Abstract
Current models for flu-like epidemics insufficiently explain multi-cycle seasonality. Meteorological factors alone, including the associated behavior, do not predict seasonality, given substantial climate differences between countries that are subject to flu-like epidemics or COVID-19. Pollen is documented to be allergenic, it plays a role in immuno-activation and defense against respiratory viruses, and seems to create a bio-aerosol that lowers the reproduction number of flu-like viruses. Therefore, we hypothesize that pollen may explain the seasonality of flu-like epidemics, including COVID-19, in combination with meteorological variables.
We have tested the Pollen-Flu Seasonality Theory for 2016–2020 flu-like seasons, including COVID-19, in the Netherlands, with its 17.4 million inhabitants. We combined changes in flu-like incidence per 100 K/Dutch residents (code: ILI) with pollen concentrations and meteorological data. Finally, a predictive model was tested using pollen and meteorological threshold values, inversely correlated to flu-like incidence.
We found a highly significant inverse correlation of r(224) = −0.41 (p < 0.001) between pollen and changes in flu-like incidence, corrected for the incubation period. The correlation was stronger after taking into account the incubation time. We found that our predictive model has the highest inverse correlation with changes in flu-like incidence of r(222) = −0.48 (p < 0.001) when average thresholds of 610 total pollen grains/m3, 120 allergenic pollen grains/m3, and a solar radiation of 510 J/cm2 are passed. The passing of at least the pollen thresholds, preludes the beginning and end of flu-like seasons. Solar radiation is a co-inhibitor of flu-like incidence, while temperature makes no difference. However, higher relative humidity increases with flu-like incidence.
We conclude that pollen is a predictor of the inverse seasonality of flu-like epidemics, including COVID-19, and that solar radiation is a co-inhibitor, in the Netherlands.
Keywords: Pollen, Flu-like seasonality, Meteorological variables, Hay fever, Epidemiological study
Graphical abstract

Highlights
-
•
Testing pollen-flu seasonality theory for 2016–2020 in the Netherlands, overlapping COVID-19
-
•
Pollen have allergenic and immuno-activating properties.
-
•
Highly significant inverse correlation between pollen and flu-like incidence.
-
•
Solar radiation is a co-inhibitor of flu-like epidemics.
-
•
COVID-19 does not break with seasonality pattern, but more data are needed for conclusion.
1. Introduction
Current models for flu-like epidemics insufficiently explain multi-cycle seasonality. Meteorological factors alone do not fully explain the seasonality of flu-like epidemics (Tamerius et al., 2011) or COVID-19 (Yao et al., 2020). Pollen is documented to be allergenic (Klemens et al., 2007; Rosenwasser, 2011; Howarth, 2000), and it plays a role in immuno-activation (Brandelius et al., 2020). Furthermore, allergic diseases are absent as a comorbid condition of COVID-19 (Zhang et al., 2020; Dong et al., 2020). Explaining this, Licari et al. (2020) found that allergic children have a significantly higher eosinophil count than COVID-19 patients, whereby eosinophils are known to clear viral load, and contribute to the recovery from viral infections, supposedly including COVID-19 (Lindsley et al., 2020). A further explanation is provided by Jackson et al. (2020), who proved that allergic sensitization and allergen natural exposure are inversely related to membrane-bound angiotensin-converting enzyme 2 (ACE-2) expression, whereby it is known that severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) uses the ACE-2 receptor to gain cell entry, leading to COVID-19 (Wan et al., 2020). Finally, histamine and IgE serum levels are elevated in allergic rhinitis and asthma patients – an allergic disease that is also underreported as comorbid condition for COVID-19 – which downplay other anti-viral responses (plasmacytoid dendritic cells and interferon-α) but might thus prevent the cytokine storm and hyper-inflammation that typically mark severe respiratory diseases, including COVID-19 (Carli et al., 2020). Similarly, it is reported that pollen might suppress interferon-λ1 and -λ3 and pro-inflammatory chemokine responses in non-allergic subjects and it is found to be correlated to rhinovirus-positive cases (Gilles et al., 2019), but not to other flu-like virus-positive cases (Nivel.nl, 2020). It is hypothesized that exposure to and immunization for such ordinary cold viruses, especially corona cold viruses, could provide some protection against COVID-19, in the same way that the uptake of the common influenza vaccine seems to be inversely correlated to COVID-19 deaths (Marín-Hernández et al., 2020).
Recently, we identified pollen bio-aerosol as a discrete seasonal factor in inhibiting flu-like epidemics during the period 2016 to 2019 in the Netherlands (Hoogeveen, 2020). In this epidemiological study, we found strong inverse correlations between allergenic pollen concentrations and hay fever on the one hand, and flu-like incidence on the other. The study was based on the persistent observation that the pollen and flu season predictably alternate each other in moderate climate zones, and the absence of sufficient meteorological explanations (Tamerius et al., 2011). We further observed that the passing of pollen threshold values of around 100 allergenic pollen grains/m3, reliably mark the onset and decline of moderate flu-like epidemic lifecycles, and thus might be used as predictor. Such a concentration of allergenic pollen makes sense, as in an overview of real-life studies, clinical pollen threshold values are observed between 1 and 400 grains/m3, whereby the first symptoms are typically observed in the range of 1–50 grains/m3 (De Weger et al., 2013), depending on the country, period and vegetation, and, probably, the susceptibility of the subjects to allergens.
The seasonality of respiratory viral infections has been recognized for thousands of years in temperate regions (Moriyama et al., 2020). Seen in more in detail, virologists observed that cold, and flu-like epidemics (e.g., influenza and corona-caused) “go away in May” in the Northern Hemisphere, while emerging in the Southern Hemisphere with its opposite seasonality, only to re-emerge in the Northern Hemisphere during its next autumn and winter, but in a slightly mutated form. Furthermore, all new flu-like pandemics since 1889 typically emerged in the Northern Hemisphere at the tail-end of respective flu-seasons (Fox et al., 2017). Clearly, the current COVID-19 pandemic is no exception. The emergence of COVID-19 and other pandemics at the tail-end of the flu season makes sense. It takes time for a spontaneous new crossover virus with a sufficiently high reproduction number (Ro) – for SARS-CoV-2 it is estimated to be initially around 3 (Liu et al., 2020) – to develop from patient 0 to become a fully-fledged pandemic during the flu season in the Northern Hemisphere. The Northern Hemisphere, with its larger and denser populations, is more likely to be the initial breeding ground for a new flu-like pandemic than the Southern Hemisphere. Furthermore, Fox et al. showed that most flu-like pandemics are multi-wave, whereby the initial wave at the tail-end of flu season is typically short-lived. This gives rise to the idea that COVID-19 is also subject to such multi-wave seasonality (Kissler et al., 2020), because the distribution of community outbreaks is consistent with the behavior of seasonal respiratory viruses (Sajadi et al., 2020), and has a short wave at the tail end of the 2019/2020 flu-like season in the Northern Hemisphere in the temperate climate zone.
Numerous studies try to explain flu-like seasonality with meteorological factors such as sunlight, including UV radiation (Schuit et al., 2020), temperature and humidity (Chong et al., 2020; Shaman et al., 2011). However, Postnikov (2016) concluded that ambient temperature is not a good predictor for influenza seasonality in the Netherlands, and inconsistent correlations also exist for the relationships between COVID-19 and temperature (Tosepu et al., 2020; Xie and Zhu, 2020; Ma et al., 2020; Qi et al., 2020). Furthermore, findings about the relationships between humidity and influenza (Soebiyanto et al., 2014), and humidity and COVID-19 (Ahmadi et al., 2020; Ma et al., 2020; Qi et al., 2020) are equally inconsistent. Although UV light is detrimental for the flu-like virus aerosol under laboratory conditions, associated with immuno-activation (Abhimanyu and Coussens, 2017; Tan and Ruegiger Hardeland, 2020) and circadian rhythms regulating lung immunity (Nosal et al., 2020), the onset of the flu season, from mid-August in the Netherlands, coincides with an annual peak in hot, sunny days and is still in the middle of the summer season. According to Yao et al. (2020), for a decrease of COVID-19 infections neither high UV values nor high temperatures are good predictors. The contradictory findings related to COVID-19, understandably based on the analysis of a limited part of the year and disease cycle, might be partly due to sub-seasonal bias and unstandardized data-collection methods. By sub-seasonal bias we mean that if only a part of a season or cycle is analyzed, overly specialized conclusions can be drawn that cannot be generalized to the whole season or cycle.
Nevertheless, these meteorological variables are known factors in flowering and pollen maturation and dispersion. Meteorological variables, such as increased solar radiation and temperature – among others the absence of frost – not only trigger flowering and pollen maturation, they also affect the pollen bio-aerosol formation: dry and warm conditions stimulate pollen to become airborne. Rain, in contrast, makes pollen less airborne, and cools the bio-aerosol down. Very high humidity levels (RH 98%) are even detrimental to pollen (Guarnieri et al., 2006). An RH 98% effect on pollen could thus provide an alternative explanation as to why flu-like incidence in tropical countries is higher during the rainy season, and reduced during the rest of the year.
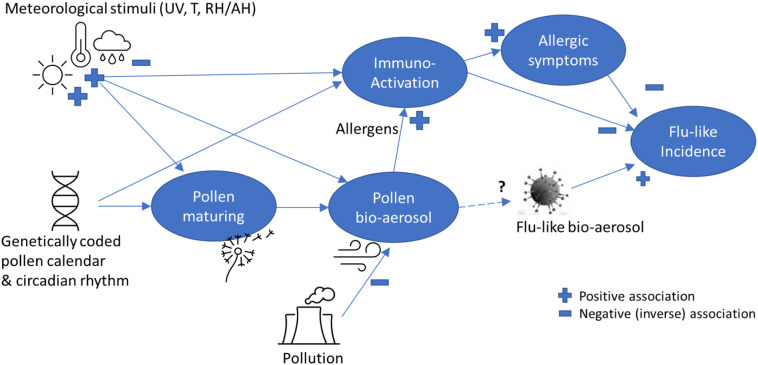
We hypothesize that pollen bio-aerosol has an inverse effect on flu-like incidence, including COVID-19 (see Fig. 1 ), whereby pollen is known to be triggered and influenced by meteorological variables, which can then jointly explain the seasonality of flu-like incidence. This indirect explanation of the pollen effect is based on the fact that pollen bio-aerosol and UV light exposure lead to immuno-activation, and sometimes allergic symptoms, which seem to protect against flu-like viruses, or at least severe outcomes from them. The indirect pollen effect is explained by the spread of pollen bio-aerosol under sunny and dry conditions. Further, it is unknown how viral bio-aerosol and pollen bio-aerosol interact with each other in the air, and whether anti-viral phytochemicals in pollen could then play a role in an alternative explanation.
Fig. 1.
A simplified theoretic model explaining pollen-flu inverse seasonality, whereby pollen might have an allergenic (I) and/or immune-triggering (II) function, inhibiting flu-like epidemic incidence in combination with meteorological conditions and triggers. The direct effect (III) on flu-like bio-aerosol is currently unknown.
To further understand the impact of pollen as an environmental factor influencing the life cycle of flu-like epidemics, the objective of this study is to determine the correlations of pollen and meteorological variables with (changes in) flu-like incidence and develop and test a discrete predictive model that combines pollen and meteorological co-inhibitors. Our main hypothesis, therefore, is that pollen is the missing link, jointly explaining with certain meteorological variables, flu-like seasonality, and that a compound threshold based factor – combining detected flu-inhibitors – is a good unified predictor of such seasonality. Regarding COVID-19, we have limited ourselves to observing whether or not COVID-19 at the tail-end of the 2019/2020 flu-like season is able to break with the flu-like seasonality pattern.
2. Methods
To study the relationship between pollen and flu-like incidence in the Netherlands, we used the public datasets of Elkerliek Hospital (Elkerliek.nl) about the weekly allergenic, low-level allergenic and total pollen concentrations in the Netherlands in grains/m3, whereby for 42 types of pollen particles the numbers are counted and averaged per day per 1m3 of air. The common Burkard spore trap was used, through which a controlled amount of air was ingested. The applied classification and analysis method conforms to the EAACI (European Academy of Allergology and Clinical Immunology) and the EAN (European Allergy Network) standards. Allergenic pollen includes nine types of particles that are classified as moderate (Corylus, Alnus, Rumex, Plantago and Cedrus Libani), strong (Betula and Artemisia), or very strong allergenic (Poaceae and Ambrosia). Additionally, we included low-level allergenic pollen concentrations in addition to the allergenic ones because we assume that they may also have effects. Low-level allergenic pollen includes the other 33 particles that are classified as non-allergenic to low-level allergenic (Cupressaceae, Ulmus, Populus, Fraxinus, Salix, Carpinus, Hippophae, Fagus, Quercus, Aesculus, Juglans, Acer, Platanus, Pinus, Ilex, Sambucus, Tilia, Ligustrum, Juncaceae, Cyperaceae, Ericaceae, Rosaceae, Asteraceae, Ranunculaceae, Apiaceae, Brassicaceae, Urtica, Chenopodiaceae, Fabaceae, Humulus, Filipendula, and Indet). Total pollen concentration is the sum of the average allergenic and low-level allergenic pollen concentrations. Advantages of using the total pollen metric are that there are hardly any 0 values (only 3 out of 266), and we did not need to limit ourselves to just parts of the seasonal cycle, which might introduce sub-seasonal bias into our research. We also assumed that long-distance pollen transport is accounted for, as foreign pollen will also be counted by a pollen measuring station that works all year round.
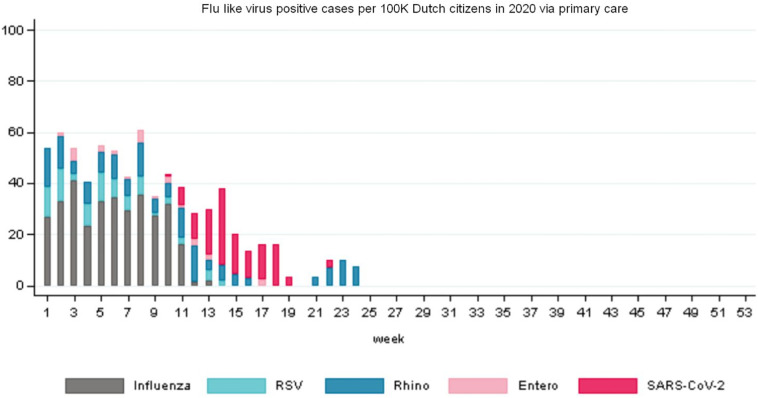
Furthermore, we used the data from the Dutch State Institute for Public Health (RIVM.nl) gathered by Nivel (Nivel.nl) about weekly flu-like incidence (WHO code “ILI” - Influenza Like Illnesses) reports at primary medical care level, per 100,000 citizens in the Netherlands. Primary medical care is the day-to-day, first-line healthcare given by local healthcare practitioners to their registered clients as typical for the Netherlands, with its current population of 17.4 million. The reports relate to a positive RIVM laboratory test for ILI after a medical practitioner diagnosed ILI after a consultation, whether that leads to hospitalization or not. The ILI metric is according to a standardized WHO method, given that ILI data is gathered and compared globally. ILI is defined by the WHO as a combination of a measured fever of ≥38 °C, and a cough, with an onset within the last 10 days. The flu-like incidence metric is a weekly average based on a representative group of 40 primary care units, and calculated using the number of influenza-like reports per primary care unit divided by the number of patients registered at that unit. This is then averaged for all primary care units and then extrapolated to the complete population. The datasets run from week 1 of 2016 up to week 18 of 2020 (n = 226 data points) to include the recent COVID-19 pandemic at the tail-end of the 2019/2020 flu-like season. To underpin the relative importance of COVID-19: SARS-CoV-2 has been detected in the Netherlands since week 9 2020. According to the figures of Nivel.nl (2020, see Fig. 2 ), from week 13 onward SARS-CoV-2 is the outcome of the (vast) majority of positive tests for patients at primary care level with flu-like complaints, and by week 18,100% of positive tests indicate SARS-CoV-2 (other tested viruses are five Influenza A and B subtypes, RSV, Rhinovirus and Enterovirus).
Fig. 2.
The overview of flu-like virus positive cases in 2020 (till week 24) shows that from week 13 onwards SARS-CoV-2 is dominant. The Rhinovirus cases spike is outside the timeline of our research (source: Nivel.nl, 2020).
Furthermore, we also included meteorological datasets from the Royal Dutch Meteorological Institute (KNMI.nl), including average relative humidity/day, average temperature/day and global solar radiation in J/cm2 per day as an indicator of UV radiation. These datasets were obtained from the KNMI's centrally located De Bilt weather station. Next, we calculated the weekly averages for the same periods that featured in the other datasets. De Bilt is traditionally chosen as it provides an approximation of modal meteorological parameters in the Netherlands, which is a small country. Furthermore, all major population centers in the Netherlands, which account for around 70% of the total Dutch population, are within a radius of only 60 km from De Bilt. We therefore assumed in this study that the measurements from De Bilt are sufficiently representative for the meteorological conditions typically experienced by the Dutch population.
To test allergenic versus low-level allergenic pollen assumptions, against hay fever and pre-COVID-19 flu-like incidence, we made use of the hay fever index. The hay fever index is defined as the turnover for over-the-counter hay fever medication, as reported by all Dutch pharmacies to the Dutch Central Bureau of Statistics (CBS.nl) and based on respective ATC codes (especially R01A/R01AC). This concerns so-called self-medication products which are available without prescription at a pharmacy, and thus might exclude certain types of medication for which prescription is needed. We used a dataset from week 1 of 2016 up to week 10 of 2019 (n = 166 data points), because no further data was made available. For the interpretation of our findings, we assumed for the Netherlands a prevalence of allergic rhinitis that is more or less similar to that in Western Europe, being around 23%, and frequently undiagnosed (Bauchau and Durham, 2004). Furthermore, it can be noted that the prevalence of allergic diseases in general in the Netherlands is around 52% (Van De Ven et al., 2006).
Datasets were complete, except that three weekly pollen concentration measurements were missing (1.3%). This was due to a malfunctioning monitoring station during week 26 of 2016, week 21 of 2017 and week 22 of 2019. These missing measurements appeared to be completely random. We imputed missing values to avoid bias and maintain power. We used a four-week surrounding average to estimate the three missing data points and thus avoid breaking lines in visuals. We checked that the missing data has no material impact on the results by comparing these averages with the data of previous years for similar periods, and by observing whether removal from statistical tests had any effect on outcomes and conclusions.
Regarding the incidence of flu-like symptoms, we calculated the weekly change compared to the previous week (ΔILI=ILIt – ILIt-1). This was to obtain an indication of the flu-like epidemic life cycle progression, whereby a decline is interpreted as Ro < 1 and an increase as Ro > 1 (Ro is the reproduction number of flu-like viruses). Furthermore, to cater, in one time-series metric, for changes in flu-like incidence as well as for an incubation period of up to two weeks, we calculated a three-week moving average (3WMA) of changes in flu-like incidence, of which two weeks are forward looking:
Thus, ΔILI3WMA has on average a one week lag. A general advantage of a moving average is that it reduces statistical noise. It should be noted that whenever we use the term incubation time, we also mean to include reporting delay (estimated to be around 4.5 days). We have not assumed delay effects for meteorological variables or pollen concentrations, so we have not calculated moving averages for other time series.
Compared with our previous study (Hoogeveen, 2020), there is an overlap in datasets of less than 10%. The datasets are extended by the extension in time, the addition of meteorological datasets and non-allergenic pollen, and the introduction of newly calculated variables, such as total pollen concentration, ΔILI3WMA, the compound predictor and the log10 transformations on pollen, ILI and the hay fever index.
We formulated the following statistical null hypotheses for falsification.
H10
there are no inverse correlations for total pollen concentrations with flu-like incidence (corrected for incubation period).
H20
there are no inverse correlations between pollen and changes in flu-like incidence (ΔILI or corrected for incubation time: ΔILI3WMA).
H30
there is no predictive significance of a discrete model's compound value, based on thresholds for pollen and meteorological co-inhibitors, related to changes in flu-like incidence (ΔILI3WMA).
To understand the role of meteorological variables, to check whether – in our datasets – meteorological variables show their well-established effects on pollen as assumed, and to select co-inhibitors:
H40
meteorological variables – solar radiation, temperature and relative humidity – have no effect on pollen and/or flu-like incidence change (ΔILI3WMA).
Low-level allergenic pollen is sometimes known to have a slight allergenic effect. To understand how to interpret adding none-to-low-level allergenic pollen to the total pollen metric, we wanted to verify their effects on the hay fever index:
H50
low-level allergenic pollen has no effect on hay fever and (changes in) flu-like incidence.
Note that with the exception of H5, all hypotheses are related to potential causality: the temporal sequentiality (temporality) of the respective independent variables, and flu-like incidence corrected for incubation period. Whenever we refer to temporality, we mean to indicate that the datasets behave as if there is causality, on the understanding that statistics alone cannot prove causality in uncontrolled settings.
2.1. Statistical analyses
Variables are presented with their means (M) and standard deviations (SD).
We calculated correlation coefficients to test the hypotheses and to assess the strength and direction of relationships. As a sensitivity analysis, we also calculated the bootstrapped correlation coefficients. We used the full datasets, to avoid sub-seasonal bias, and by extending the number of years the distortions by incidental and uncontrolled events are supposed to be minimized. However, as a second sensitivity analysis, we removed from the datasets the autumn weeks between 42 and 50, which typically show low pollen concentrations of up to 20 grains/m3, which are applied to analyze the main outcome (H20). Further, as a third sensitivity analysis we calculate correlations per individual time lag included in ΔILI3WMA in relation to H20.
Next, linear regression (F-test) on identified inhibitors and interactions was used descriptively to determine whether the relationship can (statistically) be described as linear, and to determine the equation using estimates and intercept values, and produce probability, significance level, F-value, and the Multiple R squared correlation to understand the predictive power of the respective inhibitor. Standard deviations and errors, and degrees of freedom (DF) were used as input for calculating the 95% probability interval. We have reported in the text the outcome of statistical tests in APA style, adapted to journal requirements. For relationships that appear non-linear – logarithmic or exponential – we have used the log10 function to transform the data if that makes the relationship appear linear, before re-applying linear regression. We have also used the log10 transformed datasets for the calculation of correlation coefficients, to correct for skewness.
Finally, we created a simple, discrete model resulting in one compound value, using selected flu-like inhibitors. This was to determine the optimal average threshold values for these inhibitors, which have the highest joint correlation with changes in flu-like incidence (ILI3WMA). We applied linear regression (F-test) to understand the predictive power of the compound value, and to determine the linear equation when significant. By constructing one compound, independent variable, we covered for collinearity or interaction effects between joined co-inhibitors. In our analysis, we based the compound value on three selected thresholds. For example, when one threshold value is passed, this leads to a compound value = 1, and when all three threshold values are passed, this leads to a compound value = 3. Therefore, the compound values are in the range of [0,3].
The compound value equation can be expressed as shown below, where iv = the respective independent variable that acts as inhibitor of flu-like incidence, and k relates to the respective calculated threshold value. For each respective threshold passed (iv > k), +1 is added to the compound value (CV):
For the three selected co-inhibitors (n = 3), this takes in Excel the form of , whereby we have used a threshold value for solar radiation (kr), and both pollen threshold values for allergenic (kap) and total pollen (kp) for K1, K2 and K3 respectively, as is shown in Section 3.
It is outside of the scope of this research to verify the underlying datasets of Elkerliek Ziekenhuis, RIVM/Nivel, CBS, and KNMI by examining the validity and reliability of their data collection methods. These institutes have well-established and internationally standardized protocols for data collection and verification.
All regression analyses have been carried out using the statistical package R version 3.5.
3. Results
The means and standard deviations per variable are given in Table 1 . For the correlation coefficients below, we have used the log10 transformed data for respective variables, to correct for skewness.
Table 1.
Overview of means (M) and standard deviations (SD) per variable in the dataset, including Log10 transformed data, and datasets that are reduced for sensitivity analysis.
| Variable | Mean | SD | Log10 transformed |
|
|---|---|---|---|---|
| Mean | SD | |||
| Allergenic pollen concentration in grains/m3 | 349 | 987 | 1.84 | 0.86 |
| Total pollen concentration in grains/m3 | 732 | 1368 | 2.17 | 0.98 |
| Total pollen concentration in grains/m3 (minus weeks 42 to 50) | 870 | 1452 | 2.45 | 0.76 |
| Low-level allergenic pollen concentration in grains/m3 | 383 | 626 | 1.85 | 1.03 |
| Flu-like incidence (ILI) per 100 K citizens | 47 | 40.2 | 1.54 | 0.35 |
| ΔILI | −0.25 | 15.4 | ||
| ΔILI3WMA | −0.26 | 8.9 | ||
| ΔILI3WMA (minus weeks 42 to 50) | −0.89 | 9.3 | ||
| Index hay fever | 101 | 116 | 1.81 | 0.39 |
| Relative humidity (%) | 79 | 8.27 | ||
| Temperature in °C | 10.8 | 5.82 | ||
| Solar radiation in J/cm2 | 1047 | 709 | ||
| Compound Model in thresholds passed | 1.4 | 1.10 | ||
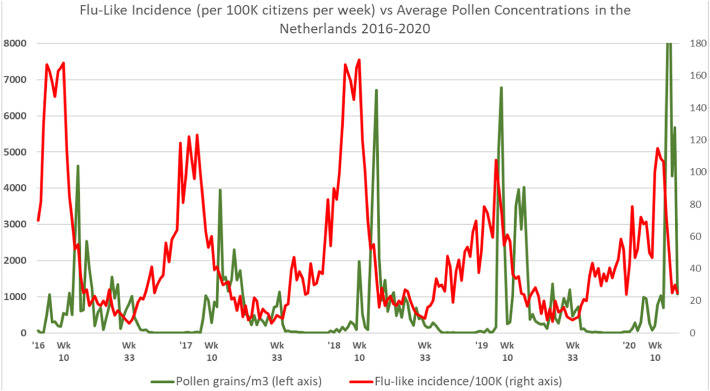
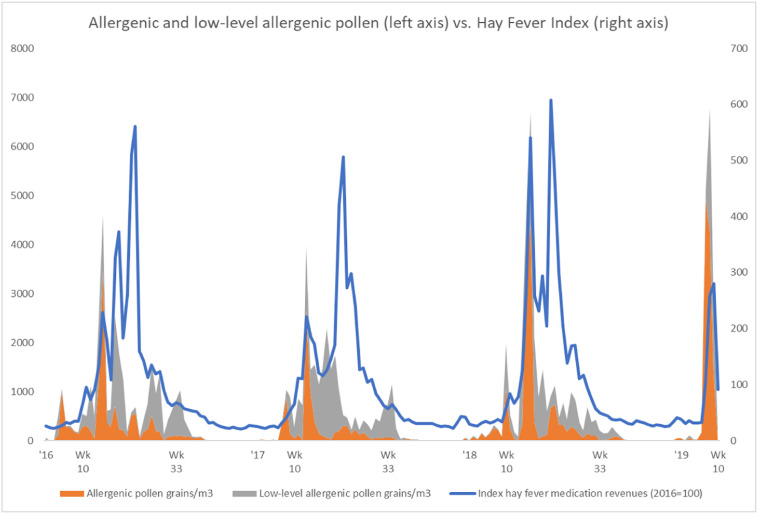
When further inspecting the datasets regarding pollen concentrations and flu-like incidence reported by primary medical care in the Netherlands, it was clear that there are continuous pollen bursts (Fig. 3 ), whereby only a few of these pollen bursts are classified as more allergenic (Fig. 6). These pollen bursts, allergenic or low-level allergenic, typically coincide with and precede a decline in flu-like incidence.
Fig. 3.
Total pollen concentrations vs. flu-like incidence in the Netherlands, whereby especially passing the 610 total pollen grains/m3 threshold marks the seasonal switches around week 10 (±5 weeks) and week 33 (±2 weeks). For the interpretation of the relationship, an incubation time of up to 2 weeks should be taken into account, and the change in flu-like incidence (Ro >1 or Ro <1) should be considered.
Fig. 6.
Both allergenic and low-level allergenic pollen are positively correlated to hay fever.
The correlation for total pollen and flu-like incidence is highly significant when taking into account incubation time: r(222) = −0.40, p < 0.001. We can thus reject the null-hypotheses H10 in favor of the alternative hypothesis that, when taking into account incubation time, there is a negative correlation between total pollen and flu-like incidence, including the first cycle of the COVID-19 pandemic. Furthermore, we can reject H50 in favor of our assumption that it makes sense to also include low-level allergenic pollen concentrations in our study. Low-level allergenic pollen is inversely correlated to flu-like incidence (r(221) = −0.37, p < 0.00001), especially when corrected for the 2 weeks incubation time (r(219) = −0.53, p < 0.00001).
The fact that the correlations become stronger when taking into account incubation time, implies temporality. Furthermore, we can also observe from Fig. 3 that flu-like incidence starts to decline after the first pollen bursts. Moreover, flu-like incidence starts to increase sharply after pollen concentrations become very low or close to zero. This is a qualitative indication of temporality. Furthermore, we can notice that the first COVID-19 cycle behaved according to pollen-flu seasonality, at least does not break with it.
When testing the impact on ΔILI, the weekly changes in medical flu-like incidence, the extended dataset till 2020, including COVID-19, shows a strong and highly significant inverse correlation with total pollen (r(226) = −0.26, p = 0.000063). Therefore, we can falsify the null-hypothesis (H20) that there is no inverse correlation between the weekly pollen concentrations and weekly changes in flu-like incidence (ΔILI), including the period covering the first cycle of the COVID-19 pandemic. This inverse correlation therefore provides further support for the alternative hypothesis that the presence of an elevated level of pollen has an inhibiting effect on flu-like incidence, and starts to immediately influence the direction and course of the epidemic life cycle. Also, during the COVID-19 dominated period of the last 9 weeks, it appears that flu-like incidence behaves according to the expected pollen-flu seasonality. This strengthens the idea that COVID-19 might itself be seasonal, like all other flu-like pandemics since the end of the 19th century. Also when studying other data from RIVM.nl about COVID-19 hospitalizations, we cannot conclude that COVID-19 breaks through the seasonal barrier. For example, new COVID-19 hospitalizations decreased from a peak of 611 on March 27 to just 33 on May 3, the last day of week 18.
Using the three-week moving average (ΔILI3WMA) of changes in flu-like incidence, the correlation coefficients become stronger and are again highly significant for total pollen concentration (r(223) = −0.41, p < 0.00001). The bootstrapped correlation coefficient calculation gives a comparable outcome (r(223) = −0.38, p < 0.0001). As a second sensitivity analysis, we used the reduced dataset (minus the weeks of low pollen activity) and again found similar correlations (r(191) = −0.44, p < 0.0001; bootstrapped r(188) = −0.44, p < 0.0001)). Finally, as a third sensitivity analysis, we analyze each time lag included in the ΔILI3WMA calculation separately. Per individual time lag there are as well highly significant inverse correlations: as given before r(226) = −0.26, p = 0.000063 in case of no time lag (ΔILIt); r(225) = −0.22, p = 0.000713 in case of a time lag of one week (ΔILIt+1); r(225) = −0.23, p = 0.000552 in case of a time lag of two weeks (ΔILIt+2); and the bootstrapped correlations for these are similar. We can thus also reject the null-hypothesis (H20) that there is no inverse relationship between pollen and changes in flu-like incidence including incubation time (ΔILI3WMA or ΔILIt+1 or ΔILIt+2). These correlations (see also Fig. 4 ) are a further indication of temporality and does not contradict the idea that COVID-19 is subject to pollen induced flu-seasonality. The fact that the correlation with ΔILI3WMA is stronger than those for each of the included time lags might be an indication of the noise reduction effect of this moving average, and makes thus the compound effect of the three covered time lags more visible.
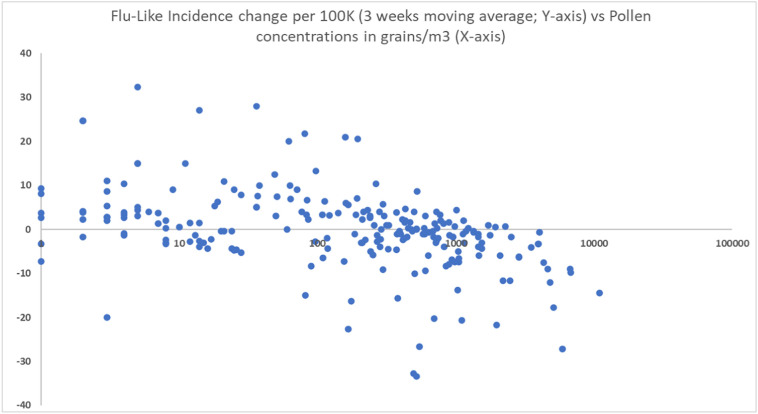
Fig. 4.
Scatter diagram showing the inverse relationship between changes in flu-like incidence (ILI3WMA) and log10(pollen concentrations in grains/m3).
Linear regression analysis shows that pollen has a highly significant inhibitory effect on flu-like incidence change (ΔILI3WMA) of F(1,222) = 37.1, p < 0.001 (see Table 2 , line 1), as a further basis for using total pollen concentration as a predictor. A Log10 transformation of pollen to compensate for visual non-linearity leads to a similar outcome: F(1, 219) = 43.87, p < 0.001 (see Table 2, line 4). At least visually, it is a good fit (see Fig. 4).
Table 2.
Summary of univariate regression analyses of pollen (1), solar radiation (2) and our compound pollen/solar radiation predictor (3) on changes in flu-like incidence (ΔILI3WMA). This shows all highly significant (p < 0.001) results, but the correlation for solar radiation is weak (0.06) and the compound predictor is the strongest (0.23). The log10 (4) analysis is a check on the non-linearity of pollen concentrations in relation to ΔILI3WMA, leading to the same conclusion.
| Δ Flu-change (ΔILI3WMA) | Estimate | 95%CI | Intercept | Multiple R sq. | F-stat on DF | P< |
|---|---|---|---|---|---|---|
| 1. Total pollen per 100/m3 incr. | −0.253 | −0.334 to −0.171 | 1.53 | 0.14 | 37.1 (1, 222) | 0.001 |
| 2. Solar radiation per 100 J/cm2 incr. | −0.312 | −0.475 to −0.153 | 2.98 | 0.06 | 14.43 (1, 222) | 0.001 |
| 3. Compound predictor per incr. of 1 | −3.88 | −4.82 to −2.94 | 4.95 | 0.23 | 65.59 (1, 222) | 0.001 |
| 4. Log10 (Total pollen grains/m3) incr. of 1 | −3.73 | −4.84 to −2.63 | 7.71 | 0.17 | 43.87 (1, 219) | 0.001 |
In line with the correlation between pollen and flu-like incidence, the correlation between total pollen concentration and hay fever is stronger (r(162) = 0.76, p < 0.00001) than it is for allergenic and low-level allergenic pollen individually. This confirms that we can best use total pollen concentration as a predictor. Univariate regression analyses show that total pollen has a highly significant positive effect on hay fever incidence, which in turn has a highly significant inhibitory effect on flu-like incidence (see Table 3 ).
Table 3.
Summary of univariate regression analyses of total pollen concentration on hay fever (p < 0.001) and hay fever on flu-like incidence/100 K citizens per week (p < 0.001), whereby pollen leads to an increase in hay fever, which in turn is associated with a decrease in flu-like incidence. To compensate for non-linearity, the regression of log10(hay fever) on log10(flu-like incidence) is added, with a similar, highly significant outcome.
| Flu-like incidence per 100 K p.w. | Estimate | 95%CI | Intercept | Multiple R sq. | F-stat on DF | P< |
|---|---|---|---|---|---|---|
| Hay fever index per 1% incr. | −0.12677 | −0.181 to −0.0723 | 63.81 | 0.11 | 20.74 (1, 164) | 0.001 |
| Δ Hay Fever Index (2016 = 100) | Estimate | 95%CI | Intercept | Multiple R sq. | F-stat on DF | P< |
| Total pollen per 100/m3 incr. | 5.3049 | 3.922 to 6.688 | 67.78 | 0.26 | 56.52 (1, 164) | 0.001 |
| Log10 (Flu-like incidence per 100 K) | Estimate | 95%CI | Intercept | Multiple R sq. | F-stat on DF | P< |
| Log10(hay fever index) | −0.444 | −0.573 to −0.316 | 2.360 | 0.22 | 45.96 (1, 164) | 0.001 |
Low-level allergenic pollen also has a highly significant effect on hay fever: r(160) = 0.77, p < 0.00001. We can thus reject the null-hypothesis H50 in favor of the alternative hypothesis that low-level allergenic pollen also has a positive effect on hay fever. This might imply that pollen classified as none-to-low-level allergenic might still be responsible for certain allergic effects, and not just the more allergenic pollen. Therefore, trying to use low-level allergenic pollen to discriminate effects outside the allergenic path regarding the immune system might be challenging. There are no significant interaction effects between low-level allergenic pollen concentration and allergenic pollen concentration in relation to hay fever.
The nature of the relationship between hay fever and flu-like incidence might be statistically described as linear. However, it could be better described as logarithmic (Fig. 5 ). In the context of this study, we have interpreted it as a further indication that it could also be described as a threshold-based switching pattern, conforming with the threshold-based approach that we have taken in our compound.
Fig. 5.
Inverse correlation between hay fever and flu-like incidence.
The expected effects of relative humidity (r(223) = −0.86, p < 0.0001), temperature (r(223) = 0.41, p < 0.0001) and solar radiation (r(223) = 0.67, p < 0.0001) on total pollen were found. So sunny, warmer and dry weather does indeed go hand-in-hand with an increase in pollen count. We can therefore reject the null-hypothesis that the selected meteorological variables (H40) have no effect on pollen, whereby relative humidity reduces the amount of aerosol pollen.
Counter to findings in other studies, relative humidity is positively associated with changes in flu-like incidence (ΔILI3WMA) in the Netherlands (r(224) = 0.34, p < 0.00001). The Dutch flu season is cold and humid, and on rainy days the effect of pollen and solar radiation are reduced. Although temperature strongly correlates with flu-like incidence (r(226) = −0.82, p < 0.0001), it has a negligible effect on ΔILI, weekly changes in flu-like incidence (r(224) = −0.02 n.s.), also when corrected for incubation time. Therefore, it seems unlikely that temperature has a direct effect on aerosol flu-like viruses and the life cycle of a flu-like epidemic. In line with this, temperature is also not a good marker for the onset or the end of the flu season. In the Netherlands the end of the flu season (Ro < 1) can coincide with an average temperature that is close to 0 °C and the start of the flu season (Ro > 1) can coincide with temperatures as high as 17 °C.
Of the meteorological variables, only solar radiation has a highly significant inverse correlation with changes in flu-like incidence (ΔILI3WMA): (r(224) = −0.25, p = 0.000156).
Thus, of the meteorological variables, when it comes to solar radiation and relative humidity the null-hypothesis (H40) can also be rejected, as they seem to effect the flu-like epidemic lifecycle. Of these two, only solar radiation is a flu-like inhibitor in line with its positive effect on pollen concentration, its association with immune-activation and the effect that UV has on viruses.
A univariate linear regression also shows the highly significant negative correlation for solar radiation on flu-like incidence change (ΔILI3WMA) (F(1, 222) = 14.43, p < 0.001 (see Table 2, line 2). As the correlation is weak (Multiple R-squared = 0.06), we have interpreted solar radiation as a co-inhibitor in relation to pollen; as a stand-alone independent variable its effect is too weak to explain flu-like seasonality.
Taking into account all these findings, we developed a discrete, compound model in which we included the changes in flu-like incidence (ΔILI3WMA), a threshold value for solar radiation (kr), and both pollen threshold values for allergenic (kap) and total pollen (kp). We found that the compound model has the highest inverse correlation (r(222) = −0.48, p < 0.001) for the following threshold values: kr: 510 J/cm2, kap: 120 allergenic pollen grains/m3, and kp: 610 total pollen grains/m3. The bootstrapped correlation coefficient calculation gives a comparable outcome (r(222) = −0.47, p < 0.0001). In line with the previous outcomes, the inclusion of relative humidity, low-level allergenic pollen or temperature did not improve the correlation strength of this model. Furthermore, given that they showed no significant interaction effects with pollen, it was not necessary to take such interactions into consideration in the model.
In each of the observed years, the now (re)defined pollen thresholds are passed in week 10 (± 5 weeks), depending on meteorological conditions controlling the pollen calendar and coinciding with reaching flu-like peaks, and again in week 33 (± 2 weeks), marking the start of the new flu-like season.
There is a highly significant inverse relationship between our compound threshold-based predictor value with flu-like incidence change (ΔILI3WMA) of F(1, 222) = 65.59, p < 0.001 and a Multiple R-squared correlation of 0.2281 (see Table 2, line 3). This confirms the usefulness of a discrete, pollen and solar radiation threshold-based model as a predictor of switches in flu-like seasonality, whereby the effect of pollen is stronger than that of solar radiation. As a consequence, we can reject the null-hypothesis (H30) that this compound pollen/solar radiation value has no predictive significance for flu-like seasonality.
4. Discussion
First of all we will discuss the possible implications of the results for our theoretic model and alternative explanations. Next, we will discuss our methods.
4.1. Theoretic model
We found highly significant inverse relationships between pollen and solar radiation and (changes in) flu-like incidence: a higher pollen concentration or an increase in solar radiation in the Netherlands is related to a decline in flu-like incidence. This inverse correlation with pollen becomes stronger when the 2019/2020 period is included, which has been increasingly dominated by COVID-19 during the last 9 weeks. Given that more time will be needed to draw conclusions about whether the spread of COVID-19 is seasonal or not, from the data in this study it can only be observed that COVID-19 is not breaking with the flu-like seasonality pattern. Alternatively, social distancing may have contributed to flattening both the flu-like epidemic and COVID-19 pandemic curves at the tail-end of the 2019/2020 flu-like season. The Dutch government imposed hygiene measures from March 9, 2020 onward and a mild form of a lockdown, that included social distancing, from March 11. Such behavioral policies will need to be included in the theoretic model, in addition to pollen and meteorological variables, to understand the relative importance of social distancing versus seasonality. We could, for example, more explicitly include behavioral variables (Gozzi et al., 2020) in the compound model, by rating lockdown regimes on a Likert-type scale [1, 5], from no lockdown (1) to a complete lockdown (5). Although seasonal behavior might be implicitly covered by the meteorological variables, it could still make sense to model them more explicitly as there might be cultural patterns in play – such as holidays or seasonal celebrations – that need to be taken into account.
The highly significant inverse correlation between hay fever and flu-like incidence confirms that allergic rhinitis makes it more difficult for flu-like viruses to propagate.
Solar radiation, the only meteorological variable that has a co-inhibitive effect on changes in flu-like incidence, has a stimulating effect on aerosol pollen formation and is responsible for melatonin-induced immuno-activation. Relative humidity reduces pollen aerosol formation, and correlates positively with flu-like incidence. We did not specifically look at precipitation, but it might make sense to explicitly consider this independent variable, given that it reduces pollen dissemination.
In our study we showed that temperature, aside from the fact that it influences pollen, has no predictive value for changes in flu-like incidence. Therefore, its inverse correlation with flu-like incidence might be interpreted in a number of ways: a) as spurious: the common causal factor is solar radiation, or b) as a stressor that has immediate effects on the functioning of the immune system of already infected persons. When discussing the influence of meteorological variables, we assume that the associated behavioral aspects are covered. These are sometimes summarized as seasonal behavior, but this independent variable might have a cultural dimension that needs to be better understood.
We showed that a compound value, based on threshold values for pollen and solar radiation, results in a stronger correlation with the flu-like lifecycle than the individual inhibitors. This model could form an empirical basis for understanding flu-like seasonality, its Ro and reliably predicting the start and end of each flu-like cycle. Given that behavior, in the form of hygiene and social distancing, is also widely seen as an inhibitor, it might be worthwhile to also include this factor in our compound value. This will probably lead to an even stronger predictor for the evolution of the reproduction number Ro of flu-like epidemics, although this might be beyond explaining the seasonality effect itself.
For as long as the level of herd immunity (Fine et al., 2011) for COVID-19 is still below required thresholds for ending pandemics (Plans-Rubió, 2012), it might make sense to also include indications of herd immunity levels in the theoretic model.
Finally, despite air pollution not been seen as an inhibitor of flu-like incidence (Coccia, 2020), it still might interact with pollen. A more complete theoretic model, controlling for the (interactions with) air pollution, could give more insight in how to interpret the findings of this or similar studies.
4.2. Methodological considerations
In general, statistical research cannot prove causal relationships in uncontrolled environments, even if datasets seem to behave as if there is causality. Such statistics, however, can provide indications and identify reliable predictors, help filter out bad ideas, and be the inspiration for testable hypotheses that can be verified in laboratory and other fully controlled experiments. With a predictor we mean that a reliable temporal relationship between two variables is identified, without yet having validated causality, i.e., a bellwether factor.
Although the datasets seem to be sufficiently representative, there appears to be room for improvement. For example, including the data of more weather stations might help to improve the approximation of the weather conditions the Dutch population experiences on average, and help to distinguish patterns per province. Furthermore, it might be useful to include wind speeds, given that these constitute a vector for the dispersal of pollen in the Netherlands, which has a maritime and temperate climate. Additionally, the effects of climate change on pollen maturation (Frei and Gassner, 2008) might also be an important factor. Another example of improving the representativeness would be by including more pollen types in the particle counts than are currently covered by the current methodology of the European Allergy Network. Further, reclassification or recalibration of pollen types on a rational scale in terms of allergenicity, let's say 0–100%, would be very useful. For example, if pollen types are identified with a reliable score of 0%, these could be used to differentiate a medical versus a non-medical explanatory pathway, including interaction effects. Reversely, the impact of the most allergenic pollen types like Betula and Poaceae could be better understood using such a rational scale.
Specifically, the validity and reliability of the hay fever index is unclear, and the dataset is not maintained. There might be lag effects, but these are unknown. Furthermore, self-prescription of over-the-counter hay fever medication might be based on an erroneous self-diagnosis. It might also be a good idea to include alternative datasets, such as search-engine based trend analysis to generate a complete dataset for a whole period of study. These could be validated separately. Alternatively, representative medical datasets could be obtained.
Finally, the ILI dataset, which is now based on a sample of 40 representative local primary care units, could be improved. For example, including all patients visiting any primary care unit in the Netherlands would reduce the need for extrapolation, with its inherent risk of bias, and information about the allergy status of patients. The use of ΔILI3WMA including incubation time, seems to be a more elegant option than carrying out multiple tests with ΔILI. At the same time, it could be argued that this metric might be better as a two-week forward-looking moving average. This is because it is unlikely that any effects will be noticed in the first week, given the average reporting delays and incubation period.
5. Conclusion
We conclude that pollen and solar radiation both have highly significant inverse correlations with changes in flu-like incidence in the Netherlands. Furthermore, the inverse correlations between pollen and flu-like incidence become stronger when the incubation time is included, and are also manifest in relation to changes in flu-like incidence. A compound variable – based on the thresholds for total pollen, allergenic pollen and solar radiation – shows the strongest correlation with changes in flu-like incidence, and appears to be useful as a predictor for switches in flu-like seasonality.
COVID-19 has dominated the tail-end of the 2019–2020 flu-like season in the Netherlands. And, although COVID-19 appears to be also subject to flu-like seasonality, like pandemics that preceded it, it is still too early to draw conclusions about this.
It will require further research to test the findings, threshold values and predictive model for flu-like seasonality in other countries with different climates. Controlled experiments are needed to deepen the biomedical understanding of how allergic rhinitis and immuno-activation by pollen protects against the spread of flu-like viruses, and to confirm and understand the assumed interaction between pollen and viral bio-aerosol.
CRediT authorship contribution statement
Martijn J. Hoogeveen: Conceptualization, Methodology, Data curation, Formal analysis, Writing - original draft, Investigation, Visualization, Resources. Eric C.M. van Gorp: Conceptualization, Supervision. Ellen K. Hoogeveen: Methodology, Writing - review & editing, Resources.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
Thanks to Sowjanya Putrevu, data scientist at Icecat, for her voluntary support in executing statistical tests.
Editor: Damia Barcelo
References
- Abhimanyu O.P., Coussens A.K. The role of UV radiation and vitamin D in the seasonality and outcomes of infectious disease. Photochem. Photobiol. Sci. 2017;16:314–338. doi: 10.1039/c6pp00355a. [DOI] [PubMed] [Google Scholar]
- Ahmadi M., Sharifi A., Dorosti S., Ghoushchi S.J., Ghanbari N. Investigation of effective climatology parameters on COVID-19 outbreak in Iran. Sci. Total Environ. 2020;729:138705. doi: 10.1016/j.scitotenv.2020.138705. 10 August. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauchau V., Durham S.R. Prevalence and rate of diagnosis of allergic rhinitis in Europe. Eur. Respir. J. 2004;24:758–764. doi: 10.1183/09031936.04.00013904. [DOI] [PubMed] [Google Scholar]
- Brandelius A., Andersson M., Uller L. Topical dsRNA challenges may induce overexpression of airway antiviral cytokines in symptomatic allergic disease. A pilot in vivo study in nasal airways. Respir. Med. 2020;108(12):1816–1819. doi: 10.1016/j.rmed.2014.10.010. [DOI] [PubMed] [Google Scholar]
- Carli G., Cecchi L., Stebbing J., Parronchi P., Farsi A. Is asthma protective against COVID-19? Allergy. 2020 doi: 10.1111/all.14426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong K.C., Liang J., Jia K.M., Kobayashi N., Wang M.W., Wei L., Lau S.Y.F., Sumi A. Latitudes mediate the association between influenza activity and meteorological factors: a nationwide modelling analysis in 45 Japanese prefectures from 2000 to 2018. Sci. Total Environ. 2020;703 doi: 10.1016/j.scitotenv.2019.134727. February. [DOI] [PubMed] [Google Scholar]
- Coccia M. Working Paper CocciaLab n. 48/2020. CNR - National Research Council of Italy; 2020. Diffusion of COVID-19 outbreaks: the interaction between air pollution-to-human and human-to-human transmission dynamics in hinterland regions with cold weather and low average wind speed. [Google Scholar]
- De Weger L.A. Impact of pollen. In: Sofiev M., Bergmann K.C., editors. Allergenic Pollen. Springer; Dordrecht: 2013. [Google Scholar]
- Dong X., Cao Y.-Y., Lu X.-X. Eleven faces of coronavirus disease 2019. Allergy. 2020;75:1699–1709. doi: 10.1111/all.14289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fine P., Eames K., Heymann D.L. “Herd immunity”: a rough guide. Clin. Infect. Dis. 2011;52(7):911–916. doi: 10.1093/cid/cir007. April. [DOI] [PubMed] [Google Scholar]
- Fox S.J., Miller J.C., Meyers L.A. Seasonality in risk of pandemic influenza emergence. PLoS Comput. Biol. 2017;13(10):e1005749. doi: 10.1371/journal.pcbi.1005749. Oct. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frei T., Gassner E. Climate change and its impact on birch pollen quantities and the start of the pollen season an example from Switzerland for the period 1969–2006. Int. J. Biometeorol. 2008;52:667. doi: 10.1007/s00484-008-0159-2. [DOI] [PubMed] [Google Scholar]
- Gilles S., Blume C., Wimmer M. Pollen exposure weakens innate defense against respiratory viruses. Allergy. 2019;75:576–587. doi: 10.1111/all.14047. [DOI] [PubMed] [Google Scholar]
- Gozzi N., Perrotta D., Paolotti D., Perra N. Cornell University; 2020. Towards a Data-driven Characterization of Behavioral Changes Induced by the Seasonal Flu. arXiv:2002.00671 [physics.soc-ph] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guarnieri M., Speranza A., Nepi M. Ripe pollen carbohydrate changes in Trachycarpus fortunei: the effect of relative humidity. Sex. Plant Reprod. 2006;19:117. doi: 10.1007/s00497-006-0027-3. [DOI] [Google Scholar]
- Hoogeveen M.J. Pollen likely seasonal factor in inhibiting flu-like epidemics. A Dutch study into the inverse relation between pollen counts, hay fever and flu-like incidence 2016–2019. Sci. Total Environ. 2020;727:2020. doi: 10.1016/j.scitotenv.2020.138543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howarth P.H. Allergic rhinitis: not purely a histamine-related disease. European Journal of Allergy and Clinical Immunology. 2000;55(s64):7–16. doi: 10.1034/j.1398-9995.2000.00802.x. [DOI] [PubMed] [Google Scholar]
- Jackson D.J., Busse W.W., Bacharier L.B. Association of respiratory allergy, asthma, and expression of the SARS-CoV-2 receptor, ACE2. J. Allergy Clin. Immunol. 2020 doi: 10.1016/j.jaci.2020.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kissler S.M., Tedijanto C., Goldstein E., Grad Y.H., Lipsitch M. Projecting the transmission dynamics of SARS-CoV-2 through the post-pandemic period. Science. 2020 doi: 10.1126/science.abb5793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klemens C., Rasp G., Jund F., Hilgert E., Devens C., Pfrogner E., Kramer M.F. Mediators and cytokines in allergic and viral-triggered rhinitis. Allergy & Asthma Proceedings. 2007;28(4):434–441. doi: 10.2500/aap.2007.28.3017. Jul/Aug. [DOI] [PubMed] [Google Scholar]
- Licari A., Votto M., Brambilla I., Castagnoli R., Piccotti E., Olcese R., Tosca M.A., Ciprandi G., Marseglia G.L. Allergy and asthma in children and adolescents during the COVID outbreak: what we know and how we could prevent allergy and asthma flares. Allergy. 2020 doi: 10.1111/all.14369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsley A.W., Schwartz J.T., Rothenberg M.E. Eosinophil responses during COVID-19 infections and coronavirus vaccination. J. Allergy Clin. Immunol. 2020 doi: 10.1016/j.jaci.2020.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Gayle A.A., Wilder-Smith A., Rocklöv J. The reproductive number of COVID-19 is higher compared to SARS coronavirus. Journal of Travel Medicine. 2020;27(2) doi: 10.1093/jtm/taaa021. March. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y., Zhao Y., Liu J., He X., Wang B., Fu S., Yan J., Niu J., Zhou J., Luo B. Effects of temperature variation and humidity on the death of COVID-19 in Wuhan, China. Sci. Total Environ. 2020;724:138226. doi: 10.1016/j.scitotenv.2020.138226. 1 July. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marín-Hernández D., Schwartz R.E., Nixon D.F. Epidemiological evidence for association between higher influenza vaccine uptake in the elderly and lower COVID-19 deaths in Italy. J. Med. Virol. 2020 doi: 10.1002/jmv.26120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriyama M., Hugentobler W.J., Iwasaki A. Seasonality of respiratory viral infections. Annual Review of Virology. 2020;7(1) doi: 10.1146/annurev-virology-012420-022445. [DOI] [PubMed] [Google Scholar]
- Nivel.nl Nivel zorgregistraties eerste lijn – Surveillance – wekelijks bulletin over symptomen en aandoeningen op basis van gegevens van huisartsen, version June 17, 2020. 2020. https://www.nivel.nl/sites/default/files/algemene-content/NivelSurveillance_2020_wk24b.pdf
- Nosal C., Ehlers A., Haspel J.A. Why lungs keep time: circadian rhythms and lung immunity. Annu. Rev. Physiol. 2020;82(1):391–412. doi: 10.1146/annurev-physiol-021119-034602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plans-Rubió P. The vaccination coverage required to establish herd immunity against influenza viruses. Prev. Med. 2012;55(1):72–77. doi: 10.1016/j.ypmed.2012.02.015. July. [DOI] [PubMed] [Google Scholar]
- Postnikov E. Dynamical prediction of flu seasonality driven by ambient temperature: influenza vs. common cold. Eur. Phys. J. B. 2016;89:13. doi: 10.1140/epjb/e2015-50845-7. [DOI] [Google Scholar]
- Qi H., Xiao S., Shi R., Ward M.P., Chen Y., Tu W., Su Q., Wang W., Wang X., Zhang Z. COVID-19 transmission in Mainland China is associated with temperature and humidity: a time-series analysis. Sci. Total Environ. 2020;728:138778. doi: 10.1016/j.scitotenv.2020.138778. 1 August. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenwasser L.J. Current understanding of the pathophysiology of allergic rhinitis. Immunology and Allergy Clinics. 2011;31(3):433–439. doi: 10.1016/j.iac.2011.05.009. [DOI] [PubMed] [Google Scholar]
- Sajadi M.M., Habibzadeh P., Vintzileos A., Shokouhi S., Miralles-Wilhelm F., Amoroso A. 2020. Temperature, Humidity and Latitude Analysis to Predict Potential Spread and Seasonality for COVID-19. March 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuit M., Gardner S., Wood S., Bower K., Williams G., Freeburger D., Dabisch P. The influence of simulated sunlight on the inactivation of influenza virus in aerosols. J. Infect. Dis. 2020;221(3):372–378. doi: 10.1093/infdis/jiz582. February. [DOI] [PubMed] [Google Scholar]
- Shaman J., Goldstein E., Lipsitch M. Absolute humidity and pandemic versus epidemic influenza. Am. J. Epidemiol. 2011;173(2):127–135. doi: 10.1093/aje/kwq347. January 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soebiyanto R.P., Clara W., Jara J., Castillo L., Sorto O.R., Marinero S., Barnett de Antinori M.E., McCracken J.P., Widdowson M.A., 9 Azziz-Baumgartner E., Kiang R.K. The role of temperature and humidity on seasonal influenza in tropical areas: Guatemala, El Salvador and Panama, 2008–2013. PLoS One. 2014;9(6):e100659. doi: 10.1371/journal.pone.0100659. Published online 2014 Jun 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamerius J., Nelson M.I., Zhou S.Z., Viboud C., Miller M.A., Alonso W.J. Global influenza seasonality: reconciling patterns across temperate and tropical regions. Environ. Health Perspect. 2011;119(4):439–445. doi: 10.1289/ehp.1002383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan D.X., Ruegiger Hardeland R. Potential utility of melatonin in deadly infectious diseases related to the overreaction of innate immune response and destructive inflammation: focus on COVID-19. Melatonin in deadly virus infection. Melatonin Research. 2020;3:1. doi: 10.32794/mr11250052. [DOI] [Google Scholar]
- Tosepu R., Gunawan J., Effendy D.S., Ahmad L.O.A.I., Lestari H., Bahar H., Asfiang P. Correlation between weather and Covid-19 pandemic in Jakarta, Indonesia. Sci. Total Environ. 2020;725:138436. doi: 10.1016/j.scitotenv.2020.138436. 10 July. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van De Ven M.O.M., Van Den Eijnden R.J.J.M., Engels R.C.M.E. Atopic diseases and related risk factors among Dutch adolescents. Eur. J. Pub. Health. 2006;16(5):549–558. doi: 10.1093/eurpub/ckl022. October. [DOI] [PubMed] [Google Scholar]
- Wan Y. J. Virol. Receptor Recognition by the Novel Coronavirus From Wuhan: An Analysis Based on Decade-long Structural Studies of SARS Coronavirus. 2020. https://jvi.asm.org/content/94/7/e00127-20.long [Epub ahead of print] [DOI] [PMC free article] [PubMed]
- Xie J., Zhu Y. Association between ambient temperature and COVID-19 infection in 122 cities from China. Sci. Total Environ. 2020;724:138201. doi: 10.1016/j.scitotenv.2020.138201. 1 July. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao Y., Pan J.H., Liu Z.X., Meng X., Wang W.D., Kan H.D., Wang W.B. No association of COVID-19 transmission with temperature or UV radiation in Chinese cities. Eur. Respir. J. 2020;2020 doi: 10.1183/13993003.00517-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J.-J., Dong X., Cao Y.-Y. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy. 2020;00:1–12. doi: 10.1111/all.14238. [DOI] [PubMed] [Google Scholar]