Abstract
To overcome the serious shortage of donor corneas for transplantation, alternatives based on tissue engineering need to be developed. Decellularized corneas are one potential alternative, but their densely packed collagen architecture inhibits recellularization in vitro. Therefore, a new rapid method of recellularizing these constructs to ensure high cellularity throughout the collagen scaffold is needed. In this study, we developed a novel method for fabricating corneal constructs by using decellularized porcine corneal sheets assembled using a bottom-up approach by layering multiple sheets between cell-laden collagen I hydrogel.
Corneal lenticules were cut from porcine corneas by cryosectioning, then decellularized with detergents and air-dried for storage as sheets. Human corneal stromal cells were encapsulated in collagen I hydrogel and cast between the dried sheets. Constructs were cultured in serum-free medium supplemented with ascorbic acid and insulin for 2 weeks. Epithelial cells were then seeded on the surface and cultured for an additional week. Transparency, cell viability, and phenotype were analyzed by qPCR, histology, and immunofluorescence. Constructs without epithelial cells were sutured onto an ex vivo porcine cornea and cultured for 1 week.
Lenticules were successfully decellularized, achieving dsDNA values of 13 ± 1.2 ng/mg dry tissue, and were more resistant to degradation than the collagen I hydrogels. Constructs maintained high cell viability with a keratocyte-like phenotype with upregulation of keratocan, decorin, lumican, collagen I, ALDH3A1, and CD34 and the corneal epithelial cells stratified with a cobblestone morphology. The construct was amenable to surgical handling and no tearing occurred during suturing. After 7 days ex vivo, constructs were covered by a neoepithelium from the host porcine cells and integration into the host stroma was observed.
This study describes a novel approach toward fabricating anterior corneal substitutes in a simple and rapid manner, obtaining mature and suturable constructs using only tissue-derived materials.
Impact statement
New strategies are needed to face the important worldwide shortage of donor tissues for corneal transplantation. This study describes a novel approach based on decellularized sheets of porcine cornea interspaced with cell-laden collagen I hydrogels. These constructs matured into a transplantable tissue.
Keywords: cornea, tissue engineering, decellularization, keratocyte, lenticules
Introduction
Several diseases affecting the cornea can lead to blindness and require a corneal transplant to regain sight. Supply of human donor tissue is limited and it is estimated that only 1 cornea is available for every 70 needed,1 highlighting the critical shortage. There is a clear necessity for alternative approaches with tissue engineering being one method of potentially generating transplantable tissue.
Among the promising materials under consideration for fabricating the cornea is decellularized animal tissue. This comprises of the extracellular matrix (ECM) of the cornea without any donor cells present to provide a scaffold for cell growth. Decellularized porcine corneas have been transplanted into humans in cases of infectious ulcers with encouraging results.2,3 However, these acellular scaffolds are composed of densely packed collagen lamellae, and thus recellularization is difficult, making it slow, both in vitro4 and in vivo.5
In attempts to improve recellularization, human lenticules extracted during myopia correction surgeries have been attached together using a fibrin glue hydrogel to prepare a decellularized scaffold.6 Although the decellularized lenticules did populate with a small number of cells, the phenotype of these was not characterized.
In another study, thin sheets of decellularized porcine cornea were seeded with stromal cells and multiple layers implanted intrastromally.7 After 6 months, the corneas had the same thickness as unoperated eyes and some cells were visible between engrafted sheets. Thus, the first study showed the feasibility of binding the decellularized lenticules without using cells, and the second showed the possibility of recellularizing each layer before implantation. The combination of both approaches where cells are embedded in the hydrogel has not been explored previously and sets the basis for this study. This novel approach would allow cells to be incorporated directly into the decellularized cornea while still binding the different layers together.
The aim of this study was therefore to develop a corneal substitute by using sheets of decellularized porcine cornea interspersed with cell-laden collagen hydrogels. The use of collagen rather than fibrin is more physiologically relevant since the cornea is primarily made up of collagen fibrils. These constructs presented high transparency, easy handling after fabrication, high viability, and in vivo-like phenotype after a culture period of 3 weeks. Furthermore, constructs containing both stromal and epithelial cells were fabricated, and remained viable when sutured into an ex vivo model of anterior lamellar keratoplasty (ALK).
Materials and Methods
Fabrication of acellular matrix sheets
Porcine eyes were obtained within 24 h from abattoir death. After trimming and under sterile conditions, the eye globes were washed twice with phosphate-buffered saline (PBS), immersed in 2% iodine solution (Videne®, Ecolab, Belgium) for 4 min under gentle agitation, and then rinsed three times with PBS for 2 min. The central corneal button from each eye was excised using a 10 mm biopsy punch. Corneal buttons were embedded in optimal cutting temperature medium (OCT; Thermo-Scientific, Ireland), snap-frozen in liquid nitrogen, and cryosectioned on a cryostat (Leica, Germany) to obtain 60-μm-thick slices. Slices were washed in PBS to remove OCT and then transferred into a decellularization solution of 0.5% (w/v) sodium dodecyl sulfate (SDS) and 1% (v/v) Triton X-100 (both Sigma-Aldrich, Ireland) in distilled water for 24 h at room temperature.
To promote penetration of the solution and removal of debris, this and further processing was carried out on an orbital shaker. The samples were treated with 10 U/mL of RNAse and DNAse in 10 mM MgCl2 solution for 1 h at 37°C (all Sigma), then decontaminated by washing in PBS supplemented with 100 U/mL penicillin, 100 mg/mL streptomycin (both Gibco, Ireland), and 250 ng/mL amphotericin B (Sigma) for another 24 h at room temperature, followed by washing in sterile distilled water three times.
The acellular sheets were air dried in a sterile biohazard cabinet and stored in a sealed container at room temperature until use. Native, decellularized and decellularized, dehydrated and rehydrated sheets were fixed in 4% paraformaldehyde for 15 min at room temperature. They were then embedded in paraffin wax and sectioned on a microtome (Leica). Slides were deparaffinized, rehydrated, and coverslipped using 4′,6-diamidino-2-phenylindole (DAPI)-containing aqueous mounting medium (Sigma). Slides were imaged with an epifluorescence microscope (Olympus IX83). The thickness of the sheets was measured in ImageJ from those images (n = 20–30). Additionally, the macroscopic appearance and transparency of the dried acellular sheets was assessed by placing them over printed text.
DNA content
Native and decellularized porcine cornea sheets were freeze dried and digested in 3.88 U/mL of papain solution rotating at 60°C for 18 h. DNA content was then quantified using a Quant-iT™ PicoGreen® dsDNA Kit (Invitrogen, Ireland) following the manufacturer's instructions. The plates were read using a spectrophotometer (BioTek™ Synergy HTX) using 480 nm excitation wavelength and 520 nm emission wavelength.
Degradation test
In vitro enzymatic digestion was performed to determine the resistance to biodegradation. Single decellularized sheets and 100 μL collagen gels (cast as described later) were tested by being incubated with 1 mL 20 U/mL collagenase I (Gibco) in PBS at 37°C. Photographs were taken periodically, before the supernatant was collected and fresh collagenase solution added. Collagen content in the supernatant was quantified by measuring the hydroxyproline content through a dimethylaminobenzaldehyde (DMBA) assay.8
Briefly, 200 μL of supernatant at each time point was digested with 12 M HCl for 18 h at 110°C. These samples were then allowed to evaporate for 48 h at 50°C in a fume hood. After evaporation, the dry samples were resuspended in deionized water and incubated for 20 min at room temperature with Chloramine T and an oxidizing buffer. DMBA reagent was added and incubated for 20 min at 60°C. After cooling, the reactants were read at 570 nm in a plate reader (BioTek Synergy HTX).
Cell culture
Human corneal stromal cells were isolated from the corneal/scleral ring remaining after a corneal transplant in accordance with the Declaration of Helsinki. The corneal/scleral rings were rinsed with sterile PBS and the epithelium and endothelium were carefully removed using a scalpel blade. After a brief wash with sterile PBS, the corneal stroma was diced into small pieces and transferred into a 25-cm2 culture flask. Media consisted of low-glucose Dulbecco's modified Eagle's medium (DMEM; HyClone, Dublin, Ireland) supplemented with 10% fetal bovine serum and 100 U/mL Penicillin/Streptomycin (Gibco). The stromal pieces were cultured at 37°C and 5% CO2 in a humidified incubator. Media were changed regularly until the cells had migrated from the tissue and reached 80–90% confluence before being passaged to allow further expansion. The cells were cryopreserved and used at passage 4.
Human telomerase-immortalized corneal epithelial cells (hTCEpi, Evercyte, Austria) were expanded and cultured using keratinocyte growth medium (PromoCell, Germany), following the manufacturer's instructions.
Construction of an engineered corneal stroma
Collagen hydrogels were prepared from rat tail collagen I (Corning, New York) as previously described.9 A 3.5 mg/mL solution was prepared from stock, salt concentration adjusted using 10 × PBS and pH raised to neutral by addition of 1 N NaOH. After this neutralization, 10,000 cells/μL gel were embedded in the solution. Twenty-five microliters of solution was deposited on a decellularized corneal sheet and a second sheet placed on top. This process was repeated until there were four gels and five sheets. This construct was then incubated for 30 min at 37°C in a humidified incubator, before being submerged in a media of DMEM/F12 (1:1; HyClone) supplemented with 1 × insulin/transferrin/selenium (Gibco) and 100 μg/mL L-ascorbic acid-2-phosphate (Sigma). This medium was chosen as it has been shown to promote a keratocyte-like phenotype.10 Constructs were fed every second day for 3 weeks.
A total of 50.000 hTCEpi cells were seeded on the construct after 2 weeks and cultured for another week in Keratinocyte growth medium.
Cell viability
Constructs were incubated with 2 μM Calcein-AM and 4 μM EthD-1 (Biotium, San Francisco, California, USA) for 1 h at 37°C in a humidified incubator. Samples were washed three times in PBS and imaged by laser scanning confocal imaging (Leica SP8, Germany).
Transparency and light transmittance
The macroscopic appearance of the constructs was assessed by placing them over printed text. Additionally, light transmittance through the constructs after 3 weeks of culture was quantified by measuring the absorbance of light at different wavelengths ranging from 300 to 700 nm that pass through each sample using a microplate reader (BioTek Synergy HTX, Winooski, Vermont, USA). The absorbance of light passing through ultrapure water was used as a control to represent 100% transmittance and this absorbance reading was subtracted from the measured absorbance of each sample to give the change in absorbance due to the sample being present (A). The transmittance of light was calculated using the following formula as described elsewhere11:
Quantitative polymerase chain reaction
RNA from constructs was extracted using TRIzol, following the manufacturer's instructions (Invitrogen). To increase lysis and release of the genetic material, samples were triturated using a tissue homogenizer (IKA T10 basic, Germany). Chloroform was added, the samples were vortexed, and then centrifuged for 15 min at 12,000 g at 4°C. The supernatant was transferred into a new tube and an equal volume of isopropanol added, along with 3 μL of GlycoBlue™ (Invitrogen) to improve precipitation. Samples were kept overnight at −20°C and then centrifuged at 12,000 g for 15 min at 4°C. The supernatant was discarded and 70% ethanol in RNAse-free water used to wash the pellet. Samples were centrifuged again, and the supernatant removed before the pellets were air dried. The pellets were dissolved in RNAse-free water and RNA yield and purity quantified using a NanoDrop-1000 (Thermo Scientific). All steps were performed on ice.
RNA was converted into cDNA using the High-Capacity cDNA Reverse Transcription Kit (Invitrogen). Real-time polymerase chain reaction was performed using TaqMan Universal Master Mix II and the following TaqMan primers: glyceraldehyde-3-phosphate dehydrogenase (GAPDH, Hs02758991_g1), aldehyde dehydrogenase 3A1 (ALDH3A1, Hs00964880_m1), alpha smooth muscle actin (α-SMA) (ACTA2, Hs00426835_g1), keratocan (KERA; Hs00559942_m1), collagen 1a1 (COL1; Hs00164004_m1), lumican (LUM, Hs00929860_m1), decorin (DCN, Hs00754870_s1), and CD34 (Hs00990732_m1). The genes of interest were normalized against GAPDH using the ΔΔCt method. Calculated values were expressed as a power of 2−ΔΔCt. For this study, all values were normalized to the serum-expanded cells.
Histological analysis and immunohistochemistry
After culture, samples were fixed with 4% paraformaldehyde for 15 min at room temperature. They were then embedded in paraffin wax and sectioned on a microtome (Leica). Routine Hematoxylin and Eosin (H&E), Alcian Blue, and Picrosirius Red staining were performed.
Slides were stained for 4 min with Harris Hematoxylin (Sigma), followed by a 10-min wash with running tap water, 30 s in acid alcohol, and further tap water for 5 min. Counterstaining was 2 min with Eosin Y (Sigma). Alcian Blue was used to assess sulfated glycoaminoglycans (sGAG) content; 1% Alcian Blue 8GX (Sigma) in 0.1 M HCl was applied for 5 min, followed by three, 30-s washes in deionized water. Picrosirius Red was used to assess collagen distribution; slides were immersed in Sirius Red (Sigma) in a saturated aqueous solution of picric acid for 1 h followed by 1 min in 0.5% acetic acid. After staining, all slides were dehydrated and coverslips attached using DPX mountant (Sigma).
For immunofluorescent staining, slides were rehydrated followed by an antigen-retrieval step. Samples were first blocked with 1% bovine serum albumin (BSA) and 10% donkey serum in 0.5% Triton X-100 for 1 h at room temperature. The following primary antibodies were used and incubated overnight at 4°C: Collagen I, Decorin, Keratocan, ALDH3A1, Lumican, CD34, and α-SMA. After incubation, three, 5-min washes with 1% BSA in PBS were performed to remove the unbound antibody. Secondary antibody was then incubated for 1 h at room temperature followed by again washing three times for 5 min with 1% BSA in PBS. Coverslips were attached using DAPI-containing aqueous mounting medium (Sigma). Slides were imaged using laser scanning confocal microscopy (Leica SP8). Table 1 contains details of the immunofluorescent staining procedure. Secondary antibody only staining was performed as negative control.
Table 1.
Antibodies and Antigen Retrieval Methods for Immunofluorescent Staining
| Primary antibody | Catalogue number (brand) | Dilution | Antigen retrieval method | Secondary antibody (dilution) |
|---|---|---|---|---|
| ALDH3A1 | Ab76976 (Abcam) | 1:200 | Heat-mediated citrate buffer pH 6 (30 min at 95°C) | Donkey anti-rabbit-AlexaFluor® 488 (1:200) |
| α-SMA | Ab7817 (Abcam) | 1:100 | Heat-mediated citrate buffer pH 6 (30 min at 95°C) | Goat anti-mouse AlexaFluor 488 (1:200) |
| Keratocan | HPA039321 (Atlas Antibodies) | 1:50 | Heat-mediated citrate buffer pH 6 (30 min at 95°C) | Donkey anti-rabbit-AlexaFluor 488 (1:200) |
| CD34 | Ab81289 (Abcam) | 1:250 | Heat-mediated citrate buffer pH 6 (30 min at 95°C) | Donkey anti-rabbit-AlexaFluor 488 (1:200) |
| Decorin | Ab189364 (Abcam) | 1:133 | Heat-mediated citrate buffer pH 6 (30 min at 95°C) | Rabbit anti-goat-AlexaFluor 594 (1:200) |
| Lumican | Ab168348 (Abcam) | 1:100 | Heat-mediated citrate buffer pH 6 (30 min at 95°C) | Donkey anti-rabbit-AlexaFluor 488 (1:200) |
| Collagen I | Ab90395 (Abcam) | 1:400 | Enzymatic-mediated 0.25 U/mL Chondroitinase ABC (1 h at 37°C) | Donkey anti-mouse AlexaFluor 488 (1.5:200) |
α-SMA, alpha smooth muscle actin.
Constructs containing epithelial and stromal cells were fixed for 15 min in 4% paraformaldehyde, permeabilized with 0.5% Triton X-100 during 10 min at room temperature, and subsequently stained with 0.5 μg/mL Phalloidin-TRITC (Sigma) and 1 μg/mL DAPI (Sigma) for 1 h protected from light. Samples were then imaged using laser scanning confocal microscopy (Leica SP8). They were further processed for wax embedding and H&E staining, as previously described.
Implantation of constructs onto an ex vivo porcine cornea
Porcine eyes were obtained within 24 h from abattoir death. A 6-mm-diameter defect of approximately half of the depth of the cornea was made using a trephine. Lamellar dissection was performed using a blunt crescent knife (Beaver-Visitec International, UK). Constructs after 3 weeks of culture (without epithelial cells) were sutured in place with 4 or 8, 10–0 nylon sutures. The corneas were resected with 5 mm of scleral rim and placed onto a hemispherical agarose mold (2% agarose w/v). Corneas were cultured for 7 days in a humidified chamber in 4 mL of serum-free DMEM/F12, supplemented with 4% dextran (MW 450,000–650,000, Sigma) to limit swelling. The medium was added to the level of the limbus, leaving the cornea at the air/liquid interface. Medium was changed daily, with three drops added onto the corneal surface to limit drying.
To determine re-epithelialization from the host tissue fluorescein staining was performed. Four–five drops of 1% fluorescein sodium salt (Sigma) in PBS solution were added to cover the surface of the corneas, after 1 min of incubation at room temperature, sterile PBS was used to wash the corneas until no more dye was visibly leaching. In the dark, samples were exposed to blue light from a handheld slit lamp (15090—PSL, Reichert) and imaged with a cell phone. This was performed at 2 and 7 days after implantation. After culture, corneas were fixed overnight in formalin and processed for wax embedding and H&E staining, as previously described.
Statistical analysis
All experiments were performed three times with a minimum of three replicates. Unpaired two-tailed t-test and two-way analysis of variance were employed, and statistical significance accepted at a level of p < 0.05.
Results
Characterization of decellularized sheets
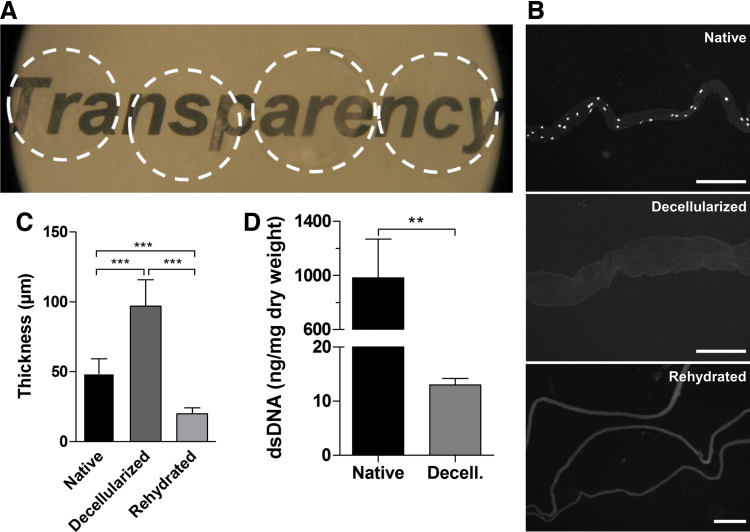
The decellularized sheets obtained were highly transparent (Fig. 1A). Sections stained with DAPI presented no cell nuclei after decellularization (Fig. 1B). Thickness measurements showed that the decellularization procedure had a swelling effect on the sections and that, when sections were air dried and rehydrated, they did not recover their original thickness (Fig. 1C). Quantification of dsDNA showed successful decellularization, with significantly lower levels of DNA remnants than the native tissue (Fig. 1D). Native sheets had 982.8 ± 286 ng/mg of DNA per dry tissue (average ± standard deviation), while decellularized sheets presented 13 ± 1.2 ng/mg of DNA per dry tissue (average ± standard deviation).
FIG. 1.
Characterization of decellularized sheets. (A) Macroscopic appearance of the dried acellular sheets, showing high transparency. (B) DAPI staining of a native sheet, a sheet after decellularization, and three sheets after dehydration and rehydration, showing the absence of cell nuclei after decellularization (scale bar = 200 μm). (C) Thickness measurements of sheets: native, after decellularization, and after dehydration and rehydration. (D) dsDNA quantification of native and decellularized sheets. **p ≤ 0.001, ***p ≤ 0.0001. DAPI, 4′,6-diamidino-2-phenylindole. Color images are available online.
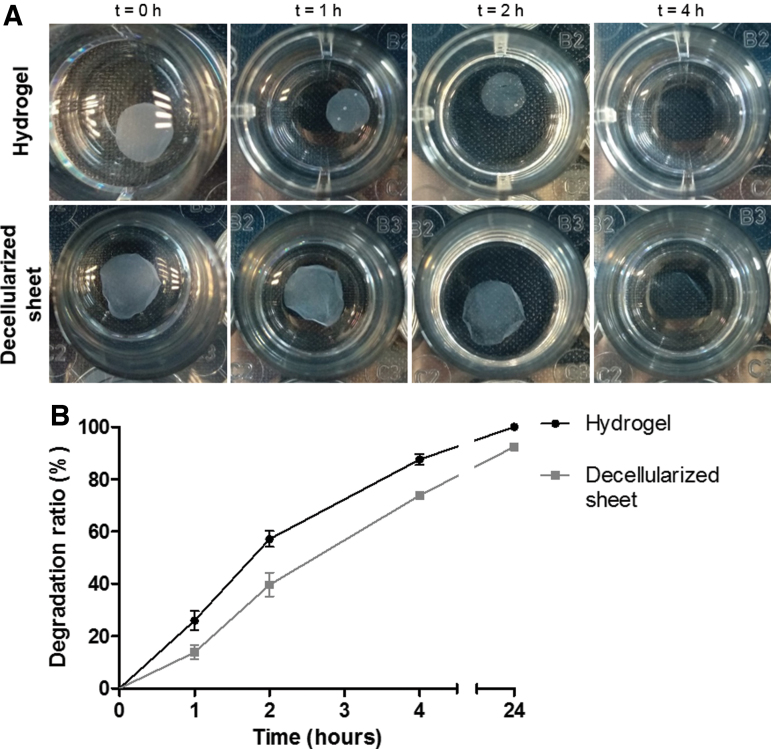
Degradation was slower in the decellularized sheets than the collagen hydrogels. After 4 h of incubation in collagenase solution, hydrogels appeared to completely degrade, while decellularized sheets were still visible (Fig. 2A). Even after 24 h, remnants of the sheets were still present. When quantified (Fig. 2B), 57% of the hydrogels was degraded by 2 h, 87% by 4 h, and 100% after 24 h. In comparison, 39%, 73%, and 92% of the decellularized sheets were degraded after 2, 4, and 24 h, respectively. After 48 h, sheets had degraded completely.
FIG. 2.
Resistance to biodegradation. (A) Macroscopic visualization and (B) quantitative analysis of degradation. Color images are available online.
Corneal stromal equivalent viability and transparency
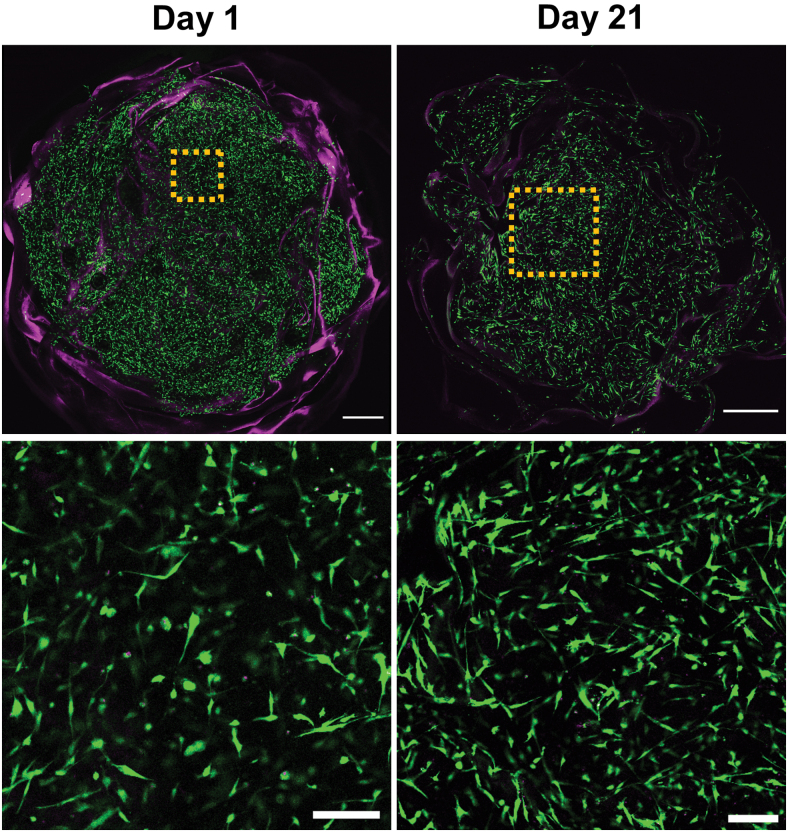
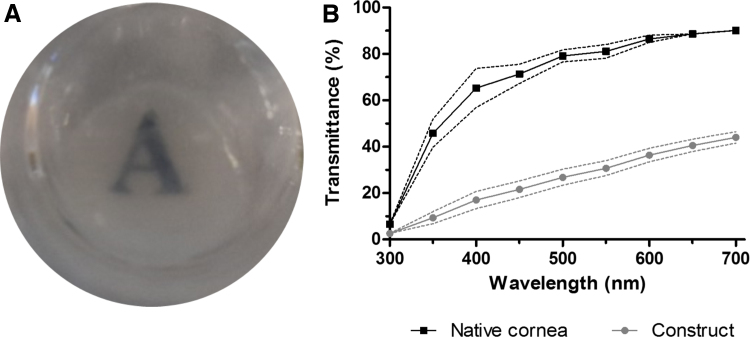
Tissue constructs were obtained by stacking dried sheets between cell-laden collagen hydrogels. Human corneal stromal cells remained viable in the constructs throughout the culturing period, even in serum-deprived conditions (Fig. 3). After 1 day in culture, some cell spreading could be detected within the scaffold. After the whole 3-week culture period, most cells displayed an elongated morphology with visible processes. Furthermore, even in this short period, constructs had acceptable optical properties when assessed macroscopically (Fig. 4A). When quantified, they allowed less light transmittance than the native cornea, but did allow >40% of transmittance in the longer wavelengths of the visible spectrum (Fig. 4B).
FIG. 3.
Cell viability in constructs after fabrication and after 3 weeks in culture (live = green, dead = magenta. Bottom row insert from top row at higher magnification. Top scale bar = 1 mm, bottom scale bar = 200 μm). Color images are available online.
FIG. 4.
Macroscopic appearance of constructs after 3 weeks of culture (A) and quantification of light transmittance (B). Color images are available online.
Corneal stromal equivalent cell phenotype
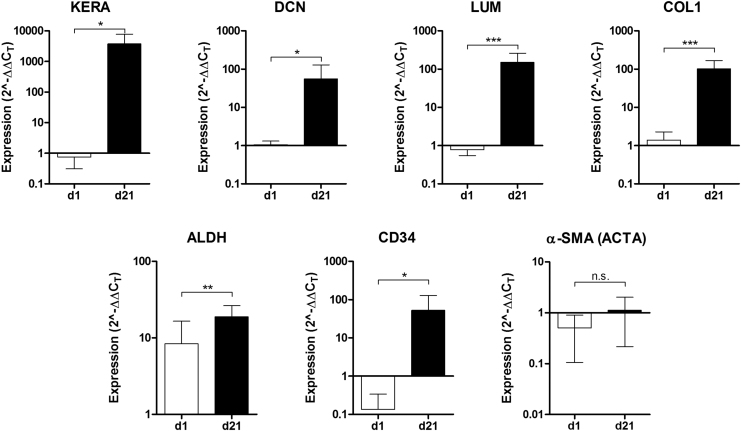
Characteristic markers of corneal stromal ECM and corneal stromal cell phenotype were analyzed by quantitative polymerase chain reaction (qPCR) (Fig. 5). Keratocyte markers ALDH3A1 and CD34 were significantly upregulated as the time in serum-free conditions increased. All ECM components analyzed were also highly upregulated. Typical small leucine-rich proteoglycans, keratocan, lumican, and decorin that are found in the corneal stroma, were expressed significantly higher. The expression of collagen I, the most common collagen in the corneal stroma, was significantly upregulated. Furthermore, the fibrotic marker α-SMA was not significantly upregulated.
FIG. 5.
Phenotypic analysis of stromal cells in the constructs by qPCR at day 1 and 21 of culture. Relative expression of keratocan (KERA), decorin (DCN), lumican (LUM), collagen I (COL1), ALDH3A1, CD34, and α-SMA (ACTA). *p ≤ 0.05, **p ≤ 0.001, ***p ≤ 0.0001. α-SMA, alpha smooth muscle actin; qPCR, quantitative polymerase chain reaction.
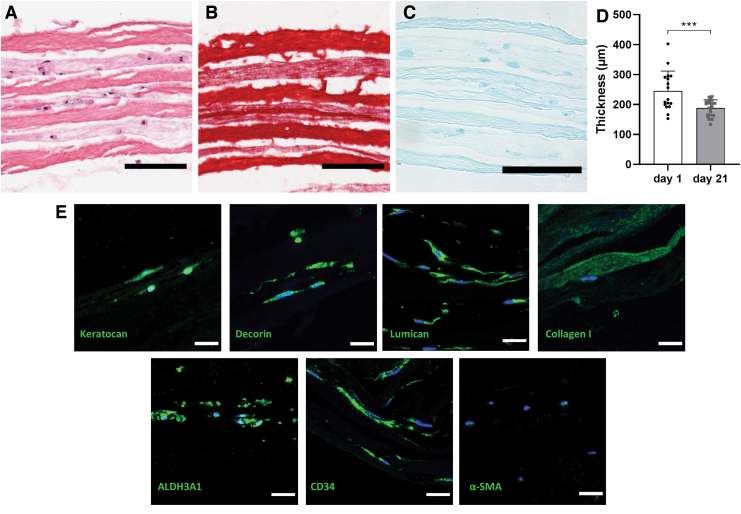
Constructs were further analyzed through histology and immunohistochemistry (Fig. 6). The thickness of the constructs decreased significantly during the culturing period, from 245.2 ± 66.50 μm to 187.9 ± 27.44 μm, presumably due to the cells remodeling the collagen hydrogel. Constructs presented cells distributed evenly in all hydrogel layers, as seen by H&E staining (Fig. 6A). Picrosirius Red staining for collagen showed differential staining in the hydrogel portion and the decellularized sheets (Fig. 6B). Despite the loss of sGAG during decellularization, upon subsequent culture, cells produced their own ECM in the hydrogel sections (Fig. 6C).
FIG. 6.
Histological examination of constructs at day 21, staining with (A) H&E (s = sheet, h = hydrogel), (B) Picrosirius Red, and (C) Alcian Blue (scale bar = 100 μm). (D) Thickness measurements of constructs at day 1 and 21. (E) Immunohistofluorescent staining of Keratocan, Lumican, Decorin, Collagen I, ALDH3A1, CD34, and α-SMA (green), and cell nuclei (blue), scale bar = 20 μm. H&E, Hematoxylin and Eosin. Color images are available online.
Immunohistostaining corroborated the results obtained from qPCR with constructs staining positive for ALDH3A1, CD34, keratocan, lumican, decorin, and collagen I, whereas α-SMA remained negative (Fig. 6D).
Epithelial cell growth on corneal stromal equivalents
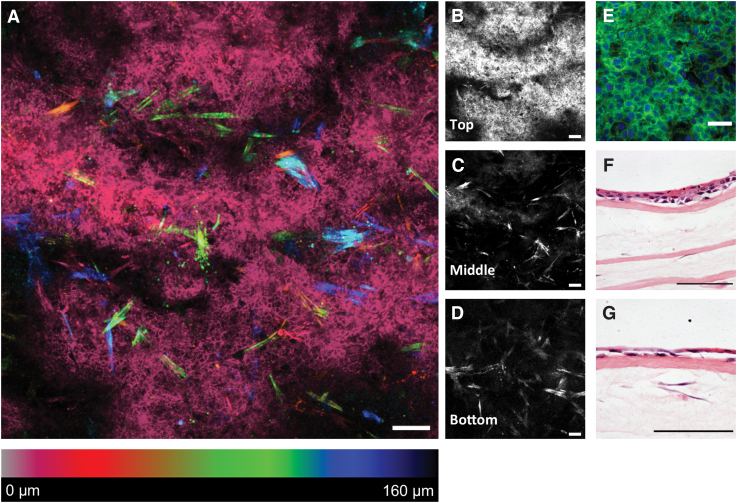
Corneal epithelial cells were cultured on the top layer of the constructs (Fig. 7). A coculture was successfully obtained using this method with stromal cells detected in the middle and lower layers. Epithelial cells had attached to the construct surface and formed a tight epithelium presenting a typical cobblestone morphology (Fig. 7E). Some degree of stratification was visible in H&E-stained sections (Fig. 7F, G).
FIG. 7.
Anterior cornea constructs with epithelium. (A) Depth color-coded Z-stack, cells at the surface (epithelium) appear pink/red, whereas stromal cells in deeper areas appear from orange to blue; (B–D) single images of the Z-stack (scale bar = 100 μm); (E) higher magnification of the epithelium F-actin (green) and nuclei (blue), scale bar = 10 μm; (F, G) H&E-stained sections show a degree of epithelial stratification (scale bar = 100 μm). Color images are available online.
Implantation of corneal stromal equivalent in an ex vivo anterior lamellar keratoplasty model
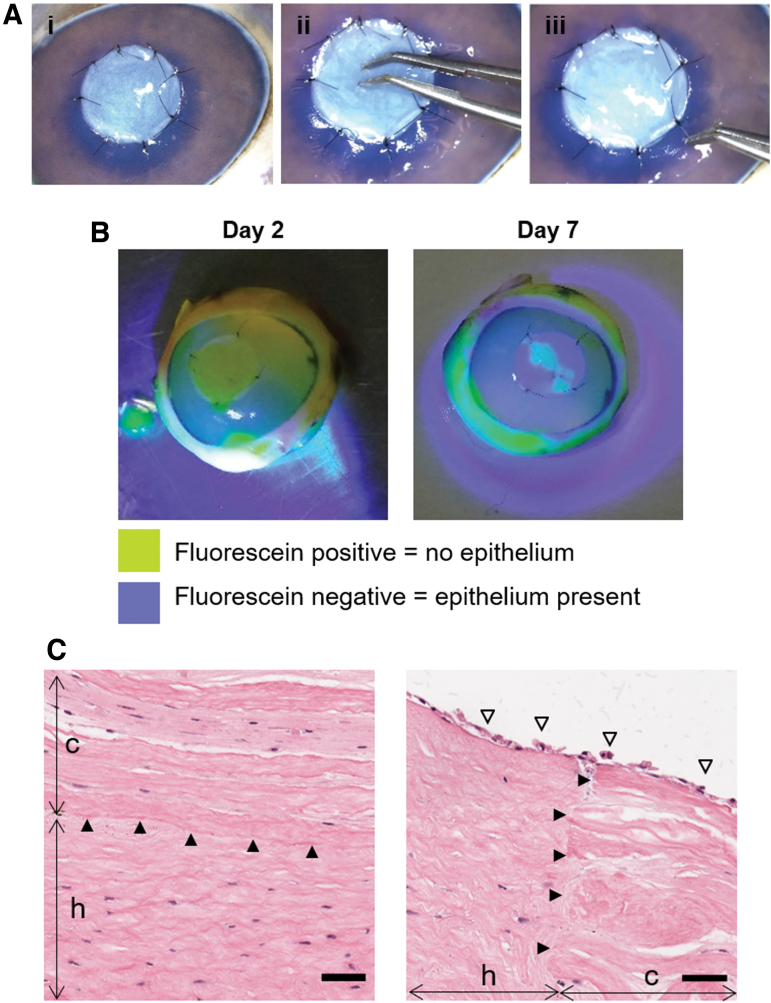
Constructs were successfully implanted onto porcine corneas in an ex vivo ALK model (Fig. 8). Constructs were amenable to surgical handling and suturing without tearing (Fig. 8A). Fluorescein staining is commonly used to assess corneal epithelial wounds. If the staining is positive (yellow), it indicates an area with damaged or absent epithelium (barrier function compromised); whereas a negative staining is an indication of a healthy epithelium as its barrier does not allow the penetration of the dye.
FIG. 8.
Implantation of constructs without epithelium onto an ex vivo porcine cornea. (A) Surgical procedure: (i) implant sutured in place using 10-0 nylon sutures, (ii) pressing onto the implant to show strength of construct and suture points and (iii) pulling of sutures to demonstrate construct does not tear. (B) Fluorescein staining of operated porcine corneas after implantation and culture. (C) H&E analysis of implanted constructs, showing good integration of construct (c) with the host (h) (arrowheads) at the center and the edge of the construct. Epithelium (empty arrowheads) from the host has grown onto the construct (scale bar = 50 μm). Color images are available online.
In this study, positive staining was seen on the constructs after 2 days of implantation, while the presence of only small islands of positive fluorescein staining at day 7 indicated that neighboring epithelial cells colonized the surface of the scaffold (Fig. 8B). Histological analysis through H&E showed good integration of the construct onto the host bed and at its periphery, while the surface was covered in a neoepithelium (Fig. 8C).
Discussion
To overcome the severe worldwide shortage of donor corneal tissue for transplantation, tissue engineering approaches are being developed. In this study, a multilayered construct fabricated with decellularized cornea sheets and cell-laden collagen hydrogels is described. These corneal stroma tissue equivalents presented high cell viability, transparency, expression of stromal cell markers, and supported epithelial regeneration.
Decellularization of the stromal sheets was successful, with values of dsDNA remaining well below the recommended maximum concentration of 50 ng/mg of dry tissue to minimize adverse host reactions.12 While a shorter decellularization protocol to that previously described was used in this study, levels of DNA were lower.13 This is probably due to the thinner tissue allowing improved penetration of the detergents.
Swelling occurred during the decellularization process, which has been previously reported.14–18 Once the endothelium is damaged or removed, the cornea swells. Furthermore, SDS is an aliphatic molecule and it binds to proteins by its hydrophobic domain that results in increased negative charge, which attracts water and leads to swelling.19 To counteract this, osmoregulators, such as dextran,13,20 glycerol,20 gelatin, and sodium chloride21 can be used to reduce swelling. Unsurprisingly, dehydration resulted in a reduction in thickness, however, the thickness only partially recovered upon rehydration. This could be attributed to the loss of sGAG during the decellularization process that would normally attract water molecules into the tissue.22,23
In this study, the sheets were dehydrated by air drying in a laminar flow hood and did not fully recover their thickness after rehydration. This would lead to a denser collagen network than found in the native tissue.
Alternative dehydration techniques such as freeze drying or critical point drying could be used but these also introduce new limitations. While freeze drying has been previously used to obtain dried sheets of decellularized cartilage,24 preliminary investigations by our group using stromal sheets showed that freeze drying did not affect the thickness but inhibited the transmission of light through the tissue. Critical point drying was not used as it could lead to degradation of the ECM following exposure to ethanol. The addition of a protecting agent to limit shrinkage was not explored in this study, but could potentially assist in controlling the sheet thickness.25 While other researchers have used freshly decellularized lenticules,6,26,27 in this study, dried lenticules were used because of their ease in handling, room temperature storage, and possibility of terminal sterilization, such as gamma irradiation.
We hypothesized that cell delivery through a gel would be a gentle, but effective, way to allow the inclusion of cells at all depths. Collagen I was chosen since it is the primary type of collagen found in the corneal stroma.28 Our approach showed homogeneous cell distribution throughout the construct. In addition, the collagen hydrogel proved to be an excellent binding agent of the dehydrated decellularized sheets, which were attracted by the moisture of the hydrogel.
The use of the decellularized sheets provided stability to the construct, making it more robust and easier to handle than a gel alone. Hong et al. reported a carrier for limbal epithelial stem cells based on embedding a single sheet of decellularized human cornea in a collagen gel that was further plastically compressed.29 With this approach they increased the suturability and resistance to biodegradation of the construct.
In our study, the sheets resisted degradation more than the hydrogels, giving an advantage for the strength and retention of an implant. Furthermore, when corneal stromal cells are embedded in collagen gel alone and cultured under free-floating conditions, they contract greatly.30 By removing the serum from the medium, this phenomenon can be diminished,30 but the present study shows that contraction can be controlled by anchoring the collagen gel to a stronger material,31 in this case, the decellularized sheets. The differences in construct thickness reported during the culture period could indicate remodeling or compaction of the collagen hydrogel, conferring the constructs more mechanical stability. Moreover, additional crosslinking, such as UV-riboflavin, genipin, or transglutaminase, could further improve mechanical properties.
Layer-by-layer is an approach that has been used for stromal replacement constructs, since it aims to replicate the lamellar structure of the corneal stroma. Wilson et al. fabricated a stromal model by orthogonal stacking of layers of cell-seeded, aligned, electrospun PLDLA mesh, using collagen gel to bind them together.32 Ghezzi et al. reported constructs based on orthogonally stacked cell-seeded patterned silk films, without the aid of any binding material.33 Che et al. have recently described the fabrication of stromal equivalents by stacking cell-seeded ultrathin amniotic membrane.34 In this case, an extended culture period of 8 weeks allowed the stromal cells to produce ECM to bind the layers. The constructs reported in our present study were fabricated with materials found in the native stroma, which might present advantages for clinical translation.
Decellularized porcine corneas have been used to treat patients suffering from corneal ulcers2,3,21 showing the feasibility of employing xenogeneic material. The use of discarded tissue from myopia correction procedures is an option to use human material6,27 as well as donated corneas unsuitable for transplantation due to low endothelial cell count, for example.26
In the present study, the keratocytes were primarily found in the gel. It is known that in vivo remodeling of thin decellularized stromal tissue is faster than that of thicker tissues.7 3 weeks of culture might not have been enough time to allow the cells to repopulate the sheets by migrating from the gels. However, the reduction in construct thickness observed can be an indication of cells remodeling the collagen hydrogel. It is necessary to remark that the nonrecovery of sheet thickness after dehydration could have led to collagen fiber compaction, which might make the sheets more dense and, therefore, more difficult for the cells to penetrate. Moreover, the culture conditions used in this study have been optimized for the recovery of a keratocyte phenotype and these cells are known to migrate slowly.10
We would, expect that given enough time, once the keratocytes have remodeled the collagen gel, they would migrate to the decellularized sheets as a suitable substrate. In the future, using corneal stromal stem cells (CSSCs) instead of corneal fibroblasts in serum-free conditions might improve the outcome further since CSSCs present a more keratocyte-like phenotype,35 produce a more natural ECM36 and possess antiscarring activity.37 These cells have also been shown to support limbal epithelial stem cells in a compressed collagen I stromal model.38
Several studies have developed hydrogels as corneal substitutes, which required implantation using overlying sutures as they are not sufficiently strong to be implanted using running sutures39–42 or relied on the use of fibrin glue.43 Alternative strategies such as the one reported by Hong et al. increased suturability by embedding a decellularized human corneal lenticule in a compressed collagen gel.29 These authors also report the fabrication of anterior stromal equivalents by embedding keratocytes in a plastically compressed gel, composed of a collagen I and decellularized corneal ECM mixture, with epithelial cells on the surface.44 In the approach we took, stacking the decellularized sheets with the normally unsuturable collagen gels provided a construct that could be sutured with conventional interrupted 10-0 sutures.
Constructs at the time of implantation had a thickness of ∼190 μm, suitable for ALK and superficial ALK. These procedures are employed to treat anterior stromal defects, such as scarring, opacification, or ulceration resulting from infection, inflammation, trauma, or inadequate healing after surgical refractive procedures.45,46 Patients suffering from lattice, granular, or Reis/Bückler corneal dystrophies could also benefit from the use of these constructs.45,46 If thicker or thinner constructs would be needed, more or less layers, respectively, should be stacked when fabricating the constructs.
The optical properties of the presented constructs were suboptimal. However, studies report a recovery of transparency upon implantation, once the hydration state of the construct matches the native rather dehydrated nature.18,47 Furthermore, decellularized cornea ECM-derived hydrogels could be employed in future studies since these are more transparent than rat tail collagen hydrogels.48
Decellularized stromal lenticules similar to the ones described in this study have been used to culture corneal endothelial cells.49–52 While not in the scope of this study, the constructs described could also be seeded with endothelial cells to obtain a full-thickness laboratory-grown corneal substitute.
In summary, the feasibility of fabricating an anterior cornea equivalent using only tissue-derived materials was demonstrated, with good cell viability, optical properties, and cell phenotype. These were assembled in a rapid process that allowed regular and dense cell distribution, independent of cell migration. These corneal substitutes show translatability since they can be sutured and support regeneration of the epithelium in an ex vivo model.
Disclosure Statement
No competing financial interests exist.
Funding Information
The research is supported by funding from the European Research Council (ERC) under the European Union's Horizon 2020 research and innovation program (grant agreement no. 637460) and from Science Foundation Ireland (15/ERC/3269). Human eye tissue was kindly supplied through the Tissue Bank of the Irish Blood Transfusion Service, Dublin, after appropriate consent.
References
- 1. Gain P., Jullienne R., He Z., et al. Global survey of corneal transplantation and eye banking. JAMA Ophthalmol 134, 167, 2016 [DOI] [PubMed] [Google Scholar]
- 2. Zhang M.-C., Liu X., Jin Y., Jiang D.-L., Wei X.-S., and Xie H.-T.. Lamellar keratoplasty treatment of fungal corneal ulcers with acellular porcine corneal stroma. Am J Transplant 15, 1068, 2015 [DOI] [PubMed] [Google Scholar]
- 3. Zheng J., Huang X., Zhang Y., et al. Short-term results of acellular porcine corneal stroma keratoplasty for herpes simplex keratitis. Xenotransplantation 26, e12509, 2019 [DOI] [PubMed] [Google Scholar]
- 4. Xu Y.-G., Xu Y.-S., Huang C., Feng Y., Li Y., and Wang W.. Development of a rabbit corneal equivalent using an acellular corneal matrix of a porcine substrate. Mol Vis 14, 2180, 2008 [PMC free article] [PubMed] [Google Scholar]
- 5. Li S., Deng Y., Tian B., et al. Healing characteristics of acellular porcine corneal stroma following therapeutic keratoplasty. Xenotransplantation 27, e12566, 2020 [DOI] [PubMed] [Google Scholar]
- 6. Yin H., Qiu P., Wu F., et al. Construction of a corneal stromal equivalent with SMILE-derived lenticules and fibrin glue. Sci Rep 6, 33848, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ma X.Y., Zhang Y., Zhu D., et al. Corneal stroma regeneration with acellular corneal stroma sheets and keratocytes in a rabbit model. PLoS One 10, 1, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kafienah W., and Sims T.J.. Biochemical methods for the analysis of tissue-engineered cartilage. Methods Mol Biol 238, 217, 2004 [DOI] [PubMed] [Google Scholar]
- 9. Ahearne M., Yang Y., Then K.Y., and Liu K.K.. Non-destructive mechanical characterisation of UVA/riboflavin crosslinked collagen hydrogels. Br J Ophthalmol 92, 268, 2008 [DOI] [PubMed] [Google Scholar]
- 10. Fernández-Pérez J., and Ahearne M.. Influence of biochemical cues in human corneal stromal cell phenotype. Curr Eye Res 44, 135, 2019 [DOI] [PubMed] [Google Scholar]
- 11. Kim H., Park M.-N., Kim J., Jang J., Kim H.-K., and Cho D.-W.. Characterization of cornea-specific bioink: high transparency, improved in vivo safety. J Tissue Eng 10, 204173141882338, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Crapo P.M., Gilbert T.W., and Badylak S.F.. An overview of tissue and whole organ decellularization processes. Biomaterials 32, 3233, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lynch A.P., Wilson S.L., and Ahearne M.. Dextran preserves native corneal structure during decellularization. Tissue Eng Part C Methods 22, 561, 2016 [DOI] [PubMed] [Google Scholar]
- 14. Dong M., Zhao L., Wang F., et al. Rapid porcine corneal decellularization through the use of sodium N-lauroyl glutamate and supernuclease. J Tissue Eng 10, 204173141987587, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Du L., and Wu X.. Development and characterization of a full-thickness acellular porcine cornea matrix for tissue engineering. Artif Organs 35, 691, 2011 [DOI] [PubMed] [Google Scholar]
- 16. Lin Y., Zheng Q., Hua S., Meng Y., Chen W., and Wang Y.. Cross-linked decellularized porcine corneal graft for treating fungal keratitis. Sci Rep 7, 9955, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pang K., Du L., and Wu X.. A rabbit anterior cornea replacement derived from acellular porcine cornea matrix, epithelial cells and keratocytes. Biomaterials 31, 7257, 2010 [DOI] [PubMed] [Google Scholar]
- 18. Wu Z., Zhou Y., Li N., et al. The use of phospholipase A2 to prepare acellular porcine corneal stroma as a tissue engineering scaffold. Biomaterials 30, 3513, 2009 [DOI] [PubMed] [Google Scholar]
- 19. Courtman D.W., Pereira C.A., Kashef V., McComb D., Lee J.M., and Wilson G.J.. Development of a pericardial acellular matrix biomaterial: biochemical and mechanical effects of cell extraction. J Biomed Mater Res 28, 655, 1994 [DOI] [PubMed] [Google Scholar]
- 20. Murab S., and Ghosh S.. Impact of osmoregulatory agents on the recovery of collagen conformation in decellularized corneas. Biomed Mater 11, 065005, 2016 [DOI] [PubMed] [Google Scholar]
- 21. Shi W., Zhou Q., Gao H., et al. Protectively decellularized porcine cornea versus human donor cornea for lamellar transplantation. Adv Funct Mater 29, 1902491, 2019 [Google Scholar]
- 22. Wang X., Xu H., Huang Y., Gu S., and Jiang J.X.. Coupling effect of water and proteoglycans on the in situ toughness of bone. J Bone Miner Res 31, 1026, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Han E., Chen S.S., Klisch S.M., and Sah R.L.. Contribution of proteoglycan osmotic swelling pressure to the compressive properties of articular cartilage. Biophys J 101, 916, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gong Y.Y., Xue J.X., Zhang W.J., Zhou G.D., Liu W., and Cao Y.. A sandwich model for engineering cartilage with acellular cartilage sheets and chondrocytes. Biomaterials 32, 2265, 2011 [DOI] [PubMed] [Google Scholar]
- 25. Allen C.L., Clare G., Stewart E.A., et al. Augmented dried versus cryopreserved amniotic membrane as an ocular surface dressing. PLoS One 8, e78441, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Alió del Barrio J.L., El Zarif M., Azaar A., et al. Corneal stroma enhancement with decellularized stromal laminas with or without stem cell recellularization for advanced keratoconus. Am J Ophthalmol 186, 47, 2018 [DOI] [PubMed] [Google Scholar]
- 27. Yam G.H.-F., Yusoff N.Z.B.M., Goh T.-W., et al. Decellularization of human stromal refractive lenticules for corneal tissue engineering. Sci Rep 6, 26339, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lee R.E., and Davison P.F.. The collagens of the developing bovine cornea. Exp Eye Res 39, 639, 1984 [DOI] [PubMed] [Google Scholar]
- 29. Hong H., Huh M.-I., Park S.M., Lee K., Kim H.K., and Kim D.S.. Decellularized corneal lenticule embedded compressed collagen: toward a suturable collagenous construct for limbal reconstruction. Biofabrication 10, 045001, 2018 [DOI] [PubMed] [Google Scholar]
- 30. Miotto M., Gouveia R.M., Ionescu A.M., Figueiredo F., Hamley I.W., and Connon C.J.. 4D Corneal tissue engineering: achieving time-dependent tissue self-curvature through localized control of cell actuators. Adv Funct Mater 29, 1807334, 2019 [Google Scholar]
- 31. Ahearne M., Liu K.-K., El Haj A.J., Then K.Y., Rauz S., and Yang Y.. Online monitoring of the mechanical behavior of collagen hydrogels: influence of corneal fibroblasts on elastic modulus. Tissue Eng Part C Methods 16, 319, 2010 [DOI] [PubMed] [Google Scholar]
- 32. Wilson S.L., Wimpenny I., Ahearne M., Rauz S., El Haj A.J., and Yang Y.. Chemical and topographical effects on cell differentiation and matrix elasticity in a corneal stromal layer model. Adv Funct Mater 22, 3641, 2012 [Google Scholar]
- 33. Ghezzi C.E., Marelli B., Omenetto F.G., Funderburgh J.L., and Kaplan D.L.. 3D functional corneal stromal tissue equivalent based on corneal stromal stem cells and multi-layered silk film architecture. PLoS One 12, 1, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Che X., Wu H., Jia C., et al. A novel tissue-engineered corneal stromal equivalent based on amniotic membrane and keratocytes. Investig Opthalmology Vis Sci. 60, 517, 2019 [DOI] [PubMed] [Google Scholar]
- 35. Wu J., Du Y., Mann M.M., Funderburgh J.L., and Wagner W.R.. Corneal stromal stem cells versus corneal fibroblasts in generating structurally appropriate corneal stromal tissue. Exp Eye Res 120, 71, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Karamichos D., Funderburgh M.L., Hutcheon A.E.K., et al. A role for topographic cues in the organization of collagenous matrix by corneal fibroblasts and stem cells. PLoS One 9, e86260, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Basu S., Hertsenberg A.J., Funderburgh M.L., et al. Human limbal biopsy-derived stromal stem cells prevent corneal scarring. Sci Transl Med 6, 266ra172, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kureshi A.K., Dziasko M.A., Funderburgh J.L., and Daniels J.T.. Human corneal stromal stem cells support limbal epithelial cells cultured on RAFT tissue equivalents. Sci Rep 5, 16186, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Rafat M., Li F., Fagerholm P., et al. PEG-stabilized carbodiimide crosslinked collagen-chitosan hydrogels for corneal tissue engineering. Biomaterials 29, 3960, 2008 [DOI] [PubMed] [Google Scholar]
- 40. Liu W., Deng C., McLaughlin C.R., et al. Collagen–phosphorylcholine interpenetrating network hydrogels as corneal substitutes. Biomaterials 30, 1551, 2009 [DOI] [PubMed] [Google Scholar]
- 41. Fagerholm P., Lagali N., Merrett K., et al. A biosynthetic alternative to human donor tissue for inducing corneal regeneration: 24-month follow-up of a phase 1 clinical study. Sci Transl Med 2, 46ra61, 2010 [DOI] [PubMed] [Google Scholar]
- 42. Fagerholm P., Lagali N., Ong J.A., et al. Stable corneal regeneration four years after implantation of a cell-free recombinant human collagen scaffold. Biomaterials 35, 2420, 2014 [DOI] [PubMed] [Google Scholar]
- 43. Massie I., Kureshi A.K., Schrader S., Shortt A.J., and Daniels J.T.. Optimization of optical and mechanical properties of real architecture for 3-dimensional tissue equivalents: towards treatment of limbal epithelial stem cell deficiency. Acta Biomater 24, 241, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hong H., Kim H., Han S.J., et al. Compressed collagen intermixed with cornea-derived decellularized extracellular matrix providing mechanical and biochemical niches for corneal stroma analogue. Mater Sci Eng C 103, 109837, 2019 [DOI] [PubMed] [Google Scholar]
- 45. Espandar L., and Carlson A.N.. Lamellar keratoplasty: a literature review. J Ophthalmol 2013, 2013, 894319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ganger A., Tandon R., Vanathi M., and Sagar P.. Superficial anterior lamellar keratoplasty (salk) for trauma-induced post refractive surgery corneal opacity. J Ophthalmic Vis Res 11, 326, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hashimoto Y., Funamoto S., Sasaki S., et al. Corneal regeneration by deep anterior lamellar keratoplasty (DALK) using decellularized corneal matrix. PLoS One 10, e0131989, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ahearne M., and Lynch A.P.. Early observation of extracellular matrix-derived hydrogels for corneal stroma regeneration. Tissue Eng Part C Methods 21, 1059, 2015 [DOI] [PubMed] [Google Scholar]
- 49. He Z., Forest F., Bernard A., et al. Cutting and decellularization of multiple corneal stromal lamellae for the bioengineering of endothelial grafts. Invest Ophthalmol Vis Sci 57, 6639, 2016 [DOI] [PubMed] [Google Scholar]
- 50. Choi J.S., Williams J.K., Greven M., et al. Bioengineering endothelialized neo-corneas using donor-derived corneal endothelial cells and decellularized corneal stroma. Biomaterials 31, 6738, 2010 [DOI] [PubMed] [Google Scholar]
- 51. Ju C., Gao L., Wu X., and Pang K.. A human corneal endothelium equivalent constructed with acellular porcine corneal matrix. Indian J Med Res 135, 887, 2012 [PMC free article] [PubMed] [Google Scholar]
- 52. Arnalich-Montiel F., Moratilla A., Fuentes-Julián S., et al. Treatment of corneal endothelial damage in a rabbit model with a bioengineered graft using human decellularized corneal lamina and cultured human corneal endothelium. PLoS One 14, e0225480, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]