaptations to assess CNS lesions in solid tumor patients. Trials have variably defined measurable lesions, the number of lesions to be evaluated, the method of measurement (e.g. linear, bi-dimensional, volumetric), cutoffs for response, requirements for corticosteroid use and neurological symptoms, and inclusion of extracranial disease status. Differences in response definitions make it difficult to place the results of clinical trials into their proper context. To address these and other issues, the RANO group is currently formulating proposed standardized response and progression criteria to carry forward in future clinical trials.
Novel Designs and Clinical Settings
The traditional setting in which new systemic agents have been evaluated in patients with breast cancer brain metastases is after progression through standard surgical and radiotherapeutic approaches. While the refractory setting can provide opportunities to screen for clinical activity using the usual metrics of response and progression-free survival, a major challenge in moving drugs from phase 2 to more definitive trials is the lack of an obvious control arm, given that there are currently no systemic agents approved in this setting. Although a control arm could consist of a radiotherapy-based approach, this introduces problems in trial design due to the likelihood that patients will receive some form of systemic therapy after radiation has been completed, and due to problems with how to consider extracranial disease in the analysis plan. One possibility could be to allow physician’s choice of systemic therapy as the control arm, with overall survival as the primary endpoint, in a definitive phase III design. Another possibility would be to mount a small, randomized phase II study which could potentially be the basis of an accelerated approval, when there are no other approved drugs for this indication.
In contrast, an entirely different strategy would be to consider a preventive approach (73, 89). For example, as shown in Table 3, preclinical models from the Steeg and other laboratories suggest that a number of compounds (e.g. pazopanib and vorinostat, among others) can prevent brain metastases even if they are not efficacious in the “treatment” setting (i.e. drug dosing begun only after macrometastases are allowed to form, with tumor shrinkage as an endpoint) (29, 31, 90, 91). In a HER2 overexpressing model, Zhang et al. have recently demonstrated a profound prevention of experimental metastasis by the combination of lapatinib and the Src inhibitor saracatinib (32).Translating this to the clinical setting, the idea would be to test the ability of a compound to prevent the emergence of CNS metastases, rather than trying to treat established metastases.
Table 3.
Experimental Brain Metastasis Preventive Activity of Compounds in the 231-BR Model.
| Drug: | Dose andSchedule2: | Expt3: | Median4 Number Large Metastases: | %Reduction: | P: | Ref.: | |
|---|---|---|---|---|---|---|---|
| Vehicle | Drug | ||||||
| Capecitabine | 400 D PO | 1 | 6.2 | 4.8 | −23 | 0.40 | |
| 2 | 2.7 | 1.7 | −37 | 0.59 | |||
| 3 | 3.0 | 1.9 | −37 | 1.0 | |||
| Carboplatin | 50 W IV | 1 | 6.2 | 3.6 | −42 | 0.32 | |
| 2 | 3.0 | 3.0 | 0 | 1.0 | |||
| Doxorubicin | 5 W IV | 1 | 6.2 | 5.4 | −13 | 0.04 | |
| Gemcitabine | 50 2xW IV | 1 | 2.7 | 0 | −100 | 0.008 | |
| 2 | 2.0 | 0 | −100 | <0.0001 | |||
| Irinotecan | 4×7 IV | 1 | 2.0 | 1.0 | −50 | 0.090 | |
| 2 | 3.8 | 1.8 | −53 | 0.010 | |||
| Paclitaxel | 6 W IV | 1 | 8.5 | 7.4 | −13 | 1.0 | |
| 2 | 3.9 | 4.8 | +23 | 0.75 | |||
| 3 | 6.2 | 4.0 | −35 | 0.65 | |||
| Lapatinib5 | 100 Dx2 PO | P | 6.8 | 3.4 | −50 | 0.0001 | (109) |
| Vorinostat | 150 D IP | P | 7.7* | 2.9* | −62 | <0.0001 | (110) |
| Pazopanib5 | 100 Dx2 PO | P | 3.9 | 1.1 | −73 | <0.0001 | (111) |
| TPI-287 | 18 3×4 IV | P | 4.3* | 1.9* | −55 | 0.025 | (112) |
Includes previously published and unpublished results, the latter without references and conducted in the Steeg laboratory.
Dose in mg/kg. D, Daily; Dx2, Twice daily; M-F, Daily- Monday through Friday; W, Weekly; 2xW, Twice weekly; 4×7, Every fourth day for 7 treatments; 4×5–2, Two doses of 5 mg/kg, reduced to 2 mg/kg thereafter for toxicity; 3×4, Three treatments-every fourth day; IV, Intravenously; PO, Oral; IP, Intraperitoneally.
P, Pooled data from multiple experiments as published.
Data published as means indicated by *
Tested on a HER2 transfectant of 231-BR.
In practice however, demonstration of CNS prevention can be logistically challenging. For example, the CEREBEL trial compared lapatinib plus capecitabine versus trastuzumab plus capecitabine with the hope that the former would prevent the development of CNS metastases (92). The study did not meet its primary endpoint. However, there were strikingly few CNS events in either arm, making the study underpowered to definitely answer the question. It is likely that the paucity of events was due to the requirement for CNS screening at baseline, which resulted in a notable 20% rate of screen failure due to the detection of asymptomatic brain metastases. This study underlines some of the practical issues in designing trials of chemoprevention for brain metastases. One potential approach for future chemoprevention studies that could avoid some of these problems would be to enroll patients with limited brain metastases immediately after SRS, as they are at risk of future CNS events over a shorter timeframe. In this trial design, patients with a limited number of brain metastases could be treated with SRS, then randomly assigned to continue standard-of-care systemic treatment for control of extracranial disease versus the same systemic treatment plus an experimental agent. Patients would be followed carefully per protocol for new or progressive CNS metastases, with the idea that the trial design could isolate the CNS preventive effect of the novel agent. Alternatively, the development of improved molecular predictors to more significantly enrich for a group of women with a high risk of CNS relapse could be used to enroll trials.
Another approach is to consider systemic therapies in lieu of radiotherapy in the upfront setting. The potential advantages of such an approach are to spare/delay patients from the toxicities of radiation therapy, to reduce the potential for post-radiation effects to cloud the interpretation of CNS response, and to study systemic therapies in a less refractory patient population who might be more likely to respond. If such therapies are associated with a high rate of durable responses with an acceptable toxicity profile, then an open question is whether randomized trials versus standard radiotherapy are required before moving such treatments into routine clinical practice. If randomized trials are deemed necessary, what systemic treatments will be allowed in the radiotherapy arm and what endpoint(s) will be considered the most clinically relevant?
Novel Imaging Techniques
Because of the location of brain metastases, serial tissue biopsies are not practical. Hence, non-invasive methods to measure drug penetration, pharmacodynamic effects, and efficacy are of heightened interest, relative to metastatic breast cancer in general.
Standard 18FDG-PET scans do not optimally image the brain, due to high background glucose update in normal brain. However, brain-specific protocols have been developed that can overcome this limitation to some extent. In a study of lapatinib among patients with HER2-positive breast cancer, reductions in the FDG uptake in brain metastases were seen in a subset of women, providing some of the first in vivo evidence that the drug reaches levels in patients with brain metastases sufficient to influence cellular signaling (93).
18F-FLT (3’-Fluoro-3’ deoxythymidine)-PET imaging is a non-invasive tool for measuring in vivo tumor cell proliferation. 18F-FLT is a pyrimidine analog. After its uptake by proliferating cells, it is phosphorylated by thymidine kinase 1 and becomes trapped in the cells (94). 18F-FLT PET imaging may allow tracking of therapeutic-associated cell-cycle arrest before morphologic changes become measurable. Several studies in breast, lung, and brain tumors have demonstrated that retention of 18F-FLT correlated with tumor proliferation and have studied it for monitoring early response to therapies (95–99). Although 18F-FLT has been used to image and stage several tumor types, the standardized uptake value is generally lower than that obtained with 18F-FDG (100). Additionally the background uptake is high in the liver, marrow, and renal system and therefore limits its use in these organs. However, importantly, 18F-FLT lacks any significant uptake in the normal brain, and thus it maybe more suitable than 18FDG-PET for molecular imaging of tumors in the brain. In several studies the usefulness of 18F-FLT-PET has been studied in detection and monitoring of response of primary brain tumors. Similarly it is being studied in secondary tumors. In two small studies at National Cancer Institute (NCT01480583, NCT01679743) the usefulness of 18F-FLT is being investigated in the detection of brain metastases from breast cancer and prediction of response to systemic therapy (Figure 4).
Figure 4. 18FLT-positron emission tomography (PET) for detection of brain metastases and evaluation of early response.
Upper panels: Post-contrast, T1-weighted magnetic resonance imaging (MRI) of brain and corresponding 18FLT-positron emission tomography (PET) images at baseline. Lower panels: Post-contrast, T1-weighted MRI of brain and corresponding 18FLT-PET images after one cycle of GRN1005.
An alternative PET-based approach has been to develop reagents to noninvasively measure drug uptake in a more direct fashion. Examples include 89Zr-trastuzumab and 89Zr-bevacizumab, and these have been shown to be feasible in breast cancer patients (54, 101) (Figure 3). With this approach, in addition to being able to visualize the accumulation of drug into tumors in vivo, there is the potential to use baseline testing to select patients in whom adequate brain metastasis drug levels would be predicted.
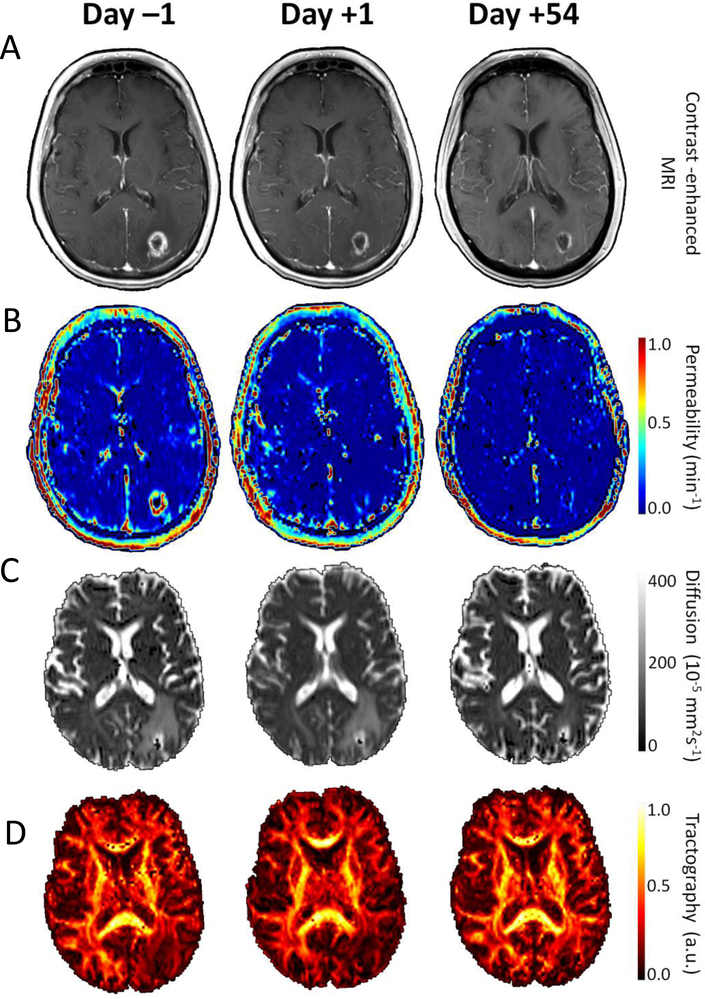
Finally, MR-based techniques have been explored in both glioblastoma multiforme (GBM) and breast cancer. In patients with GBM, treatment with the VEGFR inhibitor cedirinib is associated with rapid vascular changes, as assessed by MRI, and these changes are associated with overall survival (102, 103). Similar studies are ongoing in breast cancer patients to determine whether early vascular changes in response to anti-angiogenic therapy might be predictive of clinical outcomes (Figure 5).
Figure 5. Advanced magnetic resonance (MR)-based techniques for evaluation of early response to anti-angiogenic agents.
A) Baseline (day −1), after a single dose of bevacizumab (day +1), and after 2 cycles of carboplatin plus bevacizumab (day +54) showing a) post-contrast, T1 weighted brain MRI, B) permeability maps (Ktrans), C) apparent diffusion coefficient (ADC) maps showing water movement and D) fractional anisotropy (FA) maps from white matter diffusion tensor imaging (DTI) illustrating the degree of anisotropy in the water diffusion process [a.u. arbitrary units].
Summary and Conclusions
Brain metastases represent a common and growing problem among patients with advanced breast cancer. In patients with advanced triple-negative or HER2-positive breast cancer, brain metastases are not a rare occurrence, but instead, affect up to half of patients, often with considerable morbidity and mortality. Somewhat paradoxically, with improvements in systemic therapy leading to longer overall survival, particularly for patients with HER2-positive breast cancer, we believe that expanded efforts to study the pathophysiology of brain metastases and to develop preventive and therapeutic approaches are more important now than ever before.
Acknowledgements
The statistical assistance of Seth Steinberg, Biostatistics and Data Management Section, Center for Cancer Research, National Cancer Institute, Bethesda, MD, is appreciated. We thank the following individuals for providing figures: National Cancer Institute Molecular Imaging Program Staff (M. Liza Lindenberg, M.D., Esther Mena Gonzalex, Ph.D., Peter L. Choyke, M.D., Karen A. Kurdziel, M.D.), Kyrre E. Emblem, Ph.D (Massachusetts General Hospital, Boston, MA)., Elizabeth Gerstner, M.D. (Massachusetts General Hospital, Boston, MA), and Professor E.G. Elisabeth de Vries, M.D, Ph.D. (University Medical Centre, Groningen, the Netherlands).This work was funded, in part, by the Intramural Program of the National Cancer Institute, DOD Breast Cancer Research Program grant W81XWH-062-0033 (PS, DP), and the Breast Cancer Research Foundation (NL).
Conflicts of interest:
NL: Research funding (Genentech, GlaxoSmithKline, Array Biopharma, Novartis); Consultant (Novartis, GlaxoSmithKline, Genentech [uncompensated], to-BBB [uncompensated])
PS, Commercial Research Grant: Sanofi, GlaxoSmithKline
LAK, DP, DJL: None
References
- 1.Barnholtz-Sloan JS, Sloan AE, Davis FG, Vigneau FD, Lai P, Sawaya RE. Incidence proportions of brain metastases in patients diagnosed (1973 to 2001) in the Metropolitan Detroit Cancer Surveillance System. J Clin Oncol. 2004;22:2865–72. [DOI] [PubMed] [Google Scholar]
- 2.Fokstuen T, Wilking N, Rutqvist LE, Wolke J, Liedberg A, Signomklao T, et al. Radiation therapy in the management of brain metastases from breast cancer. Breast cancer research and treatment. 2000;62:211–6. [DOI] [PubMed] [Google Scholar]
- 3.Kennecke H, Yerushalmi R, Woods R, Cheang MC, Voduc D, Speers CH, et al. Metastatic Behavior of Breast Cancer Subtypes. J Clin Oncol. 2010. [DOI] [PubMed] [Google Scholar]
- 4.Lin NU, Vanderplas A, Hughes ME, Theriault RL, Edge SB, Wong YN, et al. Clinicopathologic features, patterns of recurrence, and survival among women with triple-negative breast cancer in the National Comprehensive Cancer Network. Cancer. 2012;118:5463–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pestalozzi BC, Zahrieh D, Price KN, Holmberg SB, Lindtner J, Collins J, et al. Identifying breast cancer patients at risk for Central Nervous System (CNS) metastases in trials of the International Breast Cancer Study Group (IBCSG). Ann Oncol. 2006;17:935–44. [DOI] [PubMed] [Google Scholar]
- 6.Dawood S, Lei X, Litton JK, Buchholz TA, Hortobagyi GN, Gonzalez-Angulo AM. Incidence of brain metastases as a first site of recurrence among women with triple receptor-negative breast cancer. Cancer. 2012;118:4652–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pestalozzi BC, Holmes E, de Azambuja E, Metzger-Filho O, Hogge L, Scullion M, et al. CNS relapses in patients with HER2-positive early breast cancer who have and have not received adjuvant trastuzumab: a retrospective substudy of the HERA trial (BIG 1–01). The lancet oncology. 2013;14:244–8. [DOI] [PubMed] [Google Scholar]
- 8.Brufsky AM, Mayer M, Rugo HS, Kaufman PA, Tan-Chiu E, Tripathy D, et al. Central nervous system metastases in patients with HER2-positive metastatic breast cancer: incidence, treatment, and survival in patients from registHER. Clin Cancer Res. 2011;17:4834–43. [DOI] [PubMed] [Google Scholar]
- 9.Bendell JC, Domchek SM, Burstein HJ, Harris L, Younger J, Kuter I, et al. Central nervous system metastases in women who receive trastuzumab-based therapy for metastatic breast carcinoma. Cancer. 2003;97:2972–7. [DOI] [PubMed] [Google Scholar]
- 10.Olson EM, Najita JS, Sohl J, Arnaout A, Burstein HJ, Winer EP, et al. Clinical outcomes and treatment practice patterns of patients with HER2-positive metastatic breast cancer in the post-trastuzumab era. Breast (Edinburgh, Scotland). 2013;22:525–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Olson EM, Abdel-Rasoul M, Maly J, Wu CS, Lin NU, Shapiro CL. Incidence and risk of central nervous system metastases as site of first recurrence in patients with HER2-positive breast cancer treated with adjuvant trastuzumab. Ann Oncol. 2013;24:1526–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lin NU. Brain metastases in HER2-positive breast cancer. The lancet oncology. 2013;14:185–6. [DOI] [PubMed] [Google Scholar]
- 13.Vaz-Luis I, Ottesen RA, Hughes ME, Marcom PK, Moy B, Rugo HS, et al. Impact of hormone receptor status on patterns of recurrence and clinical outcomes among patients with human epidermal growth factor-2-positive breast cancer in the National Comprehensive Cancer Network: a prospective cohort study. Breast Cancer Res. 2012;14:R129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heyn C, Ronald JA, Ramadan SS, Snir JA, Barry AM, MacKenzie LT, et al. In vivo MRI of cancer cell fate at the single-cell level in a mouse model of breast cancer metastasis to the brain. Magn Reson Med. 2006;56:1001–10. [DOI] [PubMed] [Google Scholar]
- 15.Karam I, Hamilton S, Nichol A, Woods R, Speers C, Kennecke H, et al. Population-based outcomes after brain radiotherapy in patients with brain metastases from breast cancer in the Pre-Trastuzumab and Trastuzumab eras. Radiation oncology (London, England). 2013;8:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bartsch R, Berghoff A, Pluschnig U, Bago-Horvath Z, Dubsky P, Rottenfusser A, et al. Impact of anti-HER2 therapy on overall survival in HER2-overexpressing breast cancer patients with brain metastases. British journal of cancer. 2012;106:25–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sperduto PW, Kased N, Roberge D, Xu Z, Shanley R, Luo X, et al. Summary Report on the Graded Prognostic Assessment: An Accurate and Facile Diagnosis-Specific Tool to Estimate Survival for Patients With Brain Metastases. J Clin Oncol. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee LJ, Alexander B, Schnitt SJ, Comander A, Gallagher B, Garber JE, et al. Clinical outcome of triple negative breast cancer in BRCA1 mutation carriers and noncarriers. Cancer. 2011;117:3093–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lin NU, Claus E, Sohl J, Razzak AR, Arnaout A, Winer EP. Sites of distant recurrence and clinical outcomes in patients with metastatic triple-negative breast cancer: high incidence of central nervous system metastases. Cancer. 2008;113:2638–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arvold ND, Oh KS, Niemierko A, Taghian AG, Lin NU, Abi-Raad RF, et al. Brain metastases after breast-conserving therapy and systemic therapy: incidence and characteristics by biologic subtype. Breast cancer research and treatment. 2012. [DOI] [PubMed] [Google Scholar]
- 21.Dawood S, Broglio K, Esteva FJ, Yang W, Kau SW, Islam R, et al. Survival among women with triple receptor-negative breast cancer and brain metastases. Ann Oncol. 2009;20:621–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Anders CK, Deal AM, Miller CR, Khorram C, Meng H, Burrows E, et al. The prognostic contribution of clinical breast cancer subtype, age, and race among patients with breast cancer brain metastases. Cancer. 2010;117:1602–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nanni P, Nicoletti G, Palladini A, Croci S, Murgo A, Ianzano ML, et al. Multiorgan metastasis of human HER-2+ breast cancer in Rag2−/−;Il2rg−/− mice and treatment with PI3K inhibitor. PloS one. 2012;7:e39626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hoenerhoff MJ, Chu I, Barkan D, Liu ZY, Datta S, Dimri GP, et al. BMI1 cooperates with H-RAS to induce an aggressive breast cancer phenotype with brain metastases. Oncogene. 2009;28:3022–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bos PD, Zhang XH, Nadal C, Shu W, Gomis RR, Nguyen DX, et al. Genes that mediate breast cancer metastasis to the brain. Nature. 2009;459:1005–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lockman PR, Mittapalli RK, Taskar KS, Rudraraju V, Gril B, Bohn KA, et al. Heterogeneous blood-tumor barrier permeability determines drug efficacy in experimental brain metastases of breast cancer. Clin Cancer Res. 2010;16:5664–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fitzgerald DP, Palmieri D, Hua E, Hargrave E, Herring JM, Qian Y, et al. Reactive glia are recruited by highly proliferative brain metastases of breast cancer and promote tumor cell colonization. Clinical & experimental metastasis. 2008;25:799–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rye PD, Norum L, Olsen DR, Garman-Vik S, Kaul S, Fodstad O. Brain metastasis model in athymic nude mice using a novel MUC1-secreting human breast-cancer cell line, MA11. International journal of cancer. 1996;68:682–7. [DOI] [PubMed] [Google Scholar]
- 29.Gril B, Palmieri D, Qian Y, Smart D, Ileva L, Liewehr DJ, et al. Pazopanib reveals a role for tumor cell B-Raf in the prevention of HER2+ breast cancer brain metastasis. Clin Cancer Res. 2011;17:142–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Palmieri D, Bronder JL, Herring JM, Yoneda T, Weil RJ, Stark AM, et al. Her-2 overexpression increases the metastatic outgrowth of breast cancer cells in the brain. Cancer Res. 2007;67:4190–8. [DOI] [PubMed] [Google Scholar]
- 31.Gril B, Palmieri D, Bronder JL, Herring JM, Vega-Valle E, Feigenbaum L, et al. Effect of lapatinib on the outgrowth of metastatic breast cancer cells to the brain. J Natl Cancer Inst. 2008;100:1092–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang S, Huang W-C, Zhang L, Zhang C, Lowery F, Ding Z, et al. Src family kinases as novel therapeutic targets to treat breast cancer brain metastases. Cancer Res. 2013;73:5764–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hinton CV, Avraham S, Avraham HK. Role of the CXCR4/CXCL12 signaling axis in breast cancer metastasis to the brain. Clinical & experimental metastasis. 2010;27:97–105. [DOI] [PubMed] [Google Scholar]
- 34.Kienast Y, von Baumgarten L, Fuhrmann M, Klinkert WE, Goldbrunner R, Herms J, et al. Real-time imaging reveals the single steps of brain metastasis formation. Nature medicine. 2010;16:116–22. [DOI] [PubMed] [Google Scholar]
- 35.Fidler IJ. The role of the organ microenvironment in brain metastasis. Seminars in cancer biology. 2011;21:107–12. [DOI] [PubMed] [Google Scholar]
- 36.Nam DH, Jeon HM, Kim S, Kim MH, Lee YJ, Lee MS, et al. Activation of notch signaling in a xenograft model of brain metastasis. Clin Cancer Res. 2008;14:4059–66. [DOI] [PubMed] [Google Scholar]
- 37.Adamo B, Deal AM, Burrows E, Geradts J, Hamilton E, Blackwell KL, et al. Phosphatidylinositol 3-kinase pathway activation in breast cancer brain metastases. Breast Cancer Res. 2011;13:R125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Seike T, Fujita K, Yamakawa Y, Kido MA, Takiguchi S, Teramoto N, et al. Interaction between lung cancer cells and astrocytes via specific inflammatory cytokines in the microenvironment of brain metastasis. Clinical & experimental metastasis. 2011;28:13–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sierra A, Price JE, Garcia-Ramirez M, Mendez O, Lopez L, Fabra A. Astrocyte-derived cytokines contribute to the metastatic brain specificity of breast cancer cells. Laboratory investigation; a journal of technical methods and pathology. 1997;77:357–68. [PubMed] [Google Scholar]
- 40.Tsao MN, Rades D, Wirth A, Lo SS, Danielson BL, Gaspar LE, et al. Radiotherapeutic and surgical management for newly diagnosed brain metastasis(es): an American Society for Radiation Oncology evidence-based guideline. Practical Radiat Oncol. 2012;2:210–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Morikawa A, Peereboom DM, Smith QR, Thorsheim H, Lockman PR, Simmons AJ, et al. Clinical evidence for drug penetration of capecitabine and lapatinib uptake in resected brain metastases from women with metastatic breast cancer. J Clin Oncol. 2013:abstract 514. [Google Scholar]
- 42.Yuan H, Gaber MW, Boyd K, Wilson CM, Kiani MF, Merchant TE. Effects of fractionated radiation on the brain vasculature in a murine model: blood-brain barrier permeability, astrocyte proliferation, and ultrastructural changes. International journal of radiation oncology, biology, physics. 2006;66:860–6. [DOI] [PubMed] [Google Scholar]
- 43.Muldoon LL, Soussain C, Jahnke K, Johanson C, Siegal T, Smith QR, et al. Chemotherapy delivery issues in central nervous system malignancy: a reality check. J Clin Oncol. 2007;25:2295–305. [DOI] [PubMed] [Google Scholar]
- 44.McDannold N, Arvanitis CD, Vykhodtseva N, Livingstone MS. Temporary disruption of the blood-brain barrier by use of ultrasound and microbubbles: safety and efficacy evaluation in rhesus macaques. Cancer Res. 2012;72:3652–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Freedman RA, Bullitt E, Sun L, Gelman R, Harris G, Ligibel JA, et al. A Phase II Study of Sagopilone (ZK 219477; ZK-EPO) in Patients With Breast Cancer and Brain Metastases. Clinical breast cancer. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Murphy C, Nulsen B, Rump M. Phase II trial of patupilone in patients (pts) with breast cancer brain metastases (BCBM) progressing or recurring after whole brain radiotherapy (WBXRT). ASCO Breast Cancer Symposium. 2009. [Google Scholar]
- 47.Kurzrock R, Gabrail N, Chandhasin C, Moulder S, Smith C, Brenner A, et al. Safety, pharmacokinetics, and activity of GRN1005, a novel conjugate of angiopep-2, a peptide facilitating brain penetration, and paclitaxel, in patients with advanced solid tumors. Molecular cancer therapeutics. 2012;11:308–16. [DOI] [PubMed] [Google Scholar]
- 48.Regina A, Demeule M, Che C, Lavallee I, Poirier J, Gabathuler R, et al. Antitumour activity of ANG1005, a conjugate between paclitaxel and the new brain delivery vector Angiopep-2. British journal of pharmacology. 2008;155:185–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Thomas FC, Taskar K, Rudraraju V, Goda S, Thorsheim HR, Gaasch JA, et al. Uptake of ANG1005, a novel paclitaxel derivative, through the blood-brain barrier into brain and experimental brain metastases of breast cancer. Pharmaceutical research. 2009;26:2486–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sarantopoulos J, Gabrail N, Moulder S, Brenner AJ, Smith CL, Bouchard D, et al. ANG1005: Results of a phase I study in patients with advanced solid tumors and brain metastases. J Clin Oncol. 2010;28:abstract 2556. [Google Scholar]
- 51.Lin NU, Schwartzberg LS, Kesari S, Yardley D, Verma S, Anders CK, et al. A phase 2, multi-center, open label study evaluating the efficacy and safety of GRN1005 alone or in combination with trastuzumab in patients with brain metastases from breast cancer. Cancer Res. 2012;72:Abstract P3–12-04. [Google Scholar]
- 52.Pestalozzi BC, Brignoli S. Trastuzumab in CSF. J Clin Oncol. 2000;18:2349–51. [DOI] [PubMed] [Google Scholar]
- 53.Stemmler HJ, Schmitt M, Willems A, Bernhard H, Harbeck N, Heinemann V. Ratio of trastuzumab levels in serum and cerebrospinal fluid is altered in HER2-positive breast cancer patients with brain metastases and impairment of blood-brain barrier. Anti-cancer drugs. 2007;18:23–8. [DOI] [PubMed] [Google Scholar]
- 54.Dijkers EC, Oude Munnink TH, Kosterink JG, Brouwers AH, Jager PL, de Jong JR, et al. Biodistribution of 89Zr-trastuzumab and PET imaging of HER2-positive lesions in patients with metastatic breast cancer. Clinical pharmacology and therapeutics. 2010;87:586–92. [DOI] [PubMed] [Google Scholar]
- 55.Lin NU, Carey LA, Liu MC, Younger J, Come SE, Ewend M, et al. Phase II trial of lapatinib for brain metastases in patients with human epidermal growth factor receptor 2-positive breast cancer. J Clin Oncol. 2008;26:1993–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lin NU, Dieras V, Paul D, Lossignol D, Christodoulou C, Stemmler HJ, et al. Multicenter phase II study of lapatinib in patients with brain metastases from HER2-positive breast cancer. Clin Cancer Res. 2009;15:1452–9. [DOI] [PubMed] [Google Scholar]
- 57.Boccardo F, Kaufman B, Baselga J, Dieras V, Link J, Casey MA, et al. Evaluation of lapatinib (Lap) plus capecitabine (Cap) in patients with brain metastses (BM) from HER2+ breast cancer (BC) enrolled in the Lapatinib Expanded Access Program (LEAP) and French Authorisation Temporaire d’Utilisation (ATU). J Clin Oncol. 2008;26:abstract 1094. [Google Scholar]
- 58.Metro G, Foglietta J, Russillo M, Stocchi L, Vidiri A, Giannarelli D, et al. Clinical outcome of patients with brain metastases from HER2-positive breast cancer treated with lapatinib and capecitabine. Ann Oncol. 2010;22:625–30. [DOI] [PubMed] [Google Scholar]
- 59.Sutherland S, Ashley S, Miles D, Chan S, Wardley A, Davidson N, et al. Treatment of HER2-positive metastatic breast cancer with lapatinib and capecitabine in the lapatinib expanded access programme, including efficacy in brain metastases--the UK experience. British journal of cancer. 2010;102:995–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lin NU, Eierman W, Greil R, Campone M, Kaufman B, Steplewski K, et al. Randomized phase II study of lapatinib plus capecitabine or lapatinib plus topotecan for patients with HER2-positive breast cancer brain metastases. Journal of neuro-oncology. 2011. [DOI] [PubMed] [Google Scholar]
- 61.Bachelot T, Romieu G, Campone M, Dieras V, Cropet C, Dalenc F, et al. Lapatinib plus capecitabine in patients with previously untreated brain metastases from HER2-positive metastatic breast cancer (LANDSCAPE): a single-group phase 2 study. The lancet oncology. 2013;14:64–71. [DOI] [PubMed] [Google Scholar]
- 62.Sambade MJ, Kimple RJ, Camp JT, Peters E, Livasy CA, Sartor CI, et al. Lapatinib in combination with radiation diminishes tumor regrowth in HER2+ and basal-like/EGFR+ breast tumor xenografts. International journal of radiation oncology, biology, physics. 2010;77:575–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Burstein HJ, Sun Y, Dirix LY, Jiang Z, Paridaens R, Tan AR, et al. Neratinib, an irreversible ErbB receptor tyrosine kinase inhibitor, in patients with advanced ErbB2-positive breast cancer. J Clin Oncol. 2010;28:1301–7. [DOI] [PubMed] [Google Scholar]
- 64.Chow LW, Xu B, Gupta S, Freyman A, Zhao Y, Abbas R, et al. Combination neratinib (HKI-272) and paclitaxel therapy in patients with HER2-positive metastatic breast cancer. British journal of cancer. 2013;108:1985–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Awada A, Dirix L, Manso Sanchez L, Xu B, Luu T, Dieras V, et al. Safety and efficacy of neratinib (HKI-272) plus vinorelbine in the treatment of patients with ErbB2-positive metastatic breast cancer pretreated with anti-HER2 therapy. Ann Oncol. 2013;24:109–16. [DOI] [PubMed] [Google Scholar]
- 66.Moulder SL, Baetz TD, D’Aloisio S, Fernetich G, Sasjan b, Freeman bB, et al. A phase 1 study to assess the safety, tolerability, and PK of ARRY-380, an oral HER2 inhibitor. American Society of Clinical Oncology Breast Cancer Symposium. 2010:Abstract 262. [Google Scholar]
- 67.Dinkel V, Anderson D, Winski S, Winkler J, Koch K, Lee PA. ARRY-380, a potent, small molecule inhibitor of ErbB2, increases survival in intracranial ErbB2+ xenograft models in mice. Cancer Res. 2012;72:abstr 852. [Google Scholar]
- 68.Platini C, Long J, Walter S. Meningeal carcinomatosis from breast cancer treated with intrathecal trastuzumab. The lancet oncology. 2006;7:778–80. [DOI] [PubMed] [Google Scholar]
- 69.Stemmler HJ, Schmitt M, Harbeck N, Willems A, Bernhard H, Lassig D, et al. Application of intrathecal trastuzumab (Herceptintrade mark) for treatment of meningeal carcinomatosis in HER2-overexpressing metastatic breast cancer. Oncology reports. 2006;15:1373–7. [DOI] [PubMed] [Google Scholar]
- 70.Siderov J Care with intrathecal trastuzumab. The lancet oncology. 2006;7:888. [DOI] [PubMed] [Google Scholar]
- 71.Zagouri F, Sergentanis TN, Bartsch R, Berghoff AS, Chrysikos D, de Azambuja E, et al. Intrathecal administration of trastuzumab for the treatment of meningeal carcinomatosis in HER2-positive metastatic breast cancer: a systematic review and pooled analysis. Breast cancer research and treatment. 2013;139:13–22. [DOI] [PubMed] [Google Scholar]
- 72.Bullitt E, Lin NU, Smith JK, Zeng D, Winer EP, Carey LA, et al. Blood vessel morphologic changes depicted with MR angiography during treatment of brain metastases: a feasibility study. Radiology. 2007;245:824–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Steeg PS, Camphausen KA, Smith QR. Brain metastases as preventive and therapeutic targets. Nature reviews Cancer. 2011;11:352–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kim LS, Huang S, Lu W, Lev DC, Price JE. Vascular endothelial growth factor expression promotes the growth of breast cancer brain metastases in nude mice. Clinical & experimental metastasis. 2004;21:107–18. [DOI] [PubMed] [Google Scholar]
- 75.Kodack DP, Chung E, Yamashita H, Incio J, Duyverman AM, Song Y, et al. Combined targeting of HER2 and VEGFR2 for effective treatment of HER2-amplified breast cancer brain metastases. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:E3119–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Leenders WP, Kusters B, Verrijp K, Maass C, Wesseling P, Heerschap A, et al. Antiangiogenic therapy of cerebral melanoma metastases results in sustained tumor progression via vessel co-option. Clin Cancer Res. 2004;10:6222–30. [DOI] [PubMed] [Google Scholar]
- 77.Lu YS, Chen WW, Ling CH, Tseng LM, Yeh DC, Wu PF, et al. Bevacizumab, etoposide, and cisplatin (BEEP) in brain metastases of breast cancer progressing from radiotherapy: results of the first stage of a multicenter phase II study. J Clin Oncol. 2012;30:Abstract 1079. [Google Scholar]
- 78.Lin NU, Gelman R, Younger J, Sohl J, Freedman R, Sorensen AG, et al. Phase II Trial of Carboplatin (C) and Bevacizumab (BEV) in Patients (Pts) with Breast Cancer Brain Metastases (BCBM). J Clin Oncol. 2013;31:abstract 513. [Google Scholar]
- 79.Bendell JC, Rodon J, Burris HA, de Jonge M, Verweij J, Birle D, et al. Phase I, dose-escalation study of BKM120, an oral pan-Class I PI3K inhibitor, in patients with advanced solid tumors. J Clin Oncol. 2012;30:282–90. [DOI] [PubMed] [Google Scholar]
- 80.Tabchy A, Ma CX, Bose R, Ellis MJ. Incorporating genomics into breast cancer clinical trials and care. Clin Cancer Res 2013;19xx–xx. [DOI] [PubMed] [Google Scholar]
- 81.Margolin K, Ernstoff MS, Hamid O, Lawrence D, McDermott D, Puzanov I, et al. Ipilimumab in patients with melanoma and brain metastases: an open-label, phase 2 trial. The lancet oncology. 2012;13:459–65. [DOI] [PubMed] [Google Scholar]
- 82.Weber JS, Amin A, Minor D, Siegel J, Berman D, O’Day SJ. Safety and clinical activity of ipilimumab in melanoma patients with brain metastases: retrospective analysis of data from a phase 2 trial. Melanoma research. 2011;21:530–4. [DOI] [PubMed] [Google Scholar]
- 83.Hamid O, Robert C, Daud A, Hodi FS, Hwu WJ, Kefford R, et al. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. The New England journal of medicine. 2013;369:134–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wolchok JD, Kluger H, Callahan MK, Postow MA, Rizvi NA, Lesokhin AM, et al. Nivolumab plus ipilimumab in advanced melanoma. The New England journal of medicine. 2013;369:122–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zeng J, See AP, Phallen J, Jackson CM, Belcaid Z, Ruzevick J, et al. Anti-PD-1 blockade and stereotactic radiation produce long-term survival in mice with intracranial gliomas. International journal of radiation oncology, biology, physics. 2013;86:343–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Knisely JP, Yu JB, Flanigan J, Sznol M, Kluger HM, Chiang VL. Radiosurgery for melanoma brain metastases in the ipilimumab era and the possibility of longer survival. Journal of neurosurgery. 2012;117:227–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Disis ML, Stanton SE. Can immunity to breast cancer eliminate residual micrometastases? Clin Cancer Res 2013;19xx–xx. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lin NU, Lee EQ, Aoyama H, Barani IJ, Baumert BG, Brown PD, et al. Challenges relating to solid tumour brain metastases in clinical trials, part 1: patient population, response, and progression. A report from the RANO group. The lancet oncology. 2013;14:e396–406. [DOI] [PubMed] [Google Scholar]
- 89.Steeg PS. Perspective: The right trials. Nature. 2012;485:S58–9. [DOI] [PubMed] [Google Scholar]
- 90.Qian Y, Hua E, Bisht K, Woditschka S, Skordos KW, Liewehr DJ, et al. Inhibition of Polo-like kinase 1 prevents the growth of metastatic breast cancer cells in the brain. Clinical & experimental metastasis. 2011;28:899–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Palmieri D, Lockman PR, Thomas FC, Hua E, Herring J, Hargrave E, et al. Vorinostat inhibits brain metastatic colonization in a model of triple-negative breast cancer and induces DNA double-strand breaks. Clin Cancer Res. 2009;15:6148–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Pivot X, Semiglazov V, Żurawski B, Allerton R, Fabi A, Ciruelos E, et al. CEREBEL (EGF111438): An open label randomized phase III study comparing the incidence of CNS metastases in patients (pts) with HER2+ Metastatic Breast Cancer (MBC), treated with Lapatinib plus Capecitabine (LC) versus Trastuzumab plus Capecitabine (TC) ESMO 2012 Annual Meeting. 2012:LBA11. [Google Scholar]
- 93.Van den Abbeele A,D, Lin NU, Yap JT, Urban T, de Vries DJ, Barnes AS, et al. Evaluation of response to lapatinib in patients with HER2-positive metastatic breast cancer using FDG-PET. Breast cancer research and treatment. 2006;100:S67, abstract 1089. [Google Scholar]
- 94.Shields AF, Grierson JR, Dohmen BM, Machulla HJ, Stayanoff JC, Lawhorn-Crews JM, et al. Imaging proliferation in vivo with [F-18]FLT and positron emission tomography. Nature medicine. 1998;4:1334–6. [DOI] [PubMed] [Google Scholar]
- 95.Smyczek-Gargya B, Fersis N, Dittmann H, Vogel U, Reischl G, Machulla HJ, et al. PET with [18F]fluorothymidine for imaging of primary breast cancer: a pilot study. European journal of nuclear medicine and molecular imaging. 2004;31:720–4. [DOI] [PubMed] [Google Scholar]
- 96.Pio BS, Park CK, Pietras R, Hsueh WA, Satyamurthy N, Pegram MD, et al. Usefulness of 3’-[F-18]fluoro-3’-deoxythymidine with positron emission tomography in predicting breast cancer response to therapy. Molecular imaging and biology : MIB : the official publication of the Academy of Molecular Imaging. 2006;8:36–42. [DOI] [PubMed] [Google Scholar]
- 97.Contractor KB, Kenny LM, Stebbing J, Rosso L, Ahmad R, Jacob J, et al. [18F]-3’Deoxy-3’-fluorothymidine positron emission tomography and breast cancer response to docetaxel. Clin Cancer Res. 2011;17:7664–72. [DOI] [PubMed] [Google Scholar]
- 98.Frings V, van der Veldt AA, Boellaard R, Herder GJ, Giovannetti E, Honeywell R, et al. Pemetrexed induced thymidylate synthase inhibition in non-small cell lung cancer patients: a pilot study with 3’-deoxy-3’-[(1)(8)F]fluorothymidine positron emission tomography. PloS one. 2013;8:e63705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Shinomiya A, Kawai N, Okada M, Miyake K, Nakamura T, Kushida Y, et al. Evaluation of 3’-deoxy-3’-[18F]-fluorothymidine (18F-FLT) kinetics correlated with thymidine kinase-1 expression and cell proliferation in newly diagnosed gliomas. European journal of nuclear medicine and molecular imaging. 2013;40:175–85. [DOI] [PubMed] [Google Scholar]
- 100.Been LB, Suurmeijer AJ, Cobben DC, Jager PL, Hoekstra HJ, Elsinga PH. [18F]FLT-PET in oncology: current status and opportunities. European journal of nuclear medicine and molecular imaging. 2004;31:1659–72. [DOI] [PubMed] [Google Scholar]
- 101.Gaykema SB, Brouwers AH, Lub-de Hooge MN, Pleijhuis RG, Timmer-Bosscha H, Pot L, et al. 89Zr-Bevacizumab PET Imaging in Primary Breast Cancer. J Nucl Med. 2013;54:1014–8. [DOI] [PubMed] [Google Scholar]
- 102.Sorensen AG, Batchelor TT, Zhang WT, Chen PJ, Yeo P, Wang M, et al. A “vascular normalization index” as potential mechanistic biomarker to predict survival after a single dose of cediranib in recurrent glioblastoma patients. Cancer Res. 2009;69:5296–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Batchelor TT, Sorensen AG, di Tomaso E, Zhang WT, Duda DG, Cohen KS, et al. AZD2171, a pan-VEGF receptor tyrosine kinase inhibitor, normalizes tumor vasculature and alleviates edema in glioblastoma patients. Cancer cell. 2007;11:83–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Eichler AF, Kuter I, Ryan P, Schapira L, Younger J, Henson JW. Survival in patients with brain metastases from breast cancer: the importance of HER-2 status. Cancer. 2008;112:2359–67. [DOI] [PubMed] [Google Scholar]
- 105.Nam BH, Kim SY, Han HS, Kwon Y, Lee KS, Kim TH, et al. Breast cancer subtypes and survival in patients with brain metastases. Breast Cancer Res. 2008;10:R20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Anders CK, Deal AM, Miller CR, Khorram C, Meng H, Burrows E, et al. The prognostic contribution of clinical breast cancer subtype, age, and race among patients with breast cancer brain metastases. Cancer. 2011;117:1602–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Olson EM, Najita JS, Burstein HJ, Sohl J, Arnaout A, Winer EP, et al. Clinical outcomes and treatment practice patterns of patients with HER2-positive metastatic breast cancer in the post-trastuzumab era. The Breast. 2013:in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sperduto PW, Kased N, Roberge D, Chao ST, Shanley R, Luo X, et al. The effect of tumor subtype on the time from primary diagnosis to development of brain metastases and survival in patients with breast cancer. Journal of neuro-oncology. 2013;112:467–72. [DOI] [PubMed] [Google Scholar]
- 109.Gril B, Palmieri D, Bronder JL, Herring JM, Vega-Valle E, Feigenbaum L, et al. Effect of lapatinib on the outgrowth of metastatic breast cancer cells to the brain. J Natl Cancer Inst. 2008;100:1092–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Palmieri D, Lockman P, Thomas F, Hua E, Herring J, Hargrave E, et al. Vorinostat inhibits brain metastatic colonization in a model of triple-negative breast cancer. Clin Cancer Res. 2009;15:6148–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Gril B, Palmieri D, Qian Y, Ileva L, Bernardo M, Choyke P, et al. Pazopanib reveals a role for tumor cell B-Raf in the prevention of breast cancer brain metastasis. Clin Cancer Res. 2010;17:142–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Fitzgerald D, Emerson D, Qian Y, Anwar T, Liewher D, Steinberg S, et al. TPI-287, a new taxane family member, reduced the brain metastatic colonization of breast cancer cells. Mol Cancer Ther. 2012;11:1959–67. [DOI] [PMC free article] [PubMed] [Google Scholar]