Abstract
A substantial fraction of coronavirus disease 2019 (COVID-19) patients experience neurological manifestations. Nevertheless, brain changes caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) remain largely unknown. Here, we provide a brief overview of positron emission tomography (PET) applications that could advance current understanding of CNS pathophysiological alterations associated with SARS-CoV-2 infection.
Keywords: SARS-CoV-2, neurological manifestations, PET radiotracers
Neurological Manifestations in COVID-19
Neurological manifestations are highly prevalent in COVID-19 patients, ranging from mild ones, for instance dizziness and headaches, to severe complications, such as encephalitis and ischemic stroke. Yet, the pathological changes in the CNS induced by SARS-CoV-2 are still uncertain [1]. The two main hypotheses for the causes of COVID-19 neurological symptoms posit either indirect effects via peripheral inflammation, or direct effects via CNS invasion by SARS-CoV-2. The relative contributions of these two potential mechanisms are under debate. It seems that overreaction of the immune system due to SARS-CoV-2 infection, a phenomenon called the cytokine storm, may at least in part, indirectly contribute to neurological manifestations [2]. But emerging reports also suggest that SARS-CoV-2 may invade the CNS, potentially infecting brain cells via its functional receptor, the human angiotensin-converting enzyme 2 (hACE2) (Box 1 ) [3].
Box 1. Imaging Human Angiotensin-Converting Enzyme 2 (hACE2).
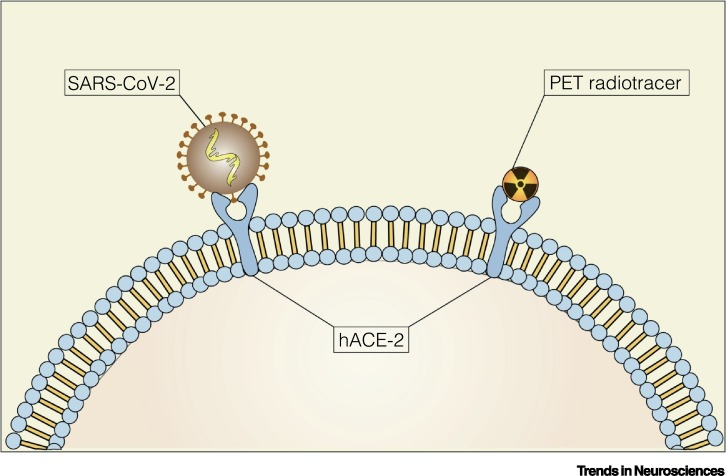
Emerging findings suggest that SARS-CoV-2 penetrates a cell via its functional receptor, the hACE2 [3]. PET imaging of hACE2 availability in the brain (Figure I) could indicate whether there is a link between hACE2 overexpression and the risk of developing neurological complications due to SARS-CoV-2 infection. Currently, there are no PET radiotracers available for assessing hACE2 availability. Thus, the design of the first generation of hACE2 radiotracers seems an attractive strategy, which could help advance our knowledge of SARS-CoV-2 brain infection susceptibility.
Figure I.
A Potential Positron Emission Tomography (PET) Radiotracer Targeting Human Angiotensin-Converting Enzyme 2 (hACE2).
Schematic representation of a potential PET radiotracer and severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) binding to the hACE2.
Alt-text: Box 1
Interestingly, a large fraction of patients with severe COVID-19 presented with abnormal brain magnetic resonance imaging (MRI), and lesions compatible with brain encephalopathy [4,5]. Although these MRI findings indicate the possibility of brain changes in individuals infected with SARS-CoV-2, larger cohorts and further studies are needed for a more comprehensive picture of brain pathophysiological alterations and their causes.
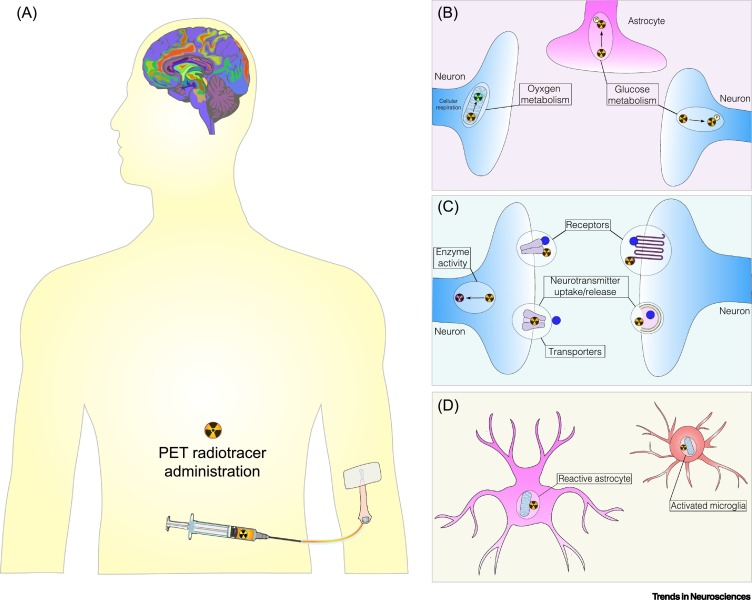
PET imaging (Figure 1A), combined with a wide range of PET radiotracers, may help us to comprehend COVID-19 neurological manifestations in patients by revealing: (i) ‘where’: brain regions affected; (ii) ‘which’: cell types involved; (iii) ‘how’: changes in neurotransmission or metabolic parameters; and (iv) ‘when’: early PET exams could identify individuals at risk of developing neurological complications [6]. In this forum article, we provide a concise overview of some widely used PET radiotracers, which can rapidly advance our understanding of acute, mid-, and long-term COVID-19 effects in the brain.
Figure 1.
Assessing Coronavirus Disease 2019 (COVID-19) Brain Changes Using Positron Emission Tomography (PET) Imaging.
(A) PET radiotracers are injected intravenously at tracer concentrations and a PET scanner records the distribution of radioactivity in the brain, which is reconstructed, analyzed, and read by imaging experts. PET radiotracers, commonly used in brain imaging research, can be used to uncover brain changes associated with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in the domains of (B) metabolic parameters, such as glucose and oxygen metabolism; (C) neurotransmission systems, by estimating neurotransmitter uptake/release/synthesis, enzyme activity, and receptor/transporter availability; and (D) brain cell types affected: neurons, astrocytes, or microglia.
Brain Metabolic Changes in COVID-19
[18F]FDG, a fluorine-18 radiolabeled glucose analog, is the most widely used PET radiotracer worldwide. Brain PET [18F]FDG hypometabolism is thought to reflect synaptic dysfunction (Figure 1B). Early PET findings detected [18F]FDG hypometabolism in multiple brain areas, including the bilateral gyrus rectus [7], prefrontal and orbitofrontal cortices, and cerebellar vermis [8], of COVID-19 patients. These initial findings provide hints as to brain regions more prone to be affected by SARS-CoV-2. Nevertheless, PET imaging studies involving larger cohorts are required to build up our knowledge of brain pathophysiological changes caused by SARS-CoV-2.
Alterations in oxygen metabolism were also found in the brain of COVID-19 patients [1]. In fact, SARS-CoV-2 is likely to induce oxygen dysmetabolism in neuronal cells [3]. In this context, the PET radiotracer [15O]O2 may help us to examine the prevalence of hypoxia in the brain of COVID-19 patients (Figure 1B). It is important to emphasize that acute oxygen deprivation may evolve into severe complications, including ischemic encephalopathy and blood–brain barrier (BBB) leakage.
Neurotransmission Changes in SARS-CoV-2
PET radiotracers are key tools to interrogate neurotransmission systems by estimating neurotransmitter uptake/release/synthesis, key enzyme activity, and receptor/transporter availability (Figure 1C) [9]. A single PET radiotracer will only provide information about a particular aspect of a neurotransmission system, although alterations in a single molecular target may suggest that the system is ‘abnormal’. In the following paragraphs, we exemplify the application of PET radiotracers for probing selected neurotransmission systems.
Tracking Glutamate and GABA Neuroreceptors
Glutamate is the primary excitatory neurotransmitter in the CNS. Interestingly, hACE2 is highly expressed in glutamatergic neurons, which are widely distributed in the human brain. PET radiotracers are available for measuring ionotropic or metabotropic glutamatergic receptor availability. An example of a PET radiotracer widely used in clinical research is [11C]ABP688, which indicates the availability of the metabotropic glutamate receptor 5 (mGluR5). Of note, aberrant activation of glutamate receptors can lead to neuronal damage/death, a phenomenon termed excitotoxicity. Based on the high density of hACE2 in glutamatergic neurons, it seems reasonable to hypothesize that excitotoxicity might be implicated in COVID-19 and possibly some of its neurological manifestations. If so, early pharmacological intervention, using approved drugs targeting glutamatergic receptors, could be a potential treatment strategy.
GABAergic neurotransmission is the major inhibitory system in the brain. The [11C]Flumazenil PET radiotracer has been used to access the availability of the α subunits of the GABAA receptor. By assessing possible changes in glutamatergic and GABAergic neurotransmission, PET imaging could be used to detect perturbations in the fine-tuned balance between the two neurotransmission systems, which may contribute to neurological manifestations such as neuronal hyperexcitability and seizures.
Imaging Other Neurotransmission Systems
Cholinergic neurotransmission plays critical roles in various cognitive functions, including memory, learning, and attention. Cholinergic dysfunction has been linked to neurodegenerative disorders such as Alzheimer’s disease (AD). More specifically, presynaptic components of cholinergic neurons, including the vesicular acetylcholine transporter (VAChT), are vulnerable targets of the aging process and neurodegeneration. Whether COVID-19 has specific impacts on the cholinergic system remains to be determined and, in that regard, the VAChT PET radiotracer [18F]FEOBV could be instrumental in testing for potential cholinergic deficits in these patients.
The serotoninergic system might be a particularly intriguing target for research in the context of COVID-19, in part, due to its involvement in mood disorders and social cognition. Several PET radiotracers have been developed for evaluation of serotoninergic homeostasis in humans. As one example, [11C]DASB is a serotonin transporter (SERT) PET radiotracer, and could be used to address the questions of whether SARS-CoV-2 infection may be the tipping point for the exacerbation of certain psychiatric conditions and if social isolation may aggravate certain mental health conditions.
Over the last decades, the PET radiotracer [18F]FDOPA has been used as a marker of dopaminergic cell loss in Parkinson’s disease (PD). A recent case report described a COVID-19 patient presenting persistent episodes of tremor [10]. Despite normal brain MRI, a PET exam found decreased uptake of [18F]FDOPA in the putamen region. Clinical interpretation of these findings suggested secondary Parkinsonism due to SARS-CoV-2 infection. Of note, this patient’s cerebrospinal fluid tested negative for anti-SARS-CoV-2 IgG antibodies, despite a positive serum test result. While much remains to be clarified, these findings seem consistent with the idea of peripheral inflammation as a possible trigger of PD.
The Cellular Origins of COVID-19 Neurological Manifestations
Recent autopsy findings from COVID-19 patients indicated reactive astrogliosis and microgliosis in many of the patients [11]. Furthermore, inflammatory CNS syndromes, including encephalitis, acute disseminated encephalomyelitis, and myelitis have been found in COVID-19 patients [1]. Neuroinflammatory changes can be assessed, for instance, using [11C]PK11195, a widely used radiotracer to track microglial activation, and [11C]DED, a radiotracer for detecting reactive astrogliosis [12]. In addition, since astrocytes play critical roles in maintaining BBB integrity, reactive astrogliosis triggered by peripheral inflammation may contribute to BBB breakdown and leakage.
Concluding Remarks
We have presented an overview of PET radiotracer applications for providing pathophysiological insights into the effects of SARS-CoV-2 on the brain. Apart from testing for brain changes during the acute phase of the disease, longitudinal assessment of COVID-19 patients using PET may be conducted to examine whether brain changes are transient or long-lasting. If the changes evolve into a chronic state, attention should be directed towards understanding the likelihood of neurodegeneration associated with COVID-19. In this case, PET scanning of individuals at risk might be used for detecting pathological features of neurodegenerative disorders, for instance those of AD (using amyloid-β and tau radiotracers) and PD (using radiotracers for dopaminergic function, receptors, and transporters). One should keep in mind that PET examinations are relatively costly and their availability is limited. However, larger studies are needed and may help develop our understanding of COVID-19 brain pathophysiological changes.
Acknowledgments
I.C.F. and D.O.S. are supported by CAPES [88887.185806/2018-00]. D.O.S. is supported by CNPQ/INCT [465671/2014-4], CNPQ/ZIKA [440763/2016-9], CNPQ/FAPERGS/PRONEX [16/2551-0000475-7], FAPERGS [19/2551-0000700-0], CAPES [88887.507218/2020-00] [88887.507161/2020-00]. E.R.Z. is supported by CNPq [435642/2018-9] [312410/2018-2] and Instituto Serrapilheira [Serra-1912-31365]. S.B. and A.G. are supported by EPSRC Programme Grant [EP/S032789/1], Wellcome Trust Multi-User Equipment grant [212885/Z/18Z], the National Institute for Health Research (NIHR) Biomedical Research Centre at Guy's and St Thomas' NHS Foundation Trust and King's College London.
References
- 1.Paterson R.W. The emerging spectrum of COVID-19 neurology: clinical, radiological and laboratory findings. Brain. 2020 doi: 10.1093/brain/awaa240. Published online July 8, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mehta P. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Song E. Neuroinvasion of SARS-CoV-2 in human and mouse brain. bioRxiv. 2020 doi: 10.1101/2020.06.25.169946. Published online September 8, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Helms J. Neurologic features in severe SARS-CoV-2 infection. N. Engl. J. Med. 2020;382:2268–2270. doi: 10.1056/NEJMc2008597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kremer S. Brain MRI findings in severe COVID-19: a retrospective observational study. Radiology. 2020;297:242–251. doi: 10.1148/radiol.2020202222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hooker J.M., Carson R.E. Human positron emission tomography neuroimaging. Annu. Rev. Biomed. Eng. 2019;21:551–581. doi: 10.1146/annurev-bioeng-062117-121056. [DOI] [PubMed] [Google Scholar]
- 7.Guedj E. (18)F-FDG brain PET hypometabolism in post-SARS-CoV-2 infection: substrate for persistent/delayed disorders? Eur. J. Nucl. Med. Mol. Imaging. 2020 doi: 10.1007/s00259-020-04973-x. Published online July 30, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Delorme C. Covid-19-related encephalopathy: a case series with brain FDG-PET/CT findings. Eur. J. Neurol. 2020 doi: 10.1111/ene.14478. Published online August 15, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sander C.Y., Hesse S. News and views on in-vivo imaging of neurotransmission using PET and MRI. Q. J. Nucl. Med. Mol. Imaging. 2017;61:414–428. doi: 10.23736/S1824-4785.17.03019-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cohen M.E. A case of probable Parkinson's disease after SARS-CoV-2 infection. Lancet. 2020;19:804–805. doi: 10.1016/S1474-4422(20)30305-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Matschke J. Neuropathology of patients with COVID-19 in Germany: a post-mortem case series. Lancet. 2020 doi: 10.1016/S1474-4422(20)30308-2. Published online October 5, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Narayanaswami V. Emerging PET radiotracers and targets for imaging of neuroinflammation in neurodegenerative diseases: outlook beyond TSPO. Mol. Imaging. 2018;17:1–25. doi: 10.1177/1536012118792317. [DOI] [PMC free article] [PubMed] [Google Scholar]