Abstract
Background
This study reports the accelerometer-based physical activity (PA) and sedentary behavior (SB) before and during the COVID-19 pandemic in hypertensive older adults.
Methods
Thirty-five hypertensive older adults were included in this observational study. Accelerometer-based PA and SB measures were assessed before (January to March 2020) and during (June 2020) the COVID-19 pandemic. Linear mixed models were used to assess within-group changes in PA and SB measures, adjusted by accelerometer wear time.
Results
Before COVID-19 pandemic participants presented: 5809 steps/day (SE = 366), 303.1 min/day (SE = 11.9) of light PA, 15.5 min/day (SE = 2.2) of moderate-vigorous PA, and 653.0 min/day (SE = 12.6) of SB. During COVID-19 pandemic there was a decrease in steps/day (β = −886 steps/day, SE = 361, p = 0.018), in moderate-vigorous PA (β = −2.8 min/day, SE = 2.4, p = 0.018), and a trend in light PA (β = −26.6 min/day, SE = 13.4, p = 0.053). In addition, SB increased during the COVID-19 pandemic (β = 29.6 min/day, SE = 13.4, p = 0.032). The magnitude of changes was greater on the weekend, mainly for steps/day (β = −1739 steps/day, SE = 424, p < 0.001) and the SB pattern (more time spent in bouts of ≥10 and 30 min, less breaks/day and breaks/h).
Conclusions
The COVID-19 pandemic may elicit unhealthy changes in movement behavior in hypertensive older adults. Lower PA, higher and more prolonged SB on the weekend are the main features of the behavioral changes.
Keywords: Coronavirus, Hypertension, Aging, Movement behavior, Walking, Sitting
Highlights
-
•
Unhealthy changes in movement behavior were observed during the COVID-19 pandemic.
-
•
Hypertensive older adults decreased steps/day and time spent in physical activities.
-
•
An increase in time spent in sedentary behavior (SB) was observed.
-
•
The time spent in prolonged SB increased and the breaks in SB decreased.
-
•
Greater unhealthy changes in movement behavior occurred on weekend.
1. Introduction
The coronavirus disease (COVID-19) pandemic has completed nine months since the outbreak in Wuhan, China. To date, it has infected almost 33 million individuals and caused more than 991 thousand deaths across the globe (World Health Organization, 2020). Older age and cardiovascular risk factors such as hypertension and diabetes are associated with an increased risk of infection and severity of COVID-19 (Espinosa et al., 2020; Yang et al., 2020). Comprehensive social distancing policies, which include closing schools, prohibiting agglomerations, travelling restrictions, and staying-at-home recommendations, have been adopted by several countries to prevent introducing the disease to new areas or reduce human-to-human transmission in areas where COVID-19 is already circulating (Aquino et al., 2020). Despite the benefits of social distancing as a public health strategy during the COVID-19 pandemic, its consequences on daily lifestyle have negatively impacted the cardiovascular (Mattioli et al., 2020) and mental health of individuals (Dubey et al., 2020).
Although changes in physical activity (PA) and sedentary behavior (SB) are expected (Hall et al., 2020), the impact of COVID-19 pandemic on objectively measured PA and SB is still unclear. For older adults with hypertension who represent a high-risk group (Espinosa et al., 2020; Yang et al., 2020), the description of movement behavior during the COVID-19 pandemic may play a pivotal role for better understanding their health-related consequences and guide feasible future preventive and therapeutic actions. Herein, we report the accelerometer-based PA and SB measures before and during the COVID-19 pandemic in hypertensive older adults involved in an interrupted clinical trial.
2. Materials and methods
2.1. Participants and study design
This observational study was approved by the Research Ethics Board at the Onofre Lopes University Hospital (protocol 4.005.835/2020). The study was conducted according to the Declaration of Helsinki. The participants were informed about all study procedures and provided written informed consent. A sample of Brazilian hypertensive older adults who were screened for the Hypertension EXercise Approaches study (HEXA study; for more details: http://ensaiosclinicos.gov.br/rg/RBR-4ntszb/) were assessed before and during a period of social distancing policy imposed due to the COVID-19 pandemic. This study was conducted in the city of Natal, the capital city of the state of Rio Grande do Norte, Brazil. A total of 41 participants were screened for the HEXA study before the social distancing period (from January 24th to March 15th, 2020), including accelerometer-based PA and SB measures. The HEXA study was interrupted immediately after the first case of COVID-19 in the city of Natal on March 12th, 2020, following the recommendations of the state authorities and the Federal University of Rio Grande do Norte. All participants have been receiving weekly phone calls from the research staff since March 17th to be monitored regarding COVID-19 symptoms. The participants were invited by phone calls to participate in the present study after a 10-week period from the first case of COVID-19 in the city of Natal. All participants who agreed to participate in this study received sterilized accelerometers in their homes between June 1st and June 3rd, 2020, to be used during a one-week period. When the HEXA study was interrupted on March 17th, the national epidemiological bulletin reported 291 cases and one death by COVID-19 in Brazil, and one case in the city of Natal (0 deaths) (Brazil. Ministry of Health, 2020; Laboratory of Technological Innovation in Health, 2020). On June 1st, the first day of the accelerometer-based PA and SB measures, the national epidemiological bulletin reported 526,447 cases in Brazil (29,937 deaths) and 3103 cases in the city of Natal (105 deaths) (Brazil. Ministry of Health, 2020; Laboratory of Technological Innovation in Health, 2020). On June 4th, the governor of the state of Rio Grande do Norte implemented a more rigid social distancing policy, especially for older adults and high-risk individuals. Therefore, the accelerometer-based PA and SB measures occurred during a period of high mobility restriction in the public areas within the city of Natal and with a clear ‘stay-at-home’ message for population.
2.2. Physical activity and sedentary behavior measures
Accelerometer-based PA and SB measures were assessed by the Actigraph GT3X (Actigraph LLC, Pensacola, USA). Participants wore a hip accelerometer during seven consecutive days, including awake and asleep periods. They also filled out a diary describing the time they took off the accelerometer during the awake period, went to bed to sleep and wake up. A sampling rate of 60 Hz with epoch of 60 s was used. Non-wearing time was defined as ≥90 consecutive minutes of zero counts with a tolerance of up to 2 min of ≥100 cpm (Choi et al., 2011). Participants with at least three valid days of accelerometer wear time (≥600 min/day) with at least one weekend day were included in the data analysis (Trost et al., 2005). The cut-offs to define SB, light PA, and moderate-vigorous PA were: 0–99 cpm, 100–1951 cpm, ≥1952 cpm, respectively (Freedson et al., 1998; Matthews et al., 2008). Accelerometer-based PA and SB measures were analyzed as total period (weighted average; weekdays and weekend), weekdays and weekend using the ActiLife version 6.13.3.2 software program. The following variables were considered for data analysis: steps/day; time spent in SB, light PA, and moderate-vigorous PA (min/day and accelerometer wear time %); bouts ≥10 and 30 consecutive minutes of SB, light PA, moderate-vigorous PA (min/day and bouts/day); length of sedentary bouts (min/day; defined as time spent in SB ÷ number of sedentary bouts ≥1 min); sedentary breaks of ≥1 and ≥5 min (breaks/day; defined as ≥100 cpm following a sedentary bout); break rate (breaks/h; defined as number of breaks ÷ accelerometer wear time) (Boerema et al., 2020).
2.3. Statistical analysis
Descriptive data are presented as mean ± standard deviation, absolute and relative frequencies. Linear mixed models with subject as a random effect and time and covariates as fixed effects were used to assess within-group changes in accelerometer-based PA and SB measures, controlling for the following covariates: a) only by accelerometer wear time; b) age, sex and accelerometer wear time. The subject was included as a random effect due to the high intra-subject variability, as observed by the intra-subject intraclass correlation coefficient (ICC) between 0.26 and 0.64 for volume-related variables of SB and PA and by the intra-subject ICC between 0.08 and 0.73 for pattern-related variables of SB and PA, respectively. Maximum likelihood estimation was used to calculate the coefficient estimates (β), estimated marginal means (EMM), standard error (SE), and 95% confidence interval (CI). Residuals distribution was verified using the normal Q-Q plot. The variables that were not normally distributed were square root transformed. In these cases, the model analysis was performed only with the transformed variables, but the original descriptive results were presented for a better clinical interpretation. A two-tailed p < 0.05 was considered statistically significant for all analyses. Statistical analyses were performed using IBM SPSS Statistics for Win/v.25.0 (IBM Corp., Armonk, NY).
3. Results
3.1. Sample characteristics
Five participants declined to participate in the study (three for personal reasons, one was sick with dizziness symptoms, and one was in quarantine due to direct contact with a relative infected by COVID-19). One participant was excluded due to a technical issue with the accelerometer. Thus, a total of 35 participants were included in the final analysis. Table 1 shows the characteristics of the included participants. Most participants were women (65.7%) and married (48.6%). All participants were taking antihypertensive medication(s). In addition, most participants had dyslipidemia (51.4%) and were overweight/obese (48.6%). Approximately one-third had type 2 diabetes (34.3%) and one-fourth were ex-smokers (22.9%).
Table 1.
Characteristics of the participants (n = 35).
| Mean ± SD or n (%) | |
|---|---|
| Age, yrs | 65.6 ± 3.8 |
| Females, n (%) | 23 (65.7) |
| Married, n (%) | 17 (48.6) |
| Living alone, n (%) | 5 (14.3) |
| Post-secondary education, n (%) | 10 (28.6) |
| Body mass index, kg/m2 | 27.3 ± 3.4 |
| Resting SBP, mmHg | 134.3 ± 18.3 |
| Resting DBP, mmHg | 74.1 ± 9.4 |
| Resting HR, bpm | 71.1 ± 12.0 |
| Hypertension diagnosis, yrs | 12.1 ± 9.1 |
| Risk factors, n (%) | |
| Diabetes | 12 (34.3) |
| Dyslipidemia | 18 (51.4) |
| Overweight | 12 (34.3) |
| Obesity | 5 (14.3) |
| Ex-smoker | 8 (22.9) |
| Medication, n (%) | |
| ACE inhibitor | 5 (14.3) |
| Angiotensin-receptor antagonists | 26 (74.3) |
| Calcium-channel blocker | 7 (20.0) |
| Diuretics | 12 (34.3) |
| Beta-blockers | 9 (25.7) |
| Statins | 13 (37.1) |
| Hypoglycemics | 14 (40.0) |
| Anxiolytic/antidepressant | 10 (28.6) |
Values are expressed as mean ± standard deviation (SD) or absolute (n) and relative frequencies (%).
Abbreviations: ACE, angiotensin converting enzyme; DBP, diastolic blood pressure; HR, heart rate; SBP, systolic blood pressure.
All participants had 6–7 valid days of accelerometer use before and during the COVID-19 pandemic. Accelerometer wear time before and during the COVID-19 pandemic were: total period (weighted average), 997.7 ± 71.1 vs. 945.7 ± 71.2 min/day (p = 0.003); weekdays, 1011.6 ± 79.3 vs. 953.3 ± 77.4 min/day (p = 0.002); weekend, 963.0 ± 117.8 vs. 927.3 ± 78.2 min/day (p = 0.134).
3.2. Volume of physical and sedentary behavior
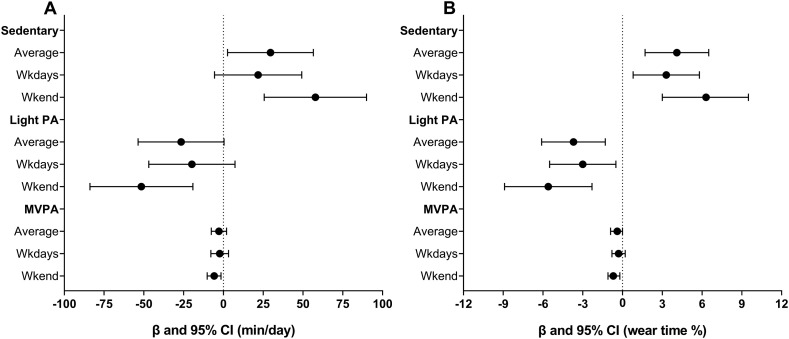
The PA and SB volumes before and during the COVID-19 pandemic are shown in Table 2 and Fig. 1 . Considering the total period (weekdays and weekend), there was a significant increase in SB (β = 29.6 min/day, p = 0.032), a decrease in steps/day (β = −886, p = 0.018), moderate-vigorous PA (β = −2.8 min/day, p = 0.018), and a trend for light PA (β = −26.6 min/day, p = 0.053). Similar results were observed in the models using the accelerometer wear time %. There was a significant decrease in moderate-vigorous PA (β = −2.3 min/day, p = 0.030) and a decreasing trend for steps/day (β = −721, p = 0.081) on weekdays. No significant changes were observed for SB and light PA. However, there was a significant increase in SB and a decrease in light PA and moderate-vigorous PA in the models using the accelerometer wear time %. Greater changes in movement behavior were observed on the weekend. There was a significant increase in SB (β = 57.8 min/day, p < 0.001) and a decrease in light PA (β = −51.6 min/day, p = 0.003) and moderate-vigorous PA (β = −5.8 min/day, p = 0.003) in both models (min/day and accelerometer wear time %). There was also a significant decrease in steps/day on the weekend (β = −1739, p < 0.001). In addition, it was tested the within-group changes in PA and SB volumes controlling for age and sex (Supplementary Table 1). After the inclusion of these covariates, the results remained unchanged.
Table 2.
Volume of physical and sedentary behavior before and during the COVID-19 pandemic in hypertensive older adults (n = 35).
| Before |
During |
βa | SE | 95% CI | p-Value | |
|---|---|---|---|---|---|---|
| EMM (95% CI) | EMM (95% CI) | |||||
| Sedentary behavior | ||||||
| Average | ||||||
| Sedentary time, min/day | 653.0 (627.7, 678.3) | 682.6 (657.3, 707.9) | 29.6 | 13.4 | 2.6, 56.5 | 0.0321 |
| Sedentary, wear time % | 66.8 (64.2, 69.4) | 70.9 (68.3, 73.6) | 4.1 | 1.2 | 1.7, 6.5 | 0.0013 |
| Weekdays | ||||||
| Sedentary time, min/day | 665.7 (640.5, 691.0) | 687.6 (662.3, 712.8) | 21.8 | 13.6 | −5.6, 49.2 | 0.1156 |
| Sedentary, wear time % | 67.4 (64.8, 69.9) | 70.7 (68.1, 73.2) | 3.3 | 1.2 | 0.8, 5.8 | 0.0106 |
| Weekend | ||||||
| Sedentary time, min/day | 616.8 (586.6, 647.1) | 674.6 (644.3, 704.8) | 57.8 | 15.9 | 25.6, 89.9 | 0.0009 |
| Sedentary, wear time % | 65.4 (62.2, 68.6) | 71.7 (68.4, 74.9) | 6.3 | 1.6 | 3.0, 9.5 | 0.0004 |
| Physical activity | ||||||
| Average | ||||||
| Steps/day | 5809 (5075, 6543) | 4922 (4188, 5656) | −886 | 361 | −1612, −161 | 0.0177 |
| Light PA, min/day | 303.1 (279.3, 326.9) | 276.5 (252.7, 300.4) | −26.6 | 13.4 | −53.6, 0.4 | 0.0531 |
| Light PA, wear time % | 31.6 (29.1, 34.0) | 27.8 (25.4, 30.3) | −3.7 | 1.2 | −6.1, −1.3 | 0.0038 |
| MVPA, min/day | 15.5 (10.5, 20.5) | 12.7 (7.7, 17.7) | −2.8 | 2.4 | −7.6, 2.0 | – |
| Sqrt MVPA, min/day | 3.6 (3.0, 4.2) | 2.8 (2.2, 3.5) | −0.8 | 0.3 | −1.4, −0.1 | 0.0181 |
| MVPA, wear time % | 1.6 (1.1, 2.1) | 1.2 (0.7, 1.7) | −0.4 | 0.2 | −0.9, 0.0 | – |
| Sqrt MVPA, wear time % | 1.2 (1.0, 1.4) | 0.9 (0.7, 1.1) | −0.3 | 0.1 | −0.5, −0.1 | 0.0015 |
| Weekdays | ||||||
| Steps/day | 5887 (5113, 6660) | 5165 (4391, 5939) | −721 | 406 | −1538, 95 | 0.0819 |
| Light PA, min/day | 300.6 (276.7, 324.5) | 280.8 (256.9, 304.6) | −19.8 | 13.5 | −46.9, 7.3 | 0.1480 |
| Light PA, wear time % | 31.0 (28.6, 33.4) | 28.0 (25.6, 30.4) | −3.0 | 1.2 | −5.5, −0.5 | 0.0197 |
| MVPA, min/day | 16.2 (10.9, 21.5) | 13.9 (8.6, 19.2) | −2.3 | 2.8 | −7.9, 3.2 | – |
| Sqrt MVPA, min/day | 3.7 (3.1, 4.4) | 3.0 (2.3, 3.6) | −0.8 | 0.3 | −1.5, −0.1 | 0.0301 |
| MVPA, wear time % | 1.7 (1.2, 2.2) | 1.4 (0.8, 1.9) | −0.3 | 0.2 | −0.8, 0.2 | – |
| Sqrt MVPA, wear time % | 1.2 (1.0, 1.4) | 0.9 (0.7, 1.1) | −0.3 | 0.1 | −0.5, −0.1 | 0.0062 |
| Weekend | ||||||
| Steps/day | 5839 (5046, 6632) | 4100 (3307, 4893) | −1739 | 424 | −2599, −879 | 0.0002 |
| Light PA, min/day | 313.7 (285.0, 342.4) | 262.1 (233.4, 290.8) | −51.6 | 15.9 | −83.9, −19.2 | 0.0026 |
| Light PA, wear time % | 33.1 (30.1, 36.2) | 27.5 (24.4, 30.5) | −5.6 | 1.6 | −8.9, −2.3 | 0.0014 |
| MVPA, min/day | 14.5 (9.6, 19.4) | 8.6 (3.8, 13.5) | −5.8 | 2.1 | −10.2, −1.5 | – |
| Sqrt MVPA, min/day | 3.2 (2.5, 3.9) | 2.1 (1.5, 2.8) | −1.1 | 0.3 | −1.8, −0.4 | 0.0030 |
| MVPA, wear time % | 1.6 (1.0, 2.1) | 0.9 (0.4, 1.4) | −0.7 | 0.2 | −1.1, −0.2 | – |
| Sqrt MVPA, wear time % | 1.1 (0.9, 1.3) | 0.7 (0.5, 0.9) | −0.4 | 0.1 | −0.6, −0.2 | 0.0015 |
Values are expressed as estimated marginal means (EMM), coefficient estimates (β), standard error (SE), and 95% confidence interval (CI).
Bold values indicate significance at p < 0.05.
Abbreviations: PA, physical activity; MVPA, moderate to vigorous physical activity; Sqrt, square root.
Model adjusted for accelerometer wear time, except for the models of measures of wear time %.
Fig. 1.
Change in volume of physical activity and sedentary behavior during the COVID-19 pandemic in hypertensive older adults (n = 35). Panel A: physical activity and sedentary behavior volume in minutes per day. Panel B: physical activity and sedentary behavior volume in accelerometer wear time %. Values are expressed as coefficient estimates (β) and 95% confidence interval (CI). MVPA, moderate to vigorous physical activity; PA, physical activity.
3.3. Pattern of physical and sedentary behavior
The PA and SB patterns before and during the COVID-19 pandemic are shown in Table 3 . Considering the total period, there was a significant increase in sedentary bouts ≥10 and 30 min (β = 42.0 min/day; β = 37.7 min/day, respectively; p < 0.05) and the length of sedentary bouts. Also, there was a decrease in the number of breaks ≥1 and 5 min from SB (β = −6.0 breaks/day; β = −2.3 breaks/day, respectively; p < 0.05) and the break rate. There was a significant increase in sedentary bouts ≥30 min (β = 33.4 min/day, p = 0.023) and the length of sedentary bouts, as well as a decrease in the break rate on weekdays. The greater changes in the SB pattern were observed on the weekend. There was a significant increase in sedentary bouts ≥10 and 30 min (β = 73.9 min/day; β = 54.4 min/day, respectively; p < 0.05) and the length of sedentary bouts. In addition, there was a decrease in the number of breaks ≥1 and 5 min from SB (β = −8.0 breaks/day; β = −4.6 breaks/day, respectively; p < 0.05) and the break rate. No significant changes were observed in the PA pattern on weekdays or on the weekend. In addition, it was tested the within-group changes in PA and SB patterns controlling for age and sex (Supplementary Table 2). After the inclusion of these covariates, the results remained unchanged.
Table 3.
Pattern of physical and sedentary behavior before and during the COVID-19 pandemic in hypertensive older adults (n = 35).
| Before |
During |
βa | SE | 95% CI | p-Value | |
|---|---|---|---|---|---|---|
| EMM (95% CI) | EMM (95% CI) | |||||
| Sedentary behavior | ||||||
| Average | ||||||
| Sedentary bouts ≥10 min, bouts/day | 18.7 (17.6, 19.8) | 19.8 (18.7, 20.8) | 1.1 | 0.6 | −0.1, 2.3 | 0.0783 |
| Sedentary bouts ≥30 min, bouts/day | 3.7 (3.2, 4.3) | 4.4 (3.9, 4.9) | 0.7 | 0.3 | 0.1, 1.2 | 0.0138 |
| Sedentary time bouts ≥10 min, min/day | 410.9 (378.5, 443.3) | 452.9 (420.5, 485.3) | 42.0 | 16.8 | 8.3, 75.8 | 0.0158 |
| Sedentary time bouts ≥30 min, min/day | 170.3 (143.2, 197.5) | 208.0 (180.8, 235.1) | 37.7 | 12.1 | 13.2, 62.1 | 0.0033 |
| Length of sedentary bouts, min/day | 6.3 (5.8, 6.8) | 7.2 (6.7, 7.7) | 0.9 | 0.2 | 0.4, 1.3 | 0.0006 |
| Breaks ≥1 min, breaks/day | 104.3 (99.8, 108.8) | 98.3 (93.8, 102.8) | −6.0 | 2.4 | −10.8, −1.2 | 0.0157 |
| Breaks ≥5 min, breaks/day | 17.9 (15.8, 19.9) | 15.6 (13.5, 17.6) | −2.3 | 1.0 | −4.4, −0.2 | 0.0294 |
| Break rate ≥ 1 min, breaks/h | 6.4 (6.2, 6.7) | 6.1 (5.8, 6.3) | −0.3 | 0.1 | −0.6, −0.1 | 0.0097 |
| Break rate ≥ 5 min, breaks/h | 1.1 (1.0, 1.3) | 0.9 (0.8, 1.0) | −0.2 | 0.1 | −0.3, −0.1 | 0.0004 |
| Weekdays | ||||||
| Sedentary bouts ≥10 min, bouts/day | 19.2 (18.1, 20.3) | 19.7 (18.6, 20.8) | 0.5 | 0.6 | −0.8, 1.8 | 0.4177 |
| Sedentary bouts ≥30 min, bouts/day | 3.8 (3.2, 4.4) | 4.4 (3.9, 5.0) | 0.6 | 0.3 | 0.0, 1.2 | 0.0415 |
| Sedentary time bouts ≥10 min, min/day | 421.2 (387.7, 454.7) | 451.8 (418.3, 485.3) | 30.6 | 17.4 | −4.3, 65.6 | 0.0846 |
| Sedentary time bouts ≥30 min, min/day | 175.2 (147.0, 203.3) | 208.6 (180.4, 236.7) | 33.4 | 14.3 | 4.7, 62.1 | 0.0234 |
| Length of sedentary bouts, min/day | 6.4 (5.9, 6.9) | 7.1 (6.6, 7.6) | 0.7 | 0.2 | 0.2, 1.2 | 0.0054 |
| Breaks ≥1 min, breaks/day | 105.1 (100.3, 109.8) | 100.1 (95.4, 104.8) | −5.0 | 2.5 | −10.1, 0.2 | 0.0573 |
| Breaks ≥5 min, breaks/day | 17.9 (15.8, 19.9) | 16.2 (14.1, 18.3) | −1.6 | 1.1 | −3.8, 0.6 | 0.1416 |
| Break rate ≥ 1 min, breaks/h | 6.4 (6.1, 6.7) | 6.1 (5.8, 6.4) | −0.3 | 0.1 | −0.6, −0.02 | 0.0370 |
| Break rate ≥ 5 min, breaks/h | 1.1 (1.0, 1.3) | 0.9 (0.8, 1.1) | −0.2 | 0.1 | −0.3, −0.1 | 0.0046 |
| Weekend | ||||||
| Sedentary bouts ≥10 min, bouts/day | 17.6 (16.2, 18.9) | 19.8 (18.5, 21.2) | 2.3 | 0.8 | 0.6, 3.9 | 0.0085 |
| Sedentary bouts ≥30 min, bouts/day | 3.5 (2.8, 4.1) | 4.4 (3.8, 5.1) | 1.0 | 0.4 | 0.2, 1.8 | 0.0137 |
| Sedentary time bouts ≥10 min, min/day | 383.4 (346.4, 420.5) | 457.3 (420.3, 494.3) | 73.9 | 20.8 | 31.7, 116.0 | 0.0011 |
| Sedentary time bouts ≥30 min, min/day | 155.4 (121.7, 189.1) | 209.8 (176.1, 243.5) | 54.4 | 17.9 | 18.2, 90.7 | 0.0044 |
| Length of sedentary bouts, min/day | 6.2 (5.6, 6.8) | 7.5 (6.9, 8.1) | 1.3 | 0.4 | 0.5, 2.0 | 0.0018 |
| Breaks ≥1 min, breaks/day | 102.0 (96.8, 107.2) | 94.0 (88.8, 99.2) | −8.0 | 3.5 | −15.1, −0.9 | 0.0284 |
| Breaks ≥5 min, breaks/day | 18.3 (16.0, 20.7) | 13.7 (11.3, 16.1) | −4.6 | 1.2 | −7.0, −2.2 | 0.0003 |
| Break rate ≥ 1 min, breaks/h | 6.5 (6.1, 6.8) | 6.0 (5.6, 6.3) | −0.5 | 0.2 | −0.9, −0.05 | 0.0310 |
| Break rate ≥ 5 min, breaks/h | 1.2 (1.0, 1.3) | 0.9 (0.7, 1.0) | −0.3 | 0.1 | −0.4, −0.2 | 0.0001 |
| Physical activity | ||||||
| Average | ||||||
| Light PA in bouts ≥10 min, bouts/day | 4.0 (3.2, 4.8) | 3.8 (3.0, 4.6) | −0.2 | 0.4 | −1.1, 0.7 | 0.6973 |
| Light PA in bouts ≥10 min, min/day | 60.9 (47.6, 74.3) | 56.6 (43.3, 70.0) | −4.3 | 7.5 | −19.4, 10.8 | – |
| Sqrt Light PA in bouts ≥10 min, min/day | 7.4 (6.5, 8.3) | 6.9 (6.1, 7.8) | −0.5 | 0.5 | −1.4, 0.5 | 0.3164 |
| MVPA in bouts ≥10 min, bouts/day | 0.1 (0.0, 0.2) | 0.2 (0.1, 0.3) | 0.1 | 0.1 | −0.1, 0.2 | – |
| Sqrt MVPA in bouts ≥10 min, bouts/day | 0.2 (0.1, 0.3) | 0.2 (0.1, 0.4) | 0.0 | 0.1 | −0.1, 0.1 | 0.7545 |
| MVPA in bouts ≥10 min, min/day | 2.6 (−0.4, 5.6) | 4.6 (1.5, 7.6) | 2.0 | 1.4 | −0.9, 4.9 | – |
| Sqrt MVPA in bouts ≥10 min, min/day | 0.9 (0.4, 1.5) | 1.1 (0.5, 1.6) | 0.2 | 0.3 | −0.4, 0.7 | 0.5916 |
| Weekdays | ||||||
| Light PA in bouts ≥10 min, bouts/day | 3.9 (3.1, 4.7) | 3.7 (2.9, 4.5) | −0.2 | 0.4 | −1.1, 0.7 | 0.6910 |
| Light PA in bouts ≥10 min, min/day | 58.0 (45.3, 70.7) | 54.4 (41.7, 67.1) | −3.5 | 7.5 | −18.6, 11.5 | – |
| Sqrt Light PA in bouts ≥10 min, min/day | 7.2 (6.3, 8.1) | 6.8 (5.9, 7.7) | −0.4 | 0.5 | −1.5, 0.6 | 0.4008 |
| MVPA in bouts ≥10 min, bouts/day | 0.2 (0.0, 0.3) | 0.2 (0.1, 0.3) | 0.1 | 0.1 | −0.1, 0.2 | – |
| Sqrt MVPA in bouts ≥10 min, bouts/day | 0.2 (0.1, 0.4) | 0.2 (0.1, 0.4) | 0.0 | 0.1 | −0.1, 0.1 | 0.9831 |
| MVPA in bouts ≥10 min, min/day | 3.0 (−0.2, 6.2) | 4.7 (1.5, 8.0) | 1.8 | 1.6 | −1.5, 5.0 | – |
| Sqrt MVPA in bouts ≥10 min, min/day | 1.0 (0.4, 1.6) | 1.1 (0.5, 1.6) | 0.1 | 0.3 | −0.6, 0.7 | 0.8635 |
| Weekend | ||||||
| Light PA in bouts ≥10 min, bouts/day | 4.5 (3.4, 5.6) | 3.9 (2.8, 5.0) | −0.6 | 0.6 | −1.7, 0.6 | – |
| Sqrt Light PA in bouts ≥10 min, bouts/day | 2.0 (1.7, 2.2) | 1.7 (1.5, 2.0) | −0.2 | 0.1 | −0.5, 0.1 | 0.1451 |
| Light PA in bouts ≥10 min, min/day | 72.4 (53.0, 91.7) | 58.5 (39.2, 77.8) | −13.9 | 10.5 | −35.2, 7.4 | – |
| Sqrt Light PA in bouts ≥10 min, min/day | 7.7 (6.6, 8.9) | 6.6 (5.4, 7.8) | −1.1 | 0.6 | −2.4, 0.1 | 0.0807 |
| MVPA in bouts ≥10 min, bouts/day | 0.1 (0.0, 0.3) | 0.1 (0.0, 0.3) | 0.0 | 0.1 | −0.1, 0.1 | – |
| Sqrt MVPA in bouts ≥10 min, bouts/day | 0.1 (0.0, 0.3) | 0.1 (0.0, 0.2) | −0.0 | 0.1 | −0.1, 0.1 | 0.8382 |
| MVPA in bouts ≥10 min, min/day | 2.6 (−0.3, 5.4) | 3.0 (0.2, 5.9) | 0.5 | 1.1 | −1.7, 2.6 | – |
| Sqrt MVPA in bouts ≥10 min, min/day | 0.6 (0.1, 1.1) | 0.6 (0.1, 1.1) | 0.0 | 0.2 | −0.5, 0.5 | 0.9153 |
Values are expressed as estimated marginal means (EMM), coefficient estimates (β), standard error (SE), and 95% confidence interval (CI).
Bold values indicate significance at p < 0.05.
Abbreviations: PA, physical activity; MVPA, moderate to vigorous physical activity; Sqrt, square root.
Model adjusted for accelerometer wear time, except for the variables of length of sedentary bouts and break rate.
4. Discussion
This study aimed to report the objectively measured movement behavior of hypertensive older adults before and during the COVID-19 pandemic. The main findings showed that the individuals increased the time spent in SB, decreased the amount of steps/day and the time spent in light and moderate-vigorous PA during the COVID-19 pandemic. In addition, the SB pattern was modified, whereas prolonged/uninterrupted sedentary time increased and breaks in SB decreased. Importantly, the greater changes in PA and SB occurred on the weekend.
A comprehensive social distancing policy has been adopted during the COVID-19 pandemic worldwide, including high-, middle- and low-income countries (Aquino et al., 2020). Particularly in middle- and low-income countries where individuals often live in houses with limited space shared with other family members (Organisation for Economic Co-operation and Development, 2020), it seems reasonable to expect that a period of high mobility restriction in the public areas and a ‘stay-at-home’ recommendation might impact the PA and SB levels (Hall et al., 2020). Indeed, our findings confirm the expected unhealthy changes in movement behavior during the COVID-19 pandemic. We highlight that the decreases in PA and the increases in SB levels occurred in a sample of hypertensive older adults who already had low PA (~5800 steps/day, ~5 h/day of light PA, and ~ 15 min/day of moderate-vigorous PA) and high SB (~11 h/day) levels before the COVID-19 pandemic.
Hypertensive older adults are a high-risk group for COVID-19 severity (Espinosa et al., 2020; Yang et al., 2020). Since the interruption of the HEXA study in March 2020, the participants have been asked about COVID-19 symptoms and their compliance with the social distancing policy by weekly phone calls. From March 2020 to the end of the data collection on June 2020, no participants reported COVID-19 symptoms and all of them reported that they are following the social distancing policy imposed in the city of Natal, which included staying at home. Interestingly, the greater changes in PA and SB were observed on the weekend, which may be partially explained by higher restrictions for activities commonly performed on Saturday and Sunday outside home, such as playing with grandchildren, going to a park, attending religious activities, and shopping at malls and grocery stores. Our findings suggest that there was a reallocation of time spent in light PA and steps for time spent in SB with a more prolonged/uninterrupted pattern especially on the weekend.
A high amount of time spent in SB, especially in a prolonged/uninterrupted sitting pattern, might have several cardiovascular- and metabolic-related implications (Biswas et al., 2015). Although it is not possible to determine how much time the participants spent sitting in the present study, this is the most common SB in humans. Prolonged/uninterrupted sitting reduces the blood flow of the lower limbs, decreasing the shear stress, which attenuates nitric oxide and increases endothelin 1 production, in turn promoting endothelial dysfunction due to a decreased flow-mediated dilation (Carter et al., 2017). These alterations can contribute to increase the arterial stiffness, which is a strong predictor of major cardiovascular diseases/events (Ben-Shlomo et al., 2014; Vlachopoulos et al., 2010). In addition, experimental studies have showed impairments in post-prandial insulin sensitivity and glucose levels using prolonged/uninterrupted sitting models (Loh et al., 2020). Taken together, our findings suggest that the participants are more vulnerable to a cardiometabolic disturbance cascade during the COVID-19 pandemic, which may potentially increase their risk for cardiovascular and metabolic diseases. Thus, prolonged/uninterrupted SB should be considered as a target for management during the COVID-19 pandemic in hypertensive older adults.
Additional changes in movement behavior which have occurred in the present study were a reduction in steps per day and time spent in light PA. It seems that the majority of time spent in steps per day and light PA before the COVID-19 pandemic was reallocated for SB, given that the reduction in time spent in moderate-vigorous PA was modest and the individuals already had a very low PA level in this intensity. Evidence has emerged in more recent years showing that in addition to moderate-vigorous PA, the amount of steps per day (Saint-Maurice et al., 2020) and time spent in light PA seem to play a role in reducing mortality risk (Ku et al., 2020), although its protective mechanisms are less known. In a prospective population-based cohort study of older men from the UK, each additional 30 min spent in SB and light PA were associated with a hazard ratio for all-cause mortality of 1.17 (95% CI, 1.10 to 1.25) and 0.83 (95% CI 0.77 to 0.90), respectively (Jefferis et al., 2019). In addition, each additional 1000 steps/day were associated with a hazard ratio for all-cause mortality of 0.84 (95% CI 0.78 to 0.91) (Jefferis et al., 2019). Therefore, in addition to decreasing SB, two additional targets for management during the COVID-19 pandemic are the increase in the amount of steps per day and time spent in light PA, which were dramatically decreased on the weekend (~1700 steps/day and ~51 min/day, respectively).
Moderate-vigorous PA is the core component of PA guidelines given its well-known benefits. Despite this, the majority of older adults do not perform the recommended amount of moderate-vigorous PA (i.e. <150 min/week) (Lee et al., 2012). In our study only 22.9% (n = 8) of the participants performed 150 min/week of moderate-vigorous PA before and during COVID-19 pandemic. A longitudinal analysis of ~150,000 individuals aged 45+ years about the joint and stratified associations of time spent sitting and in moderate-vigorous PA with all-cause and cardiovascular disease mortality recently showed that a high sitting time (≥8 h/day) among inactive individuals (i.e. <150 min/week of moderate-vigorous PA) was associated with the highest all-cause and cardiovascular mortality risk (Stamatakis et al., 2019). Interestingly, such association was attenuated in active (150–299 min/week) individuals, while it was eliminated in more active (300–419 min/week) and highly active (≥420 min/week) individuals (Stamatakis et al., 2019). Similar results were previously found in a harmonized meta-analysis including more than 1 million individuals (Ekelund et al., 2016). Together, these evidences (Ekelund et al., 2016; Stamatakis et al., 2019) suggest that high moderate-vigorous PA levels might have a protective effect against the harmful impact of SB. However, the potential protective effect of PA against SB-induced risk seems to be dependent of a high amount of moderate-vigorous PA (≥60 min/day) (Ekelund et al., 2016; Stamatakis et al., 2019), which is very uncommon in hypertensive older adults. Therefore, it seems unrealistic to propose home-based countermeasures delivered to increase the moderate-vigorous PA levels of these individuals in a great magnitude during the COVID-19 pandemic. Feasible home-based actions focusing on reducing the time spent in prolonged/uninterrupted SB, reallocating time for PA independent of its intensity, seems to be more realistic actions.
Based on our findings, a rule of thumb seems to be “move more, sit less, more often” (Dempsey et al., 2018), particularly at home. Although challenging, some actions might be implemented to elicit healthier movement behavior: i) increase time spent in moderate-vigorous PA by home-based aerobic exercises (e.g. walking in place, walking, dancing), bodyweight exercises (e.g. chair squat, wall push-ups, calf raises), and household chores (e.g. cleaning, dusting or vacuuming), trying to meet 150 min/week (Pescatello et al., 2015); and ii) decrease the time spent in SB (i.e. <8 h/day) (Ekelund et al., 2019; Ku et al., 2019; Patterson et al., 2018) and breaking up prolonged SB with light PA (3–5 min every 20–30 min of SB) (Loh et al., 2020). The accumulation of PA at home elicits a blood pressure-lowering effect in individuals with hypertension, contributing to better BP control (Padilla et al., 2005). Regular breaks in SB every 30 min with 3–5 min of light PA (aerobic or resistance-based activities) are able to attenuate post-prandial glucose, insulin, and triglycerides (Dempsey et al., 2016; Loh et al., 2020). The combination of moderate walking in the morning with regular breaks in SB (3 min of light walking every 30 min) may have an additional blood pressure-lowering effect in comparison to walking without breaks in SB throughout the day (Wheeler et al., 2019). In addition, other home-based countermeasures to attenuate the deleterious effects of prolonged sitting time on vascular health could be implemented by hypertensive older adults such as warm water immersion (ankle level, 42 °C, 30 min), fidgeting/shaking legs (1 min on/4 min off), and adjustments in their seated position (Padilla and Fadel, 2017).
This study has limitations which should be mentioned. First, this study was not a priori designed to investigate the impact of COVID-19 pandemic on movement behavior in hypertensive older adults. It is an exploratory analysis that has included a sample of participants who have been screed for a clinical trial that was interrupted due to the COVID-19 pandemic. Second, this study included medicated hypertensive older adults aged 60–80 years without known cardiovascular diseases (i.e. coronary heart disease, chronic heart failure). Therefore, our results should be interpreted with caution and not generalized for other hypertensive populations. Despite the above-mentioned limitations, our preliminary findings might contribute to better understanding of the unhealthy changes in movement behavior during the COVID-19 pandemic and guide future feasible preventive and therapeutic actions for hypertensive older adults.
5. Conclusion
The COVID-19 pandemic may elicit unhealthy changes in movement behavior in hypertensive older adults, characterized by an increase in time spent in SB and a decrease in time spent in PA, especially on weekend. Further studies involving larger samples are needed to confirm our preliminary results.
Funding sources
This study was supported by the Brazilian Council for Scientific and Technological Development (CNPq; 427729/2018-1). The first author is supported by a PhD scholarship from the Brazilian Coordination of Improvement of Higher Education Personnel (CAPES; 88882.375386/2019-01). The last author is supported by a research productivity grant from CNPq (306744/2019-8).
CRediT authorship contribution statement
Rodrigo A.V. Browne: Conceptualization, Methodology, Formal analysis, Investigation, Data curation, Project administration, Writing – original draft. Geovani A.D. Macêdo: Methodology, Formal analysis, Data curation, Writing – original draft. Ludmila L.P. Cabral: Methodology, Investigation, Data curation, Writing – review & editing. Gledson T.A. Oliveira: Investigation, Data curation, Writing – review & editing. Andres Vivas: Investigation, Data curation, Writing – review & editing. Eduardo B. Fontes: Conceptualization, Supervision, Resources, Writing – review & editing. Hassan M. Elsangedy: Conceptualization, Supervision, Resources, Writing – review & editing. Eduardo C. Costa: Conceptualization, Methodology, Supervision, Project administration, Resources, Funding acquisition, Writing – original draft.
Declaration of competing interest
The authors have no conflicts of interest to declare.
Acknowledgements
The authors would like to thank the hypertensive older adults who volunteered to participate in this study. We also thank Maria Beatriz F. Araújo and Júlio Sócrates for their support in data collection.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.exger.2020.111121.
Appendix A. Supplementary data
Supplementary tables
References
- Aquino E.M.L., Silveira I.H., Pescarini J.M., Aquino R., Souza-Filho J.A. de, Rocha A. dos S., Ferreira A., Victor A., Teixeira C., Machado D.B., Paixão E., Alves F.J.O., Pilecco F., Menezes G., Gabrielli L., Leite L., Almeida M. da C.C. de, Ortelan N., Fernandes Q.H.R.F., Ortiz R.J.F., Palmeira R.N., Pinto Junior E.P., Aragão E., Souza L.E.P.F. de, Barral Netto M., Teixeira M.G., Barreto M.L., Ichihara M.Y., Lima R.T. dos R.S. Social distancing measures to control the COVID-19 pandemic: potential impacts and challenges in Brazil. Cien. Saude Colet. 2020;25:2423–2446. doi: 10.1590/1413-81232020256.1.10502020. [DOI] [PubMed] [Google Scholar]
- Ben-Shlomo Y., Spears M., Boustred C., May M., Anderson S.G., Benjamin E.J., Boutouyrie P., Cameron J., Chen C.-H., Cruickshank J.K., Hwang S.-J., Lakatta E.G., Laurent S., Maldonado J., Mitchell G.F., Najjar S.S., Newman A.B., Ohishi M., Pannier B., Pereira T., Vasan R.S., Shokawa T., Sutton-Tyrell K., Verbeke F., Wang K.-L., Webb D.J., Willum Hansen T., Zoungas S., McEniery C.M., Cockcroft J.R., Wilkinson I.B. Aortic pulse wave velocity improves cardiovascular event prediction. J. Am. Coll. Cardiol. 2014;63:636–646. doi: 10.1016/j.jacc.2013.09.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswas A., Oh P.I., Faulkner G.E., Bajaj R.R., Silver M.A., Mitchell M.S., Alter D.A. Sedentary time and its association with risk for disease incidence, mortality, and hospitalization in adults. Ann. Intern. Med. 2015;162:123. doi: 10.7326/M14-1651. [DOI] [PubMed] [Google Scholar]
- Boerema S.T., van Velsen L., Vollenbroek M.M., Hermens H.J. Pattern measures of sedentary behaviour in adults: a literature review. Digit. Heal. 2020;6 doi: 10.1177/2055207620905418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brazil. Ministry of Health Coronavirus disease case panel (COVID-19) in Brazil. 2020. https://covid.saude.gov.br/ [WWW Document]. URL. accessed 7.10.20.
- Carter S., Hartman Y., Holder S., Thijssen D.H., Hopkins N.D. Sedentary behavior and cardiovascular disease risk. Exerc. Sport Sci. Rev. 2017;45:80–86. doi: 10.1249/JES.0000000000000106. [DOI] [PubMed] [Google Scholar]
- Choi L., Liu Z., Matthews C.E., Buchowski M.S. Validation of accelerometer wear and nonwear time classification algorithm. Med. Sci. Sports Exerc. 2011;43:357–364. doi: 10.1249/MSS.0b013e3181ed61a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dempsey P.C., Larsen R.N., Sethi P., Sacre J.W., Straznicky N.E., Cohen N.D., Cerin E., Lambert G.W., Owen N., Kingwell B.A., Dunstan D.W. Benefits for type 2 diabetes of interrupting prolonged sitting with brief bouts of light walking or simple resistance activities. Diabetes Care. 2016;39:964–972. doi: 10.2337/dc15-2336. [DOI] [PubMed] [Google Scholar]
- Dempsey P.C., Larsen R.N., Dunstan D.W., Owen N., Kingwell B.A. Sitting less and moving more. Hypertension. 2018;72:1037–1046. doi: 10.1161/HYPERTENSIONAHA.118.11190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubey S., Biswas P., Ghosh R., Chatterjee Subhankar, Dubey M.J., Chatterjee Subham, Lahiri D., Lavie C.J. Psychosocial impact of COVID-19. Diabetes Metab. Syndr. Clin. Res. Rev. 2020;14:779–788. doi: 10.1016/j.dsx.2020.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekelund U., Steene-Johannessen J., Brown W.J., Fagerland M.W., Owen N., Powell K.E., Bauman A., Lee I.-M., Lancet Physical Activity Series 2 Executive Committe, Lancet Sedentary Behaviour Working Group Does physical activity attenuate, or even eliminate, the detrimental association of sitting time with mortality? A harmonised meta-analysis of data from more than 1 million men and women. Lancet. 2016;388:1302–1310. doi: 10.1016/S0140-6736(16)30370-1. [DOI] [PubMed] [Google Scholar]
- Ekelund U., Tarp J., Steene-Johannessen J., Hansen B.H., Jefferis B., Fagerland M.W., Whincup P., Diaz K.M., Hooker S.P., Chernofsky A., Larson M.G., Spartano N., Vasan R.S., Dohrn I.-M., Hagströmer M., Edwardson C., Yates T., Shiroma E., Anderssen S.A., Lee I.-M. Dose-response associations between accelerometry measured physical activity and sedentary time and all cause mortality: systematic review and harmonised meta-analysis. BMJ. 2019;366 doi: 10.1136/bmj.l4570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espinosa O.A., Zanetti A. dos S., Antunes E.F., Longhi F.G., Matos T.A. de, Battaglini P.F. Prevalence of comorbidities in patients and mortality cases affected by SARS-CoV2: a systematic review and meta-analysis. Rev. Inst. Med. Trop. Sao Paulo. 2020;62:e43. doi: 10.1590/s1678-9946202062043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedson P.S., Melanson E., Sirard J. Calibration of the Computer Science and Applications, Inc. accelerometer. Med. Sci. Sports Exerc. 1998;30:777–781. doi: 10.1097/00005768-199805000-00021. [DOI] [PubMed] [Google Scholar]
- Hall G., Laddu D.R., Phillips S.A., Lavie C.J., Arena R. A tale of two pandemics: how will COVID-19 and global trends in physical inactivity and sedentary behavior affect one another? Prog. Cardiovasc. Dis. 2020 doi: 10.1016/j.pcad.2020.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferis B.J., Parsons T.J., Sartini C., Ash S., Lennon L.T., Papacosta O., Morris R.W., Wannamethee S.G., Lee I.-M., Whincup P.H. Objectively measured physical activity, sedentary behaviour and all-cause mortality in older men: does volume of activity matter more than pattern of accumulation? Br. J. Sports Med. 2019;53:1013–1020. doi: 10.1136/bjsports-2017-098733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ku P.-W., Steptoe A., Liao Y., Hsueh M.-C., Chen L.-J. A threshold of objectively-assessed daily sedentary time for all-cause mortality in older adults: a meta-regression of prospective cohort studies. J. Clin. Med. 2019;8:564. doi: 10.3390/jcm8040564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ku P., Hamer M., Liao Y., Hsueh M., Chen L. Device-measured light-intensity physical activity and mortality: a meta-analysis. Scand. J. Med. Sci. Sports. 2020;30:13–24. doi: 10.1111/sms.13557. [DOI] [PubMed] [Google Scholar]
- Laboratory of Technological Innovation in Health Coronavirus disease case panel (COVID-19) in Rio Grande do Norte. 2020. https://covid.lais.ufrn.br/ [WWW Document]. UFRN. URL. accessed 7.10.20.
- Lee I.M., Shiroma E.J., Lobelo F., Puska P., Blair S.N., Katzmarzyk P.T., Alkandari J.R., Andersen L.B., Bauman A.E., Brownson R.C., Bull F.C., Craig C.L., Ekelund U., Goenka S., Guthold R., Hallal P.C., Haskell W.L., Heath G.W., Inoue S., Kahlmeier S., Kohl H.W., Lambert E.V., Leetongin G., Loos R.J.F., Marcus B., Martin B.W., Owen N., Parra D.C., Pratt M., Ogilvie D., Reis R.S., Sallis J.F., Sarmiento O.L., Wells J.C. Effect of physical inactivity on major non-communicable diseases worldwide: an analysis of burden of disease and life expectancy. Lancet. 2012;380:219–229. doi: 10.1016/S0140-6736(12)61031-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loh R., Stamatakis E., Folkerts D., Allgrove J.E., Moir H.J. Effects of interrupting prolonged sitting with physical activity breaks on blood glucose, insulin and triacylglycerol measures: a systematic review and meta-analysis. Sport. Med. 2020;50:295–330. doi: 10.1007/s40279-019-01183-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews C.E., Chen K.Y., Freedson P.S., Buchowski M.S., Beech B.M., Pate R.R., Troiano R.P. Amount of time spent in sedentary behaviors in the United States, 2003–2004. Am. J. Epidemiol. 2008;167:875–881. doi: 10.1093/aje/kwm390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattioli A.V., Ballerini Puviani M., Nasi M., Farinetti A. COVID-19 pandemic: the effects of quarantine on cardiovascular risk. Eur. J. Clin. Nutr. 2020;74:852–855. doi: 10.1038/s41430-020-0646-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Organisation for Economic Co-operation and Development . How’s Life? 2020: Measuring Well-being. OECD; Paris: 2020. Housing; pp. 76–87. [DOI] [Google Scholar]
- Padilla J., Fadel P.J. Prolonged sitting leg vasculopathy: contributing factors and clinical implications. Am. J. Physiol. Circ. Physiol. 2017;313:H722–H728. doi: 10.1152/ajpheart.00326.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padilla J., Wallace J.P., Park S. Accumulation of physical activity reduces blood pressure in pre- and hypertension. Med. Sci. Sports Exerc. 2005;37:1264–1275. doi: 10.1249/01.mss.0000175079.23850.95. [DOI] [PubMed] [Google Scholar]
- Patterson R., McNamara E., Tainio M., de Sá T.H., Smith A.D., Sharp S.J., Edwards P., Woodcock J., Brage S., Wijndaele K. Sedentary behaviour and risk of all-cause, cardiovascular and cancer mortality, and incident type 2 diabetes: a systematic review and dose response meta-analysis. Eur. J. Epidemiol. 2018;33:811–829. doi: 10.1007/s10654-018-0380-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pescatello L.S., MacDonald H.V., Lamberti L., Johnson B.T. Exercise for hypertension: a prescription update integrating existing recommendations with emerging research. Curr. Hypertens. Rep. 2015;17:87. doi: 10.1007/s11906-015-0600-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saint-Maurice P.F., Troiano R.P., Bassett D.R., Graubard B.I., Carlson S.A., Shiroma E.J., Fulton J.E., Matthews C.E. Association of daily step count and step intensity with mortality among US adults. JAMA. 2020;323:1151. doi: 10.1001/jama.2020.1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamatakis E., Gale J., Bauman A., Ekelund U., Hamer M., Ding D. Sitting time, physical activity, and risk of mortality in adults. J. Am. Coll. Cardiol. 2019;73:2062–2072. doi: 10.1016/j.jacc.2019.02.031. [DOI] [PubMed] [Google Scholar]
- Trost S.G., Mciver K.L., Pate R.R. Conducting accelerometer-based activity assessments in field-based research. Med. Sci. Sport. Exerc. 2005;37:S531–S543. doi: 10.1249/01.mss.0000185657.86065.98. [DOI] [PubMed] [Google Scholar]
- Vlachopoulos C., Aznaouridis K., Stefanadis C. Prediction of cardiovascular events and all-cause mortality with arterial stiffness. J. Am. Coll. Cardiol. 2010;55:1318–1327. doi: 10.1016/j.jacc.2009.10.061. [DOI] [PubMed] [Google Scholar]
- Wheeler M.J., Dunstan D.W., Ellis K.A., Cerin E., Phillips S., Lambert G., Naylor L.H., Dempsey P.C., Kingwell B.A., Green D.J. Effect of morning exercise with or without breaks in prolonged sitting on blood pressure in older overweight/obese adults. Hypertension. 2019;73:859–867. doi: 10.1161/HYPERTENSIONAHA.118.12373. [DOI] [PubMed] [Google Scholar]
- World Health Organization . WHO; 2020. Coronavirus Disease (COVID-19) Weekly Epidemiological Update - 28 September 2020 [WWW Document]https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports accessed 9.30.20. [Google Scholar]
- Yang J., Zheng Y., Gou X., Pu K., Chen Z., Guo Q., Ji R., Wang H., Wang Y., Zhou Y. Prevalence of comorbidities and its effects in patients infected with SARS-CoV-2: a systematic review and meta-analysis. Int. J. Infect. Dis. 2020;94:91–95. doi: 10.1016/j.ijid.2020.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary tables