Abstract
Many diverse strategies allow and facilitate severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) to evade antiviral innate immune mechanisms. Although the type I interferon (IFN) system has a critical role in restricting the dissemination of viral infection, suppression of IFN receptor signals by SARS-CoV-2 constitutes a checkpoint that plays an important role in the immune escape of the virus. Environmental pollution not only facilitates SARS-CoV-2 infection but also increases infection-associated fatality risk, which arises due to Systemic Aryl hydrocarbon Receptor (AhR) Activation Syndrome. The intracellular accumulation of endogenous kynurenic acid due to overexpression of the indoleamine 2,3-dioxygenase (IDO) by AhR activation induces AhR-interleukin-6 (IL-6)-signal transducers and activators of the transcription 3 (STAT3) signaling pathway. The AhR-IDO1-Kynurenine pathway is an important checkpoint, which leads to fatal consequences in SARS-CoV-2 infection and immune evasion in the context of Treg/Th17 imbalance and cytokine storm.
Keywords: Aryl hydrocarbon receptor; Environmental pollution; Indoleamine 2,3-dioxygenase; Severe acute respiratory syndrome coronavirus 2; Angiotensin-converting enzyme 2; Interferons
1. Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which was firstly identified in Wuhan, China in December 2019, rapidly spread all over the world. Whilst the number of cases to date exceeds 37 million worldwide, more than one million people have died due to this virus infection (Coronavirus Update (Live), 2020). Although the pathogenesis of SARS-CoV-2 infection is still not completely clarified, a four-stage classification is proposed for the course of disease (From the American Association of Neurological Surgeons (AANS), American Society of Neuroradiology (ASNR), Cardiovascular and Interventional Radiology Society of Europe (CIRSE), Canadian Interventional Radiology Association (CIRA), Congress of Neurological Surgeons (CNS), European Society of Minimally Invasive Neurological Therapy (ESMINT), European Society of Neuroradiology (ESNR), European Stroke Organization (ESO), Society for Cardiovascular Angiography and Interventions (SCAI), Society of Interventional Radiology (SIR), Society of NeuroInterventional Surgery (SNIS), and World Stroke Organization (WSO) et al., 2018; Rizk et al., 2020; Rodríguez et al., 2020; Siddiqi and Mehra, 2020).
SARS-CoV-2 enters cells via angiotensin-converting enzyme 2 (ACE2) by activating viral spike glycoproteins (SARS-2-S) through transmembrane protease serine 2 (TMPRSS2) (Engin et al., 2020a; Lei et al., 2020; Letko et al., 2020). However, the crosstalk between the SARS-CoV-2 and host innate immunity is poorly understood (Lei et al., 2020). Upregulation of ACE2 or higher ACE2 gene expression may increase susceptibility to infection by SARS-CoV-2 (Brake et al., 2020). Unfortunately, SARS-CoV-2 not only effectively uses a critical and unique system which is ACE2, to enter and multiply in the host (Gheblawi et al., 2020), but also its binding to ACE2 allows it to evade immune surveillance. The engulfment of ACE2 provides the virus access to the host cells system, thereby viral proliferation and immune evasion are strongly linked with a successful and potentially devastating infection (Brake et al., 2020). Thus, despite all the preventive measures, the rate of COVID-19 began to rise again, over second half of 2020. Most probably, one of the important contributing factors for the continuation of the pandemic is that environmental pollution negatively modulates the host's immune response.
In this context, epidemiological data regarding COVID-19 from 110 Italian provinces confirmed that environmental pollution facilitates SARS-CoV-2 infection and increases the infection-associated fatality risk (Borro et al., 2020). Specifically, the adverse health effects of traffic-related daily ambient particulate matter pollution result from their chemical components like polycyclic aromatic hydrocarbons (PAHs), as well as particulates (Xie et al., 2012). PAHs are well-known activators of the aryl hydrocarbon receptor (AhR), which is a latent transcription factor. Indeed, AhR is a chemical/ligand-dependent cytoplasmic receptor that responds to xenobiotics. In this respect, persistent organic pollutants, such as dibenzo-p-dioxins, dibenzofurans and non-ortho substituted (Borro et al., 2020) as well as polychlorinated biphenyl groups that can be found in particulate fractions, are high-affinity AhR ligands (Aristizábal et al., 2011; Denison and Nagy, 2003; Ma et al., 2013). Furthermore, the bioinformatic analysis of the ACE2 gene has identified nine putative motifs for the AhR. This finding not only confirms the supposed link between environmental pollution and the SARS-CoV-2 infection, but also supports the hypothesis of pollution-induced ACE2 over-expression (Borro et al., 2020). As an evidence of this, high AhR signal activity related with SARS-CoV-2 infection together have been shown to contribute to life-threatening progressive respiratory failure (Giovannoni et al., 2020).
AhR has both exogenous and endogenous ligands. Exogenous ligands includes 2,3,7,8‐tetrachlorodibenzo‐p‐dioxin (TCDD) which has a high affinity to the receptor and is found in cigarette smoke. In addition, the polychlorinated biphenyl congener 126 (PCB126), is a common pollutant and among the chlorinated biphenyls (PCBs) is the AhR agonist with the highest potency. Additionally, the novel pharmaceutical, 2‐(1′H‐indole‐3′‐carbonyl)‐thiazole‐4‐carboxylic acid methyl ester (ITE), is an indole-based ligand. The endogenous ligands include tryptophan degradation products, which originate from the kynurenine pathway. All of these can modulate the immune responses of host to viral infection (Boule et al., 2018; Franchini et al., 2019). Modulation of the host's immune response via AhR activation, leads to the expression of several effector genes, including TCDD‐inducible poly adenosine diphosphate ribose (ADP‐ribose) polymerase (TiPARP), which is required for maximal coronavirus replication (Grunewald et al., 2020). Consequently, AhRs play a critical role in immune and inflammatory processes and modulate the host’s responses to the environmental pollution (Veldhoen et al., 2008). Nevertheless, there is very scanty information on the innate immune responses to SARS-CoV-2, and on the regulatory mechanisms thereof, which impair the clearance of the virus and promote its immune escape (Maggi et al., 2020). In this review, the hypothetical mechanisms of immune evasion checkpoints adopted by SARS-CoV-2 and the impaired immune response of the host due to environmental pollution contributing the immunosuppression, which is observed in COVID-19 are discussed.
2. Type I and III interferons in SARS-CoV-2 infection
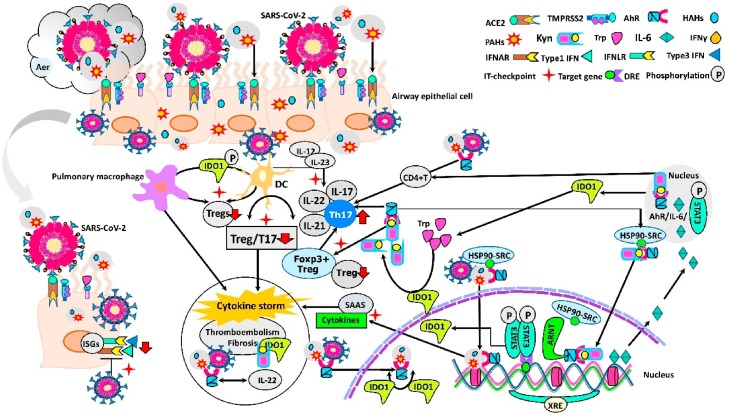
Pattern recognition receptors like Toll-like receptors (TLR-3, -7/8), and RIG-1-like receptors (RLRs), and cytokine receptors mediate the responses to RNA viruses which infect the airways (Goritzka et al., 2015). After endocytosis, RNA genome of SARS-CoV-2 binds to the TLRs 3, 7 and 8, RLRs, nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) and interferon regulatory factor 3 and 7 (IRF3 and IRF7). Via type I and type III interferons (IFNs) (interferon-gamma; IFN-γ) signaling, pro-inflammatory cytokines synthesis is initiated (Fig. 1 ) (Park and Iwasaki, 2020; Zhou et al., 2020).
Fig. 1.
Possible checkpoints that suppress the immune response of patients who are co-exposed to environmental pollution and SARS-CoV-2 aerosol.
Airway innate immune cells, such as epithelial cells, DCs and pulmonary macrophages, function at different stages of SARS-CoV-2 infection. Both xenobiotic and virus act as AhR ligands in individuals exposed to aerosol effect, which contains various environmental pollutants and SARS-CoV-2. While xenobiotics pass through the plasma membrane by passive diffusion, SARS-CoV-2 binds to ACE2 and enters into cell, thereby both form a ligand-receptor complex with AhR ("double-hit hypothesis"). After this complex is transported to nucleus, it heterodimerizes with its partner ARNT and genomic pathway is activated. This initiates the "Systemic AhR Activation Syndrome" (SAAS) via multiple AhR signaling pathways. SARS-CoV-2 blocks the production of type 1 IFNs and binding to the IFNARs, thus directly antagonizes the actions of ISG. Delayed IFN-I signaling results in excess virus replication in the epithelial cells and leads to severe complications. AhR activation by xenobiotic ligands and SARS-CoV-2 increases the expression of the IDO1 in DCs. Increase in IDO1 activity results in the production of immunosuppressive tryptophan metabolites via kynurenine pathway. The AhR-IL-6 STAT3 pathway induces the differentiation of naïve CD4+ T cells toward Th17 cells, while inhibiting Treg cells, leading to Treg/Th17 imbalance and SARS-CoV-2 immune evasion. Persistence of the inflammatory vicious circle and the IDO1-kynurenine-AhR pathway hyperactivation culminate with cytokine storm. The intensity of AhR activation, and AhR/IL-22 signaling pathway may enhance the thromboembolism and fibrosis in multiple organs (Abbreviations: ACE2: Angiotensin-converting enzyme 2; Aer: Aerosol; AhR: Aryl hydrocarbon receptor; ARNT: AhR nuclear translocator protein; DC: Dendritic cell; DRE: Dioxin responsive element; Foxp3: Forkhead box P3+; HAHs: Halogenated Aromatic Hydrocarbons; HSP90: Chaperone 90-kDa heat shock protein; IDO1: Indolamine 2,3-dioxygenase 1; IFNγ: Interferon-gamma, Type III Interferon; IFNAR: Interferon alpha and beta receptors; IFNLR: Interferon lambda receptor, Type III IFN receptor; IL-6: Interleukin-6; ISG: Interferon-1 stimulated gene; IT-checkpoint: Immune tolerance checkpoints; Kyn: Kynurenine; PAHs: Polycyclic aromatic hydrocarbons; SAAS: Systemic AhR Activation Syndrome; SARS-CoV-2: Severe acute respiratory syndrome coronavirus 2; SRC: HSP90 co-chaperone kinase (stress-regulated client protein); STAT3: Signal transducers and activators of transcription 3; TMPRSS: Transmembrane protease serine 2; Treg: T regulatory cell; Trp: Tryptophan; Type1 IFNs: Interferon alpha and beta; XRE: Xenobiotic response element).
Indeed, the type I interferon system has a critical role in restricting the dissemination of viral infection. Viruses stimulate the expression of IFN and binding of IFN to the IFN-α/β receptor (IFNAR) initiates the Janus kinase (JAK)/signal transducers and activators of transcription (STAT) signaling cascade. Subsequent activation of IFN-stimulated genes (ISGs) prevents viral replication and support the host innate antiviral response (Lenschow, 2010). Whereas RNA viruses arrest or impede IFN production via reducing the expression of ISGs. (Reid and Charleston, 2014).
A vigorous antiviral response is triggered by type I IFNs via binding its own receptor, which stimulates phosphorylation of STAT1 and STAT2. Subsequently, these transcription factors together with IRF create the IFN stimulated gene factor (ISGF) complex. This complex induces ISGs (Platanias, 2005; Schoggins et al., 2011). Despite the similarities between the type I IFNs and IFN-γ signaling pathways and ISGs, both type of IFNs’ responses are thought to be depend on virus intensity. The IFN-γ is the primary local defense against the low doses of viruses, however, the IFN-γ activation may be insufficient during the exposure to high doses of virus (Andreakos et al., 2017; Schoggins et al., 2011). Although it is claimed that IFN-γ does not trigger inflammation as much as type I IFNs (Galani et al., 2017), interestingly, serum analysis of COVID-19 patients showed that proinflammatory cytokines and chemokines are strongly elevated without a significant increase in type I IFNs and IFN-γ (Blanco-Melo et al., 2020). In view of these findings, Blanco-Melo et al. suggested that SARS-CoV-2 infection induces low IFN-I and IFN-γ with insufficient ISG response while unexpectedly vigorously stimulating the expression of chemokine and pro-inflammatory cytokine genes (Blanco-Melo et al., 2020). This is attributed to the inhibition of phosphorylation and nuclear translocation of IRF3 by SARS-CoV-2 non-structural protein 3 (nsp3) (Blanco-Melo et al., 2020). The common accessory protein of SARS-CoVs, capsid protein ORF3b suppresses type I IFN more effectively by inhibiting ISGs transcription in SARS-CoV-2 compared to other SARS-CoVs. Therefore, in COVID-19, although the cytokine signal is intact, IFN signaling is uniquely suppressed by infected airway cells (Kopecky-Bromberg et al., 2007; Schwartz et al., 2020).
Recently, Park and Iwasaki claimed that the ISGs are proportional with the viral load as well as disease severity, and accordingly, severe SARS-CoV-2 infection leads to large quantities of type I IFN expression that fail to reduce viral load (Park and Iwasaki, 2020). Although the findings on IFN expression are contradictory, in the early phase of COVID-19, high amounts of inflammatory cytokines and chemokines secretion from activated macrophages, mature dendritic cells (DCs) and epithelial cells is unchallenged (Cheung et al., 2005; Fu et al., 2020). This clearly demonstrates the presence of disequilibrium between pro-inflammatory cytokine and IFN expression in SARS-CoV-2 infection (Park and Iwasaki, 2020). Suppression of IFN receptor signals by SARS-CoV-2 constitutes a checkpoint that plays an important role in the immune evasion of the virus. As mentioned above, there is an imbalance between the amounts of IFNs and the level of highly expressed proinflammatory cytokines. Because of this conflict, it is thought that either the AhR directly or the AhR-Kynurenine pathway influence the formation of the cytokine storm, which is largely responsible for lung tissue damage and death in COVID-19 patients.
Environmental pollution enhances angiotensin I (Ang I) conversion to Ang II via promoting plasma ACE activity and results in increased COVID-19 mortality (Engin et al., 2020b). Indeed, increased Ang II promotes IL-6 expression via JAK/STAT pathway, thereby setting up a positive inflammatory feedback loop. The resultant cytokine storm causes tissue damage. Furthermore, the disintegrin and metalloproteinase 17 (ADAM17), which is activated by the Ang II/AT1 receptor axis, increases Ang II retention (Catanzaro et al., 2020).
3. Aryl hydrocarbon receptor and immune evasion of SARS-CoV-2
Environmental pollutants are very stable in the human body, and some may accumulate and remain as potent inducers of AhR for many years (Denison and Nagy, 2003). In the sampling study conducted in the fall of 2017, it was determined that particulate matter 2.5 (PM2.5) concentrations in six Chinese cities, including Wuhan, exceeded the amounts recommended by the World Health Organization regarding daily average air quality by 2.4–6.1 times (Wang et al., 2020b). The epidemiological data revealed that the air quality index was significantly and positively correlated with the daily amount of COVID-19 cases in both Wuhan and XiaoGan. In this respect, air quality index, PM2.5, nitrogen dioxide (NO2), and temperature have been established as promoting factors in the transmission of COVID-19 (Engin et al., 2020b; H. Li et al., 2020b). These particles contain AhR ligands as well as the potential to carry viable virus particles. Although the transmission of COVID-19 is dependent on human-to-human spread, the environmental pollution rapidly enhances the air-to-human contamination and forms a hazardous vicious cycle (Coccia, 2020). Thus, analysis of data of 150 patients from Wuhan, China evidently displayed that the fatal outcome in COVID-19 patients was due to uncontrollable cytokine release (Ruan et al., 2020). In fact, environmental pollutants expressing ACE2 and AhR use the same genomic pathway. Pollution-induced over-expression of ACE2 on human airways promotes SARS-CoV-2 infectivity, while inducing viral immune evasion (Borro et al., 2020). Hence, a positive correlation was found between the virus spread, environmental pollution, and ACE2 receptor expression and severity of SARS-CoV-2 infection (Comunian et al., 2020). This process is defined as the "double-hit hypothesis" by Frontera et al. (Frontera et al., 2020). Precursors to AhR ligands, which are derived from industrial waste, such as PAHs found in tobacco smoke, coal tar, grilled meats, and in many foods are potent agonists of the AhR (Machala et al., 2001; Quintana and Sherr, 2013). Following the diffusion through plasma membrane, these exogenous ligands bind to cytoplasmic AhR. Hsp90 and aryl hydrocarbon receptor interacting protein (AIP) retain AhR in the cytoplasm, and AIP prevents the degradation of AhR (Kazlauskas et al., 2000). Furthermore, AIP strongly suppresses interferon regulatory factor 7-induced type I IFN (IFNα/β) expression and negatively affects anti-viral response (Zhou et al., 2015). Ligand-AhR complex moves towards the nucleus (Larigot et al., 2018; Neavin et al., 2018). Later, the 90-kDa heat shock protein (HSP90/c)-SRC compound and AIP dissociate from the Ligand-AhR complex that translocates to the nucleus (Kazlauskas et al., 2000; Quintana and Sherr, 2013; Stockinger et al., 2014). Indeed, HSP90 release in the nuclear region is required for the generation of the AhR/AhR nuclear translocator (ARNT) heterodimer, which controls the transcriptional activity of target genes (Quintana and Sherr, 2013; Stockinger et al., 2014; Tsuji et al., 2014). AhR heterodimerizes with the ARNT protein. This heterodimer binds to xenobiotic responsive elements, which has specific DNA sequences, thereby genomic pathway is activated (Larigot et al., 2018; Neavin et al., 2018; Petrulis et al., 2003, 2000). In addition to environmental pollution, direct activation of AhRs by virus stimulates immediately simultaneous up-regulation of various distinct AhR-dependent effectors. All these, by turns consequently lead to a "Systemic AhR Activation Syndrome" (SAAS) (Fig. 1). Clinical manifestations of SAAS are inflammation, cytokine storm, thromboembolism, fibrosis, and result in multiple organ injuries and death (Larigot et al., 2018; Neavin et al., 2018). In this process, simultaneous exposure to SARS-CoV-2 with environmental pollutants initially induces activation of AhRs by a mechanism in which indolamine 2,3-dioxygenase 1 (IDO1) is not involved. (Checkpoint) (Fig. 1) (Larigot et al., 2018; Neavin et al., 2018). Subsequently, AhR activation increases the expression of the IDO1, and the essential amino acid tryptophan is metabolized throughout the kynurenine pathway. Formation of kynurenine and kynurenine metabolites initiate an important immune tolerance mechanism in DCs (Mezrich et al., 2010; Munn et al., 2002; Nguyen et al., 2010; Takikawa, 2005). Besides the environmental pollutants, activation of AhR due to extensive viral exposure leads to additional release of kynurenine (Turski et al., 2020). The downstream metabolite of kynurenine, kynurenic acid, acts as an AhR ligand (DiNatale et al., 2010; Heath-Pagliuso et al., 1998). The intracellular accumulation of endogenous kynurenine has 100–1000 fold more AhR agonist potential in comparison to classic AhR ligands (Seok et al., 2018). Kynurenine and kynurenic acid produced by IDO1 in epithelial cells, activates more AhR, thereby induce interleukin (IL)-6. IL-6 in turn drives more IDO1 expression via an AhR-IL-6-STAT3 signaling (Litzenburger et al., 2014). Thus, kynurenic acid supports the cytokine storm by inducing the production of inflammatory cytokines (DiNatale et al., 2010).
Therefore, AhR also increases its own effect via the activation of the IDO1-AhR-IDO1 positive feedback loop. The effects of AhR activation by SARS-CoV-2 with environmental pollutants together are essentially synergistic (Neavin et al., 2018). The AhR ligand-receptor interactions control the immune processes in response to environmental stimuli. Kynurenine and related metabolites increase the differentiation of forkhead box P3+ (FoxP3+) regulatory T cells (Tregs), and this effect is also dependent on AhR signaling in T cells. In this respect, it is thought that there is a positive feedback loop in that AhR ligands dramatically induce the expression of IDO1. More IDO1, in turn generates more kynurenine, as endogenous AhR ligand. Both cytokine storm, and immune escape of SARS-CoV-2 supports each other in fatal progression of COVID-19 (Rizk et al., 2020). The AhR-IDO1-Kynurenine pathway is an important checkpoint which leads to fatal consequences in SARS-CoV-2 infection. Indeed, IDO1 activity is markedly increased in sepsis, constituting an independent predictor of severity and fatality of various diseases (Huttunen et al., 2010). Moreover, increased IDO1 activity and kynurenine show a strong positive correlation with community-acquired pneumonia severity score (Meier et al., 2017). The concept stated as "AhR activation could suppress immune responses" was first defined by Kerkvliet et al. (Kerkvliet et al., 1990). In this context, AhR activation induces the differentiation to IL-17-producing CD4+ T cells. Polarization into new Th17 cells increases the production of the Th17-related inflammatory cytokines, IL-17A, IL-17F, IL-22 and IL-21 (Kimura et al., 2008; Quintana et al., 2008; Veldhoen et al., 2008). In severe SARS-CoV-2 infection Th17 cells (CCR6+ Th17) rapidly increase, while Treg cells (CD3+CD4+CD25+CD127low+) are significantly decreasing. Thereby, in lethal COVID-19, cytokine secretion from DCs and macrophages provoke the infiltration of Th17 cells to the site of infection and lead to diffuse lung injury (Qin et al., 2020; F. Wang et al., 2020a; Wu and Yang, 2020). In fact, IL‐12 predominantly induces Th1 immune responses, whereas IL‐23 contributes to Th17 immunity (Schön and Erpenbeck, 2018). An infection with SARS‐CoV‐2 likely leads to enhanced IL‐12 and IL‐23 serum concentrations (Schön et al., 2020). The effect of environmental pollution on Th17 cell systems incorporates AhR induction within intermediary DCs, rather than a direct impact on actual Th17 cells (Kazantseva et al., 2012). Since cytokines act through a common JAK-STAT signaling pathway, STAT3, a transcription factor, mediates IL-6 and IL-23 signals for the initial differentiation of Th17 cell. In this feedback cycle in patients with COVID-19, both IL-6 and IL-23 activate STAT3 through JAK2, whereas IL-21 activates STAT3 via JAK1 and JAK3 (Wu and Yang, 2020). Eventually, differentiation of Th17 represses Treg cells and this is followed by severe cytokine release (G. Li et al., 2020a). FoxP3+ Treg cells act as a key factor in the control of immune reactivity to self and non-self-antigens (Sakaguchi et al., 2010). Treg cells express anti‐inflammatory cytokines, and control excessive immune responses. Thereby, decrease in Treg/Th17 cell ratio in SARS-CoV-2 infection is a serious immunological conundrum.
In this context, the Treg/Th17 imbalance contributes to the explosion of the cytokine storm and can lead to severe multiple organ failure during the SARS-CoV-2 infection (Li et al., 2016; Muyayalo et al., 2020). In a series of twenty-one patients with COVID-19, who had pulmonary insufficiency, significantly higher cytokine expression was found despite the decreasing IFNs (Chen et al., 2020). The decrease in CD4+ and CD8+ cells, increased level of proinflammatory cytokines and chemokines, in addition to decreased Treg cells, and the resultant excessive cytokine release syndrome eliminates the patient's control over the destructive immune response and leads to the death in COVID-19 (Jesenak et al., 2020). In fact, all these alterations are more pronounced in severe SARS-CoV-2 infection than in those with mild disease (Song et al., 2020).
4. Conclusion
SARS-CoV-2 is explicitly prone to evade immune detection by suppressing human immune responses. The presence of disequilibrium between pro-inflammatory cytokine and IFN expression (Checkpoint) in SARS-CoV-2 infection may be associated with the environmental pollution-related IDO1-AhR-IDO1 pathway activation. Decreased clearance of the virus through immune evasion of SARS-CoV-2 due to AhR-Kynurenine pathway interactions plays an important role in the severity of COVID-19. The AhR-IDO1-Kynurenine pathway supports the cytokine storm by inducing the production of inflammatory cytokines. The immunomodulatory effects of kynurenine are mediated by the AhR. Thus, it seems reasonable that novel therapies of SARS-CoV-2 infection could perhaps target the IDO1 pathway and thus reverse the IDO1-mediated suppression of T cell activity. Such a treatment strategy may also include the use of IDO1 inhibitors in addition to checkpoint-related combination regimens.
Declaration of Competing Interest
The authors report no declarations of interest.
References
- Andreakos E., Salagianni M., Galani I.E., Koltsida O. Interferon-λs: front-line guardians of immunity and homeostasis in the respiratory tract. Front. Immunol. 2017;8:1232. doi: 10.3389/fimmu.2017.01232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aristizábal B.H., Gonzalez C.M., Morales L., Abalos M., Abad E. Polychlorinated dibenzo-p-dioxin and dibenzofuran in urban air of an Andean city. Chemosphere. 2011;85:170–178. doi: 10.1016/j.chemosphere.2011.06.035. [DOI] [PubMed] [Google Scholar]
- Blanco-Melo D., Nilsson-Payant B.E., Liu W.-C., Uhl S., Hoagland D., Møller R., Jordan T.X., Oishi K., Panis M., Sachs D., Wang T.T., Schwartz R.E., Lim J.K., Albrecht R.A., tenOever B.R. Imbalanced host response to SARS-CoV-2 drives development of COVID-19. Cell. 2020;181:1036–1045. doi: 10.1016/j.cell.2020.04.026. e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borro M., Di Girolamo P., Gentile G., De Luca O., Preissner R., Marcolongo A., Ferracuti S., Simmaco M. Evidence-based considerations exploring relations between SARS-CoV-2 pandemic and air pollution: involvement of PM2.5-Mediated up-regulation of the viral receptor ACE-2. Int. J. Environ. Res. Public Health. 2020:17. doi: 10.3390/ijerph17155573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boule L.A., Burke C.G., Jin G.-B., Lawrence B.P. Aryl hydrocarbon receptor signaling modulates antiviral immune responses: ligand metabolism rather than chemical source is the stronger predictor of outcome. Sci. Rep. 2018;8:1826. doi: 10.1038/s41598-018-20197-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brake S.J., Barnsley K., Lu W., McAlinden K.D., Eapen M.S., Sohal S.S. Smoking Upregulates Angiotensin-Converting Enzyme-2 Receptor: A Potential Adhesion Site for Novel Coronavirus SARS-CoV-2 (Covid-19) J. Clin. Med. 2020:9. doi: 10.3390/jcm9030841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catanzaro M., Fagiani F., Racchi M., Corsini E., Govoni S., Lanni C. Immune response in COVID-19: addressing a pharmacological challenge by targeting pathways triggered by SARS-CoV-2. Signal Transduct. Target. Ther. 2020;5:84. doi: 10.1038/s41392-020-0191-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G., Wu D., Guo W., Cao Y., Huang D., Wang H., Wang T., Zhang Xiaoyun, Chen H., Yu H., Zhang Xiaoping, Zhang M., Wu S., Song J., Chen T., Han M., Li S., Luo X., Zhao J., Ning Q. Clinical and immunological features of severe and moderate coronavirus disease 2019. J. Clin. Invest. 2020;130:2620–2629. doi: 10.1172/JCI137244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung C.Y., Poon L.L.M., Ng I.H.Y., Luk W., Sia S.-F., Wu M.H.S., Chan K.-H., Yuen K.-Y., Gordon S., Guan Y., Peiris J.S.M. Cytokine responses in severe acute respiratory syndrome coronavirus-infected macrophages in vitro: possible relevance to pathogenesis. J. Virol. 2005;79:7819–7826. doi: 10.1128/JVI.79.12.7819-7826.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coccia M. Factors determining the diffusion of COVID-19 and suggested strategy to prevent future accelerated viral infectivity similar to COVID. Sci. Total Environ. 2020;729 doi: 10.1016/j.scitotenv.2020.138474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comunian S., Dongo D., Milani C., Palestini P. Air pollution and Covid-19: the role of particulate matter in the spread and increase of Covid-19’s morbidity and mortality. Int. J. Environ. Res. Public Health. 2020:17. doi: 10.3390/ijerph17124487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coronavirus Update (Live) 2020. Coronavirus Update (Live) [WWW Document] URL https://www.worldometers.info/coronavirus/ (accessed 9.3.20) [Google Scholar]
- Denison M.S., Nagy S.R. Activation of the aryl hydrocarbon receptor by structurally diverse exogenous and endogenous chemicals. Annu. Rev. Pharmacol. Toxicol. 2003;43:309–334. doi: 10.1146/annurev.pharmtox.43.100901.135828. [DOI] [PubMed] [Google Scholar]
- DiNatale B.C., Murray I.A., Schroeder J.C., Flaveny C.A., Lahoti T.S., Laurenzana E.M., Omiecinski C.J., Perdew G.H. Kynurenic acid is a potent endogenous aryl hydrocarbon receptor ligand that synergistically induces interleukin-6 in the presence of inflammatory signaling. Toxicol. Sci. Off. J. Soc. Toxicol. 2010;115:89–97. doi: 10.1093/toxsci/kfq024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engin A.B., Engin E.D., Engin A. Dual function of sialic acid in gastrointestinal SARS-CoV-2 infection. Environ. Toxicol. Pharmacol. 2020;79 doi: 10.1016/j.etap.2020.103436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engin A.B., Engin E.D., Engin A. Two important controversial risk factors in SARS-CoV-2 infection: obesity and smoking. Environ. Toxicol. Pharmacol. 2020;78 doi: 10.1016/j.etap.2020.103411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franchini A.M., Myers J.R., Jin G.-B., Shepherd D.M., Lawrence B.P. Genome-wide transcriptional analysis reveals novel AhR targets that regulate dendritic cell function during influenza a virus infection. ImmunoHorizons. 2019;3:219–235. doi: 10.4049/immunohorizons.1900004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- From the American Association of Neurological Surgeons (AANS), American Society of Neuroradiology (ASNR), Cardiovascular and Interventional Radiology Society of Europe (CIRSE), Canadian Interventional Radiology Association (CIRA), Congress of Neurological Surgeons (CNS), European Society of Minimally Invasive Neurological Therapy (ESMINT), European Society of Neuroradiology (ESNR), European Stroke Organization (ESO), Society for Cardiovascular Angiography and Interventions (SCAI), Society of Interventional Radiology (SIR), Society of NeuroInterventional Surgery (SNIS), World Stroke Organization (WSO), Sacks D., Baxter B., Campbell B.C.V., Carpenter J.S., Cognard C., Dippel D., Eesa M., Fischer U., Hausegger K., Hirsch J.A., Shazam Hussain M., Jansen O., Jayaraman M.V., Khalessi A.A., Kluck B.W., Lavine S., Meyers P.M., Ramee S., Rüfenacht D.A., Schirmer C.M., Vorwerk D. Multisociety consensus quality improvement revised consensus statement for endovascular therapy of acute ischemic stroke. Int. J. Stroke Off. J. Int. Stroke Soc. 2018;13:612–632. doi: 10.1177/1747493018778713. [DOI] [PubMed] [Google Scholar]
- Frontera A., Cianfanelli L., Vlachos K., Landoni G., Cremona G. Severe air pollution links to higher mortality in COVID-19 patients: the “double-hit” hypothesis. J. Infect. 2020;81:255–259. doi: 10.1016/j.jinf.2020.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Y., Cheng Y., Wu Y. Understanding SARS-CoV-2-Mediated inflammatory responses: from mechanisms to potential therapeutic tools. Virol. Sin. 2020;35:266–271. doi: 10.1007/s12250-020-00207-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galani I.E., Triantafyllia V., Eleminiadou E.-E., Koltsida O., Stavropoulos A., Manioudaki M., Thanos D., Doyle S.E., Kotenko S.V., Thanopoulou K., Andreakos E. Interferon-λ mediates non-redundant front-line antiviral protection against influenza virus infection without compromising host fitness. Immunity. 2017;46:875–890. doi: 10.1016/j.immuni.2017.04.025. e6. [DOI] [PubMed] [Google Scholar]
- Gheblawi M., Wang K., Viveiros A., Nguyen Q., Zhong J.-C., Turner A.J., Raizada M.K., Grant M.B., Oudit G.Y. Angiotensin converting enzyme 2: SARS-CoV-2 receptor and regulator of the renin-angiotensin system. Circ. Res. 2020 doi: 10.1161/CIRCRESAHA.120.317015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovannoni F., Li Z., Garcia C.C., Quintana F.J. A potential role for AHR in SARS-CoV-2 pathology. Res. Sq. 2020 doi: 10.21203/rs.3.rs-25639/v1. [DOI] [Google Scholar]
- Goritzka M., Pereira C., Makris S., Durant L.R., Johansson C. T cell responses are elicited against Respiratory Syncytial Virus in the absence of signalling through TLRs, RLRs and IL-1R/IL-18R. Sci. Rep. 2015;5:18533. doi: 10.1038/srep18533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunewald M.E., Shaban M.G., Mackin S.R., Fehr A.R., Perlman S. Murine coronavirus infection activates the aryl hydrocarbon receptor in an indoleamine 2,3-Dioxygenase-Independent manner, contributing to cytokine modulation and proviral TCDD-Inducible-PARP expression. J. Virol. 2020:94. doi: 10.1128/JVI.01743-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath-Pagliuso S., Rogers W.J., Tullis K., Seidel S.D., Cenijn P.H., Brouwer A., Denison M.S. Activation of the Ah receptor by tryptophan and tryptophan metabolites. Biochemistry. 1998;37:11508–11515. doi: 10.1021/bi980087p. [DOI] [PubMed] [Google Scholar]
- Huttunen R., Syrjänen J., Aittoniemi J., Oja S.S., Raitala A., Laine J., Pertovaara M., Vuento R., Huhtala H., Hurme M. High activity of indoleamine 2,3 dioxygenase enzyme predicts disease severity and case fatality in bacteremic patients. Shock Augusta Ga. 2010;33:149–154. doi: 10.1097/SHK.0b013e3181ad3195. [DOI] [PubMed] [Google Scholar]
- Jesenak M., Brndiarova M., Urbancikova I., Rennerova Z., Vojtkova J., Bobcakova A., Ostro R., Banovcin P. Immune parameters and COVID-19 infection - associations with clinical severity and disease prognosis. Front. Cell. Infect. Microbiol. 2020;10:364. doi: 10.3389/fcimb.2020.00364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazantseva M.G., Highton J., Stamp L.K., Hessian P.A. Dendritic cells provide a potential link between smoking and inflammation in rheumatoid arthritis. Arthritis Res. Ther. 2012;14:R208. doi: 10.1186/ar4046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazlauskas A., Poellinger L., Pongratz I. The immunophilin-like protein XAP2 regulates ubiquitination and subcellular localization of the dioxin receptor. J. Biol. Chem. 2000;275:41317–41324. doi: 10.1074/jbc.M007765200. [DOI] [PubMed] [Google Scholar]
- Kerkvliet N.I., Baecher-Steppan L., Smith B.B., Youngberg J.A., Henderson M.C., Buhler D.R. Role of the Ah locus in suppression of cytotoxic T lymphocyte activity by halogenated aromatic hydrocarbons (PCBs and TCDD): structure-activity relationships and effects in C57Bl/6 mice congenic at the Ah locus. Fundam. Appl. Toxicol. Off. J. Soc. Toxicol. 1990;14:532–541. doi: 10.1016/0272-0590(90)90257-k. [DOI] [PubMed] [Google Scholar]
- Kimura A., Naka T., Nohara K., Fujii-Kuriyama Y., Kishimoto T. Aryl hydrocarbon receptor regulates Stat1 activation and participates in the development of Th17 cells. Proc. Natl. Acad. Sci. U. S. A. 2008;105:9721–9726. doi: 10.1073/pnas.0804231105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopecky-Bromberg S.A., Martínez-Sobrido L., Frieman M., Baric R.A., Palese P. Severe acute respiratory syndrome coronavirus open reading frame (ORF) 3b, ORF 6, and nucleocapsid proteins function as interferon antagonists. J. Virol. 2007;81:548–557. doi: 10.1128/JVI.01782-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larigot L., Juricek L., Dairou J., Coumoul X. AhR signaling pathways and regulatory functions. Biochim. Open. 2018;7:1–9. doi: 10.1016/j.biopen.2018.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei X., Dong X., Ma R., Wang W., Xiao X., Tian Z., Wang C., Wang Y., Li L., Ren L., Guo F., Zhao Z., Zhou Z., Xiang Z., Wang J. Activation and evasion of type I interferon responses by SARS-CoV-2. Nat. Commun. 2020;11:3810. doi: 10.1038/s41467-020-17665-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenschow D.J. Antiviral properties of ISG15. Viruses. 2010;2:2154–2168. doi: 10.3390/v2102154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letko M., Marzi A., Munster V. Functional assessment of cell entry and receptor usage for SARS-CoV-2 and other lineage B betacoronaviruses. Nat. Microbiol. 2020;5:562–569. doi: 10.1038/s41564-020-0688-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G., Cao Y., Sun Y., Xu R., Zheng Z., Song H. Ultrafine particles in the airway aggravated experimental lung injury through impairment in Treg function. Biochem. Biophys. Res. Commun. 2016;478:494–500. doi: 10.1016/j.bbrc.2016.05.059. [DOI] [PubMed] [Google Scholar]
- Li G., Fan Y., Lai Y., Han T., Li Z., Zhou P., Pan P., Wang W., Hu D., Liu X., Zhang Q., Wu J. Coronavirus infections and immune responses. J. Med. Virol. 2020;92:424–432. doi: 10.1002/jmv.25685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., Xu X.-L., Dai D.-W., Huang Z.-Y., Ma Z., Guan Y.-J. Air pollution and temperature are associated with increased COVID-19 incidence: a time series study. Int. J. Infect. Dis. IJID Off. Publ. Int. Soc. Infect. Dis. 2020;97:278–282. doi: 10.1016/j.ijid.2020.05.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litzenburger U.M., Opitz C.A., Sahm F., Rauschenbach K.J., Trump S., Winter M., Ott M., Ochs K., Lutz C., Liu X., Anastasov N., Lehmann I., Höfer T., von Deimling A., Wick W., Platten M. Constitutive IDO expression in human cancer is sustained by an autocrine signaling loop involving IL-6, STAT3 and the AHR. Oncotarget. 2014;5:1038–1051. doi: 10.18632/oncotarget.1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J., Chen Z., Wu M., Feng J., Horii Y., Ohura T., Kannan K. Airborne PM2.5/PM10-associated chlorinated polycyclic aromatic hydrocarbons and their parent compounds in a suburban area in Shanghai, China. Environ. Sci. Technol. 2013;47:7615–7623. doi: 10.1021/es400338h. [DOI] [PubMed] [Google Scholar]
- Machala M., Vondrácek J., Bláha L., Ciganek M., Neca J.V. Aryl hydrocarbon receptor-mediated activity of mutagenic polycyclic aromatic hydrocarbons determined using in vitro reporter gene assay. Mutat. Res. 2001;497:49–62. doi: 10.1016/s1383-5718(01)00240-6. [DOI] [PubMed] [Google Scholar]
- Maggi E., Canonica G.W., Moretta L. COVID-19: unanswered questions on immune response and pathogenesis. J. Allergy Clin. Immunol. 2020;146:18–22. doi: 10.1016/j.jaci.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier M.A., Ottiger M., Vögeli A., Steuer C., Bernasconi L., Thomann R., Christ-Crain M., Henzen C., Hoess C., Zimmerli W., Huber A., Mueller B., Schuetz P. Activation of the tryptophan/serotonin pathway is associated with severity and predicts outcomes in pneumonia: results of a long-term cohort study. Clin. Chem. Lab. Med. 2017;55:1060–1069. doi: 10.1515/cclm-2016-0912. [DOI] [PubMed] [Google Scholar]
- Mezrich J.D., Fechner J.H., Zhang X., Johnson B.P., Burlingham W.J., Bradfield C.A. An interaction between kynurenine and the aryl hydrocarbon receptor can generate regulatory T cells. J. Immunol. Baltim. Md. 2010;1950(185):3190–3198. doi: 10.4049/jimmunol.0903670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munn D.H., Sharma M.D., Lee J.R., Jhaver K.G., Johnson T.S., Keskin D.B., Marshall B., Chandler P., Antonia S.J., Burgess R., Slingluff C.L., Mellor A.L. Potential regulatory function of human dendritic cells expressing indoleamine 2,3-dioxygenase. Science. 2002;297:1867–1870. doi: 10.1126/science.1073514. [DOI] [PubMed] [Google Scholar]
- Muyayalo K.P., Huang D.-H., Zhao S.-J., Xie T., Mor G., Liao A.-H. COVID-19 and Treg/Th17 imbalance: potential relationship to pregnancy outcomes. Am. J. Reprod. Immunol. N. Y. N. 2020;1989 doi: 10.1111/aji.13304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neavin D.R., Liu D., Ray B., Weinshilboum R.M. The role of the aryl hydrocarbon receptor (AHR) in immune and inflammatory diseases. Int. J. Mol. Sci. 2018:19. doi: 10.3390/ijms19123851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen N.T., Kimura A., Nakahama T., Chinen I., Masuda K., Nohara K., Fujii-Kuriyama Y., Kishimoto T. Aryl hydrocarbon receptor negatively regulates dendritic cell immunogenicity via a kynurenine-dependent mechanism. Proc. Natl. Acad. Sci. U. S. A. 2010;107:19961–19966. doi: 10.1073/pnas.1014465107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park A., Iwasaki A. Type I and type III interferons - induction, signaling, evasion, and application to combat COVID-19. Cell Host Microbe. 2020;27:870–878. doi: 10.1016/j.chom.2020.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrulis J.R., Hord N.G., Perdew G.H. Subcellular localization of the aryl hydrocarbon receptor is modulated by the immunophilin homolog hepatitis B virus X-associated protein 2. J. Biol. Chem. 2000;275:37448–37453. doi: 10.1074/jbc.M006873200. [DOI] [PubMed] [Google Scholar]
- Petrulis J.R., Kusnadi A., Ramadoss P., Hollingshead B., Perdew G.H. The hsp90 Co-chaperone XAP2 alters importin beta recognition of the bipartite nuclear localization signal of the Ah receptor and represses transcriptional activity. J. Biol. Chem. 2003;278:2677–2685. doi: 10.1074/jbc.M209331200. [DOI] [PubMed] [Google Scholar]
- Platanias L.C. Mechanisms of type-I- and type-II-interferon-mediated signalling. Nat. Rev. Immunol. 2005;5:375–386. doi: 10.1038/nri1604. [DOI] [PubMed] [Google Scholar]
- Qin C., Zhou L., Hu Z., Zhang S., Yang S., Tao Y., Xie C., Ma K., Shang K., Wang W., Tian D.-S. Dysregulation of immune response in patients with coronavirus 2019 (COVID-19) in Wuhan, China. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2020;71:762–768. doi: 10.1093/cid/ciaa248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintana F.J., Sherr D.H. Aryl hydrocarbon receptor control of adaptive immunity. Pharmacol. Rev. 2013;65:1148–1161. doi: 10.1124/pr.113.007823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintana F.J., Basso A.S., Iglesias A.H., Korn T., Farez M.F., Bettelli E., Caccamo M., Oukka M., Weiner H.L. Control of T(reg) and T(H)17 cell differentiation by the aryl hydrocarbon receptor. Nature. 2008;453:65–71. doi: 10.1038/nature06880. [DOI] [PubMed] [Google Scholar]
- Reid E., Charleston B. Type I and III interferon production in response to RNA viruses. J. Interferon Cytokine Res. Off. J. Int. Soc. Interferon Cytokine Res. 2014;34:649–658. doi: 10.1089/jir.2014.0066. [DOI] [PubMed] [Google Scholar]
- Rizk J.G., Kalantar-Zadeh K., Mehra M.R., Lavie C.J., Rizk Y., Forthal D.N. Pharmaco-immunomodulatory therapy in COVID-19. Drugs. 2020 doi: 10.1007/s40265-020-01367-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez Y., Novelli L., Rojas M., De Santis M., Acosta-Ampudia Y., Monsalve D.M., Ramírez-Santana C., Costanzo A., Ridgway W.M., Ansari A.A., Gershwin M.E., Selmi C., Anaya J.-M. Autoinflammatory and autoimmune conditions at the crossroad of COVID-19. J. Autoimmun. 2020:102506. doi: 10.1016/j.jaut.2020.102506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruan Q., Yang K., Wang W., Jiang L., Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020;46:846–848. doi: 10.1007/s00134-020-05991-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaguchi S., Miyara M., Costantino C.M., Hafler D.A. FOXP3+ regulatory T cells in the human immune system. Nat. Rev. Immunol. 2010;10:490–500. doi: 10.1038/nri2785. [DOI] [PubMed] [Google Scholar]
- Schoggins J.W., Wilson S.J., Panis M., Murphy M.Y., Jones C.T., Bieniasz P., Rice C.M. A diverse range of gene products are effectors of the type I interferon antiviral response. Nature. 2011;472:481–485. doi: 10.1038/nature09907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schön M.P., Erpenbeck L. The Interleukin-23/Interleukin-17 Axis links adaptive and innate immunity in psoriasis. Front. Immunol. 2018;9:1323. doi: 10.3389/fimmu.2018.01323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schön M.P., Berking C., Biedermann T., Buhl T., Erpenbeck L., Eyerich K., Eyerich S., Ghoreschi K., Goebeler M., Ludwig R.J., Schäkel K., Schilling B., Schlapbach C., Stary G., von Stebut E., Steinbrink K. COVID-19 and immunological regulations - from basic and translational aspects to clinical implications. J. Dtsch. Dermatol. Ges. J. Ger. Soc. Dermatol. JDDG. 2020 doi: 10.1111/ddg.14169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz M.D., Emerson S.G., Punt J., Goff W.D. Decreased Naïve T-cell Production Leading to Cytokine Storm as Cause of Increased COVID-19 Severity with Comorbidities. Aging Dis. 2020;11:742–745. doi: 10.14336/AD.2020.0619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seok S.-H., Ma Z.-X., Feltenberger J.B., Chen Hongbo, Chen Hui, Scarlett C., Lin Z., Satyshur K.A., Cortopassi M., Jefcoate C.R., Ge Y., Tang W., Bradfield C.A., Xing Y. Trace derivatives of kynurenine potently activate the aryl hydrocarbon receptor (AHR) J. Biol. Chem. 2018;293:1994–2005. doi: 10.1074/jbc.RA117.000631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiqi H.K., Mehra M.R. COVID-19 illness in native and immunosuppressed states: a clinical-therapeutic staging proposal. J. Heart Lung Transplant. Off. Publ. Int. Soc. Heart Transplant. 2020;39:405–407. doi: 10.1016/j.healun.2020.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song J.-W., Zhang C., Fan X., Meng F.-P., Xu Z., Xia P., Cao W.-J., Yang T., Dai X.-P., Wang S.-Y., Xu R.-N., Jiang T.-J., Li W.-G., Zhang D.-W., Zhao P., Shi M., Agrati C., Ippolito G., Maeurer M., Zumla A., Wang F.-S., Zhang J.-Y. Immunological and inflammatory profiles in mild and severe cases of COVID-19. Nat. Commun. 2020;11:3410. doi: 10.1038/s41467-020-17240-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockinger B., Di Meglio P., Gialitakis M., Duarte J.H. The aryl hydrocarbon receptor: multitasking in the immune system. Annu. Rev. Immunol. 2014;32:403–432. doi: 10.1146/annurev-immunol-032713-120245. [DOI] [PubMed] [Google Scholar]
- Takikawa O. Biochemical and medical aspects of the indoleamine 2,3-dioxygenase-initiated L-tryptophan metabolism. Biochem. Biophys. Res. Commun. 2005;338:12–19. doi: 10.1016/j.bbrc.2005.09.032. [DOI] [PubMed] [Google Scholar]
- Tsuji N., Fukuda K., Nagata Y., Okada H., Haga A., Hatakeyama S., Yoshida S., Okamoto T., Hosaka M., Sekine K., Ohtaka K., Yamamoto S., Otaka M., Grave E., Itoh H. The activation mechanism of the aryl hydrocarbon receptor (AhR) by molecular chaperone HSP90. FEBS Open Bio. 2014;4:796–803. doi: 10.1016/j.fob.2014.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turski W.A., Wnorowski A., Turski G.N., Turski C.A., Turski L. AhR and IDO1 in pathogenesis of Covid-19 and the “Systemic AhR Activation Syndrome:” Translational review and therapeutic perspectives. Restor. Neurol. Neurosci. 2020 doi: 10.3233/RNN-201042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veldhoen M., Hirota K., Westendorf A.M., Buer J., Dumoutier L., Renauld J.-C., Stockinger B. The aryl hydrocarbon receptor links TH17-cell-mediated autoimmunity to environmental toxins. Nature. 2008;453:106–109. doi: 10.1038/nature06881. [DOI] [PubMed] [Google Scholar]
- Wang F., Hou H., Luo Y., Tang G., Wu S., Huang M., Liu W., Zhu Y., Lin Q., Mao L., Fang M., Zhang H., Sun Z. The laboratory tests and host immunity of COVID-19 patients with different severity of illness. JCI Insight. 2020:5. doi: 10.1172/jci.insight.137799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q., Fang J., Shi W., Dong X. Distribution characteristics and policy-related improvements of PM2.5 and its components in six Chinese cities. Environ. Pollut. Barking Essex. 2020;266:115299. doi: 10.1016/j.envpol.2020.115299. 1987. [DOI] [PubMed] [Google Scholar]
- Wu D., Yang X.O. TH17 responses in cytokine storm of COVID-19: an emerging target of JAK2 inhibitor Fedratinib. J. Microbiol. Immunol. Infect. Wei Mian Yu Gan Ran Za Zhi. 2020;53:368–370. doi: 10.1016/j.jmii.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie M., Coons T.L., Hemann J.G., Dutton S.J., Milford J.B., Peel J.L., Miller S.L., Kim S.-Y., Vedal S., Sheppard L., Hannigan M.P. Intra-urban spatial variability and uncertainty assessment of PM2.5 sources based on carbonaceous species. Atmospheric Environ. Oxf. Engl. 2012;1994(60):305–315. doi: 10.1016/j.atmosenv.2012.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q., Lavorgna A., Bowman M., Hiscott J., Harhaj E.W. Aryl hydrocarbon receptor interacting protein targets IRF7 to suppress antiviral signaling and the induction of type I interferon. J. Biol. Chem. 2015;290:14729–14739. doi: 10.1074/jbc.M114.633065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou P., Yang X.-L., Wang X.-G., Hu B., Zhang L., Zhang W., Si H.-R., Zhu Y., Li B., Huang C.-L., Chen H.-D., Chen J., Luo Y., Guo H., Jiang R.-D., Liu M.-Q., Chen Y., Shen X.-R., Wang X., Zheng X.-S., Zhao K., Chen Q.-J., Deng F., Liu L.-L., Yan B., Zhan F.-X., Wang Y.-Y., Xiao G.-F., Shi Z.-L. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]