Abstract
Traumatic brain injury (TBI) is a leading cause of morbidity worldwide, for which biomarkers are needed to better understand the underlying pathophysiology. Microvascular injury represents a subset of pathological mechanisms contributing to cognitive dysfunction after TBI, which may also impair subsequent neural repair thereby inhibiting cognitive recovery. Magnetic resonance imaging (MRI)-based measurement of cerebral blood flow (CBF) by arterial spin labeling (ASL) provides an appealing means of assessing microvascular disruption in TBI; however, the relationship between CBF alterations in the early chronic post-TBI setting and cognitive dysfunction as well as subsequent cognitive recovery remain poorly understood. Structural MRI and ASL were performed in 42 TBI subjects 3 months post-injury and 35 matched healthy controls. Neuropsychological testing was performed in each subject, as well as in a subset of TBI patients (n = 33) at 6 and/or 12 months post-injury. TBI and control subject CBF data were compared between groups in a voxel-wise fashion while controlling for the effects of structural atrophy. A region-of-interest approach was then used to compare CBF to clinical and neuropsychological measures within the TBI group in a cross-sectional fashion, as well as to the degree of subsequent cognitive recovery among subjects with follow-up testing. At 3 months post-injury, the TBI group demonstrated lower performance in each cognitive domain (p < 0.05), as well as widespread reductions in gray matter CBF independent of structural atrophy (p < 0.05). Within the TBI group, CBF was moderately correlated with injury severity (r = −0.43; p = 0.009) and executive function (r = 0.43; p = 0.01). In the longitudinal analysis, there was a positive correlation between initial CBF and processing speed recovery (r = 0.43; p = 0.015) independent of age, education level, and initial test score. Early chronic TBI is associated with widespread gray matter CBF deficits, which are correlated with injury severity and cognitive dysfunction. CBF may predict subsequent recovery in some cognitive domains.
Keywords: adult brain injury, ASL, blood flow, CBF, cognitive function, MRI, TBI, vascular injury
Introduction
Traumatic brain injury (TBI) is a leading cause of mortality and morbidity worldwide, which contributes to a high social and economic burden of disability,1–4 and has increasingly been recognized as a risk factor for late-life neurodegenerative disease.5,6 Moderate-to-severe TBI in particular results in a high prevalence of cognitive deficits across a number of domains, including executive function, attention, speed of information processing, learning, and memory.7,8 The degree and rate of recovery of these impairments, however, exhibits substantial variability across individuals9,10 for reasons which are incompletely understood. The application of biomarkers holds potential to address these existing knowledge gaps by elucidating the neurobiological processes underlying persistent TBI-related disability and divergent long-term post-TBI recovery patterns, ultimately aiding the development of targeted and effective rehabilitation strategies.
Injury to the cerebral microvasculature associated with TBI represents a subset of pathological mechanisms which contribute to cognitive dysfunction after TBI. Animal and pathological studies widely implicate microvascular injury in TBI across injury severity,11–15 thought to be induced by shearing forces associated with acceleration-deceleration of the brain. Microvascular injury is, in turn, related to impaired functioning of the neurovascular unit (NVU) in the maintenance of microvascular perfusion, cerebrovascular reactivity, and normal functioning of the blood–brain barrier.16 NVU dysfunction may also impair subsequent neural repair mechanisms and precipitate further neuronal loss through a range of secondary and more slowly progressive pathological processes, including mitochondrial dysfunction, calcium dysregulation, neuroinflammation, and, ultimately, deposition of tau and amyloid.17–20
Magnetic resonance imaging (MRI)-based measurement of cerebral blood flow (CBF) by arterial spin labeling (ASL) provides a means of assessing both microvascular disruption and metabolic derangement attributed to the tight coupling of CBF and metabolism in the brain. The advantages that ASL offers in the measurement of cerebral perfusion include high spatial resolution, absolute CBF quantification, and lack of exposure to contrast material or ionizing radiation. Further, ASL is readily acquired as part of a multi-modal MRI examination required to assess the breadth of heterogeneous TBI-related pathology, better account for potentially confounding structural manifestations of TBI on perfusion measurements, and accurately coregister perfusion data across subjects for quantitative analysis.
CBF has become an important biomarker of cognitive function and dysfunction in both normal aging and neurodegenerative disease, given that CBF predicts subsequent cognitive decline21–23 and, in some cases, brain atrophy.24 Given that there is increasing recognition that chronic TBI is associated with pathophysiological changes which overlap with and may accelerate neurodegenerative disorders,6,25 assessment of CBF may similarly be able to convey important prognostic information in chronic TBI. Further, NVU dysfunction represents a potentially modifiable target for intervention given its plasticity and responsiveness to pharmacological therapy.15
Several human studies to date have demonstrated that TBI is associated with focal and multi-focal reductions in CBF, particularly in the acute setting, which may be occult on conventional structural imaging, and, in some cases, predictive of outcomes.26–30 Investigations of perfusion in the chronic phase of TBI have suggested that CBF reductions are persistent,31–33 but have, however, been far fewer in number and limited by heterogeneity in potentially confounding factors, such as time from injury and structural atrophy. Thus, the relationship of CBF to cognitive functioning and post-TBI recovery remains poorly understood.
In this study, we aimed to use ASL to characterize the pattern of CBF changes independent of structural atrophy in the early chronic post-TBI setting. We further aimed to establish the relationship of reduced CBF to contemporaneous cognitive performance as well as to the subsequent cognitive recovery trajectory. We hypothesized that chronic TBI is associated with reduced CBF independent of brain atrophy, and that the degree of TBI-related perfusion alteration is related to neurocognitive deficits and recovery trajectory.
Methods
Study population
This study was approved by the institutional review board of the home institution, and written informed consent was obtained from the participants or their legally authorized representatives. Subjects were included based on age between 18 and 64 years and a history of non-penetrating TBI of at least moderate severity, as defined by at least one of the following: 1) Glasgow Coma Scale (GCS) <13 in the emergency department (not attributed to sedation, paralysis, or intoxication); 2) documented loss of consciousness for ≥12 h; or (3) duration of post-traumatic amnesia (PTA) ≥24 h. Participants were excluded for: 1) history of previous TBI, central nervous system disease, seizure disorder, schizophrenia, or bipolar disorder; 2) history of abuse of alcohol or stimulants likely to have resulted in neurological sequelae; 3) pregnancy; 4) inability to complete MRI scanning because of ferromagnetic implants, claustrophobia, or restlessness; 5) non-fluency in English; and 6) level of disability too great to allow for completion of testing and scanning 3 months post-TBI.
To maximize the likelihood of traumatic axonal injury in the absence of large focal brain lesions, participants were also excluded if the total estimated volume of focal intraparenchymal lesions was >5 cm3 for subcortical lesions and 50 cm3 for cortical lesions. In total, 42 TBI subjects met the inclusion criteria for this study. In addition, 35 healthy volunteers matched to TBI subjects for age, sex, and years of education were recruited. Exclusion criteria for controls were the same as above, with an additional exclusion for any history of TBI resulting in loss or alteration of consciousness.
Clinical and neuropsychological measures
Clinical measures were abstracted from the medical record or measured prospectively during hospitalization. Duration of PTA was used as an index of TBI severity, defined as the number of days between the TBI and the date of the first of two scores ≥25 on the Orientation Log34 administered ≤72 h apart. For patients who were discharged from rehabilitation care while still in PTA, their PTA duration was estimated conservatively at [days between TBI and rehabilitation discharge] + 1. Neuropsychological tests of processing speed, executive function, and verbal learning, domains frequently impaired in TBI, were performed in all control subjects, all TBI subjects 3 months after injury, as well as in a subset of TBI subjects at 6 and 12 months after injury. Cognitive performance was assessed across three domains, including mental processing speed as indexed by the Processing Speed Index (PSI) from the Wechsler Adult Intelligence Scale IV, verbal learning (VL) as indexed by the Rey Auditory Verbal Learning Test, and executive functioning (EF) as indexed by a composite measure consisting of an average across T-scores of the following tests: Trails-Making-Test B, color-word-interference test, backward digit span, and letter-number sequencing.35
Magnetic resonance imaging acquisition and post-processing
Brain MRI examinations were performed on a 3 Tesla MRI scanner (Siemens Trio; Siemens, Erlangen, Germany), which included a high-resolution T1 magnetization-prepared rapid gradient echo sequence acquired at 1-mm isotropic resolution using an echo time (TE) of 3.08 ms, repetition time (TR) of 1620 ms, inversion time of 950 ms, flip angle of 15 degrees, and matrix of 256 × 192. ASL imaging was performed using a pseudo-continuous labeling technique 9 cm below the center of the imaging volume with labeling duration and post-labeling delay of 1.5 sec. Images were acquired using a two-dimensional echo planar sequence with the following parameters: TR 4 sec, TE 18 ms, field of view 220 mm, matrix 64 × 64, and voxel size 3.4 × 3.4 × 7.2 mm3. Eighteen slices with a distance factor of 20% were acquired from inferior to superior direction in a sequential order. Forty-five label-control pairs were acquired for signal averaging.
Structural images from a subset of 40 study participants (20 controls and 20 TBI, with same sex balance 6 female and 14 male) were used to create a population-specific anatomical template using the longitudinal processing pipeline of the Advanced Normalization Tools (ANTs).36 Subsequently, all subjects' structural scans underwent brain extraction, six-tissue segmentation, and non-linear transformation to the group template using the ANTs structural processing pipeline.37 Macrostructural encephalomalacic lesions segmented from the structural images by a trained observer were used in masking the cost function of the registration procedure, which improves accuracy in coregistration of brains with focal lesions.38 Voxel-wise maps of the Jacobian determinant (log-transformed) were additionally calculated from the diffeomorphic registration fields. The Jacobian determinant value reflects the degree of local tissue expansion or contraction required to match the template, frequently used to assess brain atrophy.39 Anatomical labeling of each subject's structural image and the template brain was performed using the Desikan-Killiany-Tourville labeling protocol.40
ASL data were processed using SPM12, the ASL toolbox,41 and custom MATLAB scripts (The MathWorks, Inc., Natick, MA). The raw echo planar imaging label-control time series was first motion corrected and then a CBF time series was obtained using pair-wise control-label subtraction, dividing by the corresponding control image and application of the recommended model42 for CBF estimation. Subsequently, a denoised mean CBF map was obtained using a two-step data-cleaning strategy: 1) a structural correlation based outlier rejection method, which explicitly detects and discards outlier volumes iteratively, followed by 2) a voxel-wise Bayesian estimation approach.43,44 Final CBF maps were visually reviewed by a neuroradiologist and an experienced ASL data processing scientist to ensure image quality.
All CBF maps were then transformed to structural space using a functional space to structural space affine transformation computed by ANTs, as well as to group template space by applying a single transform to the initial CBF map consisting of a composition of the functional to structural affine transformation and the non-linear transform computed in the structural processing step. Standard and structural space CBF data were smoothed with a Gaussian kernel of 6 mm full width at half maximum, and standard space CBF maps were downsampled to 2-mm isotropic resolution to reduce the number of comparisons in subsequent whole-brain, voxel-wise testing.
Statistical analysis
CBF and Jacobian determinant maps were compared between the TBI and control groups in a whole-brain, voxel-wise fashion using a non-parametric permutation testing tool45 with the threshold-free cluster enhancement method of multiple comparison correction.46 Additionally, to directly account for atrophy in the CBF comparison, the group-wise testing procedure was repeated using the Jacobian determinate maps as a voxel-wise covariate. CBF reductions among the TBI group were qualitatively compared with the distribution of focal encephalomalacic lesions, displayed as an overlap map of manually segmented lesions in the template space.
Subsequent analysis within the TBI group was performed using a regions-of-interest (ROI) approach. Mean CBF and Jacobian determinant values were extracted from a “meta-ROI” comprised of all anatomical regions which contained voxels of significantly reduced CBF in the TBI group relative to the control group. The mean Jacobian determinant was also extracted from an ROI comprised of the lateral and third ventricles as a marker of diffuse atrophy. These measures were then compared with injury severity and cognitive measures among the TBI group in a cross-sectional fashion using linear correlation analysis while controlling for age and level of education. Further, the relationship of imaging measures at the initial 3-month time point was compared to cognitive recovery over the ensuing 9 months by computing the correlation between 3-month CBF and the slope change of the cognitive measures over time while controlling for the effects of age, education level, and the initial test score.
Results
A total of 9 subjects (6 TBI and 3 control) were excluded from the analysis because of poor ASL data quality, leaving a total population of 36 TBI subjects and 32 control subjects. Demographic, injury, and clinical characteristics of the TBI and control groups are listed in Table 1. There were no significant demographic differences between groups. Injury characteristics suggested that the TBI sample overall sustained moderate-severe TBI, the majority of which were attributed to traffic incidents (Table 1). With regard to neuropsychological function, the TBI group demonstrated significantly lower performance in each cognitive domain tested at 3 months post-injury.
Table 1.
Demographic Characteristics, Injury Severity among TBI Subjects, and Neuropsychological Test Results
| Control | TBI | p value | |
|---|---|---|---|
| N | 32 | 36 | |
| Age, years (SD) | 35.4 (10.1) | 33.8 (14.5) | 0.59 |
| Sex, no. male (%) | 24 (75.0) | 26 (72.2) | 1.00 |
| Education, years (SD) | 13.2 (2.2) | 13.8 (2.3) | 0.31 |
| Duration of PTA, h (SD) | — | 25.9 (21.5) | — |
| Time to follow commands, days (SD) | — | 7.2 (9.8) | — |
| Total GCS score (SD) | — | 10.0 (4.0) | — |
| Injury mechanism (traffic/fall/other) | 25/8/3 | ||
| Processing Speed Index (PSI) | 96.9 (13.9) | 85.1 (19.4) | 0.005 |
| Executive Function Composite (EF) | 49.5 (7.5) | 44.1 (9.4) | 0.013 |
| Rey Auditory Verbal Learning Test (VL) | 44.56 (9.20) | 33.54 (15.87) | 0.001 |
Bolded values indicate statistical significance.
GCS is based on a subset of TBI subjects (N = 24).
TBI, traumatic brain injury; SD, standard deviation; PTA, post-traumatic amnesia; GCS, Glasgow Coma Scale.
Focal encephalomalacia demonstrated a spatial predilection for the anterior-inferior frontal and anterior temporal lobes, and there was in general a low frequency of overlap between TBI subjects (Fig. 1). Whole-brain, voxel-wise comparison of Jacobian determinant values revealed significant elevations in the TBI group within the lateral and third ventricles (p < 0.05, corrected), indicating larger ventricular volumes among the TBI group. There were no areas of significantly reduced Jacobian determinant values in the TBI group compared to controls (p > 0.05, corrected).
FIG. 1.
Voxel-wise frequency map depicting the location and frequency of focal encephalomalacia among the TBI group. TBI, traumatic brain injury. Color image is available online.
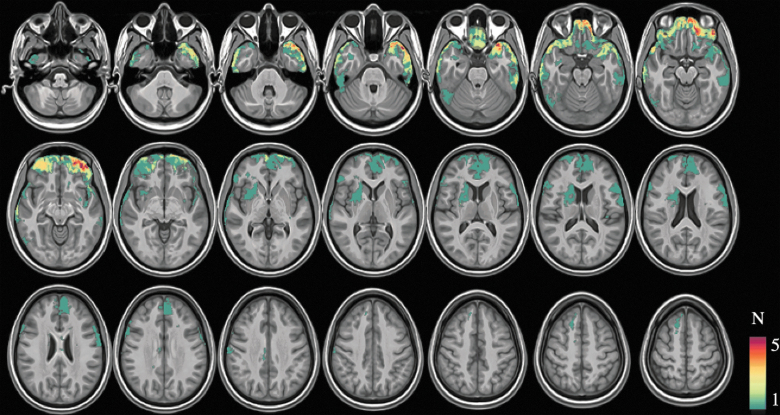
Compared to controls, resting CBF was significantly reduced in several cortical and subcortical gray matter regions in the TBI group (p < 0.05, corrected; Fig. 2). The inclusion of Jacobian determinant values as a voxel-wise confound only attenuated CBF differences to a small degree in the anterior frontal and temporal lobes (Fig. 2). In comparison to the distribution of focal lesions, the distribution of CBF showed partial overlap, but also extended beyond the location of focal lesions.
FIG. 2.
Result of group-level voxel-wise CBF comparison between TBI and control groups, depicting areas of significantly reduced CBF among the TBI group (p < 0.05, corrected). The top and bottom figures represent results without and with the use of Jacobian determinant maps as a voxel-wise confound, respectively. CBF, cerebral blood flow; TBI, traumatic brain injury. Color image is available online.
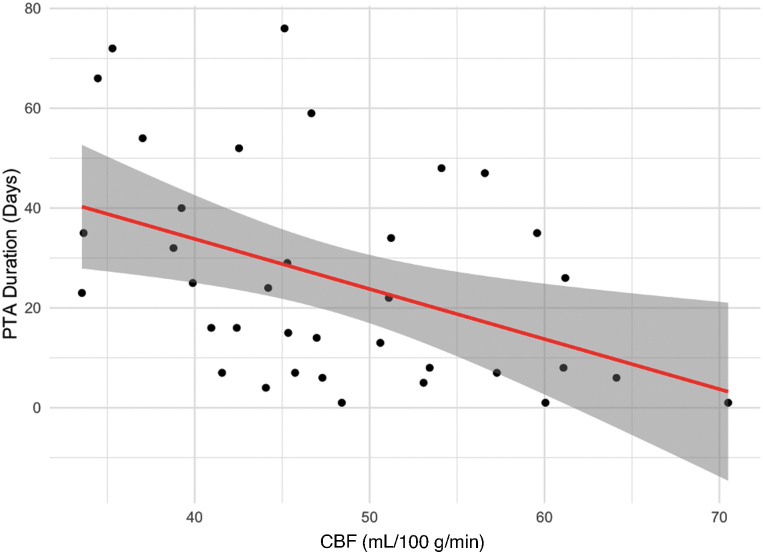
Within the TBI group, CBF in the meta-ROI was not significantly correlated with atrophy in the meta-ROI as indexed by the Jacobian determinant value (r = 0.1; p = 0.5); however, meta-ROI CBF was inversely correlated with mean Jacobian determinant of the lateral and third ventricles (r = −0.37; p = 0.02). Injury severity indexed by duration of PTA was correlated with both CBF (r = −0.43; p = 0.009; Fig. 3) and ventricular Jacobian determinant (r = 0.46; p = 0.004), but not Jacobian determinant, in the meta-ROI (r = −0.26; p = 0.11).
FIG. 3.
Relationship of CBF at 3 months post-injury to the duration of post-traumatic amnesia (index of injury severity). CBF is inversely correlated to injury severity (r = −0.43). CBF, cerebral blood flow; PTA, post-traumatic amnesia. Color image is available online.
FIG. 4.
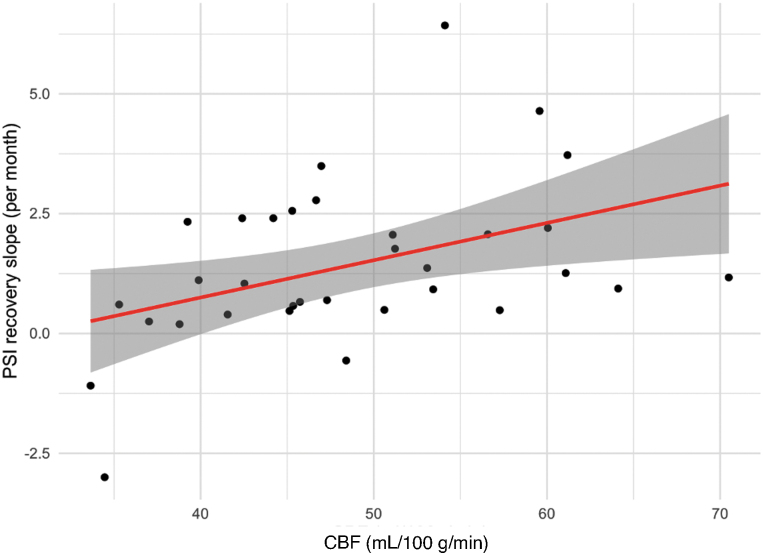
Relationship of CBF at 3 months post-injury to the slope of PSI recovery among the TBI group. The 3-month CBF is directly correlated with the recovery slope (r = 0.43). CBF, cerebral blood flow; PSI, Processing Speed Index; TBI, traumatic brain injury. Color image is available online.
The correlation between imaging measures and neuropsychological test performance is displayed in Table 2. Meta-ROI CBF showed a moderate correlation with EF (r = 0.43; p = 0.01), but was not significantly correlated with 3-month PSI (r = 0.24; p = 0.16) or VL (r = 0.20; p = 0.24). Ventricular Jacobian determinant showed an inverse correlation with both PSI (r = −0.56; p = 0.001) and EF (r = −0.48; p = 0.004), but was not correlated with VL (r = −0.11; p = 0.545). There was no significant correlation between meta-ROI Jacobian determinant and PSI (r = 0.21; p = 0.23), EF (r = 0.26; p = 0.13), or VL (r = −0.08; p = 0.64).
Table 2.
Result of Correlation Analysis between Neuropsychological Tests and ROI-Based Measures of CBF and Structural Atrophy among the TBI Group
| PSI | EF | VL | |
|---|---|---|---|
| CBF: Meta-ROI | 0.24 (p = 0.16) | 0.43 (p = 0.01) | 0.20 (p = 0.24) |
| Jacobian: Ventricles | –0.56 (p = 0.001) | –0.48 (p = 0.004) | –0.11 (p = 0.55) |
| Jacobian: Meta-ROI | 0.21 (p = 0.23) | 0.26 (p = 0.13) | –0.08 (p = 0.64) |
Bolded values indicate statistical significance.
ROI, region of interest; CBF, cerebral blood flow; TBI, traumatic brain injury; PSI, Processing Speed Index; EF, Executive Function Composite; VL, Rey Auditory Verbal Learning Test.
In the longitudinal analysis, 33 of the initial 36 TBI subjects completed at least one of the follow-up evaluations and were included (25 subjects with both 6- and 12-month post-TBI time points and 8 subjects with either the 6- or 12-month timepoint). Details of neuropsychological recovery pattern from the same cohort have been reported in our recent publication.35 As a group, TBI patients overall displayed good cognitive recovery, approaching the performance level of healthy control participants by 12 months post-injury. The recovery trajectory of neuropsychological performance was mostly linear; however, for the domain of processing speed, age moderated the trajectory such that older TBI patients were more likely to exhibit plateaued recovery or deterioration during the first year post-injury.
In the current sample, TBI subjects exhibited a positive recovery slope for PSI (mean increase, 1.41/month ±1.69), EF (mean increase, 0.21/month ±0.96), and VL (mean increase, 1.1/month ±2.1). Correlation analyses between 3-month imaging measures and slope of individual recovery curves are displayed in Table 3. There was a significant positive correlation between initial meta-ROI CBF and slope of PSI recovery (r = 0.43; p = 0.015). There was also a weak correlation between meta-ROI CBF and slope of EF recovery, which did not reach statistical significance (r = 0.33; p = 0.06). There was no significant correlation between 3-month CBF and slope of the VL recovery curve (r = 0.2; p = 0.24). Neither 3-month Jacobian determinant from the ventricles nor meta-ROI was significantly correlated with the PSI, EF, or VL recovery slope.
Table 3.
Result of Correlation Analysis between Neuropsychological Recovery Trajectory and ROI-Based Measures of CBF and Structural Atrophy among the TBI Group
| PSI recovery slope | EF recovery slope | VL recovery slope | |
|---|---|---|---|
| CBF: Meta-ROI | 0.43 (p = 0.015) | 0.33 (p = 0.06) | 0.28 (p = 0.13) |
| Jacobian: Ventricles | –0.23 (p = 0.23) | –0.07 (p = 0.7) | –0.24 (p = 0.21) |
| Jacobian: Meta-ROI | 0.19 (p = 0.31) | –0.12 (p = 0.52) | 0.07 (p = 0.72) |
Bolded values indicate statistical significance.
ROI, region of interest; CBF, cerebral blood flow; TBI, traumatic brain injury; PSI, Processing Speed Index; EF, Executive Function Composite; VL, Rey Auditory Verbal Learning Test.
Discussion
In this study, we investigated the effects of chronic TBI on resting brain perfusion as well as the relationship of TBI-related perfusion alterations to cognitive function and subsequent cognitive recovery. Generally consistent with an emerging body of literature associating chronic TBI with reduced gray matter CBF,32,33,47,48 we have found widespread reductions of cortical and subcortical CBF in the early chronic post-TBI period, in a spatial distribution, which is distinct from focal lesions, which were generally small and showed infrequent spatial overlap between subjects in our study population.
Structural analysis revealed findings consistent with diffuse brain atrophy among the TBI group, which was significantly correlated to injury severity and contemporaneous neurocognitive deficits. This is consistent with several previous studies assessing TBI-related brain volumetric changes49–51 and, additionally, suggests that diffuse brain atrophy is readily apparent as early as 3 months after injury. The lack of detection of focal gray matter volume loss in this study most likely owes to the low statistical power of our small sample size for detection of spatially heterogeneous effects; however, we were able to detect widespread reductions in CBF, suggesting that ASL is more sensitive to gray matter damage than cross-sectional tensor-based morphometry in the early chronic post-TBI period.
Our findings build upon previous work by demonstrating that CBF deficits in the early chronic post-TBI setting are directly related to injury severity and, additionally, do not appear to simply reflect regional gray matter atrophy. Further, our findings suggest that TBI-related CBF reduction carries relevance to post-traumatic neurocognitive dysfunction, particularly executive functioning, independent of both local structural changes and diffuse brain atrophy. Last, direct correlation between ensuing cognitive recovery trajectory and 3-month CBF, but not structural measures, suggests that the integrity of the cerebral microvasculature may have prognostic significance independent from early brain atrophy.
Overall, our results suggest that ASL-based measurement of CBF in the early chronic post-injury period is a promising index, both of overall severity of diffuse injury (as evidenced by its relationship to duration of PTA) as well as the potential for subsequent recovery in some cognitive domains (as evidenced by its relationship to the slope of PSI recovery). To our knowledge, this is the first longitudinal study in moderate-to-severe TBI demonstrating the relationship between early-chronic-phase CBF reduction and subsequent cognitive recovery. The natural history of regional TBI-related CBF deficits represents an important area for further study given the increasing recognition of TBI as a risk factor for later neurodegeneration,5,6 increasing recognition of the role played by chronic vascular pathology in other neurodegenerative processes,52 and the potential for intervention on this disease phenotype.15 Additional research focused on disentangling mechanisms of CBF reduction in chronic TBI, which include microvascular injury and primary metabolic dysfunction, may yield additional important insights into the nature of TBI-related neuropathology.
Our use of a meta-ROI to examine relationships between imaging and clinical measures differs from several earlier studies, which have used global and/or regional approaches based on standard anatomical parcellations to summarize imaging metrics when examining brain-behavior relationships after TBI.53 The meta-ROI, by definition, includes only brain regions exhibiting significant differences from healthy persons and thereby improves specificity for TBI-related brain-behavior effects over global approaches. Whereas additional associations might have been revealed by comparing imaging measures in different regions to cognitive scores, this approach would have limited sensitivity in our relatively small sample size, which was not well powered for performing numerous comparisons across the entire brain necessary to explore these relationships. Additionally, our TBI population was selected to enhance to prevalence of diffuse injury, which may further limit the ability to map deficits in specific cognitive domains to regionally localized injury. Further, it is increasingly recognized that TBI-related impairments of higher cognitive functions more strongly relate to derangements in large-scale brain networks resulting from injuries that are not necessarily spatially coherent.54
Although a strength of this study is a homogeneous TBI cohort at uniform time post-injury, a limitation attributable to the exclusion criteria is that these findings may not be generalizable to TBI subjects with large focal lesions or those with deficits so severe as to preclude MRI or behavioral testing. Further, our sample size was relatively small and additional work investigating the predictive power of CBF for post-TBI cognitive recovery is warranted. This study used a non-background-suppressed ASL MRI technique that is more susceptible to artifacts than background-suppressed approaches that are now being more widely adopted,42 and over 10% of study subjects were excluded because of artifacts in ASL data. Future work using background suppressed ASL MRI should improve the sensitivity and reliability of ASL MRI data.
In conclusion, early chronic TBI is associated with widespread gray matter CBF deficits, which are correlated with post-traumatic cognitive dysfunction and predictive of subsequent cognitive recovery in some domains independent of structural atrophy. ASL is a promising means of assessing TBI-related microvascular dysfunction in the brain.
Funding Information
This work was supported by the National Institutes of Health (NINDS R01NS065980; PI: J.J.K.).
Author Disclosure Statement
No competing financial disclosures exist.
References
- 1. Humphreys I., Wood R.L., Phillips C.J., and Macey S. (2013). The costs of traumatic brain injury: a literature review. Clin. Outcomes Res. 5, 281–287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hyder A.A., Wunderlich C.A., Puvanachandra P., Gururaj G., and Kobusingye O.C. (2007). The impact of traumatic brain injuries: a global perspective. NeuroRehabilitation 22, 341–353 [PubMed] [Google Scholar]
- 3. Langlois J.A., Rutland-Brown W., and Wald M.M. (2006). The epidemiology and impact of traumatic brain injury: a brief overview. J. Head Trauma Rehabil. 21, 375–378 [DOI] [PubMed] [Google Scholar]
- 4. Maas A.I.R., Menon D.K., Adelson P.D., Andelic N., Bell M.J., Belli A., Bragge P., Brazinova A., Büki A., Chesnut R.M., Citerio G., Coburn M., Cooper D.J., Crowder A.T., Czeiter E., Czosnyka M., Diaz-Arrastia R., Dreier J.P., Duhaime A.-C., Ercole A., van Essen T.A., Feigin V.L., Gao G., Giacino J., Gonzalez-Lara L.E., Gruen R.L., Gupta D., Hartings J.A., Hill S., Jiang J.-Y., Ketharanathan N., Kompanje E.J.O., Lanyon L., Laureys S., Lecky F., Levin H., Lingsma H.F., Maegele M., Majdan M., Manley G., Marsteller J., Mascia L., McFadyen C., Mondello S., Newcombe V., Palotie A., Parizel P.M., Peul W., Piercy J., Polinder S., Puybasset L., Rasmussen T.E., Rossaint R., Smielewski P., Söderberg J., Stanworth S.J., Stein M.B., von Steinbüchel N., Stewart W., Steyerberg E.W., Stocchetti N., Synnot A., Te Ao B., Tenovuo O., Theadom A., Tibboel D., Videtta W., Wang K.K.W., Williams W.H., Wilson L., and Yaffe, K.; InTBIR Participants and Investigators. (2017). Traumatic brain injury: integrated approaches to improve prevention, clinical care, and research. Lancet Neurol. 16, 987–1048 [DOI] [PubMed] [Google Scholar]
- 5. Shively S., Scher A.I., Perl D.P., and Diaz-Arrastia R. (2012). Dementia resulting from traumatic brain injury: what is the pathology? Arch. Neurol. 69, 1245–1251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wilson L., Stewart W., Dams-O'Connor K., Diaz-Arrastia R., Horton L., Menon D.K., and Polinder S. (2017). The chronic and evolving neurological consequences of traumatic brain injury. Lancet Neurol. 16, 813–825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ponsford J. (2013). Factors contributing to outcome following traumatic brain injury. NeuroRehabilitation 32, 803–815 [DOI] [PubMed] [Google Scholar]
- 8. Silver J.M., McAllister T.W., Arciniegas D.B., and American Psychiatric Association (eds). (2019). Textbook of Traumatic Brain Injury, Third ed. American Psychiatric Association: Washington, DC, 953 pp [Google Scholar]
- 9. Marsh N.V. (2019). Cognitive functioning following traumatic brain injury: the first 5 years. NeuroRehabilitation 43, 377–386 [DOI] [PubMed] [Google Scholar]
- 10. Schultz R., and Tate R.L. (2013). Methodological issues in longitudinal research on cognitive recovery after traumatic brain injury: evidence from a systematic review. Brain Impair. 14, 450–474 [Google Scholar]
- 11. DeWitt D.S., and Prough D.S. (2003). Traumatic cerebral vascular injury: the effects of concussive brain injury on the cerebral vasculature. J. Neurotrauma 20, 795–825 [DOI] [PubMed] [Google Scholar]
- 12. Kenney K., Amyot F., Haber M., Pronger A., Bogoslovsky T., Moore C., and Diaz-Arrastia R. (2016). Cerebral vascular injury in traumatic brain injury. Exp. Neurol. 275, 353–366 [DOI] [PubMed] [Google Scholar]
- 13. Castejón O.J. (2011). Ultrastructural pathology of cortical capillary pericytes in human traumatic brain oedema. Folia Neuropathol. 49, 162–173 [PubMed] [Google Scholar]
- 14. Castejón O.J. (2014). Ultrastructural alterations of human cortical capillary basement membrane in human brain oedema. Folia Neuropathol. 52, 10–21 [DOI] [PubMed] [Google Scholar]
- 15. Sandsmark D.K., Bashir A., Wellington C.L., and Diaz-Arrastia R. (2019). Cerebral microvascular injury: a potentially treatable endophenotype of traumatic brain injury-induced neurodegeneration. Neuron 103, 367–379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhao Z., Nelson A.R., Betsholtz C., and Zlokovic B.V. (2015). Establishment and dysfunction of the blood-brain barrier. Cell 163, 1064–1078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Golding E.M., Robertson C.S., and Bryan R.M. (1999). The consequences of traumatic brain injury on cerebral blood flow and autoregulation: a review. Clin. Exp. Hypertens. 21, 299–332 [DOI] [PubMed] [Google Scholar]
- 18. Golding E. (2002). Sequelae following traumatic brain injury. The cerebrovascular perspective. Brain Res. Rev. 38, 377–388 [DOI] [PubMed] [Google Scholar]
- 19. Szarka N., Pabbidi M.R., Amrein K., Czeiter E., Berta G., Pohoczky K., Helyes Z., Ungvari Z., Koller A., Buki A., and Toth P. (2018). Traumatic brain injury impairs myogenic constriction of cerebral arteries: role of mitochondria-derived H2O2 and TRPV4-dependent activation of BKca channels. J. Neurotrauma 35, 930–939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kisler K., Nelson A.R., Rege S.V., Ramanathan A., Wang Y., Ahuja A., Lazic D., Tsai P.S., Zhao Z., Zhou Y., Boas D.A., Sakadžić S., and Zlokovic B.V. (2017). Pericyte degeneration leads to neurovascular uncoupling and limits oxygen supply to brain. Nat. Neurosci. 20, 406–416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chao L.L., Buckley S.T., Kornak J., Schuff N., Madison C., Yaffe K., Miller B.L., Kramer J.H., and Weiner M.W. (2010). ASL perfusion MRI predicts cognitive decline and conversion from MCI to dementia. Alzheimer Dis. Assoc. Disord. 24, 19–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Xekardaki A., Rodriguez C., Montandon M.-L., Toma S., Tombeur E., Herrmann F.R., Zekry D., Lovblad K.-O., Barkhof F., Giannakopoulos P., and Haller S. (2015). Arterial spin labeling may contribute to the prediction of cognitive deterioration in healthy elderly individuals. Radiology 274, 490–499 [DOI] [PubMed] [Google Scholar]
- 23. Benedictus M.R., Leeuwis A.E., Binnewijzend M.A.A., Kuijer J.P.A., Scheltens P., Barkhof F., van der Flier W.M., and Prins N.D. (2017). Lower cerebral blood flow is associated with faster cognitive decline in Alzheimer's disease. Eur. Radiol. 27, 1169–1175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Olm C.A., Kandel B.M., Avants B.B., Detre J.A., Gee J.C., Grossman M., and McMillan C.T. (2016). Arterial spin labeling perfusion predicts longitudinal decline in semantic variant primary progressive aphasia. J. Neurol. 263, 1927–1938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cole J.H., Leech R., and Sharp D.J.; Alzheimer's Disease Neuroimaging Initiative. (2015). Prediction of brain age suggests accelerated atrophy after traumatic brain injury. Ann. Neurol. 77, 571–581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wintermark M., van Melle G., Schnyder P., Revelly J.-P., Porchet F., Regli L., Meuli R., Maeder P., and Chioléro R. (2004). Admission perfusion CT: prognostic value in patients with severe head trauma. Radiology 232, 211–220 [DOI] [PubMed] [Google Scholar]
- 27. Honda M., Ichibayashi R., Yokomuro H., Yoshihara K., Masuda H., Haga D., Seiki Y., Kudoh C., and Kishi T. (2016). Early cerebral circulation disturbance in patients suffering from severe traumatic brain injury (TBI): a xenon CT and perfusion CT study. Neurol. Med. Chir. (Tokyo) 56, 501–509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Fridley J., Robertson C., and Gopinath S. (2015). Quantitative lobar cerebral blood flow for outcome prediction after traumatic brain injury. J. Neurotrauma 32, 75–82 [DOI] [PubMed] [Google Scholar]
- 29. Hlatky R., Contant C.F., Diaz-Marchan P., Valadka A.B., and Robertson C.S. (2004). Significance of a reduced cerebral blood flow during the first 12 hours after traumatic brain injury. Neurocrit. Care 1, 69–83 [DOI] [PubMed] [Google Scholar]
- 30. Inoue Y., Shiozaki T., Tasaki O., Hayakata T., Ikegawa H., Yoshiya K., Fujinaka T., Tanaka H., Shimazu T., and Sugimoto H. (2005). Changes in cerebral blood flow from the acute to the chronic phase of severe head injury. J. Neurotrauma 22, 1411–1418 [DOI] [PubMed] [Google Scholar]
- 31. Stamatakis E.A., Wilson J.T.L., Hadley D.M., and Wyper D.J. (2002). SPECT imaging in head injury interpreted with statistical parametric mapping. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 43, 476–483 [PubMed] [Google Scholar]
- 32. Kim J., Whyte J., Patel S., Avants B., Europa E., Wang J., Slattery J., Gee J.C., Coslett H.B., and Detre J.A. (2010). Resting cerebral blood flow alterations in chronic traumatic brain injury: an arterial spin labeling perfusion FMRI study. J. Neurotrauma 27, 1399–1411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Amyot F., Kenney K., Moore C., Haber M., Turtzo L.C., Shenouda C., Silverman E., Gong Y., Qu B.-X., Harburg L., Lu H.Y., Wassermann E.M., and Diaz-Arrastia R. (2018). Imaging of cerebrovascular function in chronic traumatic brain injury. J. Neurotrauma 35, 1116–1123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Jackson W.T., Novack T.A., and Dowler R.N. (1998). Effective serial measurement of cognitive orientation in rehabilitation: the orientation log. Arch. Phys. Med. Rehabil. 79, 718–721 [DOI] [PubMed] [Google Scholar]
- 35. Rabinowitz A.R., Hart T., Whyte J., and Kim J. (2018). Neuropsychological recovery trajectories in moderate to severe traumatic brain injury: influence of patient characteristics and diffuse axonal injury. J. Int. Neuropsychol. Soc. 24, 237–246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Avants B.B., Epstein C.L., Grossman M., and Gee J.C. (2008). Symmetric diffeomorphic image registration with cross-correlation: evaluating automated labeling of elderly and neurodegenerative brain. Med. Image Anal. 12, 26–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Avants B.B., Tustison N.J., Song G., Cook P.A., Klein A., and Gee J.C. (2011). A reproducible evaluation of ANTs similarity metric performance in brain image registration. NeuroImage 54, 2033–2044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Andersen S.M., Rapcsak S.Z., and Beeson P.M. (2010). Cost function masking during normalization of brains with focal lesions: still a necessity? NeuroImage 53, 78–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hua X., Leow A.D., Parikshak N., Lee S., Chiang M.-C., Toga A.W., Jack C.R., Weiner M.W., and Thompson P.M.; Alzheimer's Disease Neuroimaging Initiative. (2008). Tensor-based morphometry as a neuroimaging biomarker for Alzheimer's disease: an MRI study of 676 AD, MCI, and normal subjects. NeuroImage 43, 458–469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Klein A., and Tourville J. (2012). 101 labeled brain images and a consistent human cortical labeling protocol. Front. Neurosci. 6, 171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wang Z., Aguirre G.K., Rao H., Wang J., Fernández-Seara M.A., Childress A.R., and Detre J.A. (2008). Empirical optimization of ASL data analysis using an ASL data processing toolbox: ASLtbx. Magn. Reson. Imaging 26, 261–269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Alsop D.C., Detre J.A., Golay X., Günther M., Hendrikse J., Hernandez-Garcia L., Lu H., MacIntosh B.J., Parkes L.M., Smits M., van Osch M.J.P., Wang D.J.J., Wong E.C., and Zaharchuk G. (2015). Recommended implementation of arterial spin-labeled perfusion MRI for clinical applications: a consensus of the ISMRM Perfusion Study Group and the European Consortium for ASL in Dementia: recommended implementation of ASL for clinical applications. Magn. Reson. Med. 73, 102–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Dolui S., Wang Z., Shinohara R.T., Wolk D.A., and Detre J.A.; Alzheimer's Disease Neuroimaging Initiative. (2017). Structural Correlation-based Outlier Rejection (SCORE) algorithm for arterial spin labeling time series. J. Magn. Reson. Imaging 45, 1786–1797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Dolui S., Wolk D.A., and Detre J.A. (2016). SCRUB: a structural correlation and empirical robust Bayesian method for ASL data. Presented at the 24th Annual Meeting & Exhibition of the ISMRM, Singapore [Google Scholar]
- 45. Winkler A.M., Ridgway G.R., Webster M.A., Smith S.M., and Nichols T.E. (2014). Permutation inference for the general linear model. NeuroImage 92, 381–397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Smith S.M., and Nichols T.E. (2009). Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. NeuroImage 44, 83–98 [DOI] [PubMed] [Google Scholar]
- 47. Clark A.L., Bangen K.J., Sorg S.F., Schiehser D.M., Evangelista N.D., McKenna B., Liu T.T., and Delano-Wood L. (2017). Dynamic association between perfusion and white matter integrity across time since injury in Veterans with history of TBI. NeuroImage Clin. 14, 308–315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wang Y., West J.D., Bailey J.N., Westfall D.R., Xiao H., Arnold T.W., Kersey P.A., Saykin A.J., and McDonald B.C. (2015). Decreased cerebral blood flow in chronic pediatric mild TBI: an MRI perfusion study. Dev. Neuropsychol. 40, 40–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Dennis E.L., Hua X., Villalon-Reina J., Moran L.M., Kernan C., Babikian T., Mink R., Babbitt C., Johnson J., Giza C.C., Thompson P.M., and Asarnow R.F. (2016). Tensor-based morphometry reveals volumetric deficits in moderate = severe pediatric traumatic brain injury. J. Neurotrauma 33, 840–852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ding K., Marquez de la Plata C., Wang J.Y., Mumphrey M., Moore C., Harper C., Madden C.J., McColl R., Whittemore A., Devous M.D., and Diaz-Arrastia R. (2008). Cerebral atrophy after traumatic white matter injury: correlation with acute neuroimaging and outcome. J. Neurotrauma 25, 1433–1440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Warner M.A., Youn T.S., Davis T., Chandra A., Marquez de la Plata C., Moore C., Harper C., Madden C.J., Spence J., McColl R., Devous M., King R.D., and Diaz-Arrastia R. (2010). Regionally selective atrophy after traumatic axonal injury. Arch. Neurol. 67, 1336–1344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Sweeney M.D., Kisler K., Montagne A., Toga A.W., and Zlokovic B.V. (2018). The role of brain vasculature in neurodegenerative disorders. Nat. Neurosci. 21, 1318–1331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Wallace E.J., Mathias J.L., and Ward L. (2018). The relationship between diffusion tensor imaging findings and cognitive outcomes following adult traumatic brain injury: A meta-analysis. Neurosci. Biobehav. Rev. 92, 93–103 [DOI] [PubMed] [Google Scholar]
- 54. Sharp D.J., Scott G., and Leech R. (2014). Network dysfunction after traumatic brain injury. Nat. Rev. Neurol. 10, 156–166 [DOI] [PubMed] [Google Scholar]