The concept of caries as an ecological disease implies the understanding of the intricate relationships among the populating microorganisms. Under frequent sugar exposure, some bacteria from the dental biofilm develop pathogenic traits that lead to imbalances (dysbiosis). Depending on which microorganism colonizes the dental surface first, different competition strategies may be developed. Studying the interactions in the entire dental biofilm is not an easy task. In this study, therefore, we modeled the interplay among these microorganisms using a caries-inducing species (S. mutans) and a health-associated species (S. sanguinis). Initial enamel adherence with S. sanguinis seems to induce more intense competition against typically caries-inducing species. Besides continuous exposure with sugars, early colonization of the enamel by highly cariogenic species like S. mutans appears to be needed to develop caries lesions as well. Promoting early colonization by health-associated bacteria such as S. sanguinis could help to maintain oral health, delaying dysbiosis.
KEYWORDS: cariogenicity, competition, dental caries, oral biofilm, primary colonization, Streptococcus mutans, Streptococcus sanguinis
ABSTRACT
Imbalances within the dental biofilm trigger dental caries, currently considered a dysbiosis and the most prevalent noncommunicable disease. There is still a gap in knowledge about the dynamics of enamel colonization by bacteria from the dental biofilm in caries. The aim, therefore, was to test whether the sequence of enamel colonization by a typically commensal and a cariogenic species modifies biofilm’s cariogenicity. Dual-species biofilms of Streptococcus mutans and Streptococcus sanguinis on saliva-coated enamel slabs were inoculated in different sequences: S. mutans followed by S. sanguinis (Sm-Ss), S. sanguinis followed by S. mutans (Ss-Sm), S. mutans and S. sanguinis inoculated at the same time (Sm=Ss), and the single-species controls S. mutans followed by S. mutans (Sm-Sm) and S. sanguinis followed by S. sanguinis (Ss-Ss). Biofilms were exposed to 10% sucrose 3 times per day for 5 days, and the slabs/biofilms were retrieved to assess demineralization, viable cells, biomass, proteins, polysaccharides, and H2O2 production. Compared with Sm-Sm, primary inoculation with S. sanguinis reduced demineralization (P < 0.05). Both Ss-Sm and Sm=Ss sequences showed reduction in biomass, protein, and polysaccharide content (P < 0.05). The highest S. sanguinis viable count and H2O2 production level and the lowest acidogenicity were observed when S. sanguinis colonized enamel before S. mutans (P < 0.05). Initial enamel adherence with commensal biofilms seems to induce more intense competition against more typically cariogenic species, reducing cariogenicity.
IMPORTANCE The concept of caries as an ecological disease implies the understanding of the intricate relationships among the populating microorganisms. Under frequent sugar exposure, some bacteria from the dental biofilm develop pathogenic traits that lead to imbalances (dysbiosis). Depending on which microorganism colonizes the dental surface first, different competition strategies may be developed. Studying the interactions in the entire dental biofilm is not an easy task. In this study, therefore, we modeled the interplay among these microorganisms using a caries-inducing species (S. mutans) and a health-associated species (S. sanguinis). Initial enamel adherence with S. sanguinis seems to induce more intense competition against typically caries-inducing species. Besides continuous exposure with sugars, early colonization of the enamel by highly cariogenic species like S. mutans appears to be needed to develop caries lesions as well. Promoting early colonization by health-associated bacteria such as S. sanguinis could help to maintain oral health, delaying dysbiosis.
INTRODUCTION
Dental caries and periodontal diseases have been defined as microbial dysbiosis (1), but the role played by each constituent of the multispecies microbial biofilm is far from being fully understood. It has been recognized that commensal streptococci act as early colonizers of the enamel (2), binding other early colonizers and host molecules to initiate the dental biofilm formation. Streptococcus sanguinis is a commensal member of the early colonizers in the dental biofilm that has been more abundantly recovered in caries-free children (3) and adults (4). Conversely, another important oral streptococcus, Streptococcus mutans, is not considered an early colonizer but is endowed with a powerful machinery to metabolize carbohydrates, producing critical amounts of acids as well as efficiently generating an adherent extracellular polysaccharide matrix implicated in caries development (5). An inverse relationship between S. mutans and S. sanguinis counts has been described (6), so when high numbers of colonies of S. mutans are recovered from the biofilm, relatively lower numbers of S. sanguinis are obtained. This opposite trend suggests competition between the two species. Drivers of competition between both species are nutrient availability and fitness within the ecological niche (7–9). Each species has developed strategies to mutually inhibit each other (10). Hence, S. mutans can produce bacteriocins (mutacins), which are used to inhibit competing species, including S. sanguinis (9, 11). On the other hand, S. sanguinis produces hydrogen peroxide (H2O2; generated via SpxB, which is encoded by the spxB gene [12]) as an antimicrobial compound, which, during the early stages of biofilm formation, is a powerful tool to exclude competing species, as peroxides are toxic for bacteria like S. mutans (13).
Dental caries is a disease characterized by lactic acid-induced hard dental tissue demineralization, caused by frequent carbohydrate exposure to the dental biofilm, which shifts the ecological balance toward a noninfectious polymicrobial dysbiosis (14). Despite the existence of evidence from clinical studies on the interacting relationship between commensal and cariogenic bacteria within the dental biofilm, the effect of the order in which they adhere to the enamel under environmental stressors relevant for the caries process, such as frequent sucrose exposure, has not been reported. Understanding whether primary colonization of the dental tissues by cariogenic or by commensal microorganisms promotes competition between them, and whether this competition modifies the structure and functionality of biofilm on the hard-dental tissue is of interest and has not been characterized in a caries model with dual-species biofilms. The aim of the study was, therefore, to test if the sequence of enamel adherence (colonization) by S. sanguinis and S. mutans modifies resulting cariogenicity.
RESULTS
Biofilm acidogenicity.
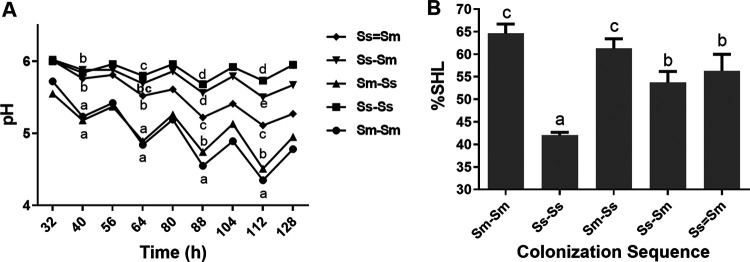
The pH decreased significantly more than the other conditions during the time of the assays when S. mutans was the initial enamel colonizer followed by S. sanguinis (Sm-Ss) and in the monospecies control Sm-Sm (P < 0.05). Both conditions showed the most acidogenic potential (pH 4.5) compared with the other groups, starting around 88 h and lasting until the end of the experimental phase (P < 0.05) (Fig. 1A). Monospecies biofilms of S. sanguinis (Ss-Ss) and the dual-species S. sanguinis followed by S. mutans (Ss-Sm) showed lower acidogenic potential (with a pH around 6.0) than any other condition (P < 0.05). Of interest, Ss-Ss showed a significantly higher pH value than Ss-Sm only after 112 h of incubation (P < 0.05), making prolonged net demineralization unlikely. Interestingly, acidogenicity seems to be intermediate (pH 5.0 to 5.5) when both species are inoculated at the same time (Ss=Sm) (Fig. 1A).
FIG 1.
(A) Biofilm acidogenicity. Biofilms were exposed to 10% sucrose for 5 min, 3 times per day under different colonization sequences (as indicated) on enamel slabs: Ss=Sm, Ss-Sm, Sm-Ss, Ss-Ss, and Sm-Sm. The medium pH was measured twice per day during the 5 days of the experiment. Each point in the plot depicts the means for two independent experiments, each in triplicate (n = 6). Different letters represent significant differences across all colonization sequences (P < 0.05). These data are adapted from reference 22 with permission of the publisher (Karger Publishers, Basel, Switzerland [copyright 2018]). (B) Enamel demineralization. Enamel slabs from each biofilm exposed to cariogenic challenges with 10% sucrose were retrieved from the orthodontic wire and cleaned of the adhered biomass. Initial and final surface microhardness (SH) were measured before and after the experiment, respectively, to assess percentage of SH loss (%SHL). Bars denote mean values of two independent experiments in triplicate (n = 6). Error bars show the standard deviation. Different letters (a to d) represent significant differences across all colonization sequences (P < 0.05).
Enamel demineralization.
The percentage of surface Knoop microhardness loss (%SHL) is also influenced by the colonization sequences (Fig. 1B). Thus, the highest %SHL was observed in the Sm-Ss sequence (P < 0.05) just above 60%, without differences with the Sm-Sm control biofilm, but higher than any other condition (Fig. 1B). When S. sanguinis was the primary colonizer (Ss-Sm), there was a significant reduction in demineralization compared to the biofilms for which the primary colonizer was S. mutans (P < 0.05) without statistical differences with Ss=Sm. However, S. sanguinis monospecies control biofilm showed the lowest %SHL. As a comparison, S. sanguinis biofilms under 0.9% NaCl with 16 h for biofilm formation had an average %SHL of 4.48 (data not shown).
Regarding the characteristics of the biofilms, there were significant variations in the properties of the different biofilms, including biomass, total protein content, and insoluble extracellular polysaccharide formation (Table 1).
TABLE 1.
Biofilm properties in different colonization sequencesa
| Colonization sequence | Biomass (mg) | Total protein (μg/mg biomass) | IEPS (%/mg biomass)b |
|---|---|---|---|
| Sm-Sm | 2.12 (0.26) D | 9.47 (1.56) B | 9.60 (3.52) A |
| Ss-Ss | 0.38 (0.21) A | 5.25 (0.52) A | 2.42 (1.36) B |
| Sm-Ss | 1.62 (0.26) C | 7.85 (0.53) B | 7.01 (1.40) A,B |
| Ss-Sm | 0.71 (0.19) AB | 5.90 (0.66) A | 3.06 (0.99) B |
| Ss=Sm | 1.00 (0.35) B | 7.77 (0.66) B | 5.00 (1.97) B |
Values are means (SDs) of two independent experiments in triplicate (n = 6). Comparisons were made vertically for each dependent variable and among the different inoculation sequences. Different letters (A through D) in the same column represent statistically significant differences (P < 0.05).
IEPS, insoluble extracellular polysaccharides.
Biomass.
When S. sanguinis adhered first to enamel (Ss-Sm), biofilms resulted in lower biomass than those where S. mutans adhered first (Sm-Ss) (P < 0.05). Both single-species controls resulted in the highest (Sm-Sm) and the lowest (Ss-Ss) biofilm formation (P < 0.05), respectively. Biofilms formed with S. sanguinis as the initial colonizer (Ss-Sm) showed lower biomass than those inoculated with both bacteria at the same time (Ss=Sm), but the difference was not statistically significant (P > 0.05).
Protein content of the biofilm.
The lowest protein content in the biofilms was detected in the Ss-Sm condition and the Ss-Ss control (P > 0.05). No differences were detected when S. mutans was the first colonizer, in the monospecies Sm-Sm control, or when both species colonized at the same time (Ss=Sm) (P > 0.05).
Insoluble extracellular polysaccharide formation.
S. mutans biofilms showed higher insoluble extracellular polysaccharide (IEPS) formation than S. sanguinis (P < 0.05). The lowest polysaccharide formation level among the dual-species groups was detected when S. sanguinis was inoculated before S. mutans, but this was still slightly higher than the Ss-Ss biofilm (P > 0.05).
Bacterial counts.
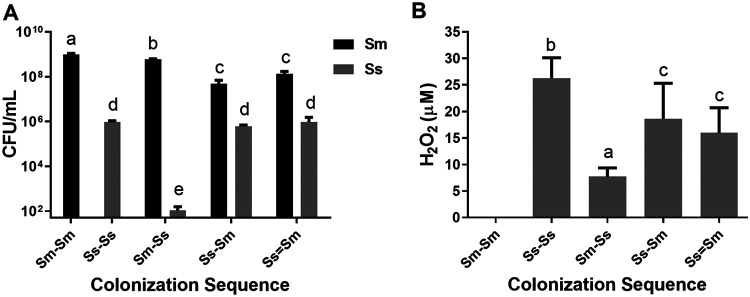
Viable cell counts (Fig. 2A) showed that S. sanguinis cells were drastically reduced when S. mutans was the initial enamel colonizer compared to any other bacterial combination (P < 0.05). Compared with the Sm-Sm monospecies control, S. mutans cells were significantly reduced in any combination when S. sanguinis was present as the first colonizer and even further in the Ss-Sm and Ss=Sm biofilms (P < 0.05), without differences between them (P > 0.05).
FIG 2.
(A) Viable microorganisms. Mean counts of S. mutans (black bar) and S. sanguinis (gray bar) expressed as CFU per milliliter were determined in each colonization sequence. Bars represent mean values of two independent experiments in triplicate (n = 6). Error bars show the standard deviation. Different letters represent significant differences across all colonization sequences (P < 0.05). These data are adapted from reference 22 with permission of the publisher (Karger Publishers, Basel, Switzerland [copyright 2018]). (B) H2O2 concentration. Production of H2O2 (μM) in each biofilm condition as described in Materials and Methods. Bars show mean values of two independent experiments in triplicate (n = 6). Error bars show the standard deviation. Different letters (a to d) represent significant differences across all colonization sequences (P < 0.05).
Hydrogen peroxide production.
Despite a decrease compared to the monospecies condition, when S. sanguinis was the first colonizer (Ss-Sm) or when both species colonized at the same time (Ss=Sm), there was sustained H2O2 production (Fig. 2B). Conversely, when S. mutans adhered to the enamel first, a drastic reduction in H2O2 was observed (P < 0.05).
DISCUSSION
In the present study, we modeled the dental biofilm by confronting a commensal species (S. sanguinis) with a cariogenic species (S. mutans) in a scenario where they compete for the same ecological niche. The opportunity of enamel colonization was used as the trigger for the competitive relationship under a steady cariogenic challenge induced by sucrose. The rationale behind these studies is that, within a cariogenic environment simulated by frequent sucrose exposure, if one of the competing species colonizes first, they can mount a response to create hostile environmental conditions for the further late colonizer microorganism. Thus, S. mutans, in this example, can initiate and mature a cariogenic biofilm with acidic characteristics, which can exclude competitors (15).
When analyzing the acidogenicity at different times for each biofilm condition, a strong decrease in pH values (<4.5) was observed in the biofilms of S. mutans as a single species in addition to their highest viable counts. Conversely, Ss-Ss and Ss-Sm sequences exhibited the highest pH values (close to 6.0), which was consistent with the highest viable cell counts of S. sanguinis. It has been described that S. sanguinis is endowed with alternative mechanisms to adapt to its environment and outcompete cariogenic competitors, such as S. mutans. For example, the arginolytic property of S. sanguinis is mediated by the arginine deiminase system (ADS). The ADS is able to generate ammonia, a metabolite that raises the pH and maintains it above the critical values of demineralization for the enamel (16–18). In addition, the ADS can be activated in slightly acidic conditions. This is consistent with the clinical data showing S. sanguinis being more abundantly isolated from caries-free children (3) and adults (4).
It should be noted that the intermediate pH that was observed in the biofilms inoculated with both bacteria at the same time could indicate only moderate competition under these conditions. This is consistent with the inhibition data obtained on agar plates between both species (9). This approach with dual-species biofilms adhering to enamel and under cariogenic environments had not been previously assayed.
Despite the lack of statistical differences, when both species adhered to enamel at the same time, demineralization increased, but not to the level of the condition with S. mutans as the pioneer colonizer. This suggests that competition is more intense when a commensal species primarily establishes biofilms and a cariogenic microorganism attempts to colonize the niche. This is consistent with previous in vitro studies showing that the inoculation sequences determine the characteristics of the oral biofilm (9).
S. sanguinis viable counts showed no significant differences when the enamel was first or at the same time colonized with S. mutans relative to its single-species biofilms. This is probably because S. sanguinis activates its ADS system, raising the pH and thus preventing it from being displaced from the biofilms. Coincidently, this occurs along with the lowest values of demineralization observed in the corresponding enamel slabs.
Cariogenic biofilms established early on enamel by S. mutans have strong adherent properties, mainly due to the production of soluble and insoluble extracellular polysaccharides (15). This property makes it difficult for other less adherent cells to colonize and displace the cariogenic species. Notably, S. sanguinis synthesizes water-insoluble glucans, but in small amounts (19).
Regarding biofilms properties, the lowest level of polysaccharide formation was detected in biofilms when S. sanguinis was the first adhering species, suggesting that S. sanguinis inhibits S. mutans colonization.
Likewise, protein and polysaccharide production followed the same trend as above, suggesting that early biofilms with S. sanguinis interfere with S. mutans colonization and the formation of thicker biofilms. When both species coexist in the dual-species biofilm, there seems to be an equilibrium in which neither manages to outcompete the other. This protective behavior may be the result of activation of virulence factors (20, 21). The expression of virulence genes associated with these species and their molecular mechanisms have been studied. Previously, our research group analyzed the transcriptional expression of the gtf genes of both bacteria and the spxB gene of S. sanguinis using the same experimental approach and design than that of this article (22). Interestingly, all genes were overexpressed when either species acted as the invading microorganism over an already formed biofilm by the antagonistic species, arguably in an attempt to colonize. Taken together, these data seem to suggest that a cariogenic environment posed by sucrose is not enough, by itself, to modify the dynamics of colonization on enamel. Although Gtf expression seems insufficient to outcompete the early colonizer, other virulent factors may be activated for competition. For example, mutacin I expressed by S. mutans may act as a potent virulent factor to maintain primary colonization and avoid competition (9, 11).
The antagonism observed may also be determined by sucrose availability and the resulting acid production. As already mentioned, acidic conditions created by S. mutans create a hostile environment for S. sanguinis, inhibiting the expression of the pyruvate oxidase enzyme, responsible for the production of H2O2 (10, 23). However, S. sanguinis ADS is acid tolerant and could contribute to maintaining H2O2 production by SpxB (24).
In this study, although S. sanguinis produced a smaller amount of hydrogen peroxide in the presence of S. mutans (and similar viable counts in Ss-Sm and Ss=Sm biofilms), there was sustained H2O2 production, creating a more competitive environment. This could explain the similar viable counts of S. mutans under the Ss-Sm and Ss=Sm biofilms conditions. As expected, the single-species control with S. mutans failed to show peroxide production, as S. mutans cannot produce H2O2 (14). Consistent with our results, H2O2 production by S. sanguinis is capable of inhibiting S. mutans (9, 25). Production of H2O2 is ubiquitous among the oral commensal streptococci. S. sanguinis, however, is resistant to its own H2O2 (10, 26), which could be a key component in the maintenance of oral ecology associated with healthy conditions (24). Although not characterized through microscopy, results indicate that S. mutans biofilms are more robust than S. sanguinis biofilms. S. mutans promptly synthesizes water-soluble and -insoluble polysaccharides using Gtfs to shape the extracellular matrix, allowing S. mutans to strongly adhere to enamel. Furthermore, S. mutans biofilms yield lower pH due to the production of organic acids, which drastically affects the viability and adaptation of S. sanguinis. In contrast, Gtf activity in S. sanguinis is restricted, with poor glucan production and biofilm formation. Despite the significant decrease in S. mutans counts when S. sanguinis was the first colonizer (relative to Sm-Ss), the highly virulent traits of S. mutans do not allow S. sanguinis to displace it.
The results from these studies contribute to shedding light on understanding the complex biological interactions in the dental biofilm under cariogenic conditions, especially when commensals are the predominant species in conditions compatible with oral health. S. sanguinis has been proposed as a model microorganism of molecular commensalism (13). In this context, it has been described that the expression of spxB is not affected by the presence of sugars (27), and the production of H2O2 is not altered by moderate pH changes (28). Thus, apparently under conditions of excess of sugars, acidic pH, and S. mutans as a first colonizer, S. sanguinis cannot compete and displace S. mutans. Under a sucrose-induced cariogenic ecological environment, initial enamel adherence by commensal biofilms seems to induce more intense competition against a canonical cariogenic species, reducing cariogenicity (acidogenicity and demineralization). Biofilm formation with cariogenic species appears to preclude the establishment of commensal-rich biofilms. These results must be interpreted as proof of principle to test a novel hypothesis in a clinical setting.
In conclusion, continuous exposure to sugars seems insufficient by itself for establishing a cariogenic biofilm. Early colonization of the enamel by highly cariogenic species, like S. mutans, appears to be needed also. Promoting early colonization by commensal species, such as S. sanguinis, could help to maintain symbiosis and delay dysbiosis.
MATERIALS AND METHODS
Enamel slab preparation and acquired pellicle formation.
Based on an established single-species caries model with biofilms of S. mutans (29), a dual-species caries model was applied. Dental enamel slabs were prepared from bovine incisors, as described before (29), and autoclaved. Slabs were mounted on metal brackets made with orthodontic wire and suspended in the wells of a 24-well plate (Costar, Corning, NY, USA). Slabs were covered with ultrafiltered stimulated saliva from two healthy donors for 30 min to simulate the formation of an acquired pellicle-like layer.
Formation of single- and dual-species biofilms of S. mutans and S. sanguinis.
Frozen stocks of S. mutans UA159 (isolated from a child with active caries and kindly donated by J. A. Cury, Unicamp, Brazil) and S. sanguinis SK36 (originally isolated from human dental plaque and donated by J. Kreth) were reactivated in brain heart infusion broth (BHI; Merck, Darmstadt, Germany) supplemented with 55.5 mM glucose and incubated at 37°C and 10% CO2 (MCO-19M; Panasonic, Osaka, Japan) for 18 h. The optical density at 600 nm (OD600) was adjusted to 0.1 (corresponding to 103 to 104 CFU/ml). A culture aliquot of 100 μl from each species was inoculated onto acquired pellicle-covered slabs with BHI medium supplemented with 1% sucrose to form adherent biofilms (30). To characterize the results of sequential colonization of enamel, the following inoculation sequences were assayed: (i) S. mutans followed by S. mutans (Sm-Sm) (control), (ii) S. sanguinis followed by S. sanguinis (Ss-Ss) (control), (iii) S. mutans followed by S. sanguinis (Sm-Ss), (iv) S. sanguinis followed by S. mutans (Ss-Sm), and (v) both species at the same time (Ss=Sm). Due to differences in biofilm formation, S. sanguinis biofilms were allowed to grow for 16 h before S. mutans was inoculated, whereas S. mutans biofilms were allowed to grow for 8 h before S. sanguinis was inoculated. Subsequently, to mimic salivary basal glucose concentration, biofilms were allowed to mature in BHI medium supplemented with 0.1 mM glucose for 24 h (31).
Sucrose exposure.
For 5 days, slabs/biofilms were exposed 3 times per day to 10% sucrose for 5 min, washed 3 times with 0.9% NaCl, and returned to a plate with BHI supplemented with 0.1 mM glucose. The culture medium was replaced twice per day, before the first and after the last exposure to sucrose (3 daily exposures), from 32 h of biofilm formation up to 128 h (5 days). At 32 h, the mature biofilm was already formed. The caries-negative control was, instead, exposed to 0.9% NaCl for 5 min with the same regime. Two independent experiments in triplicate were carried out (n = 6). The initial phase to promote adhesion and biofilm formation was carried out with BHI plus 1% sucrose, but before and during the cyclic exposures to sucrose, enamel slabs/biofilms were grown only in BHI with 0.1 mM glucose. Enamel slabs/biofilms were never simultaneously exposed to glucose and sucrose.
Biofilm acidogenicity.
To monitor acid production, medium pH was measured with a microelectrode (Orion 910500; Thermo Scientific, Waltham, MA, USA) coupled to a pH meter (Orion Star A211; Thermo Scientific). Individual measurements were made twice per day (in spent media) after each medium change from 32 h of biofilm formation up to 128 h.
Enamel demineralization assessment.
The percentage of surface Knoop microhardness loss (%SHL) was determined (31). Before the experiments, the initial surface microhardness (SHi) of the enamel slabs was determined. After completion of the 5-day experimental period, slabs were mounted on a glass plate, and a second SH measurement was obtained, considered final (SHf) (kg/mm2). Each SH test was performed with three indentations separated by 100 μm each. Mean values for SHi and SHf were used to calculate the %SHL: (SHi average − SHf average) × 100/SHi average.
Biofilm analysis.
After completion of the experiments, slabs were washed and homogenized in 0.9% NaCl for 30 s (Maxi Mix II type 37600 mixer; Thermolyne, IA, USA), which causes biofilm detachment (31). Biofilm suspensions were saved to evaluate biomass, viable microorganisms, insoluble extracellular polysaccharide formation, total protein content, and H2O2 production, all based on previously described methods, so just a brief description follows below.
Biomass.
The dry weight of the samples was used to determine the biomass (30). A volume of 200 μl of the biofilm suspension was transferred to a previously weighed tube (Wi) and incubated with absolute ethanol at −20°C for 15 min. The pellet was dried by liquid evaporation at 37°C for 24 h to obtain the final dry weight (Wf). To obtain the biomass, the following formula was applied: Wi − Wf, normalized to milligrams per milliliter of biofilm suspension.
Protein content of the biofilm.
A 50-μl aliquot of the biofilm suspension was treated with 2 M NaOH and incubated at 100°C for 15 min (31). The supernatant was used to determine the total protein concentration by the Bradford method (Bradford reagent; Merck, Darmstadt, Germany) in a microplate reader at 595 nm. Results were expressed as micrograms per milligram of biomass.
IEPS formation.
To form the IEPS (32), a 200-μl aliquot of the biofilm suspension was centrifuged, and the resulting pellet was treated with 200 μl of 1 M NaOH, homogenized, and centrifuged again. The pellet was treated with three volumes of cold absolute ethanol, and the pellet was washed with 70% cold ethanol and centrifuged again. The pellet was resuspended in 1 M NaOH, and total carbohydrates concentration was obtained by the sulfuric phenol method (33). Results were normalized by dry weight and expressed as the percentage of polysaccharides per milligram of biomass.
Counts of viable cells.
A 100-μl aliquot of the biofilm suspension was serially diluted up to 1×108 (vol/vol) in 0.9% NaCl. A drop of 50 μl of each dilution was seeded on Prussian blue agar (34) for S. sanguinis and Mitis Salivarius agar (Difco; BD, NJ, USA) supplemented with 0.2 units/ml of bacitracin for S. mutans, both in triplicate. After incubation for 48 h, phenotypic observation and counting were carried out for each plate under magnification (×4), and the numbers of colonies, corrected by the dilution factor, were normalized by biomass dry weight and expressed as CFU per milliliter.
H2O2 production.
To assess peroxide production (35), the supernatants from the single-species and dual-species biofilm cultures, at the end of the experiments, were recovered and centrifuged. Resulting pellets were resuspended in 1 ml of BHI and centrifuged, and the supernatant was filtered. The amount of H2O2 was obtained using the Amplex red hydrogen peroxide/peroxidase assay kit (Molecular Probes, Invitrogen, Burlington, ON, Canada).
Statistical analysis.
Data were analyzed using the statistical software SPSS v15.0 for Windows (SPSS Inc., Chicago, IL, USA). The variables acidogenicity, demineralization, biomass, total proteins, insoluble extracellular polysaccharides, viable microorganisms, and H2O2 production were analyzed using a multiple comparison by analysis of variance (ANOVA) with a Tukey post hoc test. Differences were considered significant if the P value was lower than 0.05.
ACKNOWLEDGMENTS
We have no conflicts of interest to declare.
R.A.G. and N.D.-G. conceived the idea and designed the experiments. N.D.-G. performed all the experiments. N.D.-G. and C.P.L. processed and analyzed the data and drafted the first manuscript. R.A.G. and C.P.L. wrote the final manuscript. J.K. critically revised and contributed new ideas to the paper. All authors revised and approved the final version of the article.
REFERENCES
- 1.Sanz M, Beighton D, Curtis MA, Cury JA, Dige I, Dommisch H, Ellwood R, Giacaman RA, Herrera D, Herzberg MC, Kononen E, Marsh PD, Meyle J, Mira A, Molina A, Mombelli A, Quirynen M, Reynolds EC, Shapira L, Zaura E. 2017. Role of microbial biofilms in the maintenance of oral health and in the development of dental caries and periodontal diseases. Consensus report of group 1 of the Joint EFP/ORCA workshop on the boundaries between caries and periodontal disease. J Clin Periodontol 44(Suppl 18):S5–S11. doi: 10.1111/jcpe.12682. [DOI] [PubMed] [Google Scholar]
- 2.Kolenbrander PE, Andersen RN, Blehert DS, Egland PG, Foster JS, Palmer RJ Jr. 2002. Communication among oral bacteria. Microbiol Mol Biol Rev 66:486–505. doi: 10.1128/mmbr.66.3.486-505.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ge Y, Caufield PW, Fisch GS, Li Y. 2008. Streptococcus mutans and Streptococcus sanguinis colonization correlated with caries experience in children. Caries Res 42:444–448. doi: 10.1159/000159608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Giacaman RA, Torres S, Gómez Y, Muñoz-Sandoval C, Kreth J. 2015. Correlation of Streptococcus mutans and Streptococcus sanguinis colonization and ex vivo hydrogen peroxide production in carious lesion-free and high caries adults. Arch Oral Biol 60:154–159. doi: 10.1016/j.archoralbio.2014.09.007. [DOI] [PubMed] [Google Scholar]
- 5.Loesche WJ. 1986. Role of Streptococcus mutans in human dental decay. Microbiol Rev 50:353–380. doi: 10.1128/MMBR.50.4.353-380.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Caufield PW, Dasanayake AP, Li Y, Pan Y, Hsu J, Hardin JM. 2000. Natural history of Streptococcus sanguinis in the oral cavity of infants: evidence for a discrete window of infectivity. Infect Immun 68:4018–4023. doi: 10.1128/iai.68.7.4018-4023.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Takahashi N, Horiuchi M, Yamada T. 1997. Effects of acidification on growth and glycolysis of Streptococcus sanguis and Streptococcus mutans. Oral Microbiol Immunol 12:72–76. doi: 10.1111/j.1399-302x.1997.tb00620.x. [DOI] [PubMed] [Google Scholar]
- 8.Takahashi N, Nyvad B. 2011. The role of bacteria in the caries process: ecological perspectives. J Dent Res 90:294–303. doi: 10.1177/0022034510379602. [DOI] [PubMed] [Google Scholar]
- 9.Kreth J, Merritt J, Shi W, Qi F. 2005. Competition and coexistence between Streptococcus mutans and Streptococcus sanguinis in the dental biofilm. J Bacteriol 187:7193–7203. doi: 10.1128/JB.187.21.7193-7203.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kreth J, Zhang Y, Herzberg MC. 2008. Streptococcal antagonism in oral biofilms: Streptococcus sanguinis and Streptococcus gordonii interference with Streptococcus mutans. J Bacteriol 190:4632–4640. doi: 10.1128/JB.00276-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Merritt J, Qi F. 2012. The mutacins of Streptococcus mutans: regulation and ecology. Mol Oral Microbiol 27:57–69. doi: 10.1111/j.2041-1014.2011.00634.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carlsson J, Edlund MB, Lundmark SK. 1987. Characteristics of a hydrogen peroxide-forming pyruvate oxidase from Streptococcus sanguis. Oral Microbiol Immunol 2:15–20. doi: 10.1111/j.1399-302x.1987.tb00264.x. [DOI] [PubMed] [Google Scholar]
- 13.Kreth J, Giacaman RA, Raghavan R, Merritt J. 2017. The road less traveled - defining molecular commensalism with Streptococcus sanguinis. Mol Oral Microbiol 32:181–196. doi: 10.1111/omi.12170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Valdebenito B, Tullume-Vergara PO, Gonzalez W, Kreth J, Giacaman RA. 2018. In silico analysis of the competition between Streptococcus sanguinis and Streptococcus mutans in the dental biofilm. Mol Oral Microbiol 33:168–180. doi: 10.1111/omi.12209. [DOI] [PubMed] [Google Scholar]
- 15.Bowen WH, Koo H. 2011. Biology of Streptococcus mutans-derived glucosyltransferases: role in extracellular matrix formation of cariogenic biofilms. Caries Res 45:69–86. doi: 10.1159/000324598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Burne RA, Marquis RE. 2000. Alkali production by oral bacteria and protection against dental caries. FEMS Microbiol Lett 193:1–6. doi: 10.1111/j.1574-6968.2000.tb09393.x. [DOI] [PubMed] [Google Scholar]
- 17.Nascimento MM, Browngardt C, Xiaohui X, Klepac-Ceraj V, Paster BJ, Burne RA. 2014. The effect of arginine on oral biofilm communities. Mol Oral Microbiol 29:45–54. doi: 10.1111/omi.12044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang X, Schulte RM, Burne RA, Nascimento MM. 2015. Characterization of the arginolytic microflora provides insights into pH homeostasis in human oral biofilms. Caries Res 49:165–176. doi: 10.1159/000365296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hamada S, Torii M, Kotani S, Tsuchitani Y. 1981. Adherence of Streptococcus sanguis clinical isolates to smooth surfaces and interactions of the isolates with Streptococcus mutans glucosyltransferase. Infect Immun 32:364–372. doi: 10.1128/IAI.32.1.364-372.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wen ZT, Yates D, Ahn SJ, Burne RA. 2010. Biofilm formation and virulence expression by Streptococcus mutans are altered when grown in dual-species model. BMC Microbiol 10:111. doi: 10.1186/1471-2180-10-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kreth J, Merritt J, Qi F. 2009. Bacterial and host interactions of oral streptococci. DNA Cell Biol 28:397–403. doi: 10.1089/dna.2009.0868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lozano CP, Diaz-Garrido N, Kreth J, Giacaman RA. 2019. Streptococcus mutans and Streptococcus sanguinis expression of competition-related genes, under sucrose. Caries Res 53:194–203. doi: 10.1159/000490950. [DOI] [PubMed] [Google Scholar]
- 23.Kreth J, Zhu L, Merritt J, Shi W, Qi F. 2008. Role of sucrose in the fitness of Streptococcus mutans. Oral Microbiol Immunol 23:213–219. doi: 10.1111/j.1399-302X.2007.00413.x. [DOI] [PubMed] [Google Scholar]
- 24.Redanz S, Cheng X, Giacaman RA, Pfeifer CS, Merritt J, Kreth J. 2018. Live and let die: hydrogen peroxide production by the commensal flora and its role in maintaining a symbiotic microbiome. Mol Oral Microbiol 33:337–352. doi: 10.1111/omi.12231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhu L, Kreth J. 2012. The role of hydrogen peroxide in environmental adaptation of oral microbial communities. Oxid Med Cell Longev 2012:717843. doi: 10.1155/2012/717843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zheng L, Itzek A, Chen Z, Kreth J. 2011. Environmental influences on competitive hydrogen peroxide production in Streptococcus gordonii. Appl Environ Microbiol 77:4318–4328. doi: 10.1128/AEM.00309-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zheng L, Chen Z, Itzek A, Ashby M, Kreth J. 2011. Catabolite control protein A controls hydrogen peroxide production and cell death in Streptococcus sanguinis. J Bacteriol 193:516–526. doi: 10.1128/JB.01131-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cheng X, Redanz S, Cullin N, Zhou X, Xu X, Joshi V, Koley D, Merritt J, Kreth J. 2017. Plasticity of the pyruvate node modulates hydrogen peroxide production and acid tolerance in multiple oral streptococci. Appl Environ Microbiol 84:e01697-17. doi: 10.1128/AEM.01697-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Diaz-Garrido N, Lozano C, Giacaman RA. 2016. Frequency of sucrose exposure on the cariogenicity of a biofilm-caries model. Eur J Dent 10:345–350. doi: 10.4103/1305-7456.184163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Koo H, Hayacibara MF, Schobel BD, Cury JA, Rosalen PL, Park YK, Vacca-Smith AM, Bowen WH. 2003. Inhibition of Streptococcus mutans biofilm accumulation and polysaccharide production by apigenin and tt-farnesol. J Antimicrob Chemother 52:782–789. doi: 10.1093/jac/dkg449. [DOI] [PubMed] [Google Scholar]
- 31.Ccahuana-Vasquez RA, Cury JA. 2010. S. mutans biofilm model to evaluate antimicrobial substances and enamel demineralization. Braz Oral Res 24:135–141. doi: 10.1590/S1806-83242010000200002. [DOI] [PubMed] [Google Scholar]
- 32.Duarte S, Klein MI, Aires CP, Cury JA, Bowen WH, Koo H. 2008. Influences of starch and sucrose on Streptococcus mutans biofilms. Oral Microbiol Immunol 23:206–212. doi: 10.1111/j.1399-302X.2007.00412.x. [DOI] [PubMed] [Google Scholar]
- 33.Dubois M, Gilles K, Hamilton J, Rebers P, Smith F. 1951. A colorimetric method for the determination of sugars. Nature 168:167. doi: 10.1038/168167a0. [DOI] [PubMed] [Google Scholar]
- 34.Saito M, Seki M, Iida K, Nakayama H, Yoshida S. 2007. A novel agar medium to detect hydrogen peroxide-producing bacteria based on the Prussian blue-forming reaction. Microbiol Immunol 51:889–892. doi: 10.1111/j.1348-0421.2007.tb03971.x. [DOI] [PubMed] [Google Scholar]
- 35.Herrero ER, Slomka V, Bernaerts K, Boon N, Hernandez-Sanabria E, Passoni BB, Quirynen M, Teughels W. 2016. Antimicrobial effects of commensal oral species are regulated by environmental factors. J Dent 47:23–33. doi: 10.1016/j.jdent.2016.02.007. [DOI] [PubMed] [Google Scholar]