Abstract
This study investigated the effects of terminal sterilization of polyvinyl alcohol (PVA) biomaterials using clinically translatable techniques, specifically ethylene oxide (EtO) and gamma (γ) irradiation. While a few studies have reported the possibility of sterilizing PVA with γ-radiation, the use of EtO sterilization of PVA requires additional study. PVA solutions were chemically crosslinked with trisodium trimetaphosphate and sodium hydroxide. The three experimental groups included untreated control, EtO, and γ-irradiation, which were tested for the degree of swelling and water content, and mechanical properties such as radial compliance, longitudinal tensile, minimum bend radius, burst pressure, and suture retention strength. In addition, samples were characterized with scanning electron microscopy, differential scanning calorimetry, X-ray photoelectron spectroscopy, and water contact angle measurements. Cell attachment was assessed using the endothelial cell line EA.hy926, and the sterilized PVA cytotoxicity was studied with a live/dead stain. Platelet and fibrin accumulation was measured using an ex vivo shunt baboon model. Finally, the immune responses of PVA implants were analyzed after a 21-day subcutaneous implantation in rats and a 30-day implantation in baboon. EtO sterilization reduced the PVA graft wall thickness, its degree of swelling, and water content compared with both γ-irradiated and untreated PVA. Moreover, EtO sterilization significantly reduced the radial compliance and increased Young's modulus. EtO did not change PVA hydrophilicity, while γ-irradiation increased the water contact angle of the PVA. Consequently, endothelial cell attachment on the EtO-sterilized PVA showed similar results to the untreated PVA, while cell attachment significantly improved on the γ-irradiated PVA. When exposing the PVA grafts to circulating whole blood, fibrin accumulation of EtO-sterilized PVA was found to be significantly lower than γ-irradiated PVA. The immune responses of γ-irradiated PVA, EtO-treated PVA, and untreated PVA were compared. Implanted EtO-treated PVA showed the least MAC387 reaction. The terminal sterilization methods in this study changed PVA hydrogel properties; nevertheless, based on the characterizations performed, both sterilization methods were suitable for sterilizing PVA. We concluded that EtO can be used as an alternative method to sterilize PVA hydrogel material.
Impact statement
Polyvinyl alcohol (PVA) hydrogels have been used for a variety of tissue replacements, including neural, cardiac, meniscal, cartilage, muscle, pancreatic, and ocular applications. In addition, PVA can be made into a tubular shape and used as a small-diameter vascular graft. Ethylene oxide (EtO) is one of the Food and Drug Administration-approved methods for sterilization, but its effect on PVA has not been studied extensively. The outcome of this study provides the effects of EtO and γ-irradiation of PVA grafts on both the material properties and the in vivo responses, particularly for vascular applications. Knowledge of these effects may ultimately improve the success rate of PVA vascular grafts.
Keywords: small-diameter vascular grafts, terminal sterilization, irradiation, hemocompatibility, cell adhesion
Introduction
Hydrogels are a class of polymeric material with water-absorbing capability and typically low elastic moduli.1 Hydrogels are usually biocompatible with tissue-mimicking properties. It is also relatively easy to incorporate ligands that promote cell adhesion into these materials.2–4 Hydrogel materials are often used in the field of tissue engineering. While many are still in the research stage and aiming to enter preclinical and clinical studies in the future,5–10 some have been commercialized in the form of contact lenses,11,12 wound healing gel,13 and nerve conduits.14 Of particular interest are polyvinyl alcohol (PVA) hydrogels, which have been widely studied due to their nontoxicity, nonimmunogenicity, noncarcinogenicity, and high biocompatibility.4,8,15–22 PVA hydrogels have been used extensively for soft tissue engineering, including vascular,8 neural,14 cardiac,23 meniscal,18 cartilage,24 muscle,25 pancreatic,26 and ocular applications.27
PVA can also be made into small-diameter tubes (<6 mm inner diameter) to be used as small-diameter vascular grafts. By varying the crosslinking method or agent, the mechanical properties of PVA hydrogel can be tuned to mimic the properties of native blood vessels. As a comparison, the compliance of human brachial artery ranges between 1.3 and 3.3%/40 mmHg,28 while the compliance of PVA has been engineered to compliances between 1.5 and 9%/40 mmHg.8,21,22 With these properties, implanted small-diameter PVA grafts in small-animal models have achieved 67% graft patency after 14 to 17 days of implantation.8
One challenge in the translation of hydrogels to clinical use is the determination of the appropriate sterilization method because the sterilization process can impact the hydrogel properties. A few studies have reported the effect of γ-radiation on PVA hydrogel properties,29–33 while other studies reported an autoclave sterilization method or the combination of autoclave and radiation for PVA sterilization.29,34,35 γ and electron beam radiation methods offer a promising hydrogel sterilizing capability because the sterilization occurs at a relatively low temperature and is shorter in duration. Also, both of these radiation methods are known to have high penetration, an ability to break down DNA and RNA, and to create reactive oxygen species that can damage cellular components.36–38
However, a known drawback of the radiation method is that the recommended dose of 25 kGy radiation can cause scission of polymer chains, which may lead to the reduction in polymer elastic modulus, and can cause crosslinking reactions, which would have the opposite effect of increasing tensile strength and elastic modulus.39–41 Steam and dry heat are high-temperature-based sterilization methods with good penetration into the material. In particular, steam heat or autoclaving destroys replication components of a microorganism, while dry heat eliminates microorganisms through direct heat and oxidation reactions.36 However, both of these sterilization methods can cause structural changes and degradation of hydrogels, which have structures similar to PVA.42,43 This may be the reason why the autoclave step in the previous studies was done before PVA crosslinking and thus not terminal sterilization methods.29,34,35
The U.S. Food and Drug Administration (FDA) has approved three terminal sterilization methods for biomedical devices, which include heat-based sterilization (steam/dry), ethylene oxide (EtO), and radiation (γ-radiation or electron beam/E-beam). EtO sterilization has not been studied in the PVA hydrogel. EtO sterilization effectively suppresses metabolism and division of microorganisms or biological agents through irreversible alkylation of cellular components.36,44 However, a few studies have shown that EtO can also accelerate polymer degradation and create toxic residuals on the implant surface.45–47 In addition, EtO was found to increase the crystallinity of poly(lactic acid) (PLA) fiber scaffolds and reduce the overall scaffold size.40
To understand how EtO sterilization affects the material properties and performance of PVA as a biomaterial, in particular with the application of PVA as vascular graft, we characterized the effects of EtO sterilization and compared it with the γ-irradiation sterilization on PVA hydrogel materials. We quantified the mechanical and surface properties, endothelialization potential (with and without surface topography), the thrombosis reactivity, and the in vivo biocompatibility. These properties are critical in small-diameter vascular grafts (<6 mm) since a small thrombus, plaque, or neointimal hyperplasia could dramatically impede blood flow and cause graft failure.
Surface topographies are known to improve cell adhesion,48 proliferation,49,50 endocytosis,51 and differentiation.49,52 Among the different types of surface topography, 2 × 2 × 2 μm grating topography is known to be the most suitable for vascular graft application and therefore studied herein. The 2 μm grating topography provides endothelial cells an orientation for alignment and it improves cell adhesion through alteration of surface apparent wettability.22,53
Considering all these, we hypothesize that EtO sterilization could alter PVA crystallinity, as was observed in PLA fiber scaffolds.40 We also speculate that while EtO could harden the hydrogel, γ-radiation could cause polymer chain scission and weaken the hydrogel. Furthermore, we hypothesize that although the sterilization could cause changes in PVA properties, the EtO-sterilized PVA hydrogel will retain suitable properties for use as a small-diameter vascular graft.
Materials and Methods
A 10% (w/v) solution of PVA was crosslinked with trisodium trimetaphosphate (STMP) and sodium hydroxide, and was used for dip coating of cylindrical molds to make vascular grafts.22 The grafts were divided into three experimental groups (untreated control, EtO, and γ) and tested for the degree of swelling and water content, and mechanical properties such as radial compliance, longitudinal tensile, minimum bend radius (MBR), burst pressure, and suture retention strength. In addition, samples were characterized with scanning electron microscopy, differential scanning calorimetry (DSC), X-ray photoelectron spectroscopy (XPS), water contact angle, X-ray diffractometry (XRD), and Fourier-transform infrared (FTIR) spectroscopy measurements. Cell attachment was assessed using the endothelial cell line EA.hy926, and the sterilized PVA cytotoxicity was studied with a live/dead stain. Platelet and fibrin accumulation was measured using an ex vivo shunt baboon model. Finally, the immune responses of PVA implants were analyzed after a 21-day subcutaneous implantation in rat and a 30-day implantation in baboon with a hematoxylin and eosin (H&E) and MAC387 antibody staining. The details of materials and methods can be found in the Supplementary Data under the Detailed Materials and Methods section.
Results
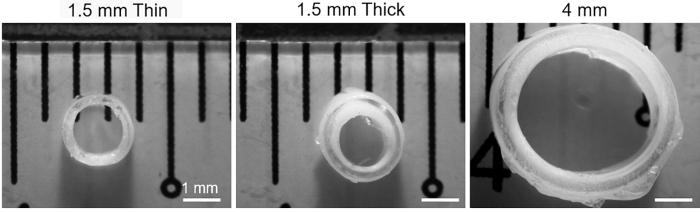
Using the 1.5 mm molds, two types of grafts were made; 1.5 mm thin had 7 layers of PVA and an outer diameter of 2.1–2.2 mm and 1.5 mm thick had 12 layers of PVA and an outer diameter of 2.5–2.7 mm. One type of graft was cast using the 4 mm molds; the graft (4 mm) had 12 layers of PVA applied and an outer diameter of 5.1–5.3 mm (Fig. 1).
FIG. 1.
Cross-sectional views of the various types of PVA grafts used in this study. Scale bar indicates 1 mm. PVA, polyvinyl alcohol.
Effect of sterilization method on structural integrity, swelling, and mechanical properties
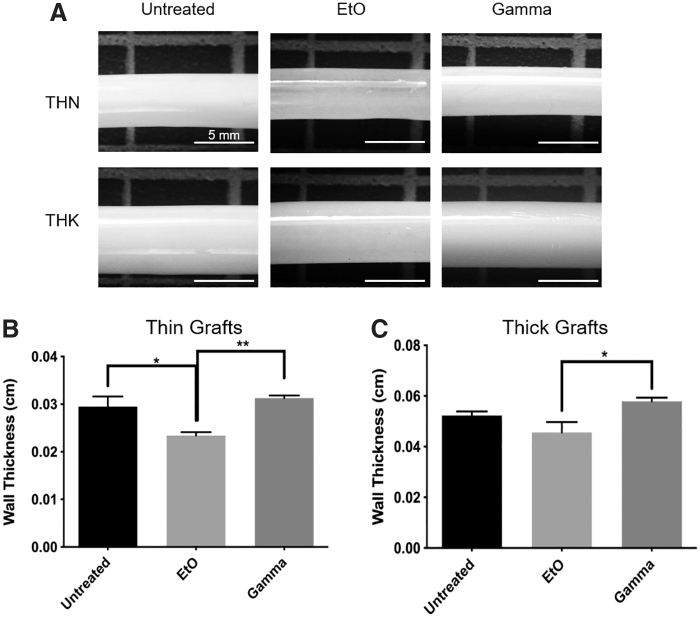
The opacity of the grafts was assessed as it is important for the ease of graft installation and handling. The opacity of grafts treated with γ-irradiation was unchanged relative to the controls; however, the EtO-treated grafts were less opaque than the control. Both 1.5-mm-thin and 1.5-mm-thick grafts treated with EtO were more translucent than their control counterparts and their wall thicknesses were found to be reduced (Fig. 2, p ≤ 0.05 for 1.5 mm thin, n = 4). In all the treatment groups, the grafts retained their structural integrity, in which none of the samples collapsed in on themselves when removed from solution.
FIG. 2.
(A) Physical appearance of 1.5-mm grafts before and after treatment; EtO-treated samples became more translucent after treatment for both thin and thick grafts. Scale bar indicates 5 mm. Quantification of change in (B) thin and (C) thick graft wall thickness due to sterilization. The wall thickness of EtO group was significantly lower than both untreated and γ groups for thin graft, as well as γ group for thick graft. *Represents statistical significance with p ≤ 0.05, **p ≤ 0.01, n = 4. EtO, ethylene oxide.
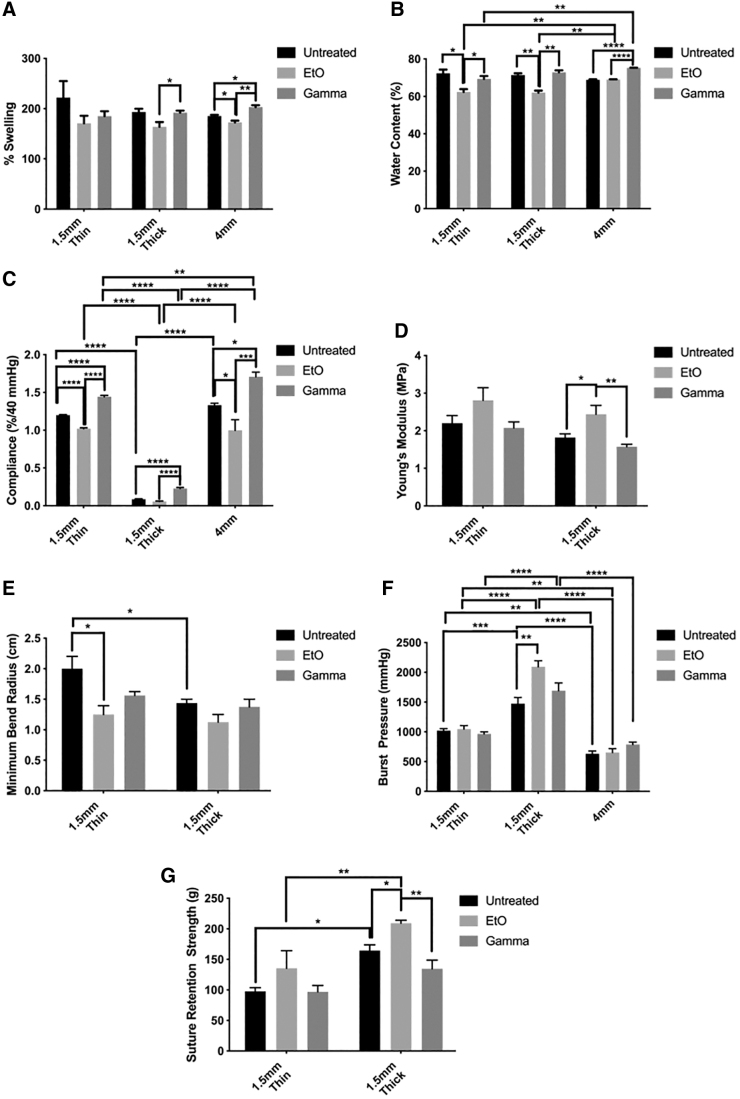
The reduction in graft wall thickness prompted us to investigate the swelling and water content of the PVA grafts before and after sterilization. PVA 1.5-mm-thin, 1.5-mm-thick, and 4-mm groups showed a similar trend. In all graft types, the average swelling percentage of EtO-treated PVA grafts was reduced compared with the untreated controls (Fig. 3A). This reduction was found to be significant for 1.5-mm-thick and 4-mm groups (p ≤ 0.05, n = 3). The swelling percentage of γ-irradiated PVA grafts was either similar to or higher than the untreated control. A significant increase in swelling percentage was observed in the 4-mm group (p ≤ 0.05, n = 3). The water contents of 1.5-mm-thin and 1.5-mm-thick grafts were shown to be significantly reduced after EtO treatment (p ≤ 0.05 and p ≤ 0.01, respectively), while the 4-mm group showed similar water content after EtO treatment compared with the untreated control (Fig. 3B). A significant increase in water content was observed in the γ-irradiated 4-mm group (p ≤ 0.0001, n = 3) compared with the untreated group.
FIG. 3.
The effect of EtO and γ irradiation on the (A) swelling percentage, (B) water content, (C) radial compliance, (D) Young's modulus (longitudinal direction), (E) minimum bend radius, (F) burst pressure, and (G) suture retention strength of PVA 1.5-mm-thin, thick, and 4-mm grafts before and after sterilization. EtO-treated grafts showed lower swelling percentage and water content, and more rigid mechanical properties compared with untreated control and γ groups. The data are presented as the average of n = 3 samples ± SE for swelling percentage and water content, and as the average of n = 4 samples ± SE for all the other data sets. *Represents statistical significance with p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001, and ****p ≤ 0.0001.
The mechanical properties, including compliance, MBR, burst pressure, Young's modulus, and suture retention strength, have a significant impact on the overall performance of the vascular graft and therefore were tested. Graft radial compliance was significantly affected by both EtO treatment and γ-irradiation (Fig. 3C). EtO treatment decreased compliance, whereas γ-irradiation increased compliance, compared with the control. The compliance of the 1.5-mm-thin grafts decreased 14% after EtO treatment and increased 24% after γ-irradiation. The compliance of the 4-mm grafts decreased 25% after EtO treatment and increased 71% after γ-irradiation. Only γ-irradiation had a significant effect on the compliance of 1.5-mm-thick grafts, increasing it by 163%, which is the largest change in compliance of all the graft types and treatment groups.
The results of compliance testing indicated that EtO treatments made grafts more rigid and γ-irradiation treatments made grafts more compliant. The difference in compliance between EtO- and γ-treated groups was also statistically significant (p ≤ 0.0001 for both thin and thick samples, and p ≤ 0.001 for 4-mm samples). This was confirmed by tensile testing. Young's modulus of samples along the graft in the longitudinal direction showed the same trend, in which EtO treatment increased Young's modulus indicating a more rigid graft and γ-irradiation decreased Young's modulus indicating a more compliant graft, compared with the control group (Fig. 3D). The only significant change in Young's modulus occurred in EtO-treated 1.5-mm-thick grafts, in which the Young's modulus increased 34% compared with the control.
The MBR of EtO-treated samples was decreased compared with the control (Fig. 3E). A decreased MBR indicates that the material had become stiffer and thus easier to bend without kinking; an increased MBR indicates the opposite. The only significant change in MBR occurred in EtO-treated 1.5-mm-thin samples, in which the MBR decreased 38% compared with the control.
The samples treated with EtO tended to have a higher burst pressure compared with the control group (Fig. 3F). The only significant change was a 42% increase in the burst pressure of EtO-treated 1.5-mm-thick grafts. The burst pressures of γ-irradiated and control samples were similar with no statistically significant differences in these tested graft types.
Suture retention strength increased after EtO treatment and remain unchanged after γ-irradiation (Fig. 3G). In 1.5-mm-thick grafts, suture retention strength of EtO-treated samples was 21% greater than that of the control. There was no significant change in suture retention strength of 1.5-mm-thin EtO-treated samples and no significant difference in the suture retention strength of untreated and γ-irradiated samples in both graft types tested. In summary, the mechanical tests showed that EtO-treated grafts exhibit stiffer, higher burst pressures, and higher suture retention properties than untreated PVA grafts, while γ-irradiated grafts were more compliant and the other material properties were unchanged compared with the control.
Effect of sterilization method on polyvinyl alcohol surface properties and crystallinity
Experimental results of surface and crystallinity testing indicated that both treatments altered the chemical properties of the PVA hydrogel. FTIR was performed to determine if a noticeable change in crosslinking occurred or if any new functional groups were introduced after treatment. Neither new peak formation nor peak loss was observed after either treatment, indicating no addition or loss of functional groups (Supplementary Fig. S1).
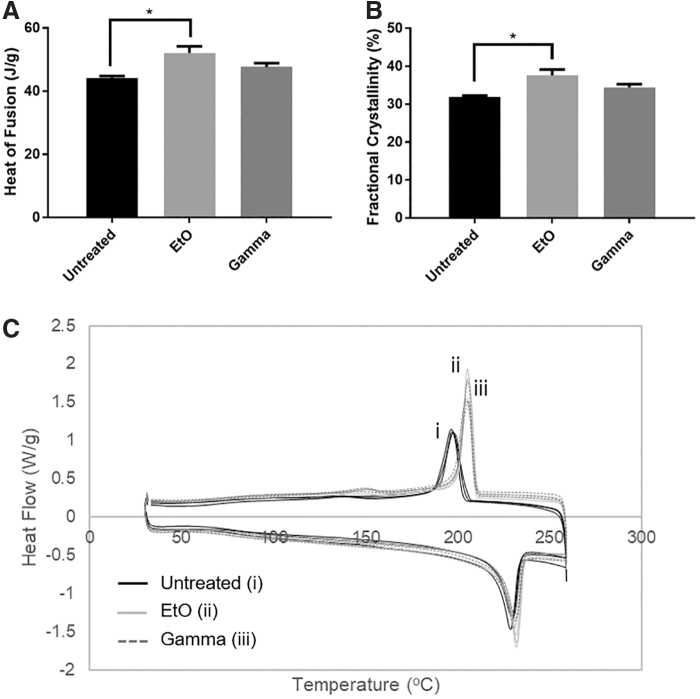
Changes to the fractional crystallinity due to EtO treatment were observed in DSC (Fig. 4). EtO treatment significantly increased the fractional crystallinity of 1.5-mm-thin grafts. The fractional crystallinity of EtO-treated grafts was 18% greater than that of the untreated samples, while γ-irradiation did not cause significant change to fractional crystallinity. To confirm the change in crystallinity, XRD of untreated and treated PVA films was measured. However, the crystalline peaks across the three experimental groups remained the same, suggesting that samples' molecular architectures remained the same irrespective of the sterilization method (Supplementary Fig. S2).
FIG. 4.
Change in (A) heat of fusion and (B) fractional crystallinity as determined by differential scanning calorimetry, (C) heating/cooling curves of 1.5-mm-thin-walled PVA grafts post-treatment. All data are presented as the average of n = 3 samples ± SE (*p ≤ 0.05).
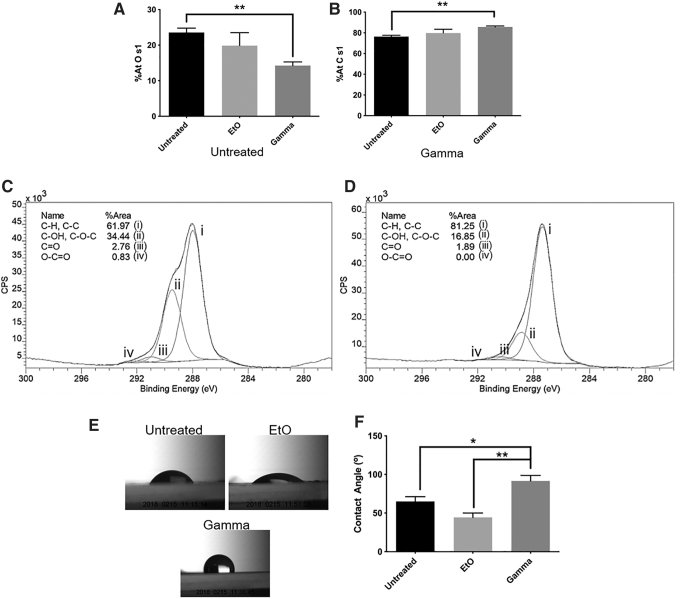
While neither treatment resulted in the change of surface patterning and texture, it was noted that γ-irradiation changed surface composition (Fig. 5A–D). XPS showed that the %-atomic concentration of oxygen (O s1) on the γ-irradiated samples was significantly lower than that of the control (p ≤ 0.01). Furthermore, the %-atomic concentration of carbon (C s1) on the γ-irradiated samples was significantly higher than that of the control (p ≤ 0.01). The hydroxyl peak area on the PVA surface was reduced and the C-H and C-C peak area was increased after γ-treatment (Fig. 5C, D), while there was no significant difference between the surface compositions of EtO-treated films and control films (Fig. 5A, B).
FIG. 5.
Surface atomic concentrations (%At) of (A) oxygen (O s1) and (B) carbon (C s1) on PVA films. (C) C s1 spectra of untreated films and (D) γ-irradiated films showed reduced surface –OH group after γ treatment. (E) Representative images of the water contact angle on PVA surfaces, and (F) average contact angle shown as a bar chart. The data are presented as the average of n = 3 samples ± SE for surface atomic concentration measurements (A, B) and n = 4 samples ± SE for water contact angle measurements (F) (*p ≤ 0.05, **p ≤ 0.01).
Contact angle testing was also performed to determine if this change in surface composition caused significant changes in surface hydrophilicity. The γ-irradiation significantly increased the water contact angle on PVA, indicating that the surface had become more hydrophobic after treatment (Fig. 5E, F). Meanwhile, EtO-treated films did not show significant change in water contact angle.
Enhancement of in vitro cell adhesion and proliferation to PVA films after γ-irradiation
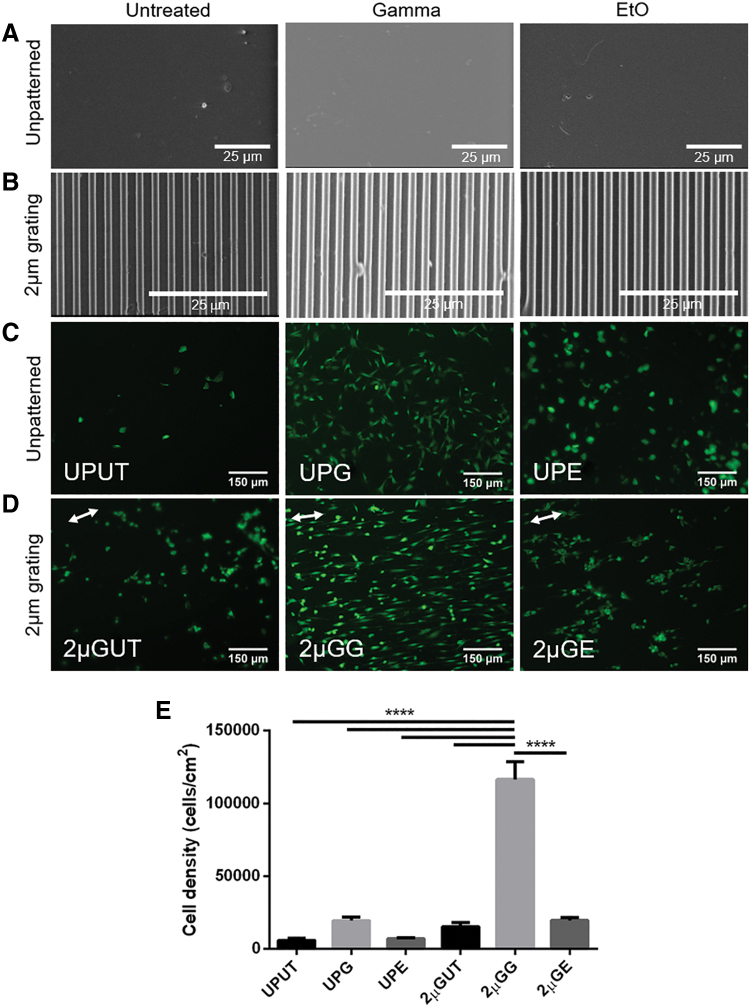
The effect of sterilization on microtopography patterning was assessed to determine if either treatment affected surface texture or patterning. We observed that the surface roughness of the unpatterned PVA was visibly similar between the treatment groups (Fig. 6A). Moreover, the fidelity of 2 μm gratings (2 μm linewidth, spacing, and height) was not affected by any of the treatments (Fig. 6B).
FIG. 6.
The effect of sterilization on cell adhesion and proliferation to unpatterned and patterned PVA films. SEM was performed on (A) unpatterned and (B) 2 μm grating PVA films before and after sterilization treatment. There was no noticeable difference in the texture of unpatterned surfaces of treated and untreated hydrogels. Gratings were also not affected by either γ or EtO treatment. Cells were seeded on these substrates and stained with live/dead assay after being cultured for 13 days; live cells were stained in green and dead cells were stained in red (no dead cells were found). (C) Unpatterned film: untreated control (UPUT), γ irradiated (UPG), and EtO treated (UPE). (D) Two micrometers grating film: untreated control (2 μGUT), γ irradiated (2 μGG), and EtO treated (2 μGE). White arrows indicate the parallel orientation of the microgratings. Scale bars represent 25 μm for SEM images and 150 μm for fluorescence images. (E) The effect of sterilization on the proliferation of cells as measured with CyQUANT assay. Quantification was performed after 13 days of culture. All data are presented as the average of n = 5 samples ± SE except for UPG and 2 μGUT where n = 4. ****Represents statistical significance with p ≤ 0.0001. SEM, scanning electron microscopy. Color images are available online.
The effect of γ-irradiation and EtO treatment on the endothelialization of unpatterned and 2 μm grating PVA films was studied via in vitro cell adhesion and toxicity studies using EA.hy926 cells (Fig. 6C–E and Supplementary Fig. S3). Of the samples tested, the largest cell count was observed on the γ-irradiated 2 μm grating (2 μGG) PVA films. After 13 days, cells on these films reached 80–90% confluency and clearly showed alignment in the parallel direction of the gratings (Fig. 6D 2 μGG), while cells on γ-treated unpatterned (UPG) PVA showed a more cobblestone shape and were randomly oriented (Fig. 6C UPG). The EA.hy926 cells on untreated and EtO-treated PVA did not proliferate as fast and had a rounder morphology compared with the cells on γ-irradiated PVA. Moreover, those seeded on untreated and EtO-treated 2 μm grating PVA films did not show a clear alignment as observed in the γ-irradiated 2 μm grating PVA films. While the γ-irradiated unpatterned PVA film still showed qualitative improvement in cell adhesion (Fig. 6C, UPG), the cell proliferation of untreated and EtO groups (Fig. 6C–E) did not improve. In agreement with the observed cell adhesion on the γ-irradiated 2 μm grating PVA films, the cells on these films showed the highest cell attachment, compared with other experimental groups (p ≤ 0.0001). These results demonstrate that γ-irradiation is nontoxic and enhances the endothelialization of the PVA. No cell death was observed after 13 days of culture in any group.
Ex vivo analysis on hemocompatibility
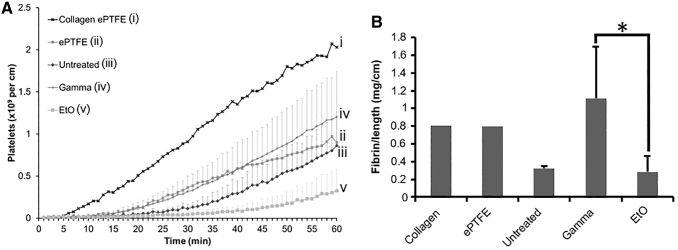
Platelet and fibrin activation data were obtained through an ex vivo shunt study using a nonhuman primate model. The platelet accumulation data (Fig. 7A) showed a sterilization treatment effect with p = 0.0001; however, Bonferroni-corrected post hoc tests showed no specific differences between various PVA samples (p = 1). Significant differences were seen for the ANOVA test (F = 4.967, p = 0.022) of fibrin accumulation between γ- and EtO-treated samples (Fig. 7B). Specifically, fibrin accumulation levels were significantly greater on γ-irradiated samples than EtO samples (Tukey's post hoc, p = 0.018). Untreated PVA was not significantly different from either γ- (Tukey's post hoc, p = 0.260) or EtO PVA (Tukey's post hoc, p = 0.559). Both untreated and EtO grafts showed low platelet and fibrin activation, indicating good hemocompatibility.
FIG. 7.
(A) Platelet accumulation and (B) fibrin accumulation data are displayed as average ± SD for PVA samples. Collagen and ePTFE controls are illustrated as means only and were not included in the statistical comparison due to low sample size. No significant differences were observed between the various PVA samples for the platelet data; however, PVA samples had significantly more fibrin on γ-treated PVA than EtO-treated PVA. (*represents statistical significance with p = 0.022, F = 4.967, n = 4–8). ePTFE, expanded polytetrafluoroethylene.
In vivo analysis on biocompatibility
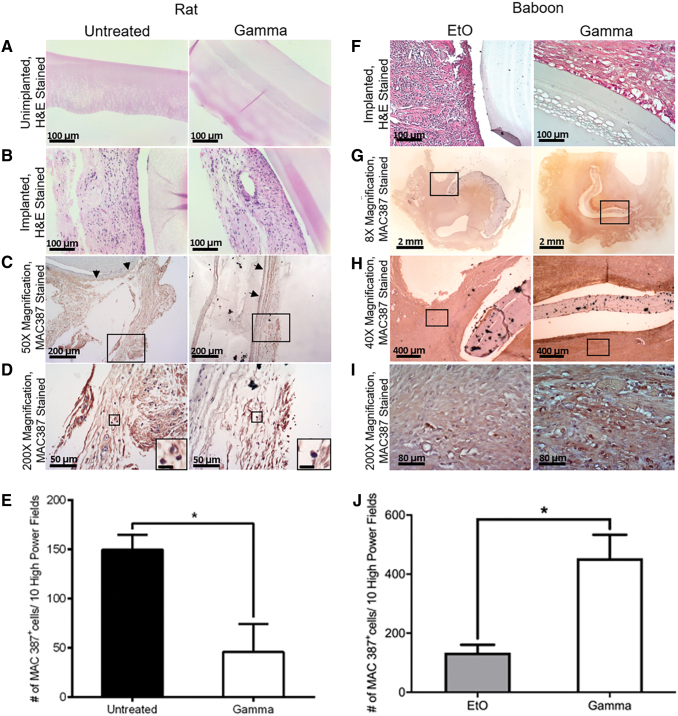
In vivo studies were performed to examine any change that the sterilization could make to PVA biocompatibility and immune response in the wound healing process (Supplementary Fig. S4). The subcutaneous implant in the rat model was excised and stained with H&E and immunostained with MAC 387 antibody (Fig. 8). The appearance of the graft/tissue interface between the untreated and γ-group remained similar after 3 weeks of implantation where there was no tissue infiltration observed. Meanwhile, from the immunostaining, it was found that only 50% of γ-irradiated PVA grafts were reactive for rat tissue growth around the grafts, compared with 100% of untreated PVA grafts. There were significantly less MAC 387+ cells in the surrounding tissues of the subcutaneously implanted γ-irradiated PVA grafts compared with the untreated/nonradiated PVA grafts in the rat studies. When comparing the macrophage activation on the baboon tissue surrounding γ- and EtO-treated grafts after 4 weeks of implantation, the implanted EtO-treated PVA showed significantly less MAC 387+ cells compared with γ-irradiated PVA.
FIG. 8.
Histology of untreated versus γ-irradiated PVA films and EtO- versus γ-treated PVA grafts after being implanted subcutaneously on the dorsal side of a rat for 21 days and at the abdominal aorta iliac bifurcation of baboons for 28 days, respectively. H&E staining of (A) unimplanted film, (B) implanted film in rat, and (F) implanted graft in baboon. H&E and immunostaining with MAC387 antibody of (C) rat tissue surrounding the film implant, 50 × magnification, with area chosen for higher magnification bounded with rectangle and arrows pointing to the graft/tissue interface, (D) 200 × magnification of (C), with inserts showing the zoomed in images of the stained macrophages. Scale bar of inserts: 10 μm. (E) The total number of rat MAC 387+ macrophages per 10 high-power fields (200 × ) in the surrounding tissues of untreated and γ-irradiated grafts. H&E and immunostaining with MAC387 antibody of baboon tissue surrounding the graft implant with (G) 8 × , (H) 40 × , and (I) 200 × magnification. Area chosen for higher magnification was bounded with rectangles. (J) The total number of baboon MAC 387+ macrophages per 10 high-power fields (200 × ) in the surrounding tissues of EtO- and γ-irradiated grafts. Statistical significance was calculated with unpaired t-test (*p < 0.05; p = 0.0346). Brightness adjustment was performed using ToupView software. H&E, hematoxylin and eosin. Color images are available online.
Discussion
While the effects of γ-radiation on PVA hydrogels for tissue engineering application have been studied,29–32 the effects of EtO sterilization, as one of the FDA-approved terminal sterilization methods, on PVA hydrogel properties were unknown. The PVA surface is known to be unfavorable for cell attachment22; however, previous studies showed the PVA materials exhibit excellent biocompatibility and hemocompatibility.4,18 The mechanical properties of the PVA material have been shown to be able to mimic the mechanical properties of native blood vessel,8,21 which compelled us to study the effect of these sterilization methods on the PVA vascular graft properties. Indeed, synthetic vascular grafts with small diameters (<6 mm) currently face many challenges to achieve high patency rates in vivo. There are at least three major factors that could determine graft success when implanted in vivo: vascular graft mechanical properties, graft endothelialization, and blood compatibility. We hypothesized that the EtO sterilization would not detrimentally affect PVA properties and, as such, the EtO-sterilized grafts would still be suitable for vascular graft applications. In this study, autoclaving was done to rapidly dissolve PVA powder in the deionized water to make a homogeneous 10% (w/v) PVA solution, as is commonly done.54–57 It is not considered part of sterilization because the subsequent steps were performed in a nonsterile environment. Autoclaving also helped to prevent fabrication inconsistencies due to solution inhomogeneity. Earlier studies from our group,58 showed that PVA films fabricated without autoclaving showed slightly lower Young's modulus, 0.43 to 0.65 MPa, while Young's modulus with autoclave step reported in this study is 1.4 to 3.7 MPa. The high Young's modulus seen in this study implies that sufficient crosslinking happens through the activation of the hydroxyl function, indicating that autoclaving is not negatively impacting the crosslinking ability of PVA. It has been reported that autoclaving could cause degradation of gelatin-methacrylate and hyaluronic acid due to peptide bond hydrolysis and splitting of (C-O-C) glycosidic bonds, respectively.59 However, the PVA backbone mainly consists of highly stable C-C bonds, which are unlikely to be disrupted through the autoclaving process. Furthermore, PVA graft storage conditions for γ-irradiation and EtO sterilization were different because EtO sterilization must be performed on dried samples to allow efficient release of excess EtO from the grafts during the aeration step, since EtO is a carcinogenic substance, while γ-irradiation on dried PVA samples would impede PVA rehydration. Moreover, unlike EtO sterilization, the dehydration step is not required for γ-radiation since no toxic residual could be formed and the sterilized hydrated grafts can be immediately used without the need of an additional hydration step with sterilized water. As such, the different storage conditions exemplified practical terminal sterilization considerations for hydrogel materials.
The mechanical testing results of the PVA grafts, treated with EtO and γ-irradiation, were compared against the untreated control grafts. The thick and thin (both having 1.5 mm diameter) and the 4-mm PVA grafts with similar thickness to the thick had changes in mechanical properties due to the treatment, yet the burst pressure values of all the grafts tested were at least five times higher than physiological resting blood pressure.60 As shown in Figure 3, the compliance of the thick graft was significantly lower than that of the thin graft. However, Young's modulus of the thick and thin was not significantly different. While these properties both describe the elastic deformation of the grafts, compliance of tubular grafts can be affected not only by the material elasticity but also by the geometry such as inner tube diameter and wall thickness.61,62 Previous studies showed that compliance is inversely proportional to wall thickness. Therefore, although the Young's moduli of thick and thin grafts were not significantly different, the compliance of the thin grafts being significantly higher than the compliance of thick grafts can be attributed to geometrical differences. The compliances of the γ-irradiated thin grafts and the γ-irradiated 4-mm grafts were also found to be significantly different, with the γ-irradiated 4-mm grafts having the highest average compliance among all other groups. The burst pressures of thin, thick, and 4-mm samples were all significantly different from one another. The least compliant group, thick, had the highest burst pressure and the most compliant group, 4 mm, had the lowest burst pressure. This is in accordance with previous studies showing that vascular grafts with lower compliance have higher burst pressures.63,64
EtO grafts were generally stiffer than the untreated control as shown in multiple mechanical property tests such as compliance, Young's modulus, burst pressure, suture retention strength, and MBR. The EtO grafts were also visually more transparent than the other two groups. These observations agreed with the significant increase in EtO-treated PVA graft crystallinity, as was measured using DSC, causing a reduction in the graft wall thickness. However, when using PVA films and the XRD method to measure crystallinity, the XRD spectra did not show any change in the crystalline peak after sterilization. However, our data also showed that bidirectionally dip cast untreated PVA films showed higher degree of crystallinity than solvent casted films based on the XRD spectra. This could explain the difference in crystallinity change between DSC and XRD results. Indeed, a bidirectional movement of the cast polymer has been reported before to induce higher polymer crystallinity.65 The increase in crystallinity of the EtO group could be a result of increased temperature and pressure during sterilization treatment. This change in crystallinity caused by EtO sterilization was interestingly observed by other groups when sterilizing PLA fibers and was speculated to be caused by an annealing effect during the sterilization process.40 The thinner graft walls of the EtO group could result from the dry condition of PVA grafts during EtO treatment, which could cause polymer chains to be in close proximity to one another and compacted even further after the treatment. This may contribute to the decrease in swelling and water content percentage of EtO-treated PVA. It was suspected that a change in crosslinking may have occurred after γ-irradiation and that the addition of an ethoxy group (ethoxylation reaction) may have occurred after EtO treatment as there is a known reaction between EtO and the -OH functional groups of uncrosslinked PVA.66 However, the EtO treatment did not cause an ethoxylation reaction as confirmed by the FTIR spectra.
The mechanical properties of γ-irradiated grafts were maintained, except for a significant increase in compliance. Hence, the γ-irradiated grafts were slightly more elastic than the untreated controls but still within the requirements of an arterial vascular graft.21 Knowing that there was no significant difference in the DSC and the FTIR spectra of the γ-irradiated grafts compared with the untreated controls, the increase in compliance could be caused by scissions at some point along the polymer chains as previously observed in other polymers.41,67,68
Another important factor to maintain graft patency is the ability to promote endothelialization while maintaining blood compatibility. Surface modifications caused by either sterilization treatment could affect these factors and were investigated in this study. Previously, our group has shown how the 2 μm grating topography could improve endothelialization on PVA while maintaining good blood compatibility.4,22 Therefore, we investigated the effect of the sterilization treatments on this topography compared with the unpatterned PVA. The fidelity of our 2 μm gratings was not affected by any of the treatments possibly because the aspect ratio of our grating topography was low (width: height = 1:1); the disruption of surface topography may be more prominent in a higher aspect ratio. The surface roughness of the unpatterned PVA was visibly similar among all the treatment groups. Nevertheless, γ-irradiation changed the surface chemistry of PVA. The treatment disrupted surface of the –OH functional groups as it was shown that the %O s1 was significantly lower compared with untreated control. The decrease in –OH group percentage on the surface may at first appear to contradict the increase in water content in the γ-irradiated 4-mm group (Fig. 3B). However, as observed in another study,47 γ-radiation could indeed create a more porous polymer, which means more room for water to be absorbed by the polymer. A similar observation regarding the increase in water uptake of γ-irradiated PVA was also reported before.30
This XPS result was further confirmed through the increase in surface hydrophobicity, which in turn led to improved protein adsorption to the surface through hydrophobic/hydrophobic interaction. Knowing that γ-irradiation was found to increase graft mechanical compliance, the increase in surface hydrophobicity further strengthened our speculation that γ-irradiation could have caused chain scissions in the PVA grafts. Scissions at the main polymer chain would expose more polymer alkyl end groups, which are more hydrophobic compared with the –OH functional group. The relationship between surface wettability and cell adhesion has been previously shown.69 Hydrophobicity promotes cell adhesion due to improvement in protein adsorption to the biomaterial surface through hydrophobic/hydrophobic interaction.
Based on the endothelial adhesion study, it was evident on both the live/dead imaging and CyQUANT measurement that endothelialization on the 2 μm grating topography γ-irradiated samples was improved significantly. There were no dead cells detected at 13 days, and the cell morphology was more elongated because of the topographical influence, compared with those on unpatterned γ-irradiated samples, which showed a more cobblestone shape. However, the morphology of cells on untreated films and EtO-treated films was similar to one another. In addition, from the live/dead imaging, we observed that cell populations on the unpatterned γ-irradiated samples were patchy with some cells having larger cell areas compared with cells on 2 μm grating topography γ-irradiated samples. These observations may explain the large difference in the cell density between 2 μm grating topography and unpatterned, both γ-irradiated, samples quantified with CyQUANT assay (Fig. 6E).
It should also be noted that to avoid contamination in cell culture of the untreated PVA, all samples were pretreated with penicillin/streptomycin solution incubation, followed by 10 washes in phosphate-buffered saline (see the Detailed Supplementary Materials and Methods section). To ensure this did not affect cell viability, proliferation assays, including Alamar blue and the live/dead assay, were done. It was found that pretreatment with penicillin/streptomycin did not affect cell viability (Supplementary Fig. S5).
Due to the enhancement in hydrophobicity and endothelialization, and changes in PVA surface functional groups of the γ-irradiated PVA, we hypothesized that the hemocompatibility of the material may be impacted after γ-radiation. The hemocompatibility quantification of treated PVA grafts showed a significant increase in fibrin accumulation of γ-irradiated PVA grafts, while the platelet data did not show statistical significance between the treatment groups. These differences should be tempered with the knowledge that significant animal variability was found in these data, but since the different graft samples were tested in the various animals it was difficult to develop a statistically valid way to control for this variability. Individual animal data are shown in Supplementary Data, Supplementary Fig. S6.
Another group has previously shown the excellent biocompatibility of PVA, which was sterilized with 70% (v/v) ethanol, where there was little foreign body reaction observed after 3-month implantations at rabbit knee joints.70 In this study, we found that in a subcutaneous rat model, the biocompatibility of γ-irradiated PVA was better than the untreated PVA control, while the EtO-treated PVA was even better than γ-irradiated PVA, as there were significantly fewer M1 expressing macrophages in the surrounding rat tissues around the PVA implant after 3 weeks, and in the surrounding baboon tissues after 4 weeks, respectively. The results from the rat study are interesting as γ-irradiated PVA was expected to induce more protein adsorption during the early stages of hemostasis than the untreated PVA control. Nevertheless, the baboon study showed a significant increase in the number of M1 expressing macrophages for γ-irradiated samples compared with EtO, which supports our hypothesis.
Conclusions
Terminal sterilization of an implant is important to prevent infectious agent buildup, which could lead to graft failure. Because sterilization can cause changes in material properties of hydrogels, it is necessary to quantify these before preclinical implantations and eventual clinical studies. Herein, we studied how EtO sterilization affected the properties of PVA vascular grafts in comparison with the γ-irradiation method. Both EtO and γ-radiation affected PVA in different ways and were shown to have different advantages and disadvantages. While EtO treatment changed PVA graft mechanical properties by making grafts stiffer and reduced PVA swelling and water content percentage, γ-radiation caused PVA to become more compliant and increased its surface hydrophobicity. The latter was also shown to cause improvement in graft endothelialization and immune response. However, EtO-treated grafts had decreased fibrin accumulation from whole blood compared with γ-irradiated grafts. Through this study, we showed that both EtO- and γ-sterilized hydrogel-based grafts remained suitable for small-diameter vascular graft applications.
Supplementary Material
Acknowledgments
We thank Dr. Boxin Zhao for giving us the permission to use the tribometer and contact angle equipment in his laboratory for the mechanical testing part of this study. We greatly appreciate the contributions of Mr. Matthew Hagen, Ms. Jennifer Johnson, Ms. Tiffany Burch, and the ONPRC staff for their help in data collection and analysis of the shunt and baboon implant studies. We also thank Ms. YeJin Jeong for helping to prepare PVA films. Finally, we thank Dr. Marek Kukumberg for performing surgery for the rat in vivo work.
Disclosure Statement
No competing financial interests exist.
Funding Information
This work was supported by the National Institutes of Health (grant numbers R01HL130274, R01HL144113, and R01DE026170), the Oregon National Primate Research Center NIH grant award P51OD011092, and partially supported by the National Research Foundation, Prime Minister's Office, Singapore, under its Research Centre of Excellence programme administered by the Mechanobiology Institute, Singapore. NSERC Canada Discovery Grant (RGPIN-2016-04043), NSERC CREATE (401207296) for S.M. and Y.Y., and NSERC USRA and CGSM for S.M.
Supplementary Material
References
- 1. Caló E., and Khutoryanskiy V.V.. Biomedical applications of hydrogels: a review of patents and commercial products. Eur Polym J 65, 252, 2015 [Google Scholar]
- 2. Hoffman A.S. Hydrogels for biomedical applications. Adv Drug Deliv Rev 64, 18, 2012 [DOI] [PubMed] [Google Scholar]
- 3. Savina I.N., Dainiak M., Jungvid H., Mikhalovsky S.V., and Galaev I.Y.. Biomimetic macroporous hydrogels: protein ligand distribution and cell response to the ligand architecture in the scaffold. J Biomater Sci Polym Ed 20, 1781, 2009 [DOI] [PubMed] [Google Scholar]
- 4. Cutiongco M.F., Anderson D.E., Hinds M.T., and Yim E.K.. In vitro and ex vivo hemocompatibility of off-the-shelf modified poly(vinyl alcohol) vascular grafts. Acta Biomater 25, 97, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Drury J.L., and Mooney D.J.. Hydrogels for tissue engineering: scaffold design variables and applications. Biomaterials 24, 4337, 2003 [DOI] [PubMed] [Google Scholar]
- 6. Liu M., Zeng X., Ma C., et al. Injectable hydrogels for cartilage and bone tissue engineering. Bone Res 5, 17014, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zhang X., Xu B., Puperi D.S., Wu Y., West J.L., and Grande-Allen K.J.. Application of hydrogels in heart valve tissue engineering. J Long Term Eff Med Implants 25, 105, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cutiongco M.F., Kukumberg M., Peneyra J.L., et al. Submillimeter diameter poly(vinyl Alcohol) vascular graft patency in rabbit model. Front Bioeng Biotechnol 4, 44, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Abidian M.R., Daneshvar E.D., Egeland B.M., Kipke D.R., Cederna P.S., and Urbanchek M.G.. Hybrid conducting polymer-hydrogel conduits for axonal growth and neural tissue engineering. Adv Healthc Mater 1, 762, 2012 [DOI] [PubMed] [Google Scholar]
- 10. Vega S.L., Kwon M.Y., and Burdick J.A.. Recent advances in hydrogels for cartilage tissue engineering. Eur Cells Mater 33, 59, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Diec J., Tilia D., and Thomas V.. Comparison of silicone hydrogel and hydrogel daily disposable contact lenses. Eye Contact Lens 44, S167, 2018 [DOI] [PubMed] [Google Scholar]
- 12. Qu X.-M., Dai J.-H., iang Z.-Y., and Qian Y.-F.. Clinic study on silicone hydrogel contact lenses used as bandage contact lenses after LASEK surgery. Int J Ophthalmol 4, 314, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yates C.C., Whaley D., Babu R., et al. The effect of multifunctional polymer-based gels on wound healing in full thickness bacteria-contaminated mouse skin wound models. Biomaterials 28, 3977, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Arslantunali D., Dursun T., Yucel D., Hasirci N., and Hasirci V.. Peripheral nerve conduits: technology update. Med Dev (Auckland, N.Z.) 7, 405, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nugent M.J., and Higginbotham C.L.. Preparation of a novel freeze thawed poly(vinyl alcohol) composite hydrogel for drug delivery applications. Eur J Pharm Biopharm 67, 377, 2007 [DOI] [PubMed] [Google Scholar]
- 16. Wan W., Dawn Bannerman A., Yang L., Helium M.. Poly(vinyl alcohol) cryogels for biomedical applications. Adv Polym Sci 263, 283, 2014 [Google Scholar]
- 17. Fathi E., Nassiri S.M., Atyabi N., et al. Induction of angiogenesis via topical delivery of basic-fibroblast growth factor from polyvinyl alcohol-dextran blend hydrogel in an ovine model of acute myocardial infarction. J Tissue Engineering Regen Med. 7, 697, 2013 [DOI] [PubMed] [Google Scholar]
- 18. Kobayashi M., Chang Y.S., and Oka M.. A two year in vivo study of polyvinyl alcohol-hydrogel (PVA-H) artificial meniscus. Biomaterials 26, 3243, 2005 [DOI] [PubMed] [Google Scholar]
- 19. Yang W.H., Smolen V.F., and Peppas N.A.. Oxygen permeability coefficients of polymers for hard and soft contact lens applications. J Membr Sci 9, 53, 1981 [Google Scholar]
- 20. Ding J., He R., Zhou G., Tang C., and Yin C.. Multilayered mucoadhesive hydrogel films based on thiolated hyaluronic acid and polyvinylalcohol for insulin delivery. Acta Biomater 8, 3643, 2012 [DOI] [PubMed] [Google Scholar]
- 21. Chaouat M., Le Visage C., Baille W.E., et al. A Novel Cross-linked poly(vinyl alcohol) (PVA) for vascular grafts. Adv Funct Mater 18, 2855, 2008 [Google Scholar]
- 22. Cutiongco M.F., Goh S.H., Aid-Launais R., Le Visage C., Low H.Y., and Yim E.K.. Planar and tubular patterning of micro and nano-topographies on poly(vinyl alcohol) hydrogel for improved endothelial cell responses. Biomaterials 84, 184, 2016 [DOI] [PubMed] [Google Scholar]
- 23. Jiang H., Campbell G., Boughner D., Wan W.-K., and Quantz M.. Design and manufacture of a polyvinyl alcohol (PVA) cryogel tri-leaflet heart valve prosthesis. Med Eng Phys 26, 269, 2004 [DOI] [PubMed] [Google Scholar]
- 24. Baker M.I., Walsh S.P., Schwartz Z., and Boyan B.D.. A review of polyvinyl alcohol and its uses in cartilage and orthopedic applications. J Biomed Mater Res Part B Appl Biomaterials 100, 1451, 2012 [DOI] [PubMed] [Google Scholar]
- 25. Suzuki M. An artificial muscle by PVA hydrogel can generate high power close to living skeletal muscles. In: Images of the Twenty-First Century. Proceedings of the Annual International Engineering in Medicine and Biology Society, vol. 3 1989, p. 916 [Google Scholar]
- 26. Yoshimatsu G., Sakata N., Tsuchiya H., et al. , Development of polyvinyl alcohol bioartificial pancreas with rat islets and mesenchymal stem cells. Transplant Proc 45, 1875, 2013 [DOI] [PubMed] [Google Scholar]
- 27. Khurana G., Arora S., and Pawar P.K.. Ocular insert for sustained delivery of gatifloxacin sesquihydrate: preparation and evaluations. Int J Pharm Invest 2, 70, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Salzer D.A., Medeiros P.J., Craen R., and Shoemaker J.K.. Neurogenic-nitric oxide interactions affecting brachial artery mechanics in humans: roles of vessel distensibility vs. diameter. Am J Physiol Regul Integr Comp Physiol 295, R1181, 2008 [DOI] [PubMed] [Google Scholar]
- 29. Varshney L. Role of natural polysaccharides in radiation formation of PVA-hydrogel wound dressing. Nucl Instrum Methods Phys Res, B 255, 343, 2007 [Google Scholar]
- 30. Oliveira M.J.A., Rodrigues K.M.S., Parra D.F., and Lugao A.B.. Effects of sterilization on Poly(Vinyl Alcohol) (PVAl) hydrogels matrices. In: Proceedings of the INAC 2007 International Nuclear Atlantic Conference Nuclear Energy and Energetic Challenges for 21st Century 15 Brazilian National Meeting on Reactor Physics and Thermal Hydraulics Brazil: 8. Brazilian National Meeting on Nuclear Applications, 2007, p. v [Google Scholar]
- 31. Yang X., Liu Q., Chen X., Yu F., and Zhu Z.. Investigation of PVA/ws-chitosan hydrogels prepared by combined γ-irradiation and freeze-thawing. Carbohydr Polym 73, 401, 2008 [Google Scholar]
- 32. Shi Y., Xiong D., and Zhang J.. Effect of irradiation dose on mechanical and biotribological properties of PVA/PVP hydrogels as articular cartilage. Tribol Int 78, 60, 2014 [Google Scholar]
- 33. Nambu M., Onishi T., and Onohara M.. Method for sterilization of polyvinyl alcohol gel by γ-ray. A61L 2/08 Radiation/WO 88/03414 19.05.88 Gazette 88/11. Sumitomo Bakelite Co Ltd, JXTG Nippon Oil and Energy Corp, European Patent Office, 1987 [Google Scholar]
- 34. Darwis D., Yoshii F., Makuuchi K., and Razzak M.T.. Improvement of hot water resistance of poly(vinyl alcohol) hydrogel by acetalization and irradiation techniques. J Appl Polym Sci 55, 1619, 1995 [Google Scholar]
- 35. Caló E., Barros J.M.S.D., Fernández-Gutiérrez M., San Román J., Ballamy L., and Khutoryanskiy V.V.. Antimicrobial hydrogels based on autoclaved poly(vinyl alcohol) and poly(methyl vinyl ether-alt-maleic anhydride) mixtures for wound care applications. RSC Adv 6, 55211, 2016 [Google Scholar]
- 36. Dai Z., Ronholm J., Tian Y., Sethi B., and Cao X.. Sterilization techniques for biodegradable scaffolds in tissue engineering applications. J Tissue Eng 7, 2041731416648810, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Cox M.M., and Battista J.R.. Deinococcus radiodurans—the consummate survivor. Nat Rev Microbiol 3, 882, 2005 [DOI] [PubMed] [Google Scholar]
- 38. Lambert P.A. Radiation sterilization. In: Fraise A.P., Maillard J-Y., Sattar A.S. (eds). Principles and Practice of Disinfection, Preservation and Sterilization. Oxford, United Kingdom: Wiley-Blackwell, 2013, pp. 294 [Google Scholar]
- 39. Gilding D.K., and Reed A.M.. Biodegradable polymers for use in surgery—polyglycolic/poly(actic acid) homo- and copolymers: 1. Polymer 20, 1459, 1979 [Google Scholar]
- 40. Valente T.A., Silva D.M., Gomes P.S., Fernandes M.H., Santos J.D., and Sencadas V.. Effect of sterilization methods on electrospun poly(lactic acid) (PLA) fiber alignment for biomedical applications. ACS Appl Mater Interf 8, 3241, 2016 [DOI] [PubMed] [Google Scholar]
- 41. Clough R.L. High-energy radiation and polymers: a review of commercial processes and emerging applications. Nucl Instrum Methods Phys Res, B 185, 8, 2001 [Google Scholar]
- 42. Zhang X., Takegoshi K., and Hikichi K.. Phase separation and thermal degradation of poly(vinyl alcohol)/poly(methacrylic acid) and poly(vinyl alcohol)/poly(acrylic acid) systems by 13C c.p./m.a.s. n.m.r. Polymer 33, 718, 1992 [Google Scholar]
- 43. Tsuchiya Y., and Sumi K.. Thermal decomposition products of poly(vinyl alcohol). J Polym Sci Part A-1 Polym Chem 7, 3151, 1969 [Google Scholar]
- 44. Gogolewski S., and Mainil-Varlet P.. Effect of thermal treatment on sterility, molecular and mechanical properties of various polylactides. 2. Poly(L/D-lactide) and poly(L/DL-lactide). Biomaterials 18, 251, 1997 [DOI] [PubMed] [Google Scholar]
- 45. Volland C., Wolff M., and Kissel T.. The influence of terminal gamma-sterilization on captopril containing poly(d,l-lactide-co-glycolide) microspheres. J Control Release 31, 293, 1994 [Google Scholar]
- 46. Zislis T., Mark D.E., Cerbas E.L., and Hollinger J.O.. Scanning electron microscopic study of cell attachment to biodegradable polymer implants. J Oral Implantol 15, 160, 1989 [PubMed] [Google Scholar]
- 47. Holy C.E., Cheng C., Davies J.E., and Shoichet M.S.. Optimizing the sterilization of PLGA scaffolds for use in tissue engineering. Biomaterials 22, 25, 2001 [DOI] [PubMed] [Google Scholar]
- 48. Vandrangi P., Gott S.C., Kozaka R., Rodgers V.G.J., and Rao M.P.. Comparative endothelial cell response on topographically patterned titanium and silicon substrates with micrometer to sub-micrometer feature sizes. PLoS One 9, e111465, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ankam S., Suryana M., Chan L.Y., et al. Substrate topography and size determine the fate of human embryonic stem cells to neuronal or glial lineage. Acta Biomater 9, 4535, 2013 [DOI] [PubMed] [Google Scholar]
- 50. Muhammad R., Peh G.S.L., Adnan K., Law J.B.K., Mehta J.S., and Yim E.K.F.. Micro- and nano-topography to enhance proliferation and sustain functional markers of donor-derived primary human corneal endothelial cells. Acta Biomater 19, 138, 2015 [DOI] [PubMed] [Google Scholar]
- 51. Teo B.K.K., Goh S.H., Kustandi T.S., Loh W.W., Yee L.H., and Yim E.K.F.. The effect of micro and nanotopography on endocytosis in drug and gene delivery systems. Biomaterials 32, 9866, 2011 [DOI] [PubMed] [Google Scholar]
- 52. Yim E.K.F., Wan A.C.A., Le Visage C., Liao I.C., and Leong K.W.. Proliferation and differentiation of human mesenchymal stem cell encapsulated in polyelectrolyte complexation fibrous scaffold. Biomaterials 27, 6111, 2006 [DOI] [PubMed] [Google Scholar]
- 53. Pohan G., Chevallier P., Anderson D., et al. Luminal plasma treatment for small diameter polyvinyl alcohol tubular scaffolds. Front Bioeng Biotechnol 7, 117, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Ishii T., Ho C.K., Nahas H., Yiu B.Y.S., Chee A.J.Y., and Yu A.C.H.. Deformable phantoms of the prostatic urinary tract for urodynamic investigations. Med Phys 46, 3034, 2019 [DOI] [PubMed] [Google Scholar]
- 55. Ma S., Wang S., Li Q., Leng Y., Wang L., and Hu G.. A novel method for preparing poly(vinyl alcohol) hydrogels: preparation, characterization, and application. Ind Eng Chem Res 56, 7971, 2017 [Google Scholar]
- 56. Hou Y., Chen C., Liu K., Tu Y., Zhang L., and Li Y.. Preparation of PVA hydrogel with high-transparence and investigations of its transparent mechanism. RSC Adv 5, 24023, 2015 [Google Scholar]
- 57. Jiang S., Liu S., and Feng W.. PVA hydrogel properties for biomedical application. J Mech Behav Biomed Mater 4, 1228, 2011 [DOI] [PubMed] [Google Scholar]
- 58. Ino J.M., Sju E., Ollivier V., Yim E.K., Letourneur D., and Le Visage C.. Evaluation of hemocompatibility and endothelialization of hybrid poly(vinyl alcohol) (PVA)/gelatin polymer films. J Biomed Mater Res Part B Appl Biomater 101, 1549, 2013 [DOI] [PubMed] [Google Scholar]
- 59. O'Connell C.D., Onofrillo C., Duchi S., et al. Evaluation of sterilisation methods for bio-ink components: gelatin, gelatin methacryloyl, hyaluronic acid and hyaluronic acid methacryloyl. Biofabrication 11, 035003, 2019 [DOI] [PubMed] [Google Scholar]
- 60. Shahoud J.S., and Aeddula N.R.. Physiology, Arterial Pressure Regulation. Treasure Island (FL): StatPearls, 2019 [PubMed] [Google Scholar]
- 61. Bouchet M., Gauthier M., Maire M., Ajji A., and Lerouge S.. Towards compliant small-diameter vascular grafts: predictive analytical model and experiments. Mater Sci Eng C Mater Biol Appl 100, 715, 2019 [DOI] [PubMed] [Google Scholar]
- 62. Gao J., Huang Z., Guo H., Tian S., Wang L., and Li Y.. Effect of wall structures on mechanical properties of small caliber PHBHHx vascular grafts. Fibers Polym 20, 2261, 2019 [Google Scholar]
- 63. Syedain Z.H., Meier L.A., Bjork J.W., Lee A., and Tranquillo R.T.. Implantable arterial grafts from human fibroblasts and fibrin using a multi-graft pulsed flow-stretch bioreactor with noninvasive strength monitoring. Biomaterials 32, 714, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. McClure M.J., Simpson D.G., and Bowlin G.L.. Tri-layered vascular grafts composed of polycaprolactone, elastin, collagen, and silk: optimization of graft properties. J Mech Behav Biomed Mater 10, 48, 2012 [DOI] [PubMed] [Google Scholar]
- 65. Dorling B., Vohra V., Dao T.T., Garriga M., Murata H., and Campoy-Quiles M.. Uniaxial macroscopic alignment of conjugated polymer systems by directional crystallization during blade coating. J Mater Chem C 2, 3303, 2014 [Google Scholar]
- 66. Jones J.I. Polyvinyl alcohol. Properties and applications. Br Polym J 5, 493, 1973 [Google Scholar]
- 67. Raghu S., Archana K., Sharanappa C., Ganesh S., and Devendrappa H.. Electron beam and gamma ray irradiated polymer electrolyte films: Dielectric properties. J Radiat Res Appl Sci 9, 117, 2016 [Google Scholar]
- 68. Otaguro H., de Lima L.F.C.P., Parra D.F., Lugão A.B., Chinelatto M.A., and Canevarolo S.V.. High-energy radiation forming chain scission and branching in polypropylene. Radiat Phys Chem 79, 318, 2010 [Google Scholar]
- 69. Lampin M., Warocquier-Clerout R., Legris C., Degrange M., and Sigot-Luizard M.F.. Correlation between substratum roughness and wettability, cell adhesion, and cell migration. J Biomed Mater Res 36, 99, 1997 [DOI] [PubMed] [Google Scholar]
- 70. Kobayashi M., and Hyu H.S.. Development and evaluation of polyvinyl alcohol-hydrogels as an artificial atrticular cartilage for orthopedic implants. Materials 3, 2753, 2010 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.