Abstract
Severe traumatic brain injury (TBI) activates a robust systemic response that involves inflammatory and other factors, including estradiol (E2), associated with increased deaths. Tumor necrosis factor-alpha (TNFα) is a significant mediator of systemic shock, and it is an extra-gonadal transcription factor for E2 production. The study objectives were to test the hypotheses: (1) a positive feedback relationship exists between acute serum TNFα and E2; and (2) acute concentrations of E2 and TNFα are prognostic indicators of death after severe TBI. This prospective cohort study included N = 157 adults with severe TBI. Serum samples were collected for the first five days post-injury. The TNFα and E2 levels were averaged into two time epochs: first 72 h (T1) and second 72 h post-injury (T2). A cross-lag panel analysis conducted between T1 and T2 TNFα and E2 levels showed significant cross-lag effects: T1 TNFα and T1 E2 were related to T2 E2 and T2 TNFα, respectively. Cox proportional hazards multi variable regression models determined that increases in T1 E2 (hazard ratio [HR] = 1.79, 95% confidence interval [CI]: 1.15, 2.81), but not T2 E2 (HR = 0.91, 95% CI: 0.56, 1.47), were associated with increased risk of death. Increased T2 TNFα (HR = 2.47, 95% CI: 1.35, 4.53), and T1 TNFα (HR = 1.47, 95% CI: 0.99, 2.19), to a lesser degree, were associated with increased risk of death. Relationships of death with T2 TNFα and T1 E2 were mediated partially by cardiovascular, hepatic, and renal dysfunction. Both E2 and TNFα are systemic, reciprocally related biomarkers that may be indicative of systemic compromise and increased risk of death after severe TBI.
Keywords: estradiol, non-neurological organ dysfunction, systemic trauma complex, traumatic brain injury, tumor necrosis factor-alpha
Introduction
Severe traumatic brain injury (TBI) constitutes a major global public health and economic burden. Individuals with severe TBI have an increased risk for premature death,1–3 and survivors often live with chronic injury-related disabilities.4 The financial burden of TBI in the United States is estimated at more than $60 billion per year.5 There have been tremendous efforts over the last several decades to identify acute neuroprotective treatments for TBI populations. Despite some progress in clinical care, mortality rates for severe TBI are largely unchanged over the last decade.6,7 There are also still no treatments that have received a Level I recommendation for efficacy in the recent 4th edition TBI Guidelines,8 and no treatments have been approved by the Food and Drug Administration after Phase III trials.
Several large clinical trials involving TBI populations have examined the efficacy of pharmacological dosing of agents, including corticosteroids and progesterone, which target inflammation9,10 and hormone physiology.11,12 These trials, however,9–12 were ineffective or were halted prematurely because of observed harm in the treatment arm.10 Unsuccessful clinical trials have occurred despite pre-clinical TBI studies that have demonstrated the efficacy of exogenous progesterone13,14 and anti-inflammatory15–17 therapies as neuroprotective agents.
Data from observational cohort studies, however, have revealed the deleterious effects of excessive peripheral hormone levels18 and acute inflammation.19–21 Interestingly, observational studies have suggested that neuroimmune and neuroendocrine networks are highly interconnected, and the regulatory connection between the two systems is associated with recovery after severe TBI.22 Little is understood about neuroendocrine-immune communication in the context of the systemic response to TBI and its associated trauma complex. The failure of previous TBI clinical trials now invites further study within an intriguing line of research involving systemic hormone and inflammatory physiology after TBI and its potential impacts on systemic compromise in the context of outcome.
Tumor necrosis factor-alpha (TNFα) and estradiol (E2) are inflammatory and endocrine markers, respectively, that are elevated acutely in serum after severe TBI23–25 and associated with morbidity26 and death.18,27 These markers are relevant to post-TBI homeostatic disturbance. After moderate-to-severe TBI, hypothalamic-pituitary-gonadotrophic (HPG) axis production of sex hormones are suppressed, with reduced luteinizing hormone (LH) and follicle stimulating hormone (FSH) noted in the setting of variable extragonadal sex hormone production, including E2.18
The autonomic nervous system (ANS) is triggered in response to trauma, which drives the sympathetic nervous system (SNS) to initiate an acute phase response that leads to peripheral TNFα production.28–30 TNFα is the body's major mediator between septic shock and secondary systemic inflammatory response syndrome.31 In the context of injury or critical illness, regulated TNFα release is physiologically supportive for healing; however, uncontrolled TNFα production can facilitate the development of systemic shock, non-neurological organ dysfunction (NNOD), and death.32
Observed elevations in TNFα and E2 in the setting of TBI are likely not independent phenomena; E2 is produced by the conversion of androgens via the aromatase gene,33 and TNFα is a tissue-specific transcription factor for the aromatase gene in adipose tissue.34–39 Increasing E2 production acutely creates a positive response that contributes to exaggerated levels of inflammation through its effects on reuptake inhibition of norepinephrine in lymphoid tissues.40,41 Evidence of this positive-feedback relationship suggests that excessive E2 in the periphery is both a cause and consequence of excessive peripheral TNFα; however, this hypothesis has not been tested specifically in prior clinical TBI studies.
A positive feedback loop between TNFα and E2 could account, in some part, for the observed increased risk of death that occurs among individuals with elevated concentrations of these markers. Thus, the primary objective of the present study was to test the hypothesis of a positive temporal feedback relationship between serum TNFα and E2 during the acute period after severe TBI using a cross-lag panel model. Secondary objectives were to test the prognostic effects of acute serum TNFα and E2 levels on risk of death in the first six months after severe TBI and to evaluate the role of systemic non-neurological compromise across multiple systems, in mediating this relationship.
Understanding evolving, reciprocal relationships between acute serum TNFα and E2 may reveal how inflammatory and endocrine levels are relevant to survival after severe TBI. Identifying the prognostic capacity of these biomarkers for risk of death has important implications for identifying individuals who may (or may not) benefit from acute neuroprotective clinical interventions.
Methods
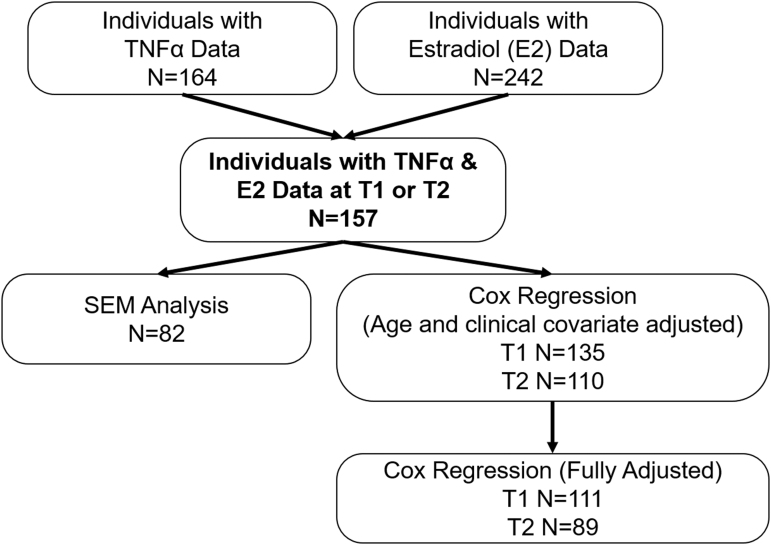
This study was approved by the Institutional Review Board for the University of Pittsburgh. The present report is a prospective observational cohort study that includes n = 157 adults with severe TBI, with injury dates from May 2004 to March 2011, with available E2 and TNFα data as summarized in Figure 1. Eligible participants were between 16 and 70 years old, had an initial Glasgow Coma Scale (GCS) score of 8 or less, and had intracranial pathology present on a computed tomography (CT) scan. Patients with TBI were excluded if they had a history of cancer or untreated thyroid disease.
FIG. 1.
Flow diagram of analytic sample. TNFα, tumor necrosis factor alpha; E2, estradiol; SEM, standard error of the mean.
Next-of-kin were approached for consent as patients were unable to self-consent. Patients with severe TBI received care consistent with the TBI Guidelines for the Management of Severe Head Injury,8 which included placement of an extraventricular device for intracranial pressure monitoring, central venous and arterial catheter placement, and neurosurgical intervention for the decompression of mass lesions.
Serum sample processing
Eligible and consented participants underwent blood sample collection daily for the first five days after injury. Blood sample collections were performed at 7:00 am on most mornings, unless there was a direct conflict with clinical care. In instances where it was not possible to collect a morning blood sample, a sample was gathered at 7:00 pm. For some participants, it was not possible to obtain a blood sample each day. After serum collection, samples were centrifuged, aliquoted, and stored at -80°C until assay completion.
Serum samples were assayed for E2 using radioimmunoassay with Coat-A-Count® In-vitro Diagnostic Test Kit (Siemens Healthcare Diagnostics Inc. Los Angeles, CA). The E2 was measured using a 125I radioimmunoassay using 100 μL sample aliquots. Serum sample measurements for TNFα were completed using a LuminexTM bead array assay (Millipore, Billerica, MA; catalog number HSCYTO-60SK). The minimum detectable limit for TNFα was 0.05 pg/mL. The TNFα levels were scaled utilizing concentration standards and quality controls before the analysis because of observed variability across plates. The interassay and intraassay coefficients of variation were <10% for both the hormone and inflammatory assays.
E2 and TNFα classification: acute response
Both E2 and TNFα were grouped into two epochs: time 1 (T1) and time 2 (T2); T1 consisted of data averaged over the first 72 h after injury, and T2 consisted of data averaged over the second 72 h after injury. Because individuals had missing values at various time points in the first week because of clinical care requirements, it was not possible to conduct analyses using daily levels.
Aromatase genetics: rs2470152
Deoxyribonucleic acid (DNA) was extracted from participants' whole-blood samples before transfusion. Blood samples were centrifuged to retrieve the buffy coat. DNA was extracted using a salting out procedure.42 The single nucleotide polymorphism (SNP) rs2470152 was genotyped as part of a larger genomic analysis of the aromatase gene (CYP19A1) that included four functional SNPs and 18 tagging SNPs. A primary article examining the effects of aromatase genetics, and the impact of rs2470152 genotype on cerebrospinal fluid (CSF) E2 levels and TBI outcomes was published previously.43
Clinical and demographic variables
Clinical and demographic variables collected in this study included: age, sex, race, best 24-h GCS score, Injury Severity Score (ISS), non-head ISS, mechanism of injury, and injury type based on review of available CT scans obtained during acute care. The GCS is a functional measure of TBI injury severity, made up of three components: motor responsiveness, verbal performance, and eye opening.44 Scores range from 3 to 15, with lower scores corresponding to more severe injuries; however, for the present study, only individuals with an initial GCS score between 3 and 8 were included. The best GCS score in the first 24 h was utilized for analysis purposes, because initial GCS values can often be confounded by paralytics and sedatives at initial hospital presentation with severe TBI.
The ISS is an anatomical trauma scoring scale that is a function of the three most severely injured body systems from the Abbreviated Injury Scale (AIS).45 Also, a non-head ISS was recalculated after removing the head-neck body region. Presence of abdominal, extremity, and thoracic (or chest) injuries was derived from AIS region scores ≥1 for the respective regions. Maximum head and neck AIS was included as the highest severity AIS score for a participant's coded head-neck injuries. Presence of splenic injury was derived using International Classification of Diseases injury codes version 9 (ICD-9) code “865.” The CT injury types included subdural hematoma (SDH), subarachnoid hemorrhage (SAH), intraventricular hemorrhage (IVH), epidural hematoma (EDH), diffuse axonal injury (DAI), contusion, or other.
Primary outcome: death
The primary outcome for this study was time until death. When applicable, these data were extracted from the Social Security Death Index. Death dates were subtracted from the date of the incident TBI to calculate a time until death in days. The data were right censored at 6 months post-injury.
Secondary outcome: non-neurological organ dysfunction
Cardiovascular (CV), hematological, renal, hepatic, and respiratory dysfunction, common NNOD components, were assessed as a secondary outcome and as possible mediators between the relationships of E2 and TNFα with 6-month death, respectively. Dysfunction in each system was categorized beginning at least 72 h post-injury, after T1. Common definitions using a modified version of the Sequential Organ Failure Assessment (SOFA)46 were evaluated using laboratory and physiological monitoring values that were available readily in clinical charts for individuals with available data for two or more days. The NNOD system definitions adopted for this analysis and associated literature sources are provided in Table 1.
Table 1.
Definitions of Non-Neurologic Organ Dysfunction
| Organ system | Dysfunction criteria |
|---|---|
| Renal | Creatinine levels >1.2 mg/dL |
| Hematologic | Platelets <150 103/mm3 |
| Respiratory | Ratio of PaO2/FiO2 <400 |
| Hepatic | Total bilirubin levels ≥1.2 mg/dL |
| Cardiovascular | Mean arterial pressure <70 mm Hg OR Administration of vasopressors/inotropes, evidenced by physician note or pharmacy order independent of surgery and not in conjunction with anesthetic |
To qualify as meeting threshold for “dysfunction” for any system, biomarker thresholds must have been met for at least two consecutive days of clinically available data (beginning on day 3 through day 15).
Definitions of dysfunction are modified from existing Sequential Organ Failure Assessment criteria.46
Statistical analysis
Demographic and clinical patient characteristics at the time of TBI were examined by E2 and TNFα level at T1 and at T2, divided at the median, and by six-month mortality status. Categorical variables were compared using a chi-square test, and continuous variables were compared using a two-sample t test or a Mann Whitney U test, where appropriate. Biomarkers were treated continuously for the primary analysis but were divided at the median level only for purposes of presenting grouped data. The distributions of E2 and TNFα were assessed, and log transformations were applied where appropriate.
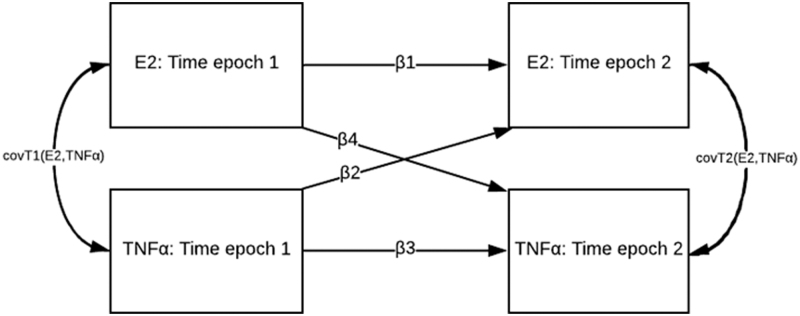
The primary objective of this study was to identify evidence of a positive temporal feedback relationship between TNFα and E2. A cross-lagged panel model was used to assess reciprocal relationships between E2 and TNFα at T1 and T2 in the first week after TBI, described using the two equations below:
In these models, the subscripts 1 and 2 represent two time intervals, and C represents a matrix of relevant confounders. The corresponding beta coefficients (i.e., β1 to β4) represent the different paths in the cross-lag panel, as shown conceptually in Figure 2.
FIG. 2.
A conceptual representation of the cross-lag panel design. β1 and β3 represent autoregressive effects, or the association of a single variable over time. β2 and β4 represent cross-lag effects, or the association of one variable at an earlier time with the other variable at the second time point, after adjustment for autoregressive paths. E2, estradiol; TNFα, tumor necrosis factor alpha.
In this panel, β1 and β3 represent the effect of a variable on itself at a later time epoch, known as autoregressive effects. The autoregressive effects represent the stability of individual differences in a variable over time.47 The coefficients β2 and β4 represent the cross-lag effects, or the relationship between a single variable at one time epoch on another variable at a later time.47 Crucially, cross-lag effects are estimated adjusting for the previous level of each variable itself. For example, the estimated association of TNFα at T1 on E2 at T2 is independent of the effect of E2 at T1 on E2 at T2. The residual covariance of E2 and TNFα at the same cross-sectional time point was also estimated; this represents the correlation between the errors; that is, the differences between the observed values and the predicted values from the fitted cross-lag model.
We aimed to determine the association between E2 and TNFα on mortality risk in the first six months post-TBI. Time until death was right censored at six months post-TBI. Separate Cox proportional hazards regression models were created for T1 biomarkers and for T2 biomarkers. Variables associated at a p < 0.20 threshold with E2 or TNF or with six-month death were considered potential confounders and were included as covariates in the cross-lag panel and Cox regression models.
The covariates included in the cross-lag model were: age, GCS, contusion, DAI, SDH, and rs2470152 genotype. For the Cox regression models, a series of four hierarchical models were created: model 1 (unadjusted), model 2 (adjusted for age only), model 3 (adjusted for age, GCS, non-head ISS CT abnormalities: contusion, SDH, and DAI), and model 4 (adjusted for age, GCS, CT abnormalities: contusion, SDH, and DAI, and rs2470152 genotype). The Harrell Concordance Statistic, a measure for model fit for Cox regression,48 was reported for all models.
To assess whether dysfunction across each of the five NNOD domains (CV, hematological, renal, hepatic, and pulmonary dysfunction) mediates biomarker-death associations, we conducted a series of mediation analyses following processes outlined by Baron and Kenny49 and by Mackinnon and Dwyer.22,50,51 We also tested the total count of NNOD domains with dysfunction as a mediator. These mediation models tested and estimated the age and sex adjusted associations between T1 and T2 E2 and TNFα and six-month death with (direct effect) and without (total effect) adjustment for each NNOD domain, and total count of NNOD domains.
We used multi-variable logistic regression for all binary outcome paths (each NNOD domain and six-month death), and a negative binomial model for total count of NNOD domain. We calculated the total mediation percentage for regression paths using the Y-standardization, a method proposed by Rijinhard and colleagues.52 We reported the mediation percentage in instances where the total effect was significant and greater than the direct effect. If the confidence interval of the direct effect overlapped 1, then the mediator was considered a full mediator; otherwise, the relationship was considered a partial mediator. Statistical analyses were performed using Stata 1553 and SAS 9.4.54
Results
Clinical and demographic variables by E2 and TNFα
Clinical and demographic variables were compared by E2 level at T1 and at T2 (Table 2) and by TNFα at T1 and at T2 (Table 3). Median E2 was 60.0 pg/mL at T1 and 35.6 pg/mL at T2. Median TNFα was 7.7 pg/mL at T1 and 8.5 pg/mL at T2. Patients with high E2 at T2 were older (p = 0.023). Individuals with high TNFα at T1 had significantly greater non-head ISS (p = 0.006). At both time points, a significantly higher proportion of individuals with high E2 had thoracic injuries and had cortical contusions (p < 0.05). Individuals with high TNFα had a higher likelihood of having abdominal injuries (p = 0.035). Finally, high E2 was associated with rs2470152 genotype at T1 (p = 0.028) and at T2 (p = 0.053). Specifically, participants with high E2 more often had the TC genotype.
Table 2.
Demographic and Clinical Characteristics in Traumatic Brain Injury Cohort by T1 and T2 Estradiol
| |
T1 Epoch |
|
T2 Epoch |
|
||
|---|---|---|---|---|---|---|
| Variables | E2 Above Median (n = 75) | E2 Below Median (n = 74) | p | E2 Above Median (n = 61) | E2 Below Median (n = 62) | p |
| Age (mean, SE) | 41.71 (2.06) | 36.40 (1.69) | 0.119 | 41.54 (2.16) | 34.95 (1.99) | 0.023* |
| Sex (men, %) | 57 (79.17) | 58 (81.69) | 0.704 | 47 (77.05) | 50 (80.65) | 0.625 |
| Race, (n, %) | 0.982 | 0.299 | ||||
| White | 68 (93.15) | 69 (93.24) | 58 (95.08) | 55 (90.16) | ||
| Black | 5 (6.85) | 5 (6.76) | 3 (4.92) | 6 (9.84) | ||
| Best in 24 h GCS (Median, IQR) | 7 (6–7) | 7 (6–8) | 0.105 | 7 (5–8) | 7 (6–8) | 0.438 |
| Maximum head AIS, (n, %) | 0.3591 | 0.5274 | ||||
| 2 | 0 (0.00) | 1 (1.37) | 0 (0.00) | 1 (1.67) | ||
| 3 | 4 (5.41) | 1 (1.37) | 3 (4.92) | 1 (1.67) | ||
| 4 | 13 (17.57) | 17 (23.29) | 12 (19.67) | 10 (16.67) | ||
| 5 | 56 (75.68) | 54 (73.97) | 46 (75.41) | 47 (78.33) | ||
| 6 | 1 (1.35) | 0 (0.00) | 0 (0.00) | 1 (1.67) | ||
| ISS (Mean, SE) | 34.81 (1.23) | 32.55 (1.18) | 0.255 | 35.39 (1.31) | 33.02 (1.35) | 0.184 |
| Non-head ISS (Mean, SE) | 13.98 (1.35) | 12.29 (1.29) | 0.266 | 14.62 (1.45) | 11.73 (1.30) | 0.134 |
| Length of hospital stay (Mean, SE) | 18.79 (1.53) | 21.77 (1.48) | 0.106 | 19.62 (1.57) | 24.42 (1.72) | 0.069 |
| Mechanism of injury, (n, %) | 0.827 | 0.443 | ||||
| MVA | 32 (46.38) | 37 (50.00) | 26 (44.07) | 31 (52.54) | ||
| Motorcycle | 15 (21.74) | 14 (18.92) | 16 (27.12) | 9 (15.25) | ||
| Fall | 15 (21.74) | 13 (17.57) | 11 (18.64) | 11 (18.64) | ||
| Other | 7 (10.14) | 10 (13.51) | 6 (10.17) | 8 (13.56) | ||
| CT injury type, (n, %) | ||||||
| SDH | 48 (64.86) | 49 (66.22) | 0.863 | 39 (62.90) | 38 (62.30) | 0.944 |
| SAH | 54 (72.97) | 46 (62.16) | 0.160 | 48 (78.69) | 39 (62.90) | 0.054 |
| IVH | 17 (22.97) | 24 (32.43) | 0.199 | 15 (24.59) | 21 (33.87) | 0.258 |
| EDH | 11 (14.86) | 8 (10.81) | 0.461 | 8 (13.11) | 7 (11.29) | 0.757 |
| DAI | 17 (22.97) | 26 (35.14) | 0.103 | 15 (24.59) | 23 (37.10) | 0.133 |
| Contusion | 35 (47.30) | 23 (31.08) | 0.043* | 32 (52.46) | 20 (32.26) | 0.023* |
| Splenic injury, (n, %) | 11 (14.67) | 4 (5.41) | 0.060 | 9 (14.75) | 5 (8.06) | 0.243 |
| Abdominal injury, (n, %) | 22 (34.92) | 28 (40.00) | 0.546 | 23 (41.82) | 20 (35.71) | 0.509 |
| Thoracic injury, (n, %) | 25 (39.68) | 13 (18.57) | 0.007* | 25 (45.45) | 10 (17.86) | 0.002* |
| Extremity injury, (n, %) | 44 (59.46%) | 44 (59.46%) | 1.000 | 33 (54.10%) | 36 (59.02%) | 0.583 |
| RS2470152 genotype, (n, %) | 0.028* | 0.053 | ||||
| CC | 11 (18.33) | 22 (37.29) | 13 (25.00) | 12 (25.00) | ||
| TC | 36 (60.00) | 22 (37.29) | 30 (57.69) | 18 (37.50) | ||
| TT | 13 (21.67) | 15 (25.42) | 9 (17.31) | 18 (37.50) | ||
E2, estradiol; SE, standard error; GCS, Glasgow Coma Scale; IQR, interquartile range; AIS, Abbreviated Injury Scale; ISS, Injury Severity Score; MVA, motor vehicle accident; SDH, subdural hematoma; SAH, subarachnoid hemorrhage; IVH, intraventricular hemorrhage; EDH, epidural hematoma; DAI, diffuse axonal injury. *indicates statistically significant at α = 0.05.
E2 T1 median: 60.00 pg/mL (n = 8 missing at T1); T2 median: 35.58 pg/mL (n = 34 missing at T2).
Table 3.
Demographic and Clinical Characteristics in Traumatic Brain Injury Cohort By T1 And T2 Tumor Necrosis Factor alpha
| |
T1 Epoch |
|
T2 Epoch |
|
||
|---|---|---|---|---|---|---|
| Variables | Above Median (n = 72) | TNFα Below Median (n = 71) | p | TNFα Above Median (n = 62) | TNFα Below Median (n = 62) | p |
| Age (Mean, SE) | 41.92 (2.08) | 36.77 (1.81) | 0.099 | 38.23 (2.00) | 36.98 (2.05) | 0.631 |
| Sex (men, %) | 57 (79.17) | 58 (81.69) | 0.704 | 50 (80.65) | 50 (80.65) | 0.999 |
| Race, (n, %) | 0.074 | 0.079 | ||||
| White | 70 (97.22) | 63 (90.00) | 60 (96.77) | 54 (88.52) | ||
| Black | 2 (2.78) | 7 (10.00) | 2 (3.23) | 7 (11.48) | ||
| Best in 24 h GCS (Median, IQR) | 7 (5–8) | 7 (6–8) | 0.149 | 7 (6–8) | 7 (6–8) | 0.483 |
| Maximum head AIS, (n, %) | 0.6470 | 0.5507 | ||||
| 2 | 0 (0.00) | 1 (1.43) | 0 (0.00) | 1 (1.64) | ||
| 3 | 3 (4.23) | 2 (2.86) | 3 (4.92) | 1 (1.64) | ||
| 4 | 13 (18.31) | 15 (21.43) | 11 (18.03) | 11 (18.03) | ||
| 5 | 55 (77.46) | 51 (72.86) | 46 (75.41) | 48 (78.69) | ||
| 6 | 0 (0.00) | 1 (1.43) | 1 (1.64) | 0 (0.00) | ||
| ISS (Mean, SE) | 35.42 (1.18) | 31.65 (1.27) | 0.069 | 34.73 (1.43) | 33.25 (1.25) | 0.639 |
| Non-head ISS (Mean, SE) | 15.48 (1.39) | 10.60 (1.26) | 0.006* | 14.16 (1.50) | 11.27 (1.30) | 0.181 |
| Length of hospital stay (Mean, SE) | 19.36 (1.52) | 20.71 (1.55) | 0.460 | 21.55 (1.74) | 21.42 (1.58) | 0.828 |
| Mechanism of injury, (n, %) | 0.878 | 0.827 | ||||
| MVA | 29 (42.65) | 34 (48.57) | 29 (49.15) | 28 (27.46) | ||
| Motorcycle | 16 (23.53) | 13 (18.57) | 13 (22.03) | 13 (22.03) | ||
| Fall | 15 (22.06) | 15 (21.43) | 12 (20.34) | 10 (16.95) | ||
| Other | 8 (11.76) | 8 (11.43) | 5 (8.47) | 8 (13.56) | ||
| CT injury type, (n, %) | ||||||
| SDH | 45 (63.38) | 50 (70.42) | 0.373 | 43 (69.35) | 34 (54.84) | 0.096 |
| SAH | 53 (74.65) | 45 (63.38) | 0.147 | 49 (79.03) | 38 (61.29) | 0.031 |
| IVH | 18 (25.35) | 21 (29.58) | 0.573 | 21 (33.87) | 15 (24.19) | 0.235 |
| EDH | 11 (15.49) | 7 (9.86) | 0.313 | 6 (9.68) | 8 (12.90) | 0.570 |
| DAI | 20 (28.17) | 19 (26.76) | 0.851 | 15 (24.19) | 25 (40.32) | 0.055 |
| Contusion | 31 (43.66) | 27 (38.03) | 0.495 | 30 (48.39) | 21 (33.87) | 0.100 |
| Splenic injury, (n, %) | 8 (11.11) | 5 (7.04) | 0.397 | 8 (12.90) | 4 (6.45) | 0.224 |
| Abdominal injury, (n, %) | 28 (45.16) | 18 (27.27) | 0.035* | 24 (42.11) | 18 (33.96) | 0.380 |
| Thoracic injury, (n, %) | 22 (35.48) | 15 (22.73) | 0.112 | 22 (38.60) | 11 (20.75) | 0.041* |
| Extremity injury, (n, %) | 37 (52.11) | 50 (70.42) | 0.025* | 36 (58.06) | 37 (60.66) | 0.770 |
α, tumor necrosis factor alpha; SE, standard error; GCS, Glasgow Coma Scale; IQR, interquartile range; AIS, Abbreviated Injury Scale; ISS, Injury Severity Score; MVA, motor vehicle accident; SDH, subdural hematoma; SAH, subarachnoid hemorrhage; IVH, intraventricular hemorrhage; EDH, epidural hematoma; DAI, diffuse axonal injury. *indicates statistically significant at α = 0.05.
α T1 median: 2.04 pg/mL (n = 14 missing); T2 median: 2.14 pg/mL (n = 33 missing).
Clinical and demographic variables by mortality status
The clinical and demographic variables by six-month mortality status are provided in Table 4. The average age for non-survivors was significantly higher than for survivors (p = < 0.001). Non-survivors had lower GCS scores compared with survivors (p = 0.028). There also was a significant difference between survivors and non-survivors with respect to mechanism of injury (p = < 0.001). Non-survivors more often had contusion (p = 0.007) and SDH (p = 0.092), and survivors were more likely to have DAI (p = 0.004). There was a trend between rs2470152 genotype and mortality status (p = 0.099), wherein non-survivors had a greater likelihood of having the TC genotype.
Table 4.
Demographic and Clinical Characteristics in Traumatic Brain Injury Cohort by Six-month Mortality Status
| Variable | Non-survivors (n = 48) | Survivors (n = 109) | p |
|---|---|---|---|
| Age (Mean, SE) | 51.08 (2.29) | 33.54 (1.35) | < 0.001 |
| Sex (men, %) | 35 (72.92) | 92 (84.40) | 0.092 |
| Race, (n, %) | 0.607 | ||
| White | 45 (95.74) | 99 (91.67) | |
| Black | 2 (4.26) | 8 (7.41) | |
| Other | 0 (0) | 1 (0.93) | |
| Best in 24 h GCS (Median, IQR) | 6 (5–7) | 7 (6–8) | 0.028 |
| Maximum head AIS severity, (n, %) | 0.6353 | ||
| 2 | 0 (0.00) | 1 (0.94) | |
| 3 | 1 (2.08) | 4 (3.77) | |
| 4 | 7 (14.58) | 23 (21.70) | |
| 5 | 40 (83.33) | 77 (72.64) | |
| 6 | 0 (0.00) | 1 (0.94) | |
| ISS (Mean, SE) | 33.90 (1.48) | 33.78 (1.00) | 0.655 |
| Non-head ISS (Mean, SE) | 14.30 (1.79) | 12.89 (1.06) | 0.558 |
| Length of hospital stay (Mean, SE) | 12.53 (1.34) | 23.89 (1.23) | < 0.001 |
| Mechanism of injury, (n, %) | < 0.001 | ||
| MVA | 16 (35.56) | 56 (53.33) | |
| Motorcycle | 8 (17.78) | 23 (21.90) | |
| Fall | 18 (40.00) | 12 (11.43) | |
| Other | 3 (6.67) | 14 (13.33) | |
| CT injury type, (n, %) | |||
| SDH | 36 (75.00) | 66 (61.11) | 0.092 |
| SAH | 35 (72.92) | 71 (65.71) | 0.375 |
| IVH | 12 (25.00) | 30 (27.78) | 0.718 |
| EDH | 9 (18.75) | 13 (12.04) | 0.266 |
| DAI | 6 (12.50) | 38 (35.19) | 0.004 |
| Contusion | 27 (56.25) | 36 (33.33) | 0.007 |
| Other | 4 (8.33) | 5 (4.63) | 0.360 |
| Splenic injury, (n, %) | 4 (8.33) | 11 (10.09) | 0.730 |
| Abdominal injury, (n, %) | 15 (36.59) | 38 (39.18) | 0.775 |
| Thoracic injury, (n, %) | 10 (24.39) | 30 (30.93) | 0.439 |
| Extremity injury, (n, %) | 29 (60.42) | 62 (57.94) | 0.773 |
| RS2470152 genotype, (n, %) | 0.099 | ||
| CC | 5 (13.89) | 25 (28.74) | |
| TC | 22 (61.11) | 36 (41.38) | |
| TT | 9 (25.00) | 26 (29.89) |
SE, standard error; GCS, Glasgow Coma Scale; IQR, interquartile range; AIS, Abbreviated Injury Scale; ISS, Injury Severity Score; MVA, motor vehicle accident; SDH, subdural hematoma; SAH, subarachnoid hemorrhage; IVH, intraventricular hemorrhage; EDH, epidural hematoma; DAI, diffuse axonal injury.
Cross-lagged panel model
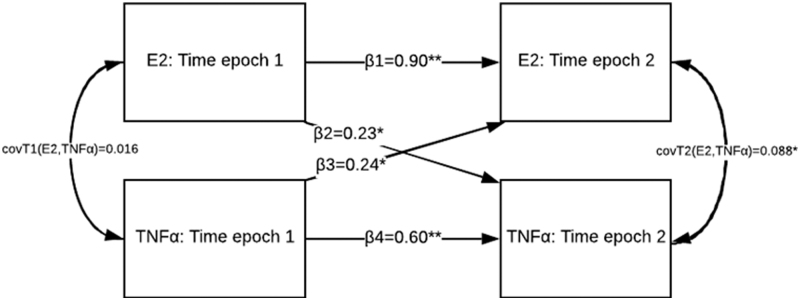
E2 and TNFα were log transformed at T1 and T2 to account for right skewedness. The cross-lagged panel is depicted in Figure 3 focusing on primary paths between E2 and TNFα at T1 to T2. The autoregressive path between E2 at T1 and E2 at T2 was statistically significant (β = 0.901, p = < 0.001), after adjustment for covariates. Likewise, the autoregressive path between TNFα at T1 and TNFα at T2 was significant (β = 0.602, p = < 0.001), after adjustment for covariates. The significance of the autoregressive paths indicates stability in the same variable with time.
FIG. 3.
The fitted cross-lag panel model. Both autoregressive paths are significant; Estradiol (E2) and tumor necrosis factor alpha (TNFα) at T1 are associated with E2 at T2 (β = 0.901, p = < 0.001) and TNFα at T2 (β = 0.602, p = < 0.001). Likewise, both cross-lag paths were statistically significant; E2 at T1 was associated with TNFα at T2 (β = 0.227, p = 0.002), and TNFα at T1 was associated with E2 and T2 (β = 0.236, p = 0.018). The significance of the cross-lag paths indicates a significant relationship between the variables over time. The covariance between E2 and TNFα at T1 was not significant (cov = 0.016, p = 0.814), but was significant at T2 (cov = 0.088, p = 0.029).
The cross-lag path between E2 at T1 and TNFα at T2 was statistically significant (β = 0.227, p = 0.002), as was the path between TNFα at T1 and E2 at T2 (β = 0.236, p = 0.018). The significance of both cross-lag paths indicates an independent relationship, with similar effect sizes, between the two variables over time. The residual covariance between E2 and TNFα at T1 was not significant (cov = 0.016, p = 0.814) and was significant at T2 (cov = 0.088, p = 0.029).
Survival analysis
Time until death ranged from two to 159 days post-injury. A total of 48 individuals (30.6%) died within six months of injury (45 died during their acute hospital stay, and three died after acute discharge). The unadjusted and covariate-adjusted Cox proportional hazards model for E2 and TNFα at T1 and at T2 is provided in Tables 5a and 5b, respectively. At T1, E2 was significantly associated with mortality risk, such that each unit increase in ln(E2) was associated with an 79% higher mortality risk, after adjustment for covariates (aHR [hazard ratio] = 1.79, 95% confidence interval [CI]: 1.15, 2.81). The E2 level at T2 was not significantly associated with mortality risk (aHR = 0.91, 95% CI: 0.56, 1.47; p = 0.691).
Table 5A.
Cox Proportional Hazards Regression for Death based on T1 Epoch (1st 72 h Post-Injury) Biomarkers
| |
Model 1£ |
Model 2§ |
|
Model 3¥ |
|
Model 4€ |
|
|
|---|---|---|---|---|---|---|---|---|
| Hazard ratio (95% CI) | p | Hazard ratio (95% CI) | p | Hazard ratio (95% CI) | p | Hazard ratio (95% CI) | p | |
| ln(E2) ln(TNFα) Harrell Concordance Statistic |
1.84 (1.31, 2.59) 1.56 (1.067, 2.27) 0.707 |
< 0.001 0.022 |
1.74 (1.22, 2.50) 1.55 (1.033, 2.31) 0.790 |
0.002 0.034 |
1.79 (1.15, 2.81) 1.44 (1.00, 2.08) 0.835 |
0.011 0.051 |
1.81 (1.14, 2.87) 1.47 (0.99, 2.19) 0.834 |
0.012 0.056 |
CI, confidence interval; E2, estradiol; TNFα, tumor necrosis factor alpha.
£: Model 1: Unadjusted.
§: Model 2: Adjusted for age only.
¥: Model 3: Adjusted for age and clinical variables (Glasgow Coma Scale, non-head Injury Severity Score, and computed tomography abnormalities: contusion, subdural hematoma, diffuse axonal injury).
€: Model 4: Adjusted for age, clinical variables, and rs2470152 genotype.
Table 5B.
Cox Proportional Hazards Regression for Death Based on T2 Epoch (2nd 72 h Post-Injury) Biomarkers
| |
Model 1£ |
Model 2§ |
|
Model 3¥ |
|
Model 4€ |
|
|
|---|---|---|---|---|---|---|---|---|
| Hazard ratio (95% CI) | p | Hazard ratio (95% CI) | p | Hazard ratio (95% CI) | p | Hazard ratio (95% CI) | p | |
| ln(E2) ln(TNFα) Harrell's Concordance Statistic |
1.36 (0.91, 2.02) 1.69 (1.03, 2.79) 0.696 |
0.133 0.039 |
1.24 (0.84, 1.83) 1.71 (1.05, 2.76) 0.756 |
0.287 0.030 |
1.10 (0.70, 1.74) 2.14 (1.22, 3.75) 0.804 |
0.680 0.008 |
0.91 (0.56, 1.47) 2.47 (1.35, 4.53) 0.819 |
0.691 0.003 |
CI, confidence interval; E2, estradiol; TNFα, tumor necrosis factor alpha.
£: Model 1: Unadjusted.
§: Model 2: Adjusted for age only.
¥: Model 3: Adjusted for age and clinical variables (Glasgow Coma Scale, non-head Injury Severity Score, and computed tomography abnormalities: contusion, subdural hematoma, diffuse axonal injury).
€: Model 4: Adjusted for age, clinical variables, and rs2470152 genotype.
At T1, a one unit increase in ln(TNFα) was associated with a 47% increased mortality risk (aHR = 1.47, 95% CI: 0.99, 2.19; p = 0.056), but this relationship did not reach statistical significance. In contrast, the TNFα level at T2 was significantly associated with mortality risk, such that one unit increase in ln(TNFα) was associated with a nearly 2.5 times higher mortality risk (aHR = 2.47, 95% CI: 1.35, 4.53; p = 0.003).
The Harrel Concordance Statistic for the unadjusted E2 and TNFα models at T1 and at T2 were 0.707 and 0.696, respectively, indicating moderately strong model discrimination. Adjusting for age improved model fit to 0.790 and 0.756, respectively. Adding clinical variables (GCS, non-head ISS, and CT abnormalities) increased the model fit to 0.835 and 0.804, respectively. The fully adjusted model, adding rs2470152 genotype, had a model fit at T1 and T2 of 0.834, and 0.819, respectively, indicating moderately strong model discrimination overall for death.
Organ dysfunction analysis
The rates of dysfunction for each system were as follows: CV (51.9%), hematological (44.9%), renal (15.5%), hepatic (10.3%), and respiratory (84.4%). The mortality rates for each dysfunction were as follows: CV (42.3%), hematology (34.0%), renal (61.1%), hepatic (53.9%), and respiratory (31.5%).
The mediation analysis regression models are adjusted for age and sex and are summarized in Tables 6a and 6b. The CV dysfunction was a partial mediator of the effects of T1 E2 (mediation percentage = 19.9%) and T2 TNFα (mediation percentage = 20.0%) on death. Renal dysfunction was a partial mediator of the effects of T1 E2 (mediation percentage = 30.7%) and T1 TNFα (mediation percentage = 30.1%) and T2 TNFα (mediation percentage = 29.7%), and a full mediator of the effects of T2 E2 (mediation percentage = 71.9%), on death.
Table 6A.
Mediation Model of the Relationship between T1 and T2 Estradiol and Six-Month Mortality
| Mediators (analyzed separately) | Association between T1 E2 and mediator |
T1 E2 |
Association between T2 E2 and mediator |
T2 E2 |
|
|
||
|---|---|---|---|---|---|---|---|---|
| Total effect | Direct effect | Mediation % | Total effect | Direct effect | Mediation % | |||
| OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | |||
| 1. Cardiovascular dysfunction | 1.58 (1.06, 2.35)* | 1.91 (1.19, 3.07)* | 1.72 (1.05, 2.81)* | 19.87¥ | 1.25 (0.88, 1.77) | 1.34 (0.91, 1.96) | 1.29 (0.87, 1.92) | †† |
| 2. Hematology dysfunction | 1.72 (1.07, 2.76)* | 2.89 (1.51, 5.53)* | 2.78 (1.44, 5.35)* | 4.52¥ | 1.79 (1.13, 2.83)* | 1.92 (1.14, 3.21)* | 1.79 (1.05, 3.07)* | 11.21¥ |
| 3. Renal dysfunction | 3.55 (1.70, 7.39)* | 2.78 (1.46, 5.31)* | 2.12 (1.03, 4.35)* | 30.69¥ | 3.98 (1.92, 8.28)* | 1.85 (1.11, 3.11)* | 1.21 (0.66, 2.20) | 71.90€ |
| 4. Hepatic dysfunction | 1.69 (0.90, 3.19) | 1.91 (1.17, 3.13)* | 1.83 (1.09, 3.08)* | 10.48¥ | 2.26 (1.26, 4.05)* | 1.44 (0.97, 2.13) | 1.28 (0.84, 1.94) | †† |
| 5. Respiratory dysfunction | 1.70 (0.97, 2.98) | 2.30 (1.36, 3.88)* | 2.32 (1.37, 3.93)* | †† | 1.39 (0.75, 2.57) | 1.48 (0.98, 2.25) | 1.50 (0.98, 2.28) | †† |
| IRR (95% CI) | OR (95% CI) | OR (95% CI) | Mediation % | IRR (95% CI) | OR (95% CI) | OR (95% CI) | Mediation % | |
| 6. Total count of system dysfunction | 1.28 (1.07, 1.52)* | 2.97 (1.46, 6.06)* | 2.50 (1.16, 5.37)* | 20.65¥ | 1.27 (1.16, 3.41)* | 1.99 (1.16, 3.41)* | 1.40 (0.74, 2.65) | 54.02€ |
E2, estradiol; OR, odds ratio; CI, confidence interval; IRR, incidence rate ratio.
Indicates p < 0.05; All paths adjusted for age and sex.
 : Total effect not significant and/or total effect ≤ direct effect, mediation % not calculated.
: Total effect not significant and/or total effect ≤ direct effect, mediation % not calculated.
¥: Indicates a partial mediator.
€: Indicates a full mediator.
Table 6B.
Mediation Model of the Relationship between T1 and T2 Tumor Necrosos Factor Alpha and Six-Month Mortality
| Mediators (analyzed separately) | Association between T1 TNFα and mediator |
T1 TNFα |
Association between T2 TNFα and mediator |
T2 TNFα |
|
|
||
|---|---|---|---|---|---|---|---|---|
| Total effect | Direct effect | Mediation % | Total effect | Direct effect | Mediation % | |||
| OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | |||
| 1. Cardiovascular dysfunction | 1.29 (0.80, 2.07) | 2.19 (1.19, 4.03)* | 2.22 (1.17, 4.24)* | †† | 2.36 (1.33, 4.19)* | 2.89 (1.47, 5.67)* | 2.47 (1.24, 4.92)* | 20.01¥ |
| 2. Hematology dysfunction | 1.49 (0.86, 2.58) | 2.64 (1.28, 5.46)* | 2.56 (1.26, 5.34)* | 3.81¥ | 1.94 (1.07, 3.53)* | 3.77 (1.67, 8.50)* | 3.63 (1.58, 8.32)* | 3.41¥ |
| 3. Renal dysfunction | 4.78 (2.00, 11.44)* | 3.20 (1.46, 6.98)* | 2.37 (1.00, 5.59)* | 30.13¥ | 4.28 (1.81, 10.14)* | 3.76 (1.64, 8.59)* | 2.68 (1.08, 6.64)* | 29.70¥ |
| 4. Hepatic dysfunction | 1.11 (0.47, 2.60) | 2.11 (1.06, 4.19)* | 2.13 (1.04, 4.35) | †† | 3.08 (1.30, 7.30)* | 3.82 (1.69, 8.65)* | 3.44 (1.47, 8.06)* | 10.34¥ |
| 5. Respiratory dysfunction | 1.02 (0.53, 1.98) | 2.36 (1.24, 4.50)* | 2.36 (1.24, 4.50)* | †† | 1.23 (0.54, 2.80) | 3.81 (1.77, 8.22)* | 3.82 (1.77, 8.26)* | †† |
| IRR (95% CI) | OR (95% CI) | OR (95% CI) | Mediation % | IRR (95% CI) | OR (95% CI) | OR (95% CI) | Mediation % | |
| 6. Total count of system dysfunction | 1.21 (0.97, 1.49) | 3.34 (1.41, 7.90)* | 3.10 (1.23, 7.78)* | 13.02¥ | 1.32 (1.07, 1.63)* | 6.34 (2.13, 18.89)* | 5.31 (1.63, 17.30)* | 15.14¥ |
TNFα, tumor necrosis factor alpha; OR, odds ratio; CI, confidence interval; IRR, incidence rate ratio.
Indicates p < 0.05; All paths adjusted for age and sex.
: Total effect not significant and/or total effect ≤ direct effect, mediation % not calculated.
¥: Indicates a partial mediator.
€: Indicates a full mediator.
Hepatic dysfunction was a partial mediator of the effects of T1 E2 (mediation percentage = 10.5%) and T2 TNFα (mediation percentage = 10.3%) on death. Hematology and respiratory dysfunction were not mediators at either time point. The total number of NNOD domains with dysfunction was a partial mediator of T1 E2 (mediation percentage = 20.7%) and T2 E2 (mediation percentage = 54.0%) and T1 TNFα (mediation percentage = 13.0%) and T2 TNFα (mediation percentage = 15.1%) effects on death.
Discussion
The physiological response to TBI is extensive and spans neurological and non-neurological systems. After severe trauma, two of the body's stress regulation networks, the hypothalamic-pituitary-adrenal axis and SNS, are triggered to drive the systemic response to injury. An overactive systemic response to TBI increases the risk for acute death. In fact, one study observed that 40% of in-hospital deaths from TBI were non-neurological in etiology.55 Yet, past studies in TBI populations have focused primarily on brain-specific biomarkers. The present study provides evidence that peripheral biomarkers, TNFα and E2, are reciprocally related and relevant to survival after severe TBI. These results provide evidence of TNFα and E2 associations over the first week after TBI, and their associations with time until death.
It is well-documented that TNFα in the brain is produced from activated microglia and acts as a mediator of the neuroinflammatory response to TBI.56–59 The systemic role of TNFα is more multi-faceted in the context of TBI, however. In response to trauma, the SNS facilitates an acute phase hepatic response that contributes to an increase in peripheral inflammation.29,30,60 Serum E2 is a systemic biomarker that we have documented previously to be increased in both sexes after severe TBI.18 Systemic TNFα directly impacts extragonadal E2 production by serving as a transcription factor for E2 in adipose tissue.34–39 Also, peripheral E2 propagates systemic inflammation, including TNFα production, by acting as a norepinephrine reuptake inhibitor in lymphoid tissue to directly amplify the SNS response to injury.40,41
These study findings support the hypothesis that E2 and TNFα are significantly related over time acutely after TBI. This finding suggests that peripheral inflammatory and hormone networks are biologically interrelated in the acute phases after TBI, which likely represents a pathological cascade that is relevant to survival after severe TBI.
These results show that both E2 and TNFα are significant mortality markers in different time epochs in the first six days after severe TBI. In the total cohort, E2 levels are associated with mortality risk in the first 72 h but not in the second 72 h after TBI. In contrast, TNFα is a highly significant mortality marker in the total cohort in the second 72 h after TBI, and modestly associated with death in the first 72 h. Also, the significance of the covariance at T2 suggests a stronger correlation between E2 and TNFα at later, rather than earlier, time points in the acute phase post-injury.
The observed associations from this study add to the existing body of clinical evidence of the lethal associations with excessive systemic E2 and TNFα. Two independent studies involving intensive care unit patients demonstrated that elevated serum TNFα levels were associated with incident sepsis/septic shock and in-hospital death.61,62 In a clinical study in a general trauma population, Zolin and colleagues41 determined that elevated systemic E2 at 24 h post-injury was associated with increased odds for multiple organ failure. Two other studies involving surgical and trauma patients determined that systemic E2 is a strong predictor of trauma-related death.63,64
In TBI, previous studies by Wagner and colleagues18 have shown that peripheral E2, along with its precursors E165 and androstenedione,66 are potent mortality markers. In addition to fueling TNFα production (a potent vasodilator), a potential mechanism for these observed harmful effects of systemic E2 after trauma could be through its direct vasodilatory actions, promoting harmful nitric oxide and hydrogen sulfide pathways.67–70
The secondary results support this hypothesis, showing that individuals with severe TBI and with higher E2 and TNFα levels have greater odds of acute dysfunction across multiple non-neurological systems. Our findings also suggest relationships of E2 and TNFα with death are mediated in part by CV, renal, and hepatic dysfunction. Higher E2 levels likely contribute to death through vasodilatory effects that exacerbate hypotension, increasing overall CV dysfunction risk and contributing to death. Importantly, E2-induced hypotension has more broad implications beyond the CV system, because it can also result in poor organ perfusion leading to global ischemia-perfusion injury to the kidney and liver.71,72
These solid organs are also vulnerable to inflammation after traumatic hemorrhagic shock and resuscitation62,73,74 secondary to TNFα production among other inflammatory mediators. The TNFα production is central to NNOD, particularly because of induction of adhesion molecule expression on endothelial cells,75 thereby contributing to cellular infiltration and proinflammation. The cumulative mediation effect observed across multiple systems in the present study (measured through the total count of NNOD domains) suggests that the E2 and TNFα response represents a broad pathophysiological response to severe TBI that may be lethal, regardless of neurological treatment/support.
The results of this study are particularly timely considering the recent findings of two large phase III trials for progesterone treatment for the TBI population.11,12 Crucially, progesterone is the biological precursor of E2 in the steroidogenesis pathway. Findings from this study indicate that individuals with higher endogenous E2, and concurrently its extragonadal transcription factor TNFα, are at great mortality risk after severe TBI. High baseline levels of these two systemic biomarkers may be relevant to post-treatment progesterone metabolism; however, this hypothesis needs to be tested formally.
In light of these clinical findings, it is important to discuss the tremendous track record of progesterone as a neuroprotectant. This hormone demonstrated appreciable neuroprotective benefits in several experimental TBI studies conducted over multiple decades.13,14,76–79 The phase III clinical trial results did not translate to human TBI, however. One reason may be that experimental TBI models do not accurately mirror what is observed clinically in critically ill, hospitalized patients with severe TBI. For instance, animal models do not include coma, do not necessitate intubation, do not typically include concomitant extracerebral injury, and animals are not as prone as humans to systemic or critical illness after injury. Clinical observational studies have documented the high rates of non-neurological deaths in severe TBI populations.55,80,81 It is possible that (for some) the contribution of the acute systemic response to severe trauma is a larger driver of survival than the CNS pathophysiological changes that occur during the initial days after TBI.
We propose that a baseline assessment of an individual's E2 and TNFα, along with clinical and demographic variables such as age and GCS score, could be important to characterize baseline mortality risk after severe TBI. This point may have specific utility to inform participant inclusion into clinical trials in general. Individuals with a high baseline risk may not be appropriate candidates for any neuroprotective clinical trial if they also are at high risk for death independent of any neuroprotective treatment received. For these individuals, concerted efforts should be focused on preventing or controlling major systemic compromise to prevent death. In contrast, individuals with low or moderate baseline risk for death because of major systemic compromise may be more appropriate candidates for inclusion into neuroprotective clinical trials, including perhaps progesterone interventional trials.
In this study, we observed that extracerebral trauma is a particularly strong risk factor for systemic increases in E2 and TNFα. Individuals with thoracic and abdominal injuries had higher levels on average of E2 and TNFα. Extremity injuries were also associated with T1 TNFα. We demonstrate that extracerebral trauma exacerbates the secondary systemic inflammatory response, perhaps through direct impact of major immunological organs, including liver, spleen, and mesenteric system lymph nodes. We adjusted for extracerebral injury severity (measured with non-head ISS), however, and the primary effects of the biomarkers on the mortality risk only marginally changed, suggesting it is not a major confounder of mortality risk.
We also observed that older age was associated with elevated E2; however, similar to previous work, we did not observe any differences in E2 by sex,18 suggesting that elevated E2 concentrations post-TBI are derived primarily from extragonadal sources. In addition, we observed from this study that the aromatase SNP rs2470152 is associated with serum E2, such that heterozygotes tend to have higher E2 levels compared with homozygotes. In a previous study,43 we observed that two SNPs on the aromatase gene (rs2470152 and rs4646) were associated with estradiol/testosterone ratios and Glasgow Outcome Scores at six months, and the current findings suggest that adjusting for E2-related personal biology factors may be relevant when considering E2 profiles and hormone treatment effects in the setting of TBI.
In our recent epidemiological work involving data from the Pennsylvania Trauma Outcomes Study, we have observed that individuals with severe AIS for head and neck showed sex differences in cardiac complications associated death.82 Women aged 50 years and higher were less likely to die from their cardiac complications then men of the same age, which is consistent with other large database studies showing a potential “protective” sex effect on complications and associated death for post-menopausal women among these populations with severe TBI.83,84
These findings suggest a potentially relevant point to consider with future studies exploring E2 and TNFα risk of death, wherein it is speculative, yet plausible, that there are sex-specific age-related differences in extragonadal hormone production and effects that might influence these relationships. Larger studies are needed to tease out age and sex relationships with E2 and TNFα and NNOD-associated death after TBI.
A cross-lag panel analysis was utilized in this article to characterize E2 and TNFα relationships over the first week post-injury. Through simultaneous modeling of autoregressive and cross-lags paths, this statistical methodology allows for the examination of longitudinal relationships of multiple variables. Historically, this method has been applied principally in developmental and social science literature.47,85 This work, however, shows that the methodology has significant utility in TBI biomarkers research to aid in the study of relationships between markers over time. Such statistical modeling can be used, in conjunction with hypothesized theories and empirical results from experimental studies, to better understand longitudinal patterns between biomarkers of interest after TBI.
There are study limitations that should be discussed. The primary analysis consisted of biomarkers averaged over the first 72 h (T1) and second 72 h (T2). Because of missing data, often due to conflicts with clinical care, we were unable to have more granularity when characterizing temporal biomarker data profiles (e.g., daily levels) and the sample size for the T2 cohort was slightly less T1 cohort due to data availability. In addition, although the cross-lag approach nicely shows the interrelatedness between E2 and TNFα across T1 and T2, even after the adjustment for confounders, the study design is still a human observational study, and we cannot conclude that the observed relationships are causal.
Further, this study focuses on TNFα as the primary systemic inflammatory marker and extragonadal transcription factor; however, other evidence suggests that cortisol86 and interleukin-687 are transcription factors for E2 and may contribute to propagation of the systemic pathophysiological response to injury.
Also, our operational definition for systemic dysfunction in this study was based on SOFA components. There are limitations with these criteria, and more direct measures of dysfunction may be more specific (e.g., troponin or brain natriuretic peptide for CV dysfunction). We adjusted for the confounders of age, GCS, CT abnormalities (contusion, SDH, DAI), and rs2470152) in our primary analyses; however, it is possible other unmeasured confounding factors could alter the observed effect estimates for the cross-lag and survival analysis models. The mediation models employed a different modeling strategy than the survival analysis, and were limited by sample size due to data availability for system dysfunction components. This may have accounted for some differences in associations observed between T2 E2 and mortality in the survival and mediation analyses.
Finally, this study was performed in a cohort with severe TBI, and thus the utility of E2 and TNFα as prognostic indicators of death are not necessarily generalizable to those with less severe injuries; however, recent studies have noted differences in neurological and quality-of-life outcomes by hormonal status in populations with mild TBI.88 Future studies should explore further the association between E2 and TNFα and multi-dimensional survivor-based outcomes.
Conclusion
The results from this study provide intriguing evidence to support the hypotheses that the peripheral inflammatory and hormone markers, E2 and TNFα, have a positive feedback relationship with each other in the first week after severe TBI, and both biomarkers have prognostic value as indicators of mortality risk after injury. These biomarkers may be relevant for both research and clinical purposes to gauge baseline risk for systemic compromise and death after TBI.
Future experimental TBI studies should consider ways to simultaneously model systemic injury and/or shock,89 in addition to the TBI itself, to more appropriately mirror common clinical conditions observed after severe TBI. In addition, future experimental studies should examine the potential benefits of aromatase and TNFα inhibitors, as well as adrenergic blockage therapies. Future clinical studies would benefit from prospectively monitoring E2 and TNFα daily while assessing subsequent risk for and development of major systemic compromise and NNOD.
Acknowledgments
Parts of this article's analyses and writing were previously included in the first author's dissertation in the University of Pittsburgh, Graduate School of Public Health's Department of Epidemiology. The authors acknowledge the UPMC Trauma Registry team for their role in clinical data collection. This work was supported in part by NIH 5P01NS030318, CDC R49 CCR 323155, DOD W81XWH-071-0701, NIDILRR 90DP0041, NIH TL1 TR001858.
Author Disclosure Statement
No competing financial interests exist.
References
- 1. Harrison-Felix C., Whiteneck G., DeVivo M., Hammond F.M., and Jha A. (2004). Mortality following rehabilitation in the traumatic brain injury model systems of care. NeuroRehabilitation 19, 45–54 [PubMed] [Google Scholar]
- 2. Harrison-Felix C., Kolakowsky-Hayner S.A., Hammond F.M., Wang R., Englander J., Dams-O'Connor K., Kreider S.E., Novack T.A., and Diaz-Arrastia R. (2012). Mortality after surviving traumatic brain injury: risks based on age groups. J. Head Trauma Rehabil. 27, E45–E56 [DOI] [PubMed] [Google Scholar]
- 3. Harrison-Felix C., Pretz C., Hammond F.M., Cuthbert J.P., Bell J., Corrigan J., Miller A.C., and Haarbauer-Krupa J. (2015). Life expectancy after inpatient rehabilitation for traumatic brain injury in the United States. J. Neurotrauma 32, 1893–1901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Roozenbeek B., Maas A.I., and Menon D.K. (2013). Changing patterns in the epidemiology of traumatic brain injury. Nat. Rev. Neurol. 9, 231–236 [DOI] [PubMed] [Google Scholar]
- 5. Thurman D. (2001). The epidemiology and economics of head trauma, in: Head Trauma: Basic Preclinical and Clinical Directions. Miller L., Hayes R. (eds). John Wiley & Sons: New York, pps. 327–348 [Google Scholar]
- 6. Maas A.I., Stocchetti N., and Bullock R. (2008). Moderate and severe traumatic brain injury in adults. Lancet Neurol. 7, 728–741 [DOI] [PubMed] [Google Scholar]
- 7. Maas A.I., Roozenbeek B., and Manley G.T. (2010). Clinical trials in traumatic brain injury: past experience and current developments. Neurotherapeutics 7, 115–126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ullman J.S., Hawryluk G.W., Bell M.J., Bratton S.L., Chesnut R., Harris Md.O.A., Kissoon N., Rubiano A.M., Shutter L., Tasker R.C., and others. (2016). Guidelines for the Management of Severe Traumatic Brain Injury. Brain Trauma Foundation: Palo Alto, CA [Google Scholar]
- 9. Alderson P., and Roberts I. (2005). Corticosteroids for acute traumatic brain injury. Cochrane Database Syst. Rev. , CD000196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Edwards P., Arango M., Balica L., Cottingham R., El-Sayed H., Farrell B., Fernandes J., Gogichaisvili T., Golden N., Hartzenberg B., Husain M., Ulloa M.I., Jerbi Z., Khamis H., Komolafe E., Laloë V., Lomas G., Ludwig S., Mazairac G., Muñoz Sanchéz M. de los A., Nasi L., Olldashi F., Plunkett P., Roberts I., Sandercock P., Shakur H., Soler C., Stocker R., Svoboda P., Trenkler S., Venkataramana N.K., Wasserberg J., Yates D., Yutthakasemsunt S., and CRASH trial collaborators. (2005). Final results of MRC CRASH, a randomised placebo-controlled trial of intravenous corticosteroid in adults with head injury-outcomes at 6 months. Lancet 365, 1957–1959 [DOI] [PubMed] [Google Scholar]
- 11. Wright D.W., Yeatts S.D., Silbergleit R., Palesch Y.Y., Hertzberg V.S., Frankel M., Goldstein F.C., Caveney A.F., Howlett-Smith H., Bengelink E.M., Manley G.T., Merck L.H., Janis L.S., and Barsan W.G. (2014). Very early administration of progesterone for acute traumatic brain injury. N. Engl. J. Med. 371, 2457–2466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Skolnick B.E., Maas A.I., Narayan R.K., van der Hoop R.G., MacAllister T., Ward J.D., Nelson N.R., and Stocchetti N. (2014). A clinical trial of progesterone for severe traumatic brain injury. N. Engl. J. Med. 371, 2467–2476 [DOI] [PubMed] [Google Scholar]
- 13. Stein D.G. (2001). Brain damage, sex hormones and recovery: a new role for progesterone and estrogen? Trends Neurosci. 24, 386–391 [DOI] [PubMed] [Google Scholar]
- 14. Stein D.G., Wright D.W., and Kellermann A.L. (2008). Does progesterone have neuroprotective properties? Ann. Emerg. Med. 51, 164–172 [DOI] [PubMed] [Google Scholar]
- 15. Maier B., Laurer H.L., Rose S., Buurman W.A., and Marzi I. (2005). Physiological levels of pro- and anti-inflammatory mediators in cerebrospinal fluid and plasma: a normative study. J. Neurotrauma 22, 822–835 [DOI] [PubMed] [Google Scholar]
- 16. Stirling D.P., Khodarahmi K., Liu J., McPhail L.T., McBride C.B., Steeves J.D., Ramer M.S., and Tetzlaff W. (2004). Minocycline treatment reduces delayed oligodendrocyte death, attenuates axonal dieback, and improves functional outcome after spinal cord injury. J. Neurosci. 24, 2182–2190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Toulmond S., and Rothwell N.J. (1995). Interleukin-1 receptor antagonist inhibits neuronal damage caused by fluid percussion injury in the rat. Brain Res. 671, 261–266 [DOI] [PubMed] [Google Scholar]
- 18. Wagner A., McCullough E.H., Niyonkuru C., Ozawa H., Loucks T.L., Dobos J.A., Brett C.A., Santarsieri M., Dixon C.E., Berga S.L., and Fabio A. (2011). Acute serum hormone levels: characterization and prognosis after severe traumatic brain injury. J. Neurotrauma 28, 871–888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kumar R.G., Diamond M.L., Boles J.A., Berger R.P., Tisherman S.A., Kochanek P.M., and Wagner A.K. (2015). Acute CSF interleukin-6 trajectories after TBI: associations with neuroinflammation, polytrauma, and outcome. Brain. Behav. Immun. 45, 253–262 [DOI] [PubMed] [Google Scholar]
- 20. Kumar R.G., Rubin J.E., Berger R.P., Kochanek P.M., and Wagner A.K. (2016). Principal components derived from CSF inflammatory profiles predict outcome in survivors after severe traumatic brain injury. Brain. Behav. Immun. 53, 183–193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ziebell J.M., and Morganti-Kossmann M.C. (2010). Involvement of pro- and anti-inflammatory cytokines and chemokines in the pathophysiology of traumatic brain injury. Neurotherapeutics 7, 22–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Santarsieri M., Kumar R.G., Kochanek P.M., Berga S., and Wagner A.K. (2015). Variable neuroendocrine-immune dysfunction in individuals with unfavorable outcome after severe traumatic brain injury. Brain Behav. Immun. 45, 15–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Liu M.D., Luo P., Wang Z.J., and Fei Z. (2014). Changes of serum Tau, GFAP, TNF-α and malonaldehyde after blast-related traumatic brain injury. Chin. J. Traumatol 17, 317–322 [PubMed] [Google Scholar]
- 24. Morganti-Kossman M.C., Lenzlinger P.M., Hans V., Stahel P., Csuka E., Ammann E., Stocker R., Trentz O., and Kossmann T. (1997). Production of cytokines following brain injury: beneficial and deleterious for the damaged tissue. Mol. Psychiatry 2, 133–136 [DOI] [PubMed] [Google Scholar]
- 25. Csuka E., Morganti-Kossmann M.C., Lenzlinger P.M., Joller H., Trentz O., and Kossmann T. (1999). IL-10 levels in cerebrospinal fluid and serum of patients with severe traumatic brain injury: relationship to IL-6, TNF-alpha, TGF-beta1 and blood-brain barrier function. J. Neuroimmunol. 101, 211–221 [DOI] [PubMed] [Google Scholar]
- 26. Juengst S.B., Kumar R.G., Arenth P.M., and Wagner A.K. (2014). Exploratory associations with tumor necrosis factor-α, disinhibition and suicidal endorsement after traumatic brain injury. Brain. Behav. Immun. 41, 134–143 [DOI] [PubMed] [Google Scholar]
- 27. Kumar R., Ritter A., Kochanek P., Berga S., and Wagner A. (2014). Serum tumor necrosis factor-α (TNFα) as a biomarker for all-cause mortality in the first six months after traumatic brain injury: mechanistic relationships with estradiol. J. Neurotrauma 32, A92 [Google Scholar]
- 28. Lund S.B., Gjeilo K.H., Moen K.G., Schirmer-Mikalsen K., Skandsen T., and Vik A. (2016). Moderate traumatic brain injury, acute phase course and deviations in physiological variables: an observational study. Scand. J. Trauma Resusc. Emerg. Med. 24, 77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Baumann H., and Gauldie J. (1994). The acute phase response. Immunol. Today 15, 74–80 [DOI] [PubMed] [Google Scholar]
- 30. Heinrich P.C., Castell J.V., and Andus T. (1990). Interleukin-6 and the acute phase response. Biochem. J. 265, 621–636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Johns D.G., and Webb R.C. (1998). TNF-α-induced endothelium-independent vasodilation: a role for phospholipase A2-dependent ceramide signaling. Am. J. Physiol. 275, H1592–H1598 [DOI] [PubMed] [Google Scholar]
- 32. Strieter R.M., Kunkel S.L., and Bone R.C. (1993). Role of tumor necrosis factor-alpha in disease states and inflammation. Crit. Care Med. 21, S447–S463 [DOI] [PubMed] [Google Scholar]
- 33. Azcoitia I., Yague J.G., and Garcia-Segura L.M. (2011). Estradiol synthesis within the human brain. Neuroscience 191, 139–147 [DOI] [PubMed] [Google Scholar]
- 34. Simpson E.R. (2003). Sources of estrogen and their importance. J. Steroid Biochem. Mol. Biol. 86, 225–230 [DOI] [PubMed] [Google Scholar]
- 35. Simpson E.R., Zhao Y., Agarwal V.R., Michael M.D., Bulun S.E., Hinshelwood M.M., Graham-Lorence S., Sun T., Fisher C.R., Qin K., and Mendelson C.R. (1997). Aromatase expression in health and disease. Recent Prog. Horm. Res. 52, 185–213 [PubMed] [Google Scholar]
- 36. Simpson E.R., Mahendroo M.S., Means G.D., Kilgore M.W., Corbin C.J., and Mendelson C.R. (1993). Tissue-specific promoters regulate aromatase cytochrome P450 expression. J. Steroid Biochem. Mol. Biol. 44, 321–330 [DOI] [PubMed] [Google Scholar]
- 37. Irahara N., Miyoshi Y., Taguchi T., Tamaki Y., and Noguchi S. (2006). Quantitative analysis of aromatase mRNA expression derived from various promoters (I.4, I.3, PII and I.7) and its association with expression of TNF-α, IL-6 and COX-2 mRNAs in human breast cancer. Int. J. Cancer 118, 1915–1921 [DOI] [PubMed] [Google Scholar]
- 38. Zhao Y., Nichols J.E., Valdez R., Mendelson C.R., and Simpson E.R. (1996). Tumor necrosis factor-alpha stimulates aromatase gene expression in human adipose stromal cells through use of an activating protein-1 binding site upstream of promoter 1.4. Mol. Endocrinol. 10, 1350–1357 [DOI] [PubMed] [Google Scholar]
- 39. Simpson E.R. (2004). Aromatase: biologic relevance of tissue-specific expression. Semin. Reprod. Med. 22, 11–23 [DOI] [PubMed] [Google Scholar]
- 40. Elenkov I.J., Wilder R.L., Chrousos G.P., and Vizi E.S. (2000). The sympathetic nerve—an integrative interface between two supersystems: the brain and the immune system. Pharmacol. Rev. 52, 595–638 [PubMed] [Google Scholar]
- 41. Zolin S.J., Vodovotz Y., Forsythe R.M., Rosengart M.R., Namas R., Brown J.B., Peitzman A.P., Billiar T.R., and Sperry J.L. (2015). The early evolving sex hormone environment is associated with significant outcome and inflammatory response differences after injury. J. Trauma Acute Care Surg. 78, 451–457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Miller S.A., Dykes D.D., and Polesky H. (1988). A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 16, 1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Garringer J.A., Niyonkuru C., McCullough E.H., Loucks T., Dixon C.E., Conley Y.P., Berga S., and Wagner A.K. (2013). Impact of aromatase genetic variation on hormone levels and global outcome after severe TBI. J. Neurotrauma 30, 1415–1425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Teasdale G., and Jennett B. (1974). Assessment of coma and impaired consciousness. A practical scale. Lancet 2, 81–84 [DOI] [PubMed] [Google Scholar]
- 45. Baker S.P., O'Neill B., Haddon W. Jr, and Long W.B. (1974). The Injury Severity Score: a method for describing patients with multiple injuries and evaluating emergency care. J. Trauma 14, 187–196 [PubMed] [Google Scholar]
- 46. Vincent J.L., Moreno R., Takala J., Willatts S., De Mendonça A., Bruining H., Reinhart C.K., Suter P., and Thijs L.G. (1996). The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. Intensive Care Med. 22, 707–710 [DOI] [PubMed] [Google Scholar]
- 47. Selig J.P., and Little T.D. (2012). Autoregressive and cross-lagged panel analysis for longitudinal data, in: Handbook of Developmental Research Methods. B. Laursen, Little T.D., and N.A. Card (eds). Guilford Press: New York [Google Scholar]
- 48. Harrell F.E., Lee K.L., and Mark D.B. (1996). Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat. Med. 15, 361–387 [DOI] [PubMed] [Google Scholar]
- 49. Baron R.M., and Kenny D.A. (1986). The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J. Pers. Soc. Psychol. 51, 1173–1182 [DOI] [PubMed] [Google Scholar]
- 50. Mackinnon D.P., and Dwyer J.H. (1993). Estimating mediated effects in prevention studies. Eval. Rev. 17, 144–158 [Google Scholar]
- 51. Macera C.A., Aralis H.J., Rauh M.J., and MacGregor A.J. (2013). Do sleep problems mediate the relationship between traumatic brain injury and development of mental health symptoms after deployment? Sleep 36, 83–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Rijnhart J.J., Twisk J.W., Eekhout I., and Heymans M.W. (2019). Comparison of logistic-regression based methods for simple mediation analysis with a dichotomous outcome variable. BMC Med. Res. Methodol. 19, 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. (2017). StataCorp. College Station, TX: StataCorp LLC
- 54. SAS Institute Inc. SAS 9.4. Cary, NC
- 55. Kemp C.D., Johnson J.C., Riordan W.P., and Cotton B.A. (2008). How we die: the impact of nonneurologic organ dysfunction after severe traumatic brain injury. Am. Surg. 74, 866–872 [PubMed] [Google Scholar]
- 56. Feuerstein G.Z., Liu T., and Barone F.C. (1994). Cytokines, inflammation, and brain injury: role of tumor necrosis factor-alpha. Cerebrovasc. Brain Metab. Rev. 6, 341–360 [PubMed] [Google Scholar]
- 57. Barone F.C., Arvin B., White R.F., Miller A., Webb C.L., Willette R.N., Lysko P.G., and Feuerstein G.Z. (1997). Tumor necrosis factor-α: a mediator of focal ischemic brain injury. Stroke 28, 1233–1244 [DOI] [PubMed] [Google Scholar]
- 58. Shohami E., Ginis I., and Hallenbeck J.M. (1999). Dual role of tumor necrosis factor alpha in brain injury. Cytokine Growth Factor Rev. 10, 119–130 [DOI] [PubMed] [Google Scholar]
- 59. Woodcock T., and Morganti-Kossmann M.C. (2013). The role of markers of inflammation in traumatic brain injury. Front. Neurol. 4, 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Dinarello C.A. (1984). Interleukin-1 and the pathogenesis of the acute-phase response. N. Engl. J. Med. 311, 1413–1418 [DOI] [PubMed] [Google Scholar]
- 61. Offner F., Philippé J., Vogelaers D., Colardyn F., Baele G., Baudrihaye M., Vermeulen A., and Leroux-Roels G. (1990). Serum tumor necrosis factor levels in patients with infectious disease and septic shock. J. Lab. Clin. Med. 116, 100–105 [PubMed] [Google Scholar]
- 62. Martin C., Boisson C., Haccoun M., Thomachot L., and Mege J.-L. (1997). Patterns of cytokine evolution (tumor necrosis factor-alpha and interleukin-6) after septic shock, hemorrhagic shock, and severe trauma. Crit. Care Med. 25, 1813–1819 [DOI] [PubMed] [Google Scholar]
- 63. Dossett L.A., Swenson B.R., Heffernan D., Bonatti H., Metzger R., Sawyer R.G., and May A.K. (2008). High levels of endogenous estrogens are associated with death in the critically injured adult. J. Trauma 64, 580–585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Dossett L.A., Swenson B.R., Evans H.L., Bonatti H., Sawyer R.G., and May A.K. (2008). Serum estradiol concentration as a predictor of death in critically ill and injured adults. Surg. Infect. 9, 41–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Rakholia M., Kumar R., Oh B., Ranganathan P., Berga S., Kochanek P., and Wagner A. (2019). Systemic estrone production and injury induced sex hormone steroidogenesis after severe traumatic brain injury: a prognostic indicator of TBI-related mortality. J Neurotrauma 36, 1156–1167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Ranganathan P., Kumar R., Oh B., Rakholia M.V., Berga S., and Wagner A. (2019). Estradiol to androstenedione ratios moderate the relationship between neurological injury severity and mortality risk after severe TBI. J. Neurotrauma 36, 538–547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Tep-areenan P., Kendall D.A., and Randall M.D. (2003). Mechanisms of vasorelaxation to 17beta-oestradiol in rat arteries. Eur. J. Pharmacol. 476, 139–149 [DOI] [PubMed] [Google Scholar]
- 68. Zhou K., Gao Q., Zheng S., Pan S., Li P., Suo K., Simoncini T., Wang T., and Fu X. (2013). 17β-estradiol induces vasorelaxation by stimulating endothelial hydrogen sulfide release. Mol. Hum. Reprod. 19, 169–176 [DOI] [PubMed] [Google Scholar]
- 69. Garbán H.J., Buga G.M., and Ignarro L.J. (2004). Estrogen receptor-mediated vascular responsiveness to nebivolol: a novel endothelium-related mechanism of therapeutic vasorelaxation. J. Cardiovasc. Pharmacol. 43, 638–644 [DOI] [PubMed] [Google Scholar]
- 70. Egami R., Tanaka Y., Nozaki M., Koera K., Okuma A., and Nakano H. (2005). Chronic treatment with 17beta-estradiol increases susceptibility of smooth muscle cells to nitric oxide. Eur. J. Pharmacol. 520, 142–149 [DOI] [PubMed] [Google Scholar]
- 71. Yokoyama Y., Schwacha M.G., Bland K.I., and Chaudry I.H. (2003). Effect of estradiol administration on splanchnic perfusion after trauma-hemorrhage and sepsis. Curr. Opin. Crit. Care 9, 137–142 [DOI] [PubMed] [Google Scholar]
- 72. Mair K.M., Johansen A.K., Wright A.F., Wallace E., and MacLean M.R. (2014). Pulmonary arterial hypertension: basis of sex differences in incidence and treatment response. Br. J. Pharmacol. 171, 567–579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Jiang J.X., Diao Y.F., Tian K.L., Chen H.S., Zhu P.F., and Wang Z.G. (1997). Effect of hemorrhagic shock on endotoxin-inducing TNF production and intra-tissue lipopolysaccharide-binding protein mRNA expression and their relationship. Shock 7, 206–210 [DOI] [PubMed] [Google Scholar]
- 74. Gilbert K., Rousseau G., Bouchard C., Dunberry-Poissant S., Baril F., Cardinal A.M., Khazoom F., Vega M.A., Brochiero E., and Charbonney E. (2019). Caspase-(8/3) activation and organ inflammation in a rat model of resuscitated hemorrhagic shock: a role for uric acid. J. Trauma Acute Care Surg. 86, 431–439 [DOI] [PubMed] [Google Scholar]
- 75. Mattila P., Majuri M.L., Mattila P.S., and Renkonen R. (1992). TNF alpha-induced expression of endothelial adhesion molecules, ICAM-1 and VCAM-1, is linked to protein kinase C activation. Scand. J. Immunol. 36, 159–165 [DOI] [PubMed] [Google Scholar]
- 76. Pettus E.H., Wright D.W., Stein D.G., and Hoffman S.W. (2005). Progesterone treatment inhibits the inflammatory agents that accompany traumatic brain injury. Brain Res. 1049, 112–119 [DOI] [PubMed] [Google Scholar]
- 77. Roof R.L., Duvdevani R., Braswell L., and Stein D.G. (1994). Progesterone facilitates cognitive recovery and reduces secondary neuronal loss caused by cortical contusion injury in male rats. Exp. Neurol. 129, 64–69 [DOI] [PubMed] [Google Scholar]
- 78. Roof R.L., Hoffman S.W., and Stein D.G. (1997). Progesterone protects against lipid peroxidation following traumatic brain injury in rats. Mol. Chem. Neuropathol. 31, 1–11 [DOI] [PubMed] [Google Scholar]
- 79. He J., Evans C.O., Hoffman S.W., Oyesiku N.M., and Stein D.G. (2004). Progesterone and allopregnanolone reduce inflammatory cytokines after traumatic brain injury. Exp. Neurol. 189, 404–412 [DOI] [PubMed] [Google Scholar]
- 80. Zygun D.A., Kortbeek J.B., Fick G.H., Laupland K.B., and Doig C.J. (2005). Non-neurologic organ dysfunction in severe traumatic brain injury. Crit. Care Med. 33, 654–660 [DOI] [PubMed] [Google Scholar]
- 81. Corral L., Javierre C.F., Ventura J.L., Marcos P., Herrero J.I., and Mañez R. (2012). Impact of non-neurological complications in severe traumatic brain injury outcome. Crit. Care 16, R44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Pinto S.M., Kumar R., Sperry J.L., and Wagner A.K. (2018). Impact of venous thromboembolism-associated complications on mortality after traumatic brain injury. Arch. Phys. Med. Rehabil. 99, e46 [Google Scholar]
- 83. Berry C., Ley E.J., Tillou A., Cryer G., Margulies D.R., and Salim A. (2009). The effect of gender on patients with moderate to severe head injuries. J. Trauma 67, 950–953 [DOI] [PubMed] [Google Scholar]
- 84. Davis D.P., Douglas D.J., Smith W., Sise M.J., Vilke G.M., Holbrook T.L., Kennedy F., Eastman A.B., Velky T., and Hoyt D.B. (2006). Traumatic brain injury outcomes in pre- and post- menopausal females versus age-matched males. J. Neurotrauma 23, 140–148 [DOI] [PubMed] [Google Scholar]
- 85. Mayer L.S. (1986). On cross-lagged panel models with serially correlated errors. J. Bus. Econ. Stat. 4, 347–357 [Google Scholar]
- 86. Zhao Y., Mendelson C.R., and Simpson E.R. (1995). Characterization of the sequences of the human CYP19 (aromatase) gene that mediate regulation by glucocorticoids in adipose stromal cells and fetal hepatocytes. Mol. Endocrinol. Baltim. Md 9, 340–349 [DOI] [PubMed] [Google Scholar]
- 87. Zhao Y., Nichols J.E., Bulun S.E., Mendelson C.R., and Simpson E.R. (1995). Aromatase P450 gene expression in human adipose tissue. Role of a Jak/STAT pathway in regulation of the adipose-specific promoter. J. Biol. Chem. 270, 16449–16457 [DOI] [PubMed] [Google Scholar]
- 88. Wunderle K., Hoeger K.M., Wasserman E., and Bazarian J.J. (2014). Menstrual phase as predictor of outcome after mild traumatic brain injury in women. J. Head Trauma Rehabil. 29, E1–E8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Lilley E., Armstrong R., Clark N., Gray P., Hawkins P., Mason K., López-Salesansky N., Stark A.K., Jackson S.K., Thiemermann C., and Nandi M. (2015). Refinement of animal models of sepsis and septic shock. Shock 43, 304–316 [DOI] [PubMed] [Google Scholar]