Abstract
Pregnant women experience weight gain, gait changes, and biochemical fluctuations that impair joint function and alter the maternal skeleton. Hormonal changes increase pelvic ligament laxity in preparation for childbirth and affect peripheral joint laxity. Calcium demands also rise during pregnancy and lactation, resulting in reduced bone mineral density (BMD) and maternal bone loss. Altered tendon properties and bone loss during pregnancy and lactation may impact tendon insertion sites, such as rotator cuff tendons where insertion site ruptures are common. However, the effects of pregnancy and lactation at the tendon-to-bone interface have not been investigated. Therefore, the objective of this study was to evaluate supraspinatus tendon mechanical properties and insertion site microstructure during pregnancy, lactation, and postweaning recovery in female rats. We hypothesized that pregnancy and lactation would compromise supraspinatus tendon mechanical properties and subchondral bone microstructure. Female rats were divided into virgin, pregnancy, lactation, and recovery groups, and supraspinatus tendons were mechanically evaluated. Surprisingly, tendon mechanics was unaffected by pregnancy and lactation. However, tendon modulus decreased two-weeks postweaning. Additionally, tendons failed by bony avulsion at the insertion site, and the lactation group exhibited reduced failure properties corresponding to decreased subchondral bone mineralization. Lactation also resulted in dramatic bone loss at the epiphysis, but trabecular bone microarchitecture recovered postweaning. In conclusion, lactation following pregnancy impaired trabecular bone microstructure and subchondral bone mineralization, leading to reduced supraspinatus tendon-to-bone insertion site failure properties. These findings will contribute toward understanding the pathogenesis of tendon-to-bone disorders.
Keywords: tendon, mechanics, pregnancy, lactation, shoulder, supraspinatus, rotator cuff, subchondral bone, mineralization, humerus, orthopedics
Introduction
Musculoskeletal disorders such as shoulder, lower back, and knee pain affect 25% of all pregnant women [1,2]. Though pregnancy-related musculoskeletal injuries are often attributed to biomechanical and gait alterations that occur with fetal weight gain, pregnant and postpartum women also experience substantial biochemical fluctuations that impair joint function and alter the maternal skeleton [3,4]. Rising estrogen and relaxin levels during pregnancy increase pelvic ligament laxity necessary for parturition [5]. These hormonal changes also have significant effects on peripheral joints with clinical data demonstrating increased laxity in the knee, fingers, and wrist [6–8]. The individual factors contributing to joint laxity are difficult to isolate, but decreased tendon and ligament stiffness [9] as well as increased tissue length [10] have been proposed as possible causes. Additionally, calcium demands from fetal growth increase during pregnancy [11] with increased demands during lactation, resulting in bone resorption, reduced bone mineral density (BMD), and substantial maternal bone loss. Despite a dramatic return to prepregnancy hormone levels postpartum, alterations in joint laxity and maternal bone properties persist [12].
Persistent joint laxity is a risk factor for joint dislocation [13–15], and the shoulder is the most frequently dislocated major joint in the body. Recurrent shoulder dislocations subject the rotator cuff to adverse loading conditions, impairing motion and predisposing tendons to degeneration and injury [16–18]. The supraspinatus tendon is of particular interest because it is the most frequently torn rotator cuff tendon, accounting for a significant percentage of shoulder pain complaints. Ruptures commonly occur at the tendon-to-bone insertion site, which is characterized by a critical mineralization gradient that transitions from tendon to regions of unmineralized and mineralized fibrocartilage before attaching to bone. Given that pregnancy and lactation are known to substantially affect calcium homeostasis, the interface between tendon and bone may also be affected.
Our previous work showed that rats with a history of two reproductive cycles exhibited reduced supraspinatus tendon insertion site modulus despite a sustained high calcium diet and a lengthy recovery period [19]. These results were accompanied by inferior trabecular bone microstructure in the humeral head, including reduced bone volume fraction, reduced trabecular number, and increased trabecular separation. These findings suggest that pregnancy and lactation may also compromise the microstructure at the tendon-to-bone insertion site. However, bone microstructural properties of insertion site region have not yet been investigated, and the transient effects of pregnancy and lactation on the rotator cuff are still unknown. Changes in this region in response to pregnancy and lactation may affect tendon mechanical properties and therefore alter shoulder function. Therefore, the objective of this study was to evaluate changes in supraspinatus tendon mechanical properties and microstructure of the bony insertion site during pregnancy, lactation, and postweaning recovery in female rats. We hypothesized that pregnancy and lactation would compromise the subchondral bone microstructure and lead to reduced supraspinatus tendon mechanical properties.
Methods
This study was approved by the University of Pennsylvania Institutional Animal Care and Use Committee. 52 Sprague-Dawley female rats were divided across four groups: virgin (n = 14), pregnancy (n = 14), lactation (n = 12), and recovery (n = 12). All rats were fed a high calcium diet throughout the study. Rats in the pregnancy group were sacrificed at parturition, while the lactation group underwent pregnancy and two weeks of lactation. Rats in the recovery group underwent pregnancy, three weeks of lactation, and two weeks of postweaning recovery (Fig. 1). Taking into account a 3-week lactation period, two weeks postweaning is five weeks after pregnancy, and this represents approximately three years postpartum on a human timeline [20]. Pregnancies were timed such that all rats were sacrificed at 7 months of age, and shoulders were harvested for supraspinatus tendon mechanical testing (n = 12–14/group) as well as subchondral and trabecular bone analysis (n = 12–14/group).
Fig. 1.
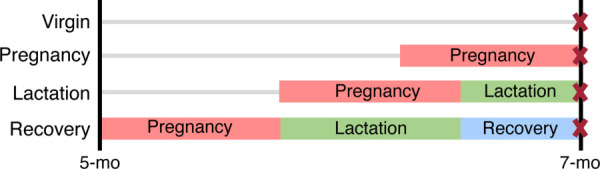
Study design. Female rats were divided into four groups: virgin, pregnancy, lactation, and recovery. Rats in the pregnancy group were sacrificed at parturition, the lactation group underwent pregnancy and 2 weeks of lactation, and the recovery group underwent pregnancy, 3 weeks of lactation, and 2 weeks of postweaning recovery. Pregnancies were timed such that all rats were sacrificed at 7 months of age.
Mechanics: Musculature and nontendinous connective tissue were removed from supraspinatus tendon-humerus complexes. Supraspinatus tendons were then marked with stain lines for optical strain tracking at 0, 2, 4, and 8 mm along the tendon length to demarcate the insertion (0–2 mm), midsubstance (2–4 mm), and 8 mm gauge length (Fig. 2(a)). Cross-sectional area was measured using a custom laser device [21], humeri were secured in polymethyl methacrylate, and the tendinous ends were sandwiched between sandpaper tabs with cyanoacrylate glue at the 8 mm gauge length mark. Tendons were submerged in a phosphate buffered saline bath maintained at 37 °C and underwent either a viscoelastic or fatigue testing protocol (Instron Electropuls E3000, Instron, Inc., Norwood, MA). Right supraspinatus tendons were subjected to viscoelastic tensile testing, consisting of a 0.05 N preload, preconditioning (0.5–1.5% strain at 0.25 Hz for 10 cycles), stress relaxation at a 5% strain hold for 600 s, a dynamic frequency sweep at 5% strain (0.125% strain amplitude at 0.1, 1, 2, and 10 Hz), and a quasi-static strain-rate controlled ramp to failure at 0.3%/s (Fig. 2(b)). Left supraspinatus tendons underwent load-controlled fatigue testing, consisting of preconditioning and fatigue loading until failure at 2 Hz between loads corresponding to 7% and 40% maximum stress, as determined from quasi-static testing [22]. Fatigue parameters, including peak cyclic strain, secant modulus, tangent modulus, hysteresis, and laxity were recorded at two breakpoints marking the ends of the primary (BP1) and secondary (BP2) phases of a triphasic fatigue life curve (Fig. 2(c)).
Fig. 2.
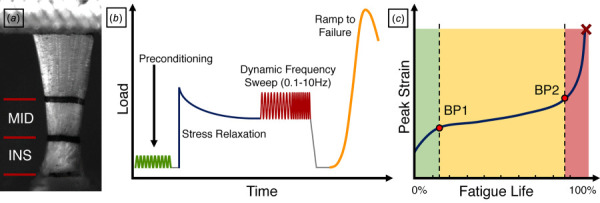
Tendon mechanical testing. (a) Supraspinatus tendons were marked with stain lines to identify the insertion site (INS) and midsubstance (MID) for mechanical testing. (b) Right tendons then underwent a testing protocol consisting of preconditioning, a stress relaxation at 5% strain hold, a dynamic frequency sweep (0.1–10 Hz) and a quasi-static ramp to failure. Left tendons were subjected to fatigue testing, where the (c) peak strain curve exhibits a triphasic response. Fatigue parameters were reported at two breakpoints marking the ends of the primary (BP1) and secondary (BP2) phases.
Trabecular and subchondral bone analysis: Prior to mechanical testing, left proximal humeri were scanned by microcomputed tomography (μCT35, Scanco Medical, Brüttisellen, Switzerland) at 6 μm isotropic voxel size (145 μA current, 55 kVp energy, 600 ms integration time). Trabecular bone within the proximal humerus epiphysis was analyzed for bone volume fraction (BV/TV), trabecular number (Tb.N), trabecular thickness (Tb.Th), trabecular separation (Tb.Sp), connectivity density (Conn.D), and structure model index (SMI). Additionally, the subchondral plate, defined as the mineralized fibrocartilage layer of the supraspinatus tendon enthesis and underlying subchondral bone (Fig. 3(a)), was analyzed to calculate average thickness and the mineralization gradient (Amira 6.7). A 600 × 720 μm area was identified in the greater tuberosity at the supraspinatus tendon insertion site (Fig. 3(b)). After global thresholding, the innermost boundary of the subchondral bone was manually defined to exclude trabecular bone. Average thickness was calculated by dividing the volume of this region by the prescribed area. Individual layers were subsequently defined outwards toward the mineralized fibrocartilage boundary (Fig. 3(c)). Layer intensity values were averaged to construct a mineralization gradient, normalized to the total subchondral plate thickness. BMD was compared at 0, 0.5, and 1.0, marking the approximate boundaries between trabecular bone, subchondral bone, mineralized fibrocartilage, and tendon.
Fig. 3.
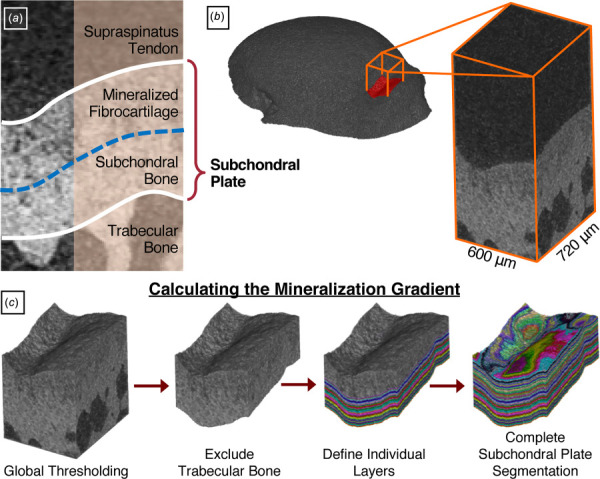
Subchondral plate mineralization. (a) The subchondral plate comprises the layers of mineralized fibrocartilage and subchondral bone underlying the supraspinatus tendon enthesis. To evaluate the mineralization gradient within this region, (b) a 600 × 720 μm area was identified in the greater tuberosity at the supraspinatus tendon insertion site. (c) After global thresholding, the innermost boundary of the subchondral bone was manually defined to exclude trabecular bone. Individual layers were subsequently defined outwards toward the mineralized fibrocartilage boundary. Intensity values within each layer were averaged to construct a mineralization gradient.
Statistics: All data were presented as mean ± standard deviation. Comparisons across groups were made using one-way analyses of variances with Tukey post hoc tests to compare all groups to each other. Significance was set at p ≤ 0.05.
Results
Structural properties remained unchanged in all groups with no differences in cross-sectional area (Fig. 4(a)) or stiffness (Table 1). Percent relaxation was greater in the recovery group compared to pregnancy (Fig. 4(b)), but there were no differences in other viscoelastic parameters including dynamic modulus and phase shift, at any frequency (Table 1). Interestingly, insertion site modulus (Fig. 4(c)) was not different across groups, while midsubstance modulus was significantly decreased in the recovery group (Fig. 4(d)). Some changes in fatigue properties were observed with decreased tangent stiffness at BP1 for both lactation and recovery groups when compared to pregnancy and remained lower in the lactation group at BP2 (Fig. 5(a)). Further, tangent modulus (Fig. 5(b)), secant stiffness (Fig. 5(c)), and secant modulus (Fig. 5(d)) at BP1 were decreased during lactation compared to virgin. Secant stiffness and modulus at BP1 were also decreased in the recovery group relative to virgin. However, there were no differences in cycles to failure, peak cyclic strain, hysteresis, or laxity (Table 2).
Fig. 4.
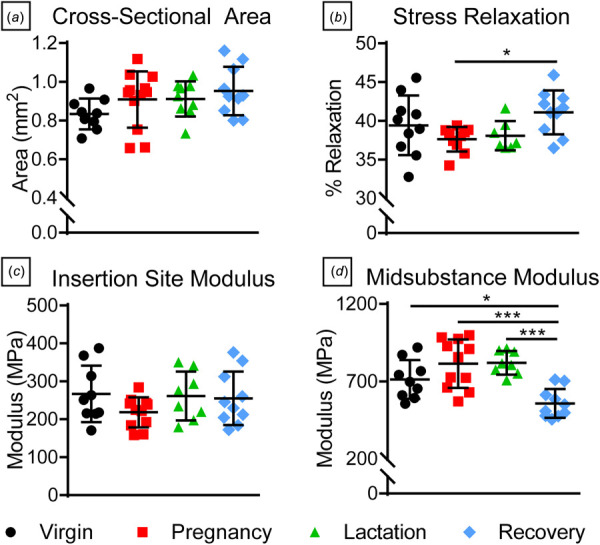
Tendon structural, quasi-static, and viscoelastic properties. There were no differences in (a) tendon cross-sectional area, and (b) percent relaxation increased during recovery compared to the pregnancy group. While there were no differences in (c) insertion site modulus, (d) midsubstance modulus in the recovery group was significantly lower compared to all other groups. Data are presented as mean ± standard deviation. Significant differences are indicated by solid bars (*p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001).
Table 1.
Tendon structural and viscoelastic properties
| Virgin | Pregnancy | Lactation | Recovery | ||
|---|---|---|---|---|---|
| Stiffness (N/mm) | 37.5 (6.3) | 39.2 (5.3) | 38.4 (5.1) | 35.1 (2.9) | |
| Dynamic modulus (MPa) | 0.1 Hz | 470.3 (80.7) | 448.2 (45.0) | 469.5 (75.0) | 392.3 (84.6) |
| 1 Hz | 488.6 (82.7) | 464.7 (48.0) | 487.1 (79.1) | 407.2 (87.1) | |
| 2 Hz | 493.9 (83.6) | 469.4 (47.5) | 491.2 (80.4) | 410.8 (87.5) | |
| 10 Hz | 497.8 (84.9) | 473.4 (49.0) | 494.6 (82.4) | 413.5 (88.7) | |
| Phase shift (tan δ) | 0.1 Hz | 0.041 (0.004) | 0.044 (0.005) | 0.040 (0.005) | 0.0444 (0.006) |
| 1 Hz | 0.035 (0.004) | 0.035 (0.003) | 0.035 (0.003) | 0.0369 (0.005) | |
| 2 Hz | 0.035 (0.003) | 0.033 (0.003) | 0.033 (0.004) | 0.0360 (0.004) | |
| 10 Hz | 0.036 (0.003) | 0.033 (0.003) | 0.035 (0.004) | 0.0372 (0.005) | |
Fig. 5.
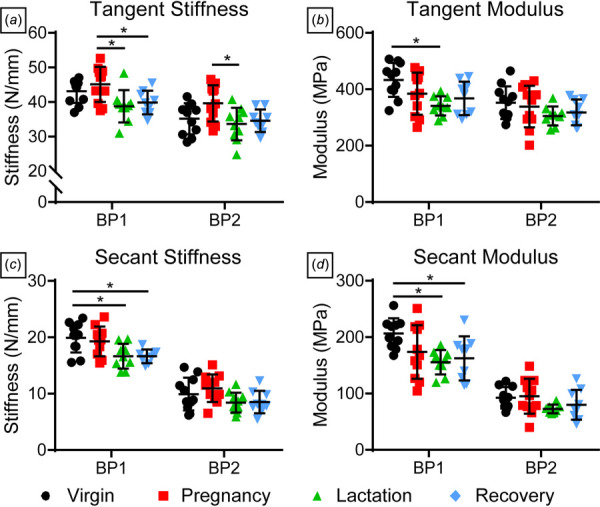
Tendon fatigue properties. (a) Tangent stiffness was higher in the pregnancy group compared to lactation at BP1 and BP2, while (b) tangent modulus was greater in virgin rats compared to lactation at BP1. (c) Secant stiffness and (d) secant modulus were also greater in virgin rats compared to lactation and recovery groups at BP1. Data are presented as mean ± standard deviation. Significant differences are indicated by solid bars (*p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001).
Table 2.
Tendon fatigue properties
| Virgin | Pregnancy | Lactation | Recovery | ||
|---|---|---|---|---|---|
| Cycles to failure (cycles) | 8434 (8068) | 9416 (6509) | 10,052 (9849) | 5672 (2174) | |
| Peak strain (%) | BP1 | 4.08 (0.580) | 4.35 (0.841) | 4.11 (0.409) | 4.41 (0.612) |
| BP2 | 8.93 (2.40) | 7.90 (1.50) | 8.34 (1.48) | 9.00 (1.46) | |
| Hysteresis (%) | BP1 | 8.03 (0.84) | 7.29 (1.18) | 7.26 (1.30) | 7.30 (1.48) |
| BP2 | 10.16 (0.97) | 9.82 (1.22) | 10.18 (1.44) | 10.29 (0.84) | |
| Laxity (%) | BP1 | 0.608 (0.149) | 0.660 (0.221) | 0.677 (0.118) | 0.791 (0.239) |
| BP2 | 5.33 (1.96) | 4.15 (1.29) | 4.96 (1.16) | 5.25 (1.32) | |
Since the predominant failure mode during quasi-static tendon testing was bony avulsion, failure properties reflect that of the supraspinatus tendon-to-bone insertion site and underlying subchondral bone. Failure stress (Fig. 6(a)) and failure strain (Fig. 6(b)) were significantly decreased during lactation compared to virgin and pregnancy groups but recovered after weaning. A similar trend was observed in subchondral plate thickness (Fig. 6(c)). The bone mineral density gradient between trabecular bone and the supraspinatus tendon was different during lactation and recovery (Fig. 6(d)). At the trabecular bone-subchondral bone boundary (normalized thickness = 0.00), BMD was significantly lower during lactation compared to virgin and pregnancy groups. During recovery, BMD remained significantly lower compared to virgin. Reduced BMD in lactation and recovery groups persisted at the subchondral bone-mineralized fibrocartilage interface (normalized thickness = 0.50), while there were no differences between groups at the mineralized fibrocartilage-tendon boundary ((normalized thickness = 1.00, Fig. 6(e)).
Fig. 6.
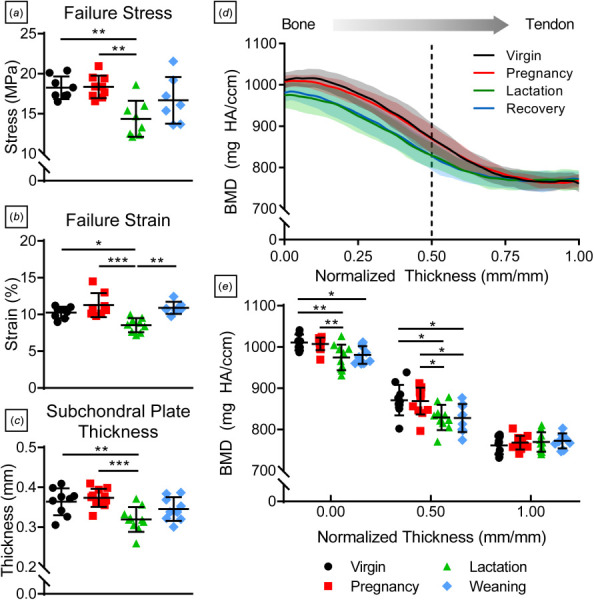
Tendon-to-bone failure and subchondral bone properties. (a) Tendon-to-bone insertion site failure stress, (b) failure strain, and (c) subchondral plate thickness were significantly lower in the lactation group compared to virgin and pregnancy rats but recovered postweaning. (d) The mineralization gradient within the subchondral plate revealed (e) reduced bone mineral density in the lactation group at the trabecular bone boundary (normalized thickness = 0.00), which persisted at the boundary between mineralized fibrocartilage and subchondral bone (normalized thickness = 0.50). Data are presented as mean ± standard deviation. Significant differences are indicated by solid bars (*p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001).
Bone volume fraction (BV/TV) (Fig. 7(a)) and Tb.N (Fig. 7(b)) were significantly lower while Tb.Sp (Fig. 7(c)) was significantly greater during lactation compared to virgin, pregnancy, and recovery groups. However, there were no differences in Tb.Th (Fig. 7(d)). Conn.D (Fig. 7(e)) was greater during recovery, while SMI (Fig. 7(f)) increased during pregnancy and lactation but later recovered.
Fig. 7.
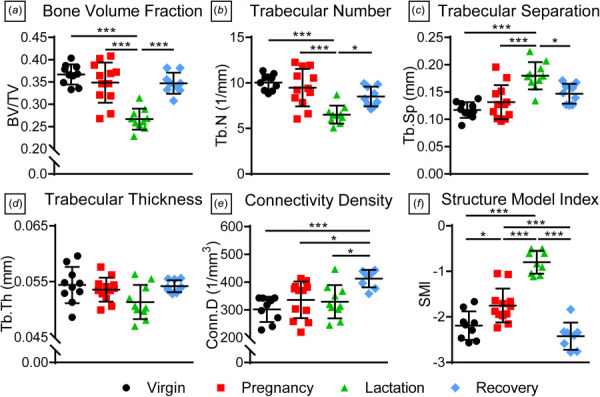
Humeral epiphysis trabecular bone properties. The lactation group had significantly lower (a) bone volume fraction and (b) trabecular number as well as greater (c) trabecular separation, and these properties recovered postweaning. However, there were no differences in (d) trabecular thickness. (e) Connectivity density increased in the recovery group, while structure model index was significantly greater in the lactation group. Data are presented as mean ± standard deviation. Significant differences are indicated by solid bars (*p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001).
Discussion
This study investigated the transient effects of pregnancy and lactation on the supraspinatus tendon, tendon-to-bone insertion site, and underlying trabecular bone. Contrary to our hypothesis, tendon mechanical properties were largely unaffected at the end of pregnancy and during lactation. However, tendon mechanical properties were altered during recovery, with a substantial reduction in both midsubstance and secant modulus compared to the virgin group as well as an increase in percent relaxation compared to the pregnancy group. This suggests that the physiological processes of pregnancy and lactation have delayed effects on tendon. Intriguingly, previous studies in the rabbit showed significantly decreased collagen I mRNA expression in the anterior cruciate ligament (ACL) and medial collateral ligament during pregnancy, increased dramatically 7-days postpartum, but then decreased again 18-days postpartum to levels below nonpregnant values [23,24]. A similar trend was observed in the ACL for fibroblast growth factor and transforming growth factor-β (TGF-β), key factors that regulate scleraxis expression [25,26] and collagen matrix production [27,28]. Whether lactation occurred in these studies is unclear; however, there may be physiological changes in the postpartum period separate from the effects of pregnancy that lead to reduced tendon mechanical properties. Further studies are required to determine the biological and structural factors driving changes in tendon modulus and viscoelasticity.
Despite the well-documented consequences of pregnancy on joint laxity, studies are conflicting and inconclusive, and the field lacks a fundamental understanding on the hormonal regulation of tendons and ligaments. Estrogen and relaxin are hormones that have been implicated in joint laxity through regulation of collagen metabolism. Estrogen is linked to increased collagen synthesis [29–31] but also to reduced collagen cross-linking and mechanical function [32]. Relaxin has been shown to increase tendon creep [33], possibly through increased expression of matrix metalloproteinases [34], and decreased collagen synthesis [35], leading to increased collagen fiber sliding. Estrogen-deficient rats exhibited reduced expression of Pcna and Timp1 [36], suggesting that estrogen also regulates cell proliferation and matrix metalloproteinase activity, respectively. Therefore, fluctuations in estrogen and relaxin levels during and following pregnancy may lead to reduced collagen synthesis and cross-linking. Clinical studies in pregnant women have demonstrated associations between increased joint laxity and serum relaxin [37,38] in the pelvis and hip, while estradiol levels [39] also correlated in the ACL. Other studies, however, have also shown increased estradiol, relaxin, progesterone, and cortisol levels as well as joint laxity in the knee, elbow, wrist, and finger but with no correlation [6–8]. These studies support that pregnancy has site-specific effects, and animal studies investigating the individual tissue contributions are vital for understanding hormonal regulation during pregnancy. However, animal studies investigating the effect of pregnancy on tendons and ligaments are limited. Pregnancy induced gene expression changes in rabbit knee ligaments [23,24] and Achilles tendon [40], while no differences were observed in medial collateral ligament mechanical properties between nonpregnant and pregnant rabbits, similar to our findings in the supraspinatus tendon. Therefore, mechanical changes in tendons and ligaments may not be the predominant driver of altered joint laxity during pregnancy. Instead, the complex interactions between estrogen and relaxin could impact other factors contributing to joint laxity such as increased tissue length [10] and inflammation of the surrounding synovial sheath [41].
Interestingly, we observed that the primary mode for supraspinatus tendon failure was by bony avulsion at the supraspinatus tendon-to-bone insertion site, and reduced failure properties during lactation corresponded with decreased thickness and mineralization of the subchondral plate. Reduced overall thickness and bone mineral density during lactation suggest that independent mechanisms modulated by osteoblast-osteoclast coupling regulation and osteocyte perilacuna–canalicular remodeling [42], respectively, may be active. Our preliminary results found that changes in bone mineral density during lactation may be associated with tartrate-resistant acid phosphatase-expressing osteocytes localized to the subchondral bone region. The number of tartrate-resistant acid phosphatase-expressing osteocytes later decreased during recovery [43]; however, the mineralization gradient in the subchondral plate showed a sustained reduction after two weeks of postweaning recovery compared to virgin rats. Evaluation of a longer recovery period will determine whether this change is fully recoverable. Previous animal studies have correlated bone mineral density and failure strength at the tendon enthesis using ovariectomy models of estrogen deficiency [44,45], noting a thinner tendon-to-bone interface with a less apparent tidemark at the enthesis. Although our results also show that reduced bone mineral density leads to impaired failure strength, the sex and calciotropic hormone fluctuations that drive lactation-induced bone loss are different from the physiological changes in an estrogen-deficient model. Further exploration into the mechanisms that govern these two different processes can have important implications for understanding how low mineral density during processes such as aging or menopause may impair the tendon-to-bone insertion site.
Our previous studies have shown that effects of reproduction on trabecular bone micro-architecture are not only tissue specific but also skeletal site specific [46]. For instance, the proximal tibia and lumbar vertebra have different skeletal roles: trabecular bone at the proximal tibia has a greater metabolic function and serves as a mineral reservoir to support homeostasis, while the lumbar vertebra primarily provides mechanical support. During pregnancy and lactation, the proximal tibia and lumbar vertebra showed similar trends of trabecular bone loss followed by recovery postweaning. However, trabecular bone at the proximal tibia exhibited greater deterioration during lactation and did not fully recover postweaning in contrast to the lumbar vertebra. In the humeral head epiphysis, lactation also resulted in dramatic bone loss, but a two-week recovery was sufficient to restore trabecular bone properties to prepregnancy levels. Taken together, the epiphysis, where four tendons converge to form the rotator cuff, serves a large mechanical role and exhibits reproduction-induced bone loss and recovery similar to the load-bearing lumbar vertebra.
Overall, this study provides novel insight into how pregnancy and lactation affect the interface between tendon and bone. However, there are several limitations to this study. First, we did not assess overall shoulder joint laxity, and evaluation of other connective tissues and joint mechanics is necessary to determine if pregnancy and lactation affect overall shoulder function. An additional limitation is that our results may not directly translate to humans because quadrupedal motion in rats would elicit differences in forelimb weight bearing, particularly in response to pregnancy-related weight gain. Nevertheless, these findings motivate investigation into the entheses of the weight-bearing lower extremities and may be translatable to other quadrupedal animals. Finally, a 2-week postweaning recovery period may not be sufficient for tendon and subchondral plate properties to recover fully despite this time period representing about three years postpartum on a human timeline [20]. Future studies will address these limitations by investigating the biological mechanisms underlying transient changes in tendon and bone properties, evaluating lower extremity tendons and ligaments and determining the long-term effects of pregnancy and lactation.
In conclusion, lactation following pregnancy induced changes in bone microstructure and mineralization, leading to reduced supraspinatus tendon-to-bone insertion site failure properties. Combined with further investigation into the cellular and structural processes that drive these changes, these findings will contribute toward understanding the pathogenesis of tendon-to-bone disorders.
Acknowledgment
This study was funded by the NIH/NIAMS supported Penn Center for Musculoskeletal Disorders, the NIH/NIAMS, and NSF. All authors were fully involved in the study and preparation of the paper. The paper has been read and approved by all of the authors.
Funding Data
-
•
National Science Foundation (No. 1653216; Funder ID: 10.13039/100000001).
National Institutes of Health (P30 AR069619, R03AR065145, R01AR071718, and T32AR007132; Funder ID: 10.13039/100000002).
References
- [1]. Kesikburun, S. , Güzelküçük, Ü. , Fidan, U. , Demir, Y. , Ergün, A. , and Tan, A. K. , 2018, “ Musculoskeletal Pain and Symptoms in Pregnancy: A Descriptive Study,” Ther. Adv. Musculoskelet. Dis., 10(12), pp. 229–234. 10.1177/1759720X18812449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2]. Young, J. , 1940, “ Relaxation of the Pelvic Joints in Pregnancy: Pelvic Arthropathy of Pregnancy,” BJOG, 47(5), pp. 493–524. 10.1111/j.1471-0528.1940.tb08842.x [DOI] [Google Scholar]
- [3]. Smith, M. W. , Marcus, P. S. , and Wurtz, L. D. , 2008, “ Orthopedic Issues in Pregnancy,” Obstet. Gynecol. Surv., 63(2), pp. 103–111. 10.1097/OGX.0b013e318160161c [DOI] [PubMed] [Google Scholar]
- [4]. Ireland, M. L. , and Ott, S. M. , 2000, “ The Effects of Pregnancy on the Musculoskeletal System,” Clinical Orthopaedics and Related Research, Lippincott Williams and Wilkins, Philadelphia, PA, pp. 169–179. 10.1097/00003086-200003000-00019 [DOI] [PubMed] [Google Scholar]
- [5]. Thoms, H. , 1936, “ Relaxation of the Symphysis Pubis in Pregnancy,” J. Am. Med. Assoc., 106(16), pp. 1364–1366. 10.1001/jama.1936.02770160022007 [DOI] [Google Scholar]
- [6]. Schauberger, C. W. , Rooney, B. L. , Goldsmith, L. , Shenton, D. , Silva, P. D. , and Schaper, A. , 1996, “ Peripheral Joint Laxity Increases in Pregnancy but Does Not Correlate With Serum Relaxin Levels,” Am. J. Obstet. Gynecol., 174(2), pp. 667–671. 10.1016/s0002-9378(96)70447-7 [DOI] [PubMed] [Google Scholar]
- [7]. Dumas, G. A. , and Reid, J. G. , 1997, “ Laxity of Knee Cruciate Ligaments During Pregnancy,” J. Orthop. Sports Phys. Ther., 26(1), pp. 2–6. 10.2519/jospt.1997.26.1.2 [DOI] [PubMed] [Google Scholar]
- [8]. Marnach, M. L. , Ramin, K. D. , Ramsey, P. S. , Song, S. W. , Stensland, J. J. , and An, K. N. , 2003, “ Characterization of the Relationship Between Joint Laxity and Maternal Hormones in Pregnancy,” Obstet. Gynecol., 101(2), pp. 331–335. 10.1016/S0029-7844(02)02447-X [DOI] [PubMed] [Google Scholar]
- [9]. Pearson, S. J. , Burgess, K. E. , and Onambélé, G. L. , 2011, “ Serum Relaxin Levels Affect the In Vivo Properties of Some but Not All Tendons in Normally Menstruating Young Women,” Exp. Physiol., 96(7), pp. 681–688. 10.1113/expphysiol.2011.057877 [DOI] [PubMed] [Google Scholar]
- [10]. Bey, M. E. , Marzilger, R. , Hinkson, L. , Arampatzis, A. , and Legerlotz, K. , 2019, “ Patellar Tendon Stiffness is Not Reduced During Pregnancy,” Front. Physiol., 10, p. 334. 10.3389/fphys.2019.00334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11]. Kovacs, C. S. , 2016, “ Maternal Mineral and Bone Metabolism During Pregnancy, Lactation, and Post-Weaning Recovery,” Physiol. Rev., 96(2), pp. 449–547. 10.1152/physrev.00027.2015 [DOI] [PubMed] [Google Scholar]
- [12]. Chu, S. R. , Boyer, E. H. , Beynnon, B. , and Segal, N. A. , 2019, “ Pregnancy Results in Lasting Changes in Knee Joint Laxity,” PM&R, 11(2), pp. 117–124. 10.1016/j.pmrj.2018.06.012 [DOI] [PMC free article] [PubMed]
- [13]. Chahal, J. , Leiter, J. , McKee, M. D. , and Whelan, D. B. , 2010, “ Generalized Ligamentous Laxity as a Predisposing Factor for Primary Traumatic Anterior Shoulder Dislocation,” J. Shoulder Elbow Surg., 19(8), pp. 1238–1242. 10.1016/j.jse.2010.02.005 [DOI] [PubMed] [Google Scholar]
- [14]. Cameron, K. L. , Duffey, M. L. , Deberardino, T. M. , Stoneman, P. D. , Jones, C. J. , and Owens, B. D. , 2010, “ Association of Generalized Joint Hypermobility With a History of Glenohumeral Joint Instability,” J. Athl. Train., 45(3), pp. 253–258. 10.4085/1062-6050-45.3.253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15]. Caplan, J. , Julien, T. P. , Michelson, J. , and Neviaser, R. J. , 2007, “ Multidirectional Instability of the Shoulder in Elite Female Gymnasts,” Am. J. Orthop., 36(12), pp. 660–665.https://pubmed.ncbi.nlm.nih.gov/18264543/ [PubMed] [Google Scholar]
- [16]. Simank, H.-G. , Dauer, G. , Schneider, S. , and Loew, M. , 2006, “ Incidence of Rotator Cuff Tears in Shoulder Dislocations and Results of Therapy in Older Patients,” Arch. Orthop. Trauma Surg., 126(4), pp. 235–240. 10.1007/s00402-005-0034-0 [DOI] [PubMed] [Google Scholar]
- [17]. Porcellini, G. , Paladini, P. , Campi, F. , and Paganelli, M. , 2006, “ Shoulder Instability and Related Rotator Cuff Tears: Arthroscopic Findings and Treatment in Patients Aged 40 to 60 Years,” Arthroscopy, 22(3), pp. 270–276. 10.1016/j.arthro.2005.12.015 [DOI] [PubMed] [Google Scholar]
- [18]. Berbig, R. , Weishaupt, D. , Prim, J. , and Shahin, O. , 1999, “ Primary Anterior Shoulder Dislocation and Rotator Cuff Tears,” J. Shoulder Elbow Surg., 8(3), pp. 220–225. 10.1016/S1058-2746(99)90132-5 [DOI] [PubMed] [Google Scholar]
- [19]. Fung, A. K. , Li, Y. , Leahy, T. P. , Shetye, S. S. , Liu, X. S. , and Soslowsky, L. J. , 2019, “ Reproduction and Lactation Lead to Long-Term Changes in Supraspinatus Tendon and Humeral Trabecular Bone Properties in a Rat Model,” Transactions of the Orthopaedic Research Society, Austin, TX, February 2–5, 2019, Paper No. 0568.https://upoj.org/wp-content/uploads/v29/143_Fung.pdf [Google Scholar]
- [20]. Sengupta, P. , 2013, “ The Laboratory Rat: Relating Its Age With Human's,” Int. J. Prev. Med., 4(6), pp. 624–630.https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3733029/ [PMC free article] [PubMed] [Google Scholar]
- [21]. Favata, M. , 2006, “ Scarless Healing in the Fetus: Implications and Strategies for Postnatal Tendon Repair,” Ph.D. dissertation, University of Pennsylvania, Philadelphia, PA: https://repository.upenn.edu/dissertations/AAI3246156/ [Google Scholar]
- [22]. Bonilla, K. A. , Pardes, A. M. , Freedman, B. R. , and Soslowsky, L. J. , 2019, “ Supraspinatus Tendons Have Different Mechanical Properties Across Sex,” ASME J. Biomech. Eng., 141(1), p. 011002. 10.1115/1.4041321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23]. Hart, D. A. , Boykiw, R. , Sciore, P. , and Reno, C. , 1998, “ Complex Alterations in Gene Expression Occur in the Knee Ligaments of the Skeletally Mature Multiparous Rabbit During Pregnancy,” Biochim. Biophys. Acta, 1397(3), pp. 331–341. 10.1016/s0167-4781(98)00018-9 [DOI] [PubMed] [Google Scholar]
- [24]. Hart, D. A. , Sciore, P. , Boykiw, R. , and Reno, C. , 1998, “ Pregnancy Induces Complex Changes in the Pattern of MRNA Expression in Knee Ligaments of the Adolescent Rabbit,” Matrix Biol., 17(1), pp. 21–34. [DOI] [PubMed] [Google Scholar]
- [25]. Brent, A. E. , and Tabin, C. J. , 2004, “ FGF Acts Directly on the Somitic Tendon Progenitors Through the Ets Transcription Factors Pea3 and Erm to Regulate Scleraxis Expression,” Development, 131(16), pp. 3885–3896. 10.1242/dev.01275 [DOI] [PubMed] [Google Scholar]
- [26]. Tokunaga, T. , Shukunami, C. , Okamoto, N. , Taniwaki, T. , Oka, K. , Sakamoto, H. , Ide, J. , Mizuta, H. , and Hiraki, Y. , 2015, “ FGF-2 Stimulates the Growth of Tenogenic Progenitor Cells to Facilitate the Generation of Tenomodulin -Positive Tenocytes in a Rat Rotator Cuff Healing Model,” Am. J. Sports Med., 43(10), pp. 2411–2422. 10.1177/0363546515597488 [DOI] [PubMed] [Google Scholar]
- [27]. Arimura, H. , Shukunami, C. , Tokunaga, T. , Karasugi, T. , Okamoto, N. , Taniwaki, T. , Sakamoto, H. , Mizuta, H. , and Hiraki, Y. , 2017, “ TGF-Β1 Improves Biomechanical Strength by Extracellular Matrix Accumulation Without Increasing the Number of Tenogenic Lineage Cells in a Rat Rotator Cuff Repair Model,” Am. J. Sports Med., 45(10), pp. 2394–2404. 10.1177/0363546517707940 [DOI] [PubMed] [Google Scholar]
- [28]. Subramanian, A. , Kanzaki, L. F. , Galloway, J. L. , and Schilling, T. F. , 2018, “ Mechanical Force Regulates Tendon Extracellular Matrix Organization and Tenocyte Morphogenesis Through TGFbeta Signaling,” eLife, 7, e38069. 10.7554/eLife.38069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29]. Hansen, M. , and Kjaer, M. , 2014, “ Influence of Sex and Estrogen on Musculotendinous Protein Turnover at Rest and After Exercise,” Exerc. Sport Sci. Rev., 42(4), pp. 183–192. 10.1249/JES.0000000000000026 [DOI] [PubMed] [Google Scholar]
- [30]. Torricelli, P. , Veronesi, F. , Pagani, S. , Maffulli, N. , Masiero, S. , Frizziero, A. , and Fini, M. , 2013, “ In Vitro Tenocyte Metabolism in Aging and Oestrogen Deficiency,” Age, 35(6), pp. 2125–2136. 10.1007/s11357-012-9500-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31]. Lee, C. Y. , Liu, X. , Smith, C. L. , Zhang, X. , Hsu, H. C. , Wang, D. Y. , and Luo, Z. P. , 2004, “ The Combined Regulation of Estrogen and Cyclic Tension on Fibroblast Biosynthesis Derived From Anterior Cruciate Ligament,” Matrix Biol., 23(5), pp. 323–329. 10.1016/j.matbio.2004.07.004 [DOI] [PubMed] [Google Scholar]
- [32]. Lee, C. A. , Lee-Barthel, A. , Marquino, L. , Sandoval, N. , Marcotte, G. R. , and Baar, K. , 2015, “ Estrogen Inhibits Lysyl Oxidase and Decreases Mechanical Function in Engineered Ligaments,” J. Appl. Physiol., 118(10), pp. 1250–1257. 10.1152/japplphysiol.00823.2014 [DOI] [PubMed] [Google Scholar]
- [33]. Wood, M. L. , Luthin, W. N. , Lester, G. E. , and Dahners, L. E. , 2003, “ Tendon Creep is Potentiated by NKISK and Relaxin Which Produce Collagen Fiber Sliding,” Iowa Orthop. J., 23, pp. 75–79.https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1888401/ [PMC free article] [PubMed] [Google Scholar]
- [34]. Qin, X. , Garibay-Tupas, J. , Chua, P. K. , Cachola, L. , and Bryant-Greenwood, G. D. , 1997, “ An Autocrine/Paracrine Role of Human Decidual Relaxin—I: Interstitial Collagenase (Matrix Metalloproteinase-1) and Tissue Plasminogen Activator1,” Biol. Reprod., 56(4), pp. 800–811. 10.1095/biolreprod56.4.800 [DOI] [PubMed] [Google Scholar]
- [35]. Konopka, J. A. , Debaun, M. R. , Chang, W. , and Dragoo, J. L. , 2016, “ The Intracellular Effect of Relaxin on Female Anterior Cruciate Ligament Cells,” Am. J. Sports Med., 44(9), pp. 2384–2392. 10.1177/0363546516646374 [DOI] [PubMed] [Google Scholar]
- [36]. Carroll, C. C. , Patel, S. H. , Simmons, J. , Gordon, B. D. , Olson, J. F. , Chemelewski, K. , Saw, S. , Hale, T. M. , Howden, R. , and Sabbaghi, A. , 2020, “ The Impact of Genistein Supplementation on Tendon Functional Properties and Gene Expression in Estrogen-Deficient Rats,” J. Med. Food, Epub. 10.1089/jmf.2019.0293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37]. MacLennan, A. H. , Nicolson, R. , Green, R. C. , and Bath, M. , 1986, “ Serum Relaxin and Pelvic Pain of Pregnancy,” Lancet, 328(8501), pp. 243–245. 10.1016/S0140-6736(86)92069-6 [DOI] [PubMed] [Google Scholar]
- [38]. Vøllestad, N. K. , Torjesen, P. A. , and Robinson, H. S. , 2012, “ Association Between the Serum Levels of Relaxin and Responses to the Active Straight Leg Raise Test in Pregnancy,” Man. Ther., 17(3), pp. 225–230. 10.1016/j.math.2012.01.003 [DOI] [PubMed] [Google Scholar]
- [39]. Charlton, W. P. H. , Coslett-Charlton, L. M. , and Ciccotti, M. G. , 2001, “ Correlation of Estradiol in Pregnancy and Anterior Cruciate Ligament Laxity,” Clin. Orthop. Relat. Res., 387, pp. 165–170. 10.1097/00003086-200106000-00022 [DOI] [PubMed] [Google Scholar]
- [40]. Hart, D. A. , Kydd, A. , and Reno, C. , 1999, “ Gender and Pregnancy Affect Neuropeptide Responses of the Rabbit Achilles Tendon,” Clin. Orthop. Relat. Res., 365, pp. 237–246. 10.1097/00003086-199908000-00029 [DOI] [PubMed] [Google Scholar]
- [41]. Shen, P.-C. , Wang, P.-H. , Wu, P.-T. , Wu, K.-C. , Hsieh, J.-L. , and Jou, I.-M. , 2015, “ The Estrogen Receptor-β Expression in De Quervain's Disease,” Int. J. Mol. Sci., 16(11), pp. 26452–26462. 10.3390/ijms161125968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42]. Qing, H. , Ardeshirpour, L. , Divieti Pajevic, P. , Dusevich, V. , Jähn, K. , Kato, S. , Wysolmerski, J. , and Bonewald, L. F. , 2012, “ Demonstration of Osteocytic Perilacunar/Canalicular Remodeling in Mice During Lactation,” J. Bone Miner. Res., 27(5), pp. 1018–1029. 10.1002/jbmr.1567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43]. Zhou, Y. , Li, Y. , Davis, Z. , Wang, W. , Fung, A. K. , Shetye, S. S. , Jiang, X. , Kuntz, A. F. , Dyment, N. A. , Soslowsky, L. J. , and Liu, X. S. , 2020, “ Structural and Cellular Responses of Supraspinatus Tendon Enthesis and Subchondral Bone to Pregnancy, Lactation, and Post-Weaning Recovery,” Transactions of the Orthopaedic Research Society, Phoenix, AZ, February 8–11, Paper No. 0407. [Google Scholar]
- [44]. Chen, X. , Giambini, H. , Ben-Abraham, E. , An, K. N. , Nassr, A. , and Zhao, C. , 2015, “ Effect of Bone Mineral Density on Rotator Cuff Tear: An Osteoporotic Rabbit Model,” PLoS One, 10(10), p. e0139384. 10.1371/journal.pone.0139384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45]. Cadet, E. R. , Vorys, G. C. , Rahman, R. , Park, S. H. , Gardner, T. R. , Lee, F. Y. , Levine, W. N. , Bigliani, L. U. , and Ahmad, C. S. , 2009, “ Improving Bone Density at the Rotator Cuff Footprint Increases Supraspinatus Tendon Failure Stress in a Rat Model,” J. Orthop. Res., 28(3), p. 314. 10.1002/jor.20972 [DOI] [PubMed] [Google Scholar]
- [46]. De Bakker, C. M. J. , Tseng, W. J. , Li, Y. , Zhao, H. , Altman-Singles, A. R. , Jeong, Y. , Robberts, J. , Han, L. , Kim, D. G. , and Sherry Liu, X. , 2017, “ Reproduction Differentially Affects Trabecular Bone Depending on Its Mechanical Versus Metabolic Role,” ASME J. Biomech. Eng., 139(11), p. 111006. 10.1115/1.4038110 [DOI] [PMC free article] [PubMed] [Google Scholar]


