Abstract
Alcohol use disorder has multiple characteristics including excessive ethanol consumption, impaired control over drinking behaviors, craving and withdrawal symptoms, compulsive seeking behaviors, and is considered a chronic condition. Relapse is common. Determining the neurobiological targets of ethanol and the adaptations induced by chronic ethanol exposure is critical to understanding the clinical manifestation of alcohol use disorders, the mechanisms underlying the various features of the disorder, and for informing medication development. In the present review, we discuss ethanol’s interactions with a variety of neurotransmitter systems, summarizing findings from preclinical and translational studies to highlight recent progress in the field. We then describe animal models of ethanol self-administration, emphasizing the value, limitations, and validity of commonly used models. Lastly, we summarize the behavioral changes induced by chronic ethanol self-administration, with an emphasis on cue-elicited behavior, the role of ethanol-related memories, and the emergence of habitual ethanol seeking behavior.
Keywords: neuroimmune, adrenergic, opioid, habitual behavior, neuropeptide, microdialysis
1. Introduction
Ethanol is a simple two carbon molecule with a single hydroxyl group bound to one of the carbons, but this simple molecule has tremendous significance for society, medicine, and pharmacology. There is a long history of the consumption of ethanol containing beverages by humans as an agent to produce intoxication (Curry, 2017). The scientific study of ethanol and its effects on the human body has been largely driven by attempts to understand the pharmacological and toxicological effects of acute and chronic ethanol exposure. Ethanol consumption provides numerous desired effects on human consciousness and social interactions due to its intoxicating nature.
The medical and social problems that are caused by the prolonged excessive consumption of ethanol in humans, known collectively as alcohol use disorders (AUD), spans a spectrum of severity that can include impaired decision making, interpersonal problems, and serious physical consequences as ethanol can have toxic effects on the liver (Rehm et al., 2010), heart (Urbano-Marquez et al., 1989), and brain (NIAAA, 2001), among numerous organ systems.
A common thread for AUD is the loss of control over consumption of ethanol that eventually leads to gross intoxication of the individual and prominent behavioral problems. Since the brain ultimately controls complex behavior including self-administration of ethanol, the study of how ethanol alters brain function is critical to understanding the mechanisms of ethanol-induced behaviors. While animal models hold tremendous value for identifying these mechanisms, ultimately, the goal of such studies is to provide new knowledge that may be harnessed to reduce or reverse the harm caused by AUD. Currently, there are only three available pharmacotherapeutic agents approved by the Food and Drug Administration of the United States for treatment of AUD (Akbar et al., 2018), and as these medications have modest efficacy, there is a critical need for new knowledge of neurobiological mechanisms of ethanol.
The present review covers the neurobiological and neurochemical substrates implicated in ethanol self-administration, summarizing findings from preclinical and translational studies to highlight recent progress in the field. We first describe animal models of ethanol self-administration, emphasizing the value, limitations, and validity of commonly used models. Next, we summarize the behavioral changes induced by chronic ethanol self-administration, with an emphasis on cue-elicited behavior, the role of ethanol-related memories, and the emergence of habitual ethanol seeking behavior. Lastly, we discuss ethanol’s interactions with specific neurochemical systems and the proposed functional implications.
2. Animal models of ethanol self-administration
2.1. Two-bottle choice models of ethanol drinking initiation
Rodents can be forced to initiate ethanol drinking by depriving them of other fluids (Veale and Myers, 1969; Wise, 1973). However, the most valid non-human models of alcohol drinking initiation process are those that incorporate a free choice between ethanol containing fluids and fluids containing substances other than ethanol. The amount of alcohol consumed by rodents in free-choice (homecage) paradigms is a function of several factors such as the concentration of alcohol in the bottle (e.g., Veale and Myers, 1969), how many bottles are presented (e.g., Palm et al., 2011), for how long alcohol is available and when (Wayner et al., 1972; Wise, 1973), whether the alcohol is sweetened (Cox and Mertz, 1985; Samson and Falk, 1974), and what other fluids are available (Colombo et al., 1997; Cox and Mertz, 1985; Loi et al., 2010; Samson and Falk, 1974). Sensitivity to these factors is also a function of rodent species, strain, and sex as well as suppliers or housing conditions (Belknap et al., 1993; Linseman, 1987; Melchior and Myers, 1976; Morales et al., 2015; Palm et al., 2011; Simms et al., 2008; Sparks et al., 2014; Wise, 1973; Yoneyama et al., 2008). The number of rodents that initiate and maintain alcohol drinking in these models may also be a function of the same factors. However, the latter is difficult to know because, as Carnicella et al. (2014) noted in a review of findings from the most popular variant of these models, researchers do not routinely report the number of rodents that they acquired vs used for their studies.
One of the mechanisms that may promote and/or maintain ethanol drinking in rodents during the initiation phase is habituation to the aversive taste of ethanol or conditioning of taste preference. Ethanol-naive rodents initially respond to the taste of unsweetened ethanol solutions with orofacial gestures indicative of disgust (Kiefer et al., 1994; Kiefer et al., 1990; Kiefer and Dopp, 1989). These negative responses (“dislike”) decrease following repeated opportunities to consume ethanol while orofacial responses that indicate “liking” remain unchanged or increase in frequency. Both “like” and “dislike” reactions revert back to initial levels after sufficient time after cessation of access (Kiefer et al., 1994; Kiefer and Dopp, 1989). Indirect measures of preference such as relative fluid consumption (e.g., ratio of consumption from the ethanol bottle versus total fluid consumption) tend to be initially low and increase over time (e.g., Simms et al., 2008). Under typical conditions, most unselected rat strains fail to show a reliable preference for ethanol over alternative fluids. In contrast, several mouse strains (e.g., C57BL/6J) will show a reliable preference for 6–10% ethanol (v/v) over plain tap water (Yoneyama et al., 2008) consuming 60% or more of their total daily fluid from the ethanol bottle.
Support for the idea that homecage drinking paradigms allow for reinforcement of ethanol seeking and drinking by ethanol’s neuropharmacological properties comes from two lines of evidence. First, these paradigms allow voluntary exposure to a wide range of blood alcohol concentrations (e.g., see Table 1 in Carnicella et al., 2014), with intermittent access protocols [i.e. alternating periods of free access to 2-bottle choice (water and ethanol) with periods during which ethanol is not available (for examples see Carnicella et al 2014; Holgate et al 2017)] generally yielding higher levels of ethanol intake than continuous access protocols (Carnicella et al, 2014). Second, the neurobiological correlates of ethanol seeking and drinking identified from analysis of rodent brains trained on the homecage paradigms overlap with the correlates identified in other paradigms (e.g., operant oral self-administration, Pavlovian conditioning). Specifically, the mesocorticolimbic systems are similarly involved in ethanol drinking across paradigms. For example, dopamine release can be observed in the ventral striatum of rats drinking ethanol in the homecage two-bottle paradigm (Ericson et al., 1998) just like it can be observed in the operant oral self-administration paradigm (see Section 4.1; Bassareo et al., 2017; Doyon et al., 2003, 2005; Howard et al., 2009; Robinson et al., 2009). Other neuromodulatory signals in the corticostriatal systems such as brain-derived neurotrophic factor are also similarly engaged by ethanol consumption in both paradigms (Jeanblanc et al., 2009, 2013; Logrip et al., 2009).
2.2. Operant self-administration in adult animals
2.2.1. Reinforcement schedules
Operant ethanol self-administration models, in which access to ethanol is contingent upon completion of a specific response or responses (i.e. lever pressing, nose-poking) (Skinner, 1938), are central to studying the behavioral pharmacology of ethanol. Preclinical operant models provide an opportunity to measure and experimentally manipulate the reinforcing effects of ethanol (or other drugs), as well as model human drug seeking.
Within an operant paradigm, appetitive/seeking and consummatory behaviors can be examined separately. The schedule of reinforcement determines the amount of work necessary to access alcohol, and different reinforcement schedules can produce different behavioral steady states (for complete review, see Panlilio and Goldberg, 2007; Cunningham et al., 2000; Leslie, 2003). The most commonly used schedules are fixed ratio, fixed interval, variable ratio, and variable interval. Ratio schedules are those in which a specified number of responses are required for access to the reinforcer and interval schedules are those in which a specified amount of time must pass before the response grants access to the reinforcer. Under fixed schedules, the response requirement remains constant throughout the operant session, while under variable schedules, the response requirement fluctuates within the operant session. Another schedule of reinforcement is the progressive ratio, in which the response requirement steadily increases for each subsequent reinforcer. The highest completed response requirement is termed the “break point” and represents the motivational salience or reinforcement efficacy of the drug (Czachowski and Samson, 1999). These fundamental schedules are frequently used to explore the neurobiological substrates and circuits involved in ethanol self-administration and in medications development for the treatment of AUD (Cunningham et al., 2000; June and Gilpin, 2010; Leslie, 2003; Panlilio and Goldberg, 2007; Samson and Czachowski, 2003).
A challenge encountered with the aforementioned reinforcement schedules is that ethanol seeking is conflated with ethanol consumption, and the rate of responding may be influenced by intoxication. Further, when operant paradigms are used in conjunction with neurochemical assays, such as in vivo voltammetry or microdialysis, it becomes impossible to distinguish the role of brain signaling molecules (i.e. neurotransmitters such as dopamine) in appetitive versus consummatory behaviors. To address this issue, Samson et al. (1998) established an appetitive-consummatory model in which a single completion of the response requirement provides ad libitum access to ethanol for a finite time period (usually 20–30 minutes). Using this model concurrently with in vivo microdialysis to quantify extracellular dopamine and ethanol concentrations facilitated the discovery that a transient, but robust accumbal dopamine response occurs at the beginning of the oral consumption period, when brain concentrations of ethanol are very low (Carrillo and Gonzales, 2011; Doyon et al., 2003; Doyon et al., 2005). Interestingly, this response declines to baseline as brain ethanol concentrations increase, suggesting that, in experienced animals, the dopamine signal may not be due to ethanol’s pharmacological actions, but instead may be an anticipatory response to the sensory cues associated with ethanol (Vena and Gonzales, 2015; Gonzales et al., 2004).
In sum, operant ethanol self-administration paradigms are excellent tools for examining and manipulating the behavioral pharmacology of alcohol in animal models. Decades of research employing operant paradigms have provided valuable insights regarding the neurobiological mechanisms of alcohol seeking behavior and reinforcement.
2.2.2. Routes of administration
A unique opportunity afforded by the use of animal models is the variety of options for the route by which ethanol is administered. Oral ethanol consumption provides the greatest face validity for human ethanol use, but rodents show an innate taste avoidance for ethanol and thus, researchers typically must incorporate strategies [i.e. food and/or fluid deprivation, adding sweeteners to the drinking solution, several weeks of acquisition of ethanol drinking in the home cage (see Section 2.1) etc.] for initiating the consumption of ethanol doses that provide pharmacological stimulation sufficient for reinforcement (Cunningham et al., 2000; Samson et al., 1988). Other commonly used routes of ethanol self-administration are intracranial microinjections, in which ethanol is locally infused into the brain, and intravenous infusions whereby ethanol is delivered directly to the bloodstream, bypassing liver metabolism and rapidly reaching the brain.
The route of ethanol administration is typically determined by the research question. For example, oral self-administration is a valid experimental model of human use, but interpretations of ethanol’s pharmacological actions may be confounded by individual variation in consumption (i.e. lick rates or quantity consumed), ethanol pharmacokinetics, and sensitivity to ethanol’s nonpharmacological effects, such as taste, odor, and caloric value (Cunningham et al., 2000; Lê and Kalant, 2017; Windisch et al., 2014). Therefore, researchers interested in examining ethanol’s specific pharmacological mechanisms of action during operant self-administration may use intravenous or intracranial route of administration; both permit delivery of precise and standardized doses of ethanol while eliminating the influence of its orosensory effects (Gass and Olive, 2007; McBride et al., 1999; Windisch et al., 2014). While intravenous administration provides systemic delivery of controlled ethanol doses, intracranial microinjections facilitate the localization of specific regions and circuits mediating ethanol’s reinforcing effects. Indeed, studies have shown that mice, rats, and non-human primates will maintain intravenous or intracranial self-administration (Grahame and Cunningham, 2002; Lê and Kalant, 2017; Rodd et al., 2004; Windisch et al., 2014).
2.2.3. Operant models of compulsive ethanol seeking and relapse
AUD in humans is characterized by its chronic and relapsing nature and the continued use of ethanol despite adverse consequences. These clinical features of AUD have been modeled in animals with a history of operant ethanol self-administration.
Using a reinstatement model, animals show relapse-like behavior by resuming ethanol seeking behavior in the presence of ethanol associated stimuli after extinction (Le and Shaham, 2002). To extinguish responding in trained animals, contextual cues remain in the operant chambers, but responses no longer provide access to the ethanol solution. Several studies have demonstrated that operant ethanol seeking behavior may be reinstated by discrete or contextual ethanol-associated cues (i.e. taste and smell of ethanol or conditioned olfactory, auditory, visual, or tactile stimuli) and by stress (i.e. intermittent footshock) (Chaudhri et al., 2008; Lê and Shaham, 2002; Lê et al., 1998). While administration of low doses of ethanol have been shown to reinstate seeking behavior, the effects in rodents are modest (Gass and Olive, 2007; Lê and Shaham, 2002; Lê et al., 1998), and there is a valid argument that non-contingent ethanol priming in animals does not parallel human lapses to ethanol use (Epstein et al., 2006).
Limited evidence suggests that after a prolonged period of ethanol self-administration or passive induction of ethanol dependence (O’Dell et al., 2004), rodents display compulsive ethanol seeking and use behaviors that may be accompanied by an escalation of ethanol self-administration. Although this remains a nascent area of research, recent studies have employed operant-based assessments of compulsive ethanol-seeking behaviors. For example, in a progressive ratio assay, rats with 3–4 months of intermittent ethanol access continue to seek ethanol despite taste adulteration with quinine. In contrast, rats with a shorter history of ethanol experience and those consuming sucrose in the operant chamber reduce seeking behavior after quinine is added to the drinking solution (Hopf et al., 2010). Similarly, rats show continued alcohol-seeking despite footshock punishments, though rats may vary in their sensitivity to punishment (Marchant et al., 2018). Collectively, this emerging body of literature indicates that operant-based assays may be effective in examining compulsive alcohol behaviors in animal models.
2.3. Self-administration of ethanol in adolescent animals
2.3.1. Ethanol self-administration in adolescent animal models compared to adults
Most animal models of alcohol use indicate that adolescents consume more ethanol per drinking session than adults, similar to human epidemiological data (Bell et al. 2011; Broadwater et al. 2011; Doremus et al. 2005; García-Burgos et al. 2009; Vetter et al. 2007). However, other studies have shown no differences or less intake in adolescents compared to adults (Doherty and Gonzales, 2015; Labots et al. 2018; Schindler et al. 2014; Schramm-Sapyta et al. 2010; Siegmund et al. 2005). A detailed review of adolescent drinking models is outside of the scope of this review, however methodological differences such as stress, housing conditions, age, and ethanol administration route or self-administration model all likely contribute to inconsistent results within the literature. Nonoperant self-administration paradigms are utilized in the majority of adolescent work, in part due to the limited timeframe (approximately 20 days in male rats) to facilitate operant training and overcome initial aversive properties of ethanol. As operant self-administration models have high predictive validity (Carter and Griffiths, 2009), more research utilizing operant models in adolescent rats would extend our knowledge on adolescent drinking behaviors and clinical utility of potential treatments in this population.
2.3.2. Modeling treatment in adolescents
The prevalence of adolescents diagnosed with AUD is rising, however only a small proportion receive treatment in part due to lack of data on the effectiveness of available medications in this age group (Miranda and Treloar, 2016; Swendsen et al., 2012). Due to ethical limitations and multiple factors that can significantly impact treatment outcomes in younger individuals (e.g. childhood trauma, age of drinking onset), bridging preclinical models with clinical findings is particularly critical during adolescence. An example from recent clinical work demonstrated that the nonselective opioid receptor antagonist naltrexone reduced heavy drinking and blunted craving in adolescents (ages 15–19) and young adults (ages 18–25), although larger trials are needed to replicate these results (Miranda et al., 2014; O’Malley et al., 2015). It is well established within the animal literature that naltrexone significantly decreases operant ethanol self-administration in adult rats (Ciccocioppo et al., 2003; Gonzales and Weiss, 1998; Hay et al., 2013; Henderson-Redmond and Czachowski, 2014). However, to our knowledge only one previous study had investigated naltrexone efficacy during adolescent ethanol self-administration, using alcohol-preferring (P) rats and a two-bottle choice paradigm (Sable et al., 2006). Recent findings from our lab expanded these results to show naltrexone significantly reduced sweetened ethanol, but not sucrose, operant self-administration during a progressive ratio schedule in adolescents at similar levels to adult rats (Figure 1; from dissertation by Zandy, S., 2016). There is some evidence suggesting opioid receptor signaling is present in adolescents like that in adults (Ellgren et al., 2008; Palm and Nylander, 2014). However, endogenous opioids have been found to differ between animal strain, housing, and ethanol exposure. Recently, forced ethanol exposure in adolescence was shown to produce residual changes in endogenous opioid peptides in brain areas associated with anxiety and stress (Granholm et al., 2018). Taken together, these results highlight one example investigating treatment effectiveness across animal and clinical models during adolescence. However, more studies are needed to determine if the proposed mechanism of action of naltrexone for reducing ethanol self-administration differs in adolescent rats compared with adults.
Figure 1.
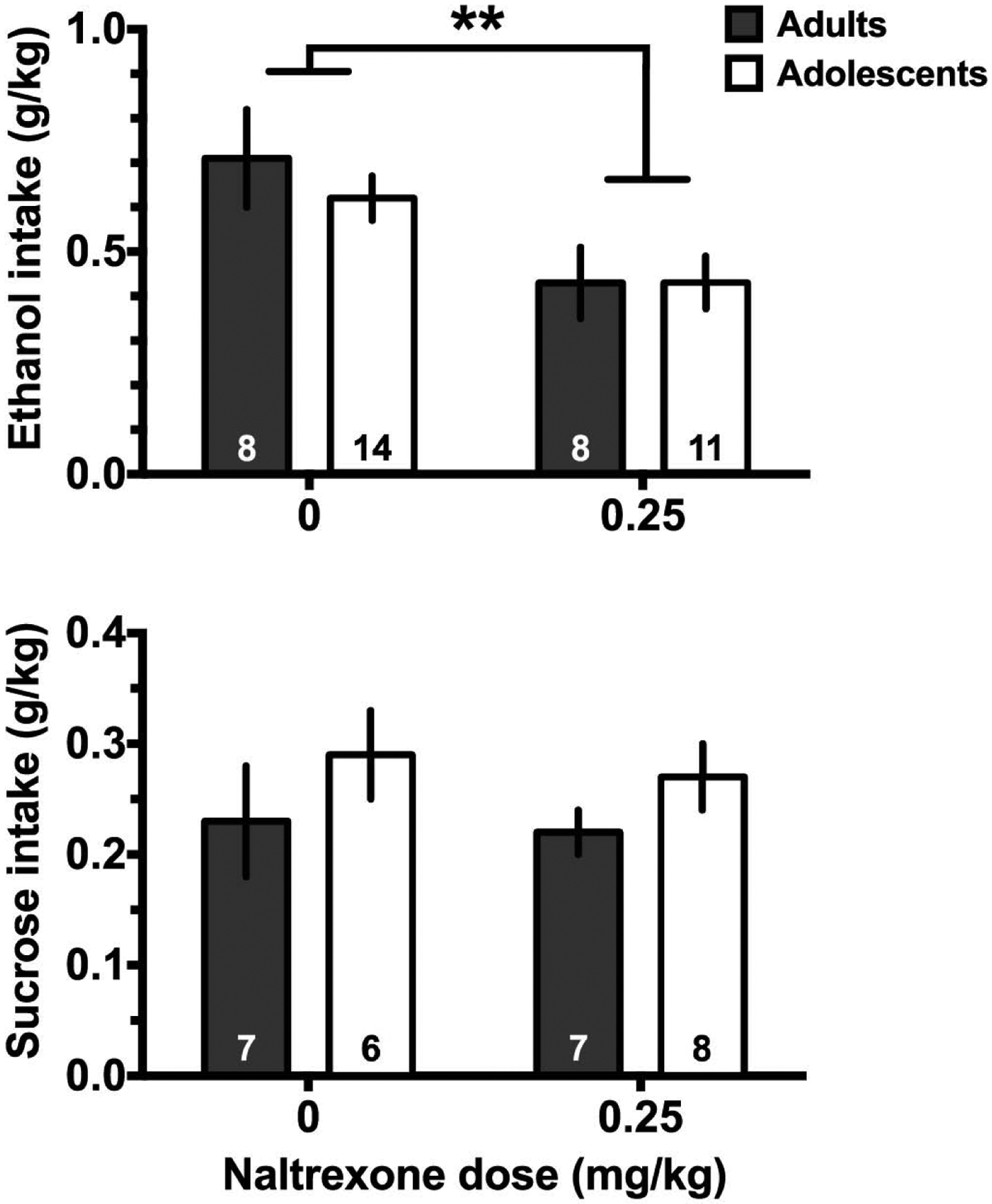
Preliminary data demonstrating that naltrexone inhibits consumption of sweetened ethanol (upper) but not sucrose (lower) intake during a progressive ratio test. (Upper panel) Naltrexone significantly reduced sweetened ethanol (10% sucrose + 10% ethanol in water) consumption in the progressive ratio test in adolescents and adults (** indicates p<0.01, main effect of naltrexone). (Lower panel) Naltrexone did not alter sucrose (10% in water) consumption during the progressive ratio test in control rats. Naltrexone or vehicle was given subcutaneously 30 min before the session. Details of the operant training and testing are in Doherty and Gonzales (2015). For both panels data presented as mean ± sem, and group n is shown within the bars.
2.3.3. Neurochemical development in adolescents
Adolescence is a period of significant development of the central nervous system including synaptic pruning, structural maturation, and changes in neurotransmitter systems, which are proposed to contribute to some of the behavioral characteristics (e.g. risk taking, reward seeking) evident during this age range (reviewed in Crews et al., 2016; Fuhrmann et al., 2015; Spear, 2018). Due to its role in processing reinforcing stimuli, the dopaminergic system has been extensively studied in adolescence in order to determine if neurochemical differences impact drinking behaviors during this developmental period. Age-dependent differences in firing rates of dopamine neurons (McCutcheon et al., 2012; Marinelli and McCutcheon, 2014) and basal extracellular dopamine concentrations (Badanich et al., 2006; Philpot et al., 2009) in the mesolimbic system appear to peak in mid to late adolescence, earlier than within the prefrontal cortex (Burke and Miczek, 2014). Binge-like ethanol exposure in adolescence has been shown to produce alterations in dopamine dynamics in adulthood within areas such as nucleus accumbens (Shnitko et al., 2016, Zandy et al., 2015) and prefrontal cortex (Trantham-Davidson et al., 2017).
Additionally, many studies examining the development of neurotransmitter systems across adolescence have focused primarily on male animals, limiting generalizability across sexes particularly with pubertal changes occurring during adolescence. Recently, Kopec et al. (2018) described sex-specific differences in microglia regulation of dopaminergic development in the nucleus accumbens. These results highlight the need for additional research to understand if distinct mechanisms underlying neurochemical development between males and females during adolescence could contribute to alterations in ethanol behaviors. Overall, multiple neurochemical differences found between adolescents and adults are hypothesized to contribute to the suggested model that adolescents may exhibit increased reward sensitivity and attenuated aversion to ethanol (Doremus-Fitzwater and Spear, 2016).
2.4. Limitations of existing animal self-administration paradigms
Central to the ecological validity of animal models of ethanol self-administration is the consumption of sufficient quantities of ethanol to produce reinforcement. Orally ingested ethanol undergoes first pass metabolism before entering systemic circulation and reaching neurobiological targets. In the absence of blood ethanol concentration (BEC) measurements, it is impossible to know whether any ingested ethanol has entered systemic circulation. Thus, the best evidence for an animal’s exposure to ethanol’s primary reinforcing properties is a non-zero blood (or brain) ethanol concentration (BEC). Yet, many studies fail to obtain BEC measurements in the animal at any point during its conditioning. Studies in our laboratory (Cofresí et al., 2018, 2019) and others (Tomie et al., 2004, 2006b, 2007; LeCocq et al., 2018; Fiorenza et al., 2018) provide direct evidence for the role of ethanol’s primary reinforcing properties in the acquisition of cue-elicited ethanol-related behavior by animals (see Section 3.1.1). Typically, BEC at the end of conditioning sessions in these studies is low (0.020–0.060 g/dL), but can be high (0.060–0.100 g/dL) under certain circumstances (e.g., Tomie et al., 2004, 2006b, 2007). In many two-bottle choice home cage paradigms, often only 50% or fewer of the rats will achieve binge levels of intoxication (BECs of 80 mg% or higher) (Carnicella et al., 2014). While it may not always be feasible to assess brain or blood ethanol concentrations either during or immediately subsequent to self-administration sessions, it is necessary to consider this parameter when interpreting findings, comparing across self-administration protocols, and developing animal models of ethanol self-administration.
Another critical issue for the interpretation of many, if not most, animal models is that the source of reinforcement remains unclear. Like the primary pharmacological agents (e.g., cocaine, heroin, nicotine) abused by humans, ethanol has central and peripheral effects on physiology; however, an important distinction is that ethanol is a source of calories. Thus, ethanol reinforcement may be attributed to its pharmacological and/or nutrient properties. Further complicating the issue, many rodent studies motivate ethanol ingestion by using fluid or food deprivation and/or naturalistic drinking solutions such as commercially available alcoholic beverages or artificially or naturally sweetened ethanol solutions prepared in the laboratory. Even in non-deprived animals, comparisons between conditioning to ethanol and isocaloric liquids remain necessary to dissociate between the two primary sources of ethanol reinforcement.
Numerous challenges exist in developing animal models of the progression from low to moderate to excessive alcohol intake, and it is infeasible for such models to mimic all of the clinical features of alcohol use and AUD. Nevertheless, existing models of self-administration, including home cage access, operant paradigms, and protocols using adolescent rodents, permit investigation of the mechanisms underlying key aspects of alcohol seeking and consumption and evaluation of the therapeutic efficacy of both pharmacological and non-pharmacological approaches for addressing pathological alcohol use behaviors in human.
3. Alterations in behavioral control following chronic ethanol self-administration
3.1. Pavlovian conditioning to ethanol
3.1.1. Cue-elicited appetitive responses in rodents
Following repeated alcohol self-administration, sensory stimuli associated with alcohol availability and intoxication can become conditioned cues that elicit appetitive, and subsequently, consummatory behaviors. The motivational properties of ethanol-related cues can be studied using Pavlovian conditioning paradigms in both rodents and humans. Rodent models provide an opportunity to dissect the neurobiological and behavioral mechanisms of ethanol cue-elicited reactivity and the extent to which it contributes to AUD development with greater experimental control over the quantity and frequency of ethanol exposure over the lifetime. Animal models of the progression to alcohol dependence have consistently demonstrated the role of Pavlovian alcohol cues in eliciting appetitive behaviors for alcohol, which can manifest as attentional bias, approach tendency, and seeking behaviors (Srey et al, 2015; Maddux and Chaudhri, 2017; Cofresi et al, 2018). However, the attribution of incentive salience and the extent to which ethanol cues elicit appetitive responses in rodents is highly dependent on the behavioral paradigm and its parameters.
In non-human animals, oral ethanol self-administration paradigms provide the greatest face-validity for human alcohol-related behavior. In an operant context, cue-elicited behavior directed toward specific stimuli in the self-administration chamber, including the magazine (the ethanol container; e.g., fluid cup, sipper tube) or lever (or other similar seeking response mechanism), serve as useful models of human ethanol seeking behaviors (e.g. approaching the beer aisle of a grocery store). Within an operant self-administration session, relatively small manipulations of alcohol delivery and availability can substantially impact approach behavior. In paradigms where fixed amounts of drinking solution are delivered into an omnipresent magazine, several factors interact to influence whether the cue acquires the ability to elicit approach behavior towards the cue itself, the magazine, or both, including the temporal relationship between cue and solution delivery, presence or absence of sweetener, food/fluid deprivation status, and the number of training sessions (Chaudhri et al., 2010; Krank, 2003; Krank et al., 2008; Srey et al., 2015; Villaruel and Chaudhri, 2016). In paradigms where time-limited opportunities to consume the reinforcer are presented via a retractable magazine (sipper) (Tomie et al., 2003; Tomie et al., 2006a; Tomie et al., 2011), the temporal relationship between cue and drinking opportunity may be the most important, if not sole, determinant of whether the cue acquires the ability to elicit approach behavior towards the cue itself, the magazine, or both. If the drinking opportunity starts at cue offset, then approach behavior tends to be directed towards the cue because it does not interfere with subsequent drinking. If the cue and drinking opportunity co-occur, then the cue tends to elicit anticipatory magazine-directed behavior because it facilitates drinking (Cofresí et al., 2018; 2019). Importantly, across the paradigms mentioned above, little to no cue-related behavior has been observed in studies using an explicitly unpaired cue-ethanol condition, indicating that cue-related behavior in these paradigms typically results from associative learning processes.
The use of Pavlovian conditioning paradigms in rodents has enabled investigation of behavioral and non-pharmacological interventions for AUD. For example, precise manipulation of memories for alcohol-related cues may help reduce reactivity to such stimuli (Hon et al., 2016). Retrieval and expression of consolidated long-term memory can, under certain conditions, destabilize the reactivated memory such that it must be reconsolidated. During the reconsolidation window, the reactivated and destabilized memory is vulnerable to interference (for review, see Lee et al., 2017). The vulnerability of maladaptive emotional memories during the reconsolidation window has been exploited to enhance the efficacy of interventions like cue extinctions in animal models, pre-clinical human laboratory models, and small clinical trials (Das et al., 2015a; for reviews, see Walsh et al., 2018; Kredlow et al., 2016; Lonergan et al., 2013). On the basis of its promise and the potential for rapid translation, our laboratory recently tested, in rodents, whether this memory retrieval-dependent reconsolidation window could be harnessed to increase the efficacy of ethanol cue exposure therapy after conditioning of alcohol cue-elicited alcohol seeking and drinking behaviors (Cofresí et al., 2017). Rats with previous alcohol self-administration experience (15 sessions; mean consumption: 3.5 g/kg/24hours) underwent 12 consecutive days of cue conditioning training followed by cue extinction training for 14 consecutive days. We found that the group treated with standard extinction was highly susceptible to post-treatment return of cue-elicited alcohol seeking and drinking behaviors whereas the group receiving the same treatment during reconsolidation of the alcohol cue memory exhibited little to no return of alcohol cue-elicited seeking and drinking behaviors (Figure 2). These studies suggest that cue exposure therapy might be optimized to help patients with AUD to prevent relapse.
Figure 2.
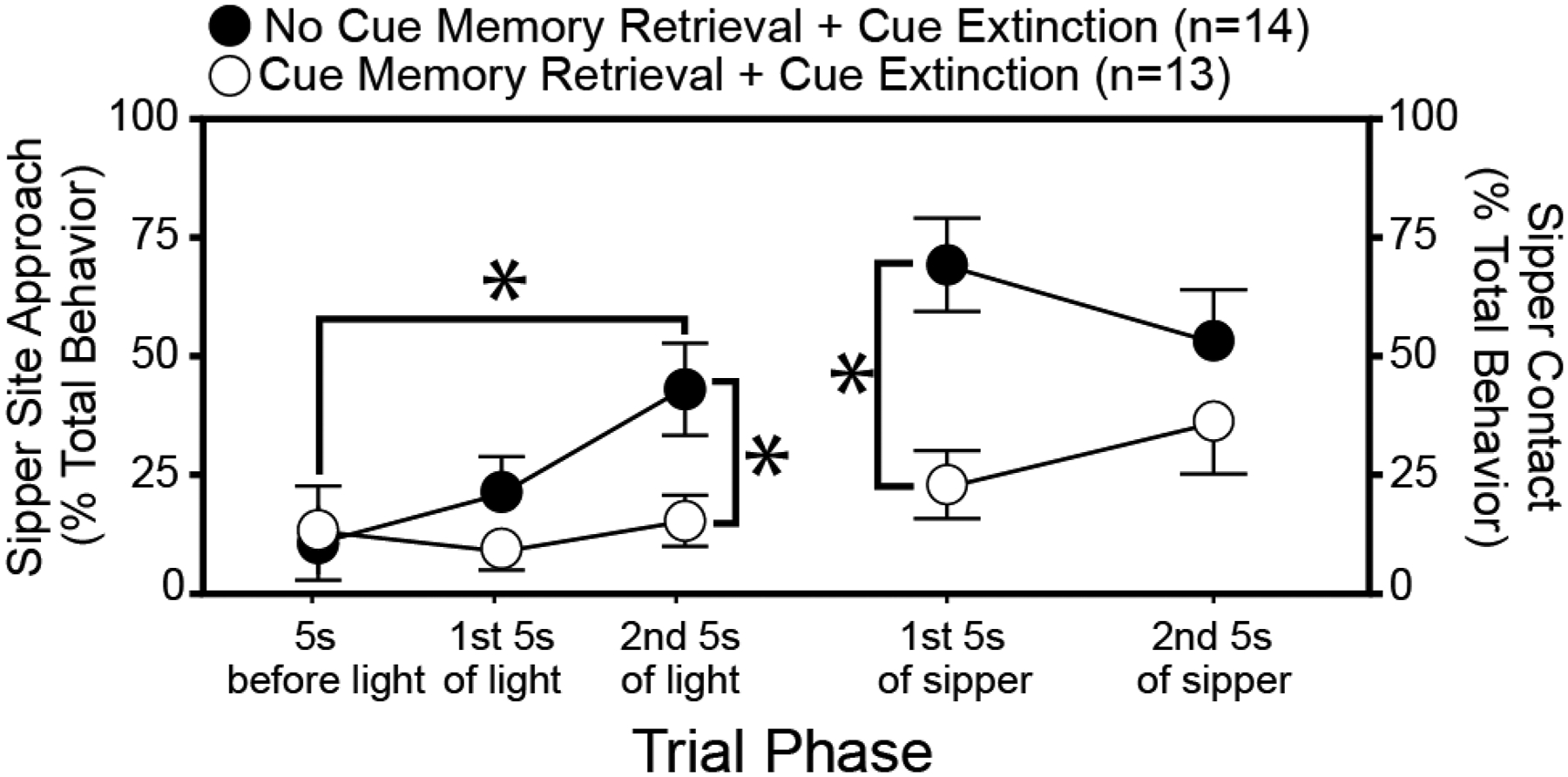
Ethanol cue extinction during cue memory retrieval-induced memory reconsolidation attenuates the ability of ethanol odor to reinstate the cue-elicited seeking-drinking response sequence. Ambient ethanol odor was re-introduced into the cue conditioning chambers after extensive cue extinction training. The degree to which responses to extinguished cues are reinstated by ambient ethanol odor is inversely related to the efficacy of cue extinction. The x-axis shows different trial phases beginning before light cue onset and extending from light cue onset through sipper access. During conditioning, the light remained illuminated during sipper access. Light and sipper presentation co-terminated. The first 3 points along the x-axis are plotted on the left y-axis and the last 2 points along the x-axis are plotted on the right y-axis. The left y-axis shows the frequency of sipper site approach states (movement toward and gnawing, nosing, or pawing the hole in the wall through which the sipper is later inserted) whereas the right y-axis shows the frequency of sipper contact states (licking the dry sipper tube while it was inserted). Both approach and contact state frequencies are expressed as a percentage of total behavioral state observations done by treatment-blinded expert raters from digital video recordings following the method of (Lee et al., 2005). Subjects were individually-housed, adult male Long-Evans rats. Mean ± sem of approach and contact state frequencies represented using black circles for the group of rats receiving our model of standard treatment and white circles for the group of rats receiving the same treatment after initial reactivation of the targeted cue memory via a single isolated cue presentation. Treatment groups were matched on: total extinction (light+dry sipper) trials, total conditioning (light+unsweetened ethanol via sipper) trials, context exposure, experimenter handling, response levels at the end of conditioning, response levels at the end extinction, ethanol doses ingested across conditioning, and ethanol doses ingested in the homecage before conditioning. Asterisks indicate p<0.05 for the indicated comparison via t-test. Data are adapted from (Cofresí et al., 2017).
3.1.2. Cue-elicited appetitive response in humans
In humans, sensory cues, such as the sight and smell of a preferred alcoholic beverage, and alcohol-related words and imagery can elicit approach, and consequently, consummatory behaviors. Similar to animals, typical ethanol cue-elicited behaviors in humans include attentional bias towards alcohol stimuli and approach tendency. Additionally, clinical research permits assessment of subjective alcohol craving, typically measured as self-reported urge or desire to drink, temptation to drink, or difficulty in resisting a drink if offered.
Ethanol-related pictures and words, as we all as the sight and smell of a person’s preferred alcoholic beverage can elicit greater attentional bias (Townshend and Duka, 2001; Field et al., 2005; Vollstädt-Klein et al., 2012; Snelleman et al., 2015; Manchery et al, 2017; Qureshi et al, 2019) and approach tendency (Field et al., 2005; Fleming and Bartholow, 2014; Hollet et al., 2017; Kreusch et al., 2017) in human drinkers than non-alcohol related stimuli. Both manifestations of cue reactivity are associated with drinking phenotypes as heavy drinkers and individuals with AUD tend to show greater reactivity towards alcohol cues as compared to occasional and non-dependent social drinkers (Field et al, 2004; Townshend and Duka, 2001; Barkby et al, 2012; Field and Cox, 2008; Fadardi and Cox, 2006; Qureshi et al, 2019). While this may be attributed to poorer cognitive performance among dependent vs non-dependent drinkers, Fadardi and Cox (2006) demonstrated that the greater attentional bias for alcohol stimuli among the former persists even after controlling for cognitive ability. However, an important caveat is the fact that the criteria defining heavy vs light drinkers varies across studies, hindering accurate comparisons between studies. Going forward, it will be important for research within this domain to achieve greater consilience in defining clinical drinking phenotypes.
Among human drinkers, subjective alcohol craving can be elicited by the sight, smell and/or taste of alcoholic beverages (Kambouropoulos and Staiger, 2004; Kareken et al., 2010; Kiefer et al., 2015; MacKillop et al., 2015; Kreusch et al, 2017; Yoder et al., 2009; Filbey et al., 2008; Oberlin et al., 2013; Oberlin et al., 2016). While cue-elicited craving has been observed in social, heavy, and AUD drinkers, few studies have directly compared drinking phenotypes to determine whether they differ in their sensitivity to sensory stimuli. One study reported that the sight and smell of alcohol elicited similar craving responses in light and heavy drinkers, but the criteria defining these two groups were unclear (Papachristou et al, 2012). With the inclusion of craving as a symptom of AUD in the most recent edition of the DSM, it is increasingly important that craving is assessed as a function of drinking behavior.
3.1.3. Neurobiological substrates of cue-elicited appetitive responses: Findings from animal and human models
Evidence from preclinical research indicates that the basolateral complex of the amygdala (BLA), nucleus accumbens, prefrontal cortex, and the insular cortex are key neurobiological substrates in the regulation of reward seeking behaviors, including ethanol cue-elicited appetitive responses (Stuber et al, 2011; Klenowski, 2018; Chaudhri et al., 2013; Sciascia et al., 2015; Millan et al., 2015; Setlow et al., 2002). Ethanol-related stimuli have been shown to induce activity in the prefrontal cortex, nucleus accumbens, and BLA (Jupp et al, 2010; Dayas et al, 2007), and ablation of prefrontal cortical inputs into the nucleus accumbens, but not the BLA, attenuates cue-induced reinstatement of alcohol seeking (Keistler et al, 2017). Similarly, pharmacological inactivation of the BLA or nucleus accumbens, reduces cue-induced alcohol seeking (Chaudhri et al, 2010; Chaudhri et al, 2013; Millan et al., 2015). Within the BLA and the nucleus accumbens, increased glutamatergic activity is associated with alcohol seeking behaviors (Gass et al., 2011; Sinclair et al, 2012). While the BLA provides glutamatergic input to the nucleus accumbens (Stuber et al, 2011), another source of excitatory drive into both the nucleus accumbens and the BLA is the insular cortex (Shi and Cassell, 1998; Reynolds and Zahm, 2005) and this circuit has been shown to regulate interoceptive cues produced by alcohol ingestion (Jaramillo et al., 2016). Initial findings also indiciate that the insular cortex may be a key component of the ability of exteroceptive alcohol-predictive cues to elicit alcohol seeking behavior in non-dependent rats (Cofresí et al., 2019). The orbitofrontal cortex is another region that is highly interconnected with the BLA and nucleus accumbens, and has long been implicated in processing reward-related cues and adaptive responding (Schoenbaum and Shaham, 2008; Lasseter et al., 2011; Takahashi et al, 2009). While initial evidence supports a role for the orbitofrontal cortex in context-induced alcohol seeking behaviors (Bianchi et al., 2018), the region has been remarkably understudied in animals with regards to alcohol cue reactivity (Moorman et al., 2018). Collectively, this emerging literature implicates several limbic, cortical, and striatal regions, which are highly interconnected, in the expression of Pavlovian conditioning to ethanol cues and appetitive behaviors, with research continuing to examine the precise functional roles of specific circuits and subregions.
Achieving consensus among findings from clinical functional neuroimaging studies has been difficult due to methodological inconsistencies [i.e. use of different cue types (gustatory, visual, olfactory), lack of a control group, and varying definitions of heavy drinking behaviors or AUD severity] and small sample sizes. Nevertheless, there are notable consistencies with the preclinical literature regarding the activation of frontal, striatal, and insular structures by ethanol-related stimuli in humans (for review, see Schacht et al., 2013). Compared to neutral cues, alcohol cues elicit activation of the ventral striatum, anterior cingulate cortex, orbitofrontal cortex, dorsolateral prefrontal cortex, and insula (Filbey et al, 2008; Bach et al, 2015; Park et al., 2007; Myrick et al, 2013; Oberlin et al., 2016; Bragulat et al., 2008; Claus et al., 2011) to a similar extent among controls, heavy drinkers, and drinkers with AUD (Schacht et al, 2013). Heavier drinking and AUD, however, may be associated with selective enhancement of cue-induced activation of parietal and temporal regions, greater connectivity between the insula and nucleus accumbens (Schacht et al., 2013; Grodin et al., 2018), and greater alcohol cue-induced activation of the dorsal striatum (Filbey et al., 2008; Vollstadt-Klein et al., 2010). Further, initial findings suggest that appetitive responses in humans (i.e. approach tendencies and subjective craving) are positively correlated with activity in the dorsal striatum, insula, ventral striatum, medial frontal cortical regions, and orbitofrontal cortex (Bach et al, 2015; Oberlin et al., 2016; Fryer et al., 2013; Weirs et al, 2014; Grodin et al, 2018).
In sum, across animal and human studies, prefrontal cortical regions, the ventral striatum (specifically the nucleus accumbens), and insula are consistently implicated in alcohol cue reactivity and appetitive responses. More broadly, these substrates, along with the basolateral amygdala, regulate the attribution of incentive salience to cue associated with rewards. Of note, however, clinical support for the basolateral amygdala in alcohol cue-elicited behaviors is generally lacking.
3.2. Emergence of “habitual” ethanol seeking behavior
3.2.1. The construct of “habitual behavior”
Behavior performed to obtain a desired outcome can be characterized as goal-directed or purposeful. After extensive repetition (Dickinson et al., 1995) or under certain schedules of reinforcement (Adams and Dickinson, 1981; Adams, 1982; Dickinson and Balleine, 1994), behavior may become less influenced by the outcome and instead, be controlled by conditioned stimuli, such as the context in which the behavior has most frequently been reinforced. Such behavior is characterized as “habitual” (Dickinson, 1985). Fundamentally, the behavior remains goal-directed and purposeful, but its expression no longer requires deliberation or sustained attention or effort; expression has simply become more automatized.
3.2.2. Ethanol seeking as a “habitual” behavior: studies in animals
In animal models, either reducing the value of the reward or manipulating the contingency between behavior performance and reward permits characterization of behavioral expression. Behaviors that persist despite these manipulations are defined as “habitual” (Dickinson, 1985; for review see Vandaele and Janak, 2018). There are two reward value manipulations common in the alcohol literature, both of which attempt to devalue alcohol in experienced animals: conditioned aversion/avoidance (e.g., Barker et al., 2010) and specific satiety (e.g., Shillinglaw et al., 2014). The conditioned aversion/avoidance paradigm pairs the reward (e.g. alcohol) with the experience of illness by injecting the animal with a noxious agent like lithium chloride. After the pairing(s), the current value of the alcohol reward is decreased as a function of its conditioned association with illness, an aversive event. Next, the animal’s expression of reward seeking behavior is tested in its usual context in a non-reinforcement condition. In the specific satiety paradigm, the current value of the alcohol reward is transiently decreased by simply allowing the animal to consume it before testing, though pre-testing consumption should be limited so that acute alcohol intoxication does not interfere with behavioral performance during testing. There are two behavior-reward contingency manipulations common in the literature: contingency degradation (e.g., Shillinglaw et al., 2014) and reversal (e.g., Mangieri et al., 2014). In the degradation paradigm, non-contingent rewards are delivered periodically to determine the sensitivity of the animal’s on-going reward seeking behavior to the relationship between performance and reward receipt. In the reversal paradigm, reward receipt is made contingent upon omission of the learned reward seeking behavior. Both forms of action-outcome contingency degradations entail multiple test sessions in the usual context.
In animals, it has been documented that ethanol seeking can become a habitual behavior after extensive training, particularly under interval reinforcement schedules (Corbit et al., 2012, 2014; Dickinson et al., 2002; López et al., 2016; Mangieri et al., 2012). In one of the first descriptions of habitual alcohol seeking behavior, Dickinson et al (2002), using a conditioned aversion paradigm, reported that in contrast to food seeking, ethanol seeking was insensitive to outcome devaluation in experienced animals. However, two critical methodological concerns limit interpretation of this findings: 1) the use of a sucrose-fading procedure for initiating alcohol self-administration and 2) the animals consumed relatively low doses of alcohol (O’Tousa and Grahame, 2014). Regarding the former, animals acquired the seeking behavior (and thus established action-outcome contingencies) with a sucrose solution as the reinforcer, and as such, habit formation was occurring before alcohol was introduced. Secondly, it is unclear, though based on the solution content it seems unlikely, whether the animals achieved intoxication and experienced any alcohol reinforcement.
More recent work has focused on models that mitigate these confounds in evaluating the time course of habitual alcohol seeking. For example, rather tha n using a sucrose-fading procedure, Corbit and colleagues (2012; 2014) used a two-bottle choice home cage paradigm to facilitate acquisition of alcohol consumption in which rats had free access to an unsweetened 10% ethanol solution and water. After the 4-week acclimation period, instrumental training commenced, during which rats self-administered on average 0.5 g/kg/1-hour session. Using a within-subject design, the authors went on to demonstrate that ethanol seeking was sensitive to devaluation by specific satiety after 2 weeks of instrumental conditioning, but after 8 weeks, the behavior was no longer sensitive to outcome devaluation, suggesting that ethanol seeking had become habitual. This effect was specific to ethanol as a separate group of rats continued to show goal-directed sucrose seeking after both 2 and 8 weeks of instrumental conditioning. A limitation of this work is that blood ethanol concentrations were not measured, and so the relationship of the findings to the central pharmacological effects of ethanol are unclear. Using Wistar rats, Lopez et al (2016) also determined that alcohol seeking behavior shows variable sensitivity to outcome devaluation by lithium chloride as a function of the length of self-administration experience in the instrumental context. However, a caveat of this latter study is the lack of data regarding the ethanol doses consumed by the rats. Nevertheless, these studies suggest that habitual alcohol seeking is an observable phenomenon that emerges after prolonged training. In addition to length of training, studies in animals suggest that other factors can influence the emergence of habitual alcohol behaviors, such as reinforcement schedules (Mangieri et al., 2012; Lopez et al, 2016), the number of action-outcome associations, and stress (For reviews see O’Tousa and Grahame, 2014; Vandaele and Janak, 2018; Corbit and Janak, 2016).
3.2.3. Ethanol seeking as a “habitual” behavior: studies in humans
To our knowledge, habitual ethanol seeking has not been demonstrated yet in human laboratory ethanol self-administration paradigms, and indirect evidence from other relevant human laboratory paradigms is mixed (Gladwin & Wiers, 2012; Sjoerds et al., 2013; but see: Hogarth et al., 2019; Rose et al., 2013; Sebold et al., 2014; de Wit et al., 2018). Using an outcome devaluation paradigm in humans, one cross-sectional study reported impaired goal directed performance in alcohol-dependent individuals compared to healthy controls (Sjoerds et al, 2013), but the primary study task required that participants memorize an assortment of symbols, and thus it is possible that effects were due to specific impairments in memory (De Houwer et al., 2018). Using a contingency reversal paradigm, Gladwin and Weirs (2012) suggested that heavier social drinkers show greater behavioral automaticity in responding to alcohol cues. However, when considering the data and participant characteristics, there are several caveats to this interpretation. Participants were young adult college drinkers with relatively low alcohol use and/or alcohol-related problems (as indicated by a mean AUDIT score of 6.2) and no participant drinking behavior data is presented so the distinction of “heavier drinker” is ambiguous.
Other human laboratory paradigms have demonstrated goal-directed alcohol choice in social drinkers (Rose et al, 2018) or have not shown a strong tendency towards habitual behaviors in humans across various alcohol use phenotypes (Sebold et al, 2014; Hogarth et al, 2018). However, this clinical research domain remains nascent and limited by existing methods of defining and assessing habitual alcohol behaviors in humans.
3.2.4. Proposed neurobiology underlying habitual ethanol behaviors
Within the past two decades, using the aforementioned paradigms in animals, significant progress has been made in understanding the neurobiological basis of habitual behavior. Most of this work has focused on food reinforcers, and though the substrates involved in habitual ethanol seeking are likely very similar, little work has explored the mechanisms by which ethanol interacts with habit circuitry (for additional review, see Corbit and Janak, 2016; Barker and Taylor, 2014). A growing consensus, based on an abundance of preclinical evidence, is that habitual behavior is associated with a shift from ventral to dorsal striatal control over behavior. As mentioned above, the ventral striatum, specifically the nucleus accumbens, plays a significant role in establishing Pavlovian-conditioned responses to alcohol, viz., associations between specific cues or contexts and alcohol. With repeated exposures to ethanol reinforcement, there is gradual recruitment of the dorsal striatum, which can be functionally and anatomically divided into the dorsomedial striatum (caudate in primates) and dorsolateral striatum (putamen in primates) (Everitt and Robbins, 2005; Everitt et al., 2008). In rats trained to lever-press to obtain ethanol, pharmacological inactivation of the dorsomedial striatum prevented the expression of goal-directed alcohol seeking, while inactivation of the dorsolateral striatum had no effect on behavior (Corbit et al., 2012). With prolonged training, alcohol seeking became inflexible, and this was blocked by inactivation of the dorsolateral striatum, indicating that as the nature of ethanol seeking behavior transitioned from goal-directed to habitual, the neuroanatomical control over behavior shifted from the dorsomedial to dorsolateral striatum. These observations are consistent with those observed with natural reinforcers (Yin et al., 2005, 2006) and cocaine (Everitt et al., 2008).
The precise mechanisms by which striatal control over behavior shifts from ventral to dorsal are unclear but are likely regulated by inputs from cortical regions and midbrain dopamine circuits (Barker et al., 2015). Indeed, corticostriatal circuits were shown to be altered by chronic ethanol exposure and this was associated with the expression of habitual behavior (Renteria et al., 2018). Dopamine may be a key mediator in the progression to habitual alcohol seeking and consumption due to the ascending serial interconnectivity of the mesolimbic and nigrostriatal dopamine systems (Haber et al., 2000; Yin et al., 2008; Ikeda et al., 2013). Midbrain dopamine systems are important for the acquisition and performance of goal-directed and habitual behaviors (Faure et al., 2005; Robinson et al., 2007; Murray et al., 2012; Willuhn et al., 2012) and for ethanol reinforcement, but additional research is necessary to determine the mechanisms by which chronic alcohol facilitates the hierarchical recruitment of midbrain dopamine neurons and how this contributes to the development of habitual alcohol seeking.
4. Neurobiological and neurochemical mechanisms of ethanol self-administration: emphasis on recent findings
As indicated above, many neurotransmitter systems have been studied with regard to potential involvement in the mechanisms of action of ethanol and ethanol self-administration. In this section, we review the neurobiological substrates and the neurochemical systems implicated in ethanol seeking and consumption, with a focus on recent updates and translational relevance.
4.1. Brief overview of the role of dopamine in ethanol self-administration
The common action of numerous drugs of abuse including ethanol to increase the concentration of dopamine in the mesolimbic system has been known since the late 1980s (Imperato and Di Chiara, 1986). The convergence of these findings with the proposed role of mesolimbic dopamine in the neurobiological mechanism of motivated behavior in general provided a strong impetus in the field for detailed studies of ethanol and dopamine (reviewed by Gonzales et al., 2004; Siciliano et al., 2018). The most parsimonious explanation is that in well-trained animals that voluntarily consume ethanol the accumbal dopamine response represents a reward prediction signal as suggested by the work of Schultz et al. (1997).
This proposed role of dopamine in the early stages of the development of the reinforcing effects of ethanol self-administration (Weiss et al., 1993) has stood up with subsequent studies. For example, on the first day that a rat has access to an ethanol solution the rat will consume a low dose, but on the second day the consumption of ethanol doubles (Carrillo et al., 2008). Likewise, the dopamine response in the nucleus accumbens that occurs upon initial licking of the spout where ethanol is being delivered in well-trained rats is not present on the first day of access to ethanol (Carrillo et al., 2011). Additional studies confirmed that dopamine release in the nucleus accumbens is an important response in rats trained to consume ethanol (Shnitko and Robinson, 2015; Bassareo et al., 2017). However, it is now clear that the dopamine response associated with ethanol consumption does not only occur in the nucleus accumbens, but evidence suggests that it also occurs in the dorsolateral striatum (Shnitko and Robinson, 2015) as well as in the medial prefrontal cortex (Doherty et al., 2016). Overall, the data obtained over several decades of research has converged on the idea that mesolimbic dopamine is an important mediator of ethanol self-administration.
4.1.1. Future directions
Interesting data is emerging from preclinical studies that indicates substantial heterogeneity exists among midbrain dopamine neurons, as projection-specific molecular, functional, and anatomical differences have recently been identified (Lammel et al., 2008; Marinelli & McCutcheon, 2014; Ford et al., 2006). Future work must consider this heterogeneity when examining dopaminergic circuits in reward-related behaviors (Juarez & Han, 2016). With the advent of newer techniques to dissect out microcircuit involvement in reinforced behavior, including alcohol reinforcement (Witten et al., 2011; Bass et al., 2013; Juarez et al., 2017), this general idea is likely to evolve as more details about the control and regulation of the mesolimbic system emerges.
Additionally, while studies in humans suggest that chronic alcohol use dysregulates the dopaminergic system (Volkow et al., 2017), this has largely been unconfirmed in animal models. Compared with controls, human alcoholics show reduced striatal dopamine signaling – positron emission topography (PET) imaging studies have linked AUD with reductions in striatal D2/D3-receptor and dopamine transporter availability (Volkow et al., 1996, 2007, 2017; but see Hirth et al., 2016 and Hansson et al., 2019). Human alcoholics (versus controls) also show reductions in methylphenidate- and amphetamine-induced dopamine release in the ventral striatum (Volkow et al., 2007; Martinez et al., 2005), despite showing a significant alcohol-induced dopamine response in the right ventral striatum (Yoder et al., 2016). As mentioned above, achieving ethanol dependence in animals via prolonged and chronic self-administration has been unsuccessful and is largely unfeasible. Therefore, preclinical studies typically induce ethanol dependence via chronic intermittent cycles of passive exposure to ethanol vapor. Indeed, animals exposed to such paradigms show altered dopaminergic signaling, but specific observations (i.e. changes in receptor or transporter availability) vary across studies and species (for review Siciliano et al., 2018). Further, passive induction of ethanol dependence lacks ecological validity and may differentially alter dopaminergic signaling relative to chronic long-term alcohol self-administration. Overall, evidence from PET imaging studies in humans suggests a hypodopaminergic state in AUD, but this effect has not been reliably reproduced in valid animal models. As such, the mechanisms by which chronic ethanol self-administration produces long-term changes in dopamine signaling remain unclear (Hansson et al, 2019).
4.2. Opioid peptides and receptors
Although the opioid system in the brain is one of the prominent neuropeptides that are involved in the actions of ethanol, we discuss it in this section separate from a few of the other neuropeptide systems (see below). The endogenous opioid system was one of the first neuropeptides to be implicated in ethanol’s mechanisms and is the most studied over the years.
4.2.1. Acute actions of ethanol on the opioid system
The acute reinforcing and behaviorally stimulating properties of ethanol have historically been associated with opioid signaling within the mesolimbic system, particularly at mu opioid receptors (Roberts et al., 2000). Acute ethanol has been shown to increase in vivo endogenous β-endorphin and dynorphin release in both rodent models and humans, while ethanol effects on enkephalin release are mixed and may be brain-region dependent (Dai et al., 2005; Jarjour et al., 2009; Lam et al., 2008; Marinelli et al., 2003; 2005; 2006; Olive et al., 2001; Mitchell et al., 2012). Notably, the preclinical microdialysis work with beta-endorphin needs to be confirmed due to the analytical sensitivity of methods used to analyze peptide concentrations (Li et al., 2009).
4.2.2. Chronic adaptation of opioid signaling
In addition to the acute effects of ethanol on opioid peptides, research has also been carried out to determine whether longer term ethanol exposure also alters these neuropeptides. Chronic ethanol self-administration has been shown to produce reduced sensitivity of the mu opioid receptor (MOR) (Saland et al., 2004; Sim-Selley et al., 2002; Chen and Lawrence, 2000). Interestingly, this was further examined to show chronic intermittent ethanol drinking interferes with MOR endocytosis, promoting tolerance to opioid administration (He and Whistler, 2011). Intragastric administration and voluntary ethanol consumption have been shown to produce elevated dynorphin levels (Chang et al., 2007; Kuzmin et al., 2013; Przewlocka et al., 1997), although ethanol injections over 14 days significantly reduced kappa opioid receptor (KOR) expression (Rosin et al., 1999), highlighting potential differences based on route of administration in rats. Increased dynorphin peptide expression and KOR signaling were reported in the amygdala using a chronic vapor model, suggesting a prominent role in ethanol withdrawal (Kissler et al. 2014). Chronic ethanol exposure has been shown to increase delta opioid receptor (DOR) expression levels in the central amygdala, hippocampus, and spinal cord (van Rijn et al., 2012; Bie et al., 2009; Saland et al., 2005), however not all studies have shown these results (reviewed in Alongkronrusmee et al., 2018). One potential explanation for the variability in DOR expression in the central nervous system after ethanol exposure is that the DOR is highly dynamic and has been found to respond to environmental influences. Inflammation, stress and ethanol exposure have all been shown to modulate DOR function (Margolis et al., 2008; Margolis et al., 2011; Morinville et al., 2004). Following stress, DOR activation can also increase GABAAR signaling in the ventral tegmental area of rats, a brain region implicated in regulation of ethanol consumption (Margolis et al., 2011).
4.2.3. Opioid modulation of drinking behaviors
It is well known that nonselective opioid receptor antagonists reduce alcohol consumption and preference in animal models (Cowen et al., 1999; Froehlich et al., 1990; Mitchell et al., 2009; Sabino et al., 2013) and alcohol dependent subjects although the clinical significance is debated (Krystal et al., 2001; O’Brien et al., 1996). Naltrexone significantly reduces alcohol seeking, consumption and cue-induced reinstatement in adult rats (Burattini et al., 2006; Ciccocioppo et al., 2003; Gonzales and Weiss, 1998; Hay et al., 2013; Henderson-Redmond and Czachowski, 2014; Katner et al., 1999). More recently, in rat’s dependent on alcohol, naltrexone displayed sex differences in the ability to reduce ethanol consumption during abstinence. Naltrexone reduced drinking at all time points for females, but only reduced drinking in males at delayed abstinence (Matzeu et al., 2018). Interestingly, studies have shown that antagonism of the opioid system may produce different results on dopamine levels and impulsivity depending on the proportion of opioid receptor subtypes present within the system (Nutt, 2014).
Delta opioid receptors have also been suggested to contribute to the reinforcing properties of ethanol, and there is some evidence beta-endorphin and enkephalins are both necessary for ethanol-induced reinforcement (Tseng et al., 2013). Mice lacking DORs show increased ethanol consumption (Roberts et al., 2001; van Rijn and Whistler, 2009), however blocking DORs either reduces (Froehlich et al., 1991; Krishnan-Sarin et al., 1995; Hyytia and Kiianmaa, 2001) or has no effect on ethanol consumption (Hyytia, 1993; Ingman et al., 2003). DORs also have an important role in cue and stress-induced reinstatement (Ciccocioppo et al., 2002; Marinelli et al., 2009; Nielsen et al., 2012). Recently, it has been suggested that DOR subtypes (DOR-1 and DOR-2) have opposing effects on ethanol intake, which may further explain the variability in past studies with respect to drinking behaviors using systemic, nonselective DOR compounds (Margolis et al., 2008; Mitchell et al., 2014; van Rijn and Whistler, 2009). There is evidence to suggest that DOR-1 forms a DOR-MOR heteromer, whereas DOR-2 does not, which may also contribute to the effects on ethanol intake by DOR-1 selective compounds (George et al., 2000; van Rijn and Whistler, 2009). These results indicate that distinct mechanisms exist between DOR-1 and DOR-2 activation to produce different effects on ethanol consumption and DOR-2 mediated reward. However, the field warrants further investigation in primate models of ethanol self-administration to determine whether targeting the DOR system has clinical value in AUD.
Most studies show KOR antagonists reduce home cage alcohol drinking (Anderson and Becker, 2017), although these effects may be specific to stressed or ethanol-dependent animals (Karkhanis et al., 2016; Sperling et al., 2010; Anderson et al., 2016; Rose et al., 2016). Similarly, KOR blockade significantly reduces operant ethanol self-administration (Schank et al., 2012; Cashman and Azar, 2014; Rorick-Kehn et al., 2014). The ability of KOR antagonism to reduce drinking appears to be greater in rats that are ethanol-dependent (Walker et al. 2011; Schank et al. 2012).
Disinhibition of dopamine neurons in the VTA through MOR activation is required for opioid reward (Fields and Margolis, 2015), however there are more complex mechanisms present for alcohol reward. Systemic or local MOR antagonism in the VTA did not prevent the initial rise in ethanol-stimulated dopamine release in the nucleus accumbens but did block release from morphine administration (Valenta et al., 2013). More recently, MORs on GABAergic forebrain neurons in the striatum were shown to be critical for alcohol drinking behavior, suggesting that VTA MORs may not be the primary opioid mechanism involved in alcohol reinforcement (Ben Hamida et al., 2019). Further work is required is determine the complex circuitry and mechanisms involved in ethanol’s effects on the opioid system, however there is substantial evidence implicating this system in alcohol drinking behaviors that supports ongoing medication development targeting the opioid system (Nutt, 2014; Ripley et al., 2015).
4.3. Neuroimmune signaling
Over the past decade, the neuroscience research community has undergone a rapid expansion of knowledge of the role of glial cells in brain function. It is now recognized that glial cells send and receive molecular signals through the extracellular space, and these signals form a complex network that interacts with neurons within the central nervous system as well as with peripheral tissues. Many of these molecules were previously known to be involved in the regulation of immune function in the periphery, and the presence of these signals within the brain has led to the idea of a neuroimmune system (Rostene et al., 2007). Investigation of the possible role of the neuroimmune system in various behavioral disorders has suggested that neuroimmune mechanisms may be involved in major psychiatric disorders (Dowlati et al., 2010; Nelson et al., in press), including AUD (see below for references).
4.3.1. Effects of ethanol on neuroimmune signaling mechanisms
Ethanol has been shown to produce inflammatory responses in brain tissue (Valles et al., 2004; Pascual et al., 2007; He and Crews, 2008; Qin et al., 2008). These findings have led to an intriguing new hypothesis that the inflammatory responses may contribute to damage in key areas of the brain that may then eventually lead to behavioral changes that promote the loss of control over drinking (Crews and Nixon, 2009). Key data that support this hypothesis include the findings tha t proinflammatory cytokines are produced in the brain after acute and chronic alcohol treatment. For example, monocyte chemoattractant protein 1 (MCP-1), a proinflammatory cytokine, was found to be increased in alcoholic brains compared to controls using tissue from a brain bank (He and Crews, 2008). Specifically, MCP-1 protein concentrations were increased by 2–3 fold in the VTA, substantia nigra, hippocampus, and amygdala in alcoholic brain tissue homogenates compared to controls. Furthermore, this finding of increased MCP-1 protein in brain has also been replicated in a mouse model of high-dose chronic alcohol exposure (Qin et al., 2008). Treatment of C57BL/6J mice with 5 g/kg ethanol (i.g.) daily for 10 days followed by 24 hours of abstinence produced a 30% increase in gene expression of MCP-1 and over 2-fold enhancement of MCP-1 protein in whole brain. In addition, other proinflammatory cytokines such as TNFα and IL-1β were also significantly increased in brain after the chronic alcohol treatment compared to controls (Valles et al., 2004; Qin et al., 2008). Moreover, protein content of MCP-1 was persistently increased for 1 week of abstinence following the high-dose 10-day alcohol treatment (Qin et al., 2008). Ethanol consumption for 5 months was also shown to increase inflammatory markers in mouse brain, and these responses were mediated in part through the toll-like receptor 4 signaling system (Alfonso-Loeches et al., 2010; Alfonso-Loeches et al., 2016). Selected markers of neuroimmune function were also found to be increased in brain tissue after a single dose of 4 g/kg ethanol (Doremus-Fitzwater et al., 2015) and also after ethanol vapor exposure (Baxter-Potter et al., 2017). Although the studies cited above show a consistent ethanol-induced neuroimmune response in brain tissue, these studies did not address whether the extracellular concentration of neuroimmune signals were also elevated. A major breakthrough has recently been published showing that an acute ethanol dose of ethanol (3 g/kg) did not alter extracellular cytokines, but that adolescent exposure blunted the time course of extracellular cytokine response to an acute ethanol dose (3 g/kg) in adulthood (Gano et al., in press). Additional studies using microdialysis to monitor cytokines in the extracellular fluid after ethanol exposure are needed.
Investigations of possible mechanisms for the ethanol-induced neuroinflammatory response have largely focused activation of toll-like receptors (Fernandez-Lizarbe et al., 2013; Coleman and Crews, 2018), which are expressed in microglia, astrocytes, and neurons. In addition, it has been suggested that ethanol-induced alterations in microRNA expression may also play a role in the mechanism of the neuroimmune response stimulated by ethanol (Crews et al., 2017).
4.3.2. Changes in neuroimmune signaling alter ethanol consumption
Although these findings that ethanol exposure stimulates a neuroimmune response are novel and intriguing, there is now also data suggesting that changes in chemokine signaling may alter ethanol self-administration. Blednov et al. (2005) studied ethanol preference with a two-bottle choice model in null mutants for various cytokines including MCP-1 and its receptor. Mutants deficient in the MCP-1 receptor showed decreased ethanol preference and intake, while in mutants deficient in the MCP-1 peptide only females exhibited a decreased preference. These data with knockouts could be influenced by compensation in other systems due to the lifelong deletion of the selected gene. However, other model systems have corroborated these initial findings that modulation of neuroimmune signaling can alter ethanol self-administration. For example, ethanol naïve P rats, which are a strain of rodents that engage in binge-like alcohol consumption, innately show GABAaR α2-mediated activation of neuronal toll-like 4 receptor signaling in the central amygdala, which is not present in their non-preferring (nP) counterparts (Liu et al. 2011; Aurelian & Balan, 2019). Both targeted inhibition of α2 expression and selective knockdown of toll-like receptor 4 in the central amygdala attenuated binge drinking in the alcohol preferring P rat (Liu et al., 2011; June et al., 2015). However, more recent findings suggest that the toll-like receptor 4 may not be a good target for pharmacotherapy (Harris et al., 2017). Furthermore, intracerebroventricular infusion of MCP-1 enhanced operant responding for ethanol (Valenta and Gonzales, 2016). Collectively, these findings that ethanol can alter neuroimmune signaling and also that interfering with neuroimmune signaling can modulate ethanol drinking behavior (for review see Coleman and Crews, 2018) has provided a rationale for testing pharmacotherapeutic agents that target the neuroimmune system as potential treatments for AUD (Crews et al., 2017; Akbar et al., 2018).
4.4. Adrenergic mechanisms
Norepinephrine containing neurons are widely distributed throughout the central nervous system, innervating many regions involved in alcohol-related behaviors and reward. Noradrenergic neurons originate from seven brainstem nuclei; the most well-characterized of which is the locus coeruleus (LC). The LC (all LC neurons produce norepinephrine) functions as a key wakefulness/arousal-promoting region (España and Berridge, 2006; Schwarz and Luo, 2015; Szabadi, 2013) and, via its projections to the amygdala and cortex, facilitates cognitive and sensory processing, as well as anxiety and stress responses (Berridge and Waterhouse, 2003; Sara, 2009; Szabadi, 2013). Additionally, the mesolimbic and mesocortical dopamine systems, which mediate ethanol’s reinforcing and motivational properties, are innervated by noradrenergic projections from the LC and medullary norepinephrine nuclei (A1 and A2 regions) (Mejias-Aponte, 2016).
Adrenoceptors are G-protein coupled receptors found throughout the central nervous system, as well as in the periphery, functioning as key mediators of sympathetic activity. Adrenoceptors are well-characterized and classified as α1- (with a, b, and d subtypes), α2- (with a, b, and c subtypes), β1-, β2-, or β3-adrenoceptors. Norepinephrine has the highest affinity for α2-adrenoceptors, which are coupled to Gi proteins and thus, their activation produces sympatholytic effects. Presynaptic α2a- and α2c-adrenoceptors regulate norepinephrine release via a negative feedback mechanism (Haass-Koffler et al., 2018). Alpha 1-adrenoceptors are the most abundant adrenergic receptor in the brain. Coupled to Gq proteins, activation of α1-adrenoceptors generally produces excitation (Piascik and Perez, 2001; Ramos and Arnsten, 2007). Norepinephrine has the lowest affinity for β-adrenoceptors, which are coupled to Gs proteins (Ramos and Arnsten, 2007). Drugs selectively targeting adrenoceptors, particularly α1-adrenoceptor antagonists and α2-adrenoceptor agonists, have been shown to alter alcohol seeking and consumption in both preclinical and clinical studies (for review, see Haass-Koffler et al., 2018).
4.4.1. Manipulations of the norepinephrine system on ethanol behavior and reward
Drugs that have the net effect of reducing central norepinephrine activity appear to reduce alcohol seeking and consummatory behaviors in rodents (for review see Haass-Koffler et al., 2018). Systemic administration of prazosin (1.0–2.0 mg/kg) or doxazosin (1.25–5.0 mg/kg), both selective α1-adrenoceptor antagonists, within 15–45 minutes of alcohol access reduced ethanol seeking and consumption in alcohol-preferring P rats (Froehlich et al., 2013; O’Neil et al., 2013; Rasmussen et al., 2009; Verplaetse et al., 2012) and non-preferring Wistar rats (Walker et al., 2008), and reduced yohimbine-induced reinstatement of alcohol seeking (Funk et al., 2016; Lê et al., 2011). The α2-adrenoceptor agonists clonidine and guanfacine produced similar effects. In P rats, clonidine [at doses of 40 and 80 μg/kg (i.p.), but not at 10 and 20 μg/kg] administered 30 minutes prior to testing robustly reduced ethanol consumption relative to rats treated with vehicle. The 40 μg/kg dose also reduced saccharin intake by half, but did not affect water intake, suggesting a non-specific effect of clonidine on alcohol and natural reward consumption (Rasmussen et al., 2014). Guanfacine (tested doses: 0.3, 0.5, and 0.6 mg/kg, i.p.) reduced responding for alcohol in a progressive ratio paradigm and reduced both cue- and yohimbine-induced reinstatement of alcohol seeking (Lê et al., 2011; Riga et al., 2014; Fredriksson et al., 2015). At doses of 0.3 and 0.6 mg/kg (i.p.), guanfacine had a greater effect than naltrexone on reducing acute ethanol consumption selectively in high-drinking Wistar rats (Fredriksson et al., 2015). Although these studies implicate adrenoceptors in the motivational properties of alcohol, most relied on systemic drug administration (the aforementioned drugs cross the blood brain barrier), which enhances face validity but undermines the ability to parse out peripheral vs central drug effects on alcohol seeking and consummatory behaviors, and thus limits interpretation of the influence of central norepinephrine signaling in ethanol reinforcement. Nevertheless, systemic blockade of α1-adrenoceptors or systemic activation of α2-adrenoceptors reduces alcohol self-administration and reinstatement of alcohol seeking behaviors, suggesting that pharmacological dampening the activity of central and peripheral noradrenergic systems can mitigate alcohol consumption, particularly in alcohol-preferring rodents.
Impairment of norepinephrine synthesis via targeted manipulations of dopamine β-hydroxylase, the enzyme that catalyzes the synthesis of norepinephrine from dopamine, provide direct evidence that central noradrenergic systems may mediate ethanol’s reinforcing properties. Dopamine β-hydroxylase knockout mice and mice with selective depletion of norepinephrine in the prefrontal cortex show reduced preference for ethanol (Ventura et al., 2006; Weinshenker et al., 2000). Similarly, systemic administration of nepicastat, a dopamine β-hydroxylase inhibitor, reduced home cage ethanol consumption, ethanol seeking in the operant chamber, and blocked a compensatory increase in alcohol consumption following deprivation in alcohol-preferring sP rats. However, these effects reached significance only at the highest tested doses (50 and 100 mg/kg, i.p.) (Colombo et al., 2014). Dopamine β-hydroxylase knockout mice also showed greater sensitivity to the sedative and hypothermic effects of ethanol, which are generally inferred to be aversive, and this was reversed by acute replacement of central norepinephrine (Weinshenker et al., 2000). Based on these findings, an emerging hypothesis is that central norepinephrine is involved in regulating the stimulating and/or sedating effects of alcohol.
Norepinephrine modulates mesolimbic dopamine activity, and this may be one mechanism by which it influences the motivational properties of alcohol. Noradrenergic afferents from the LC to the VTA have been shown to regulate dopamine neuron firing and extracellular dopamine concentrations in the nucleus accumbens, caudate, and prefrontal cortex (Mejias-Aponte, 2016), likely via activation of α1-adrenoceptors (Rommelfanger et al, 2009). Recent studies by Shelkar and colleagues (2017) explored the role of this LC-VTA circuit in ethanol reward and reinforcement via targeted pharmacological manipulations in Wistar rats trained to self-administer 200 mg% ethanol directly into the posterior VTA (pVTA). Silencing of LC neurons via lidocaine (4%, 1 μl/rat) or muscimol (100 ng/rat) reduced responding for intra-pVTA alcohol, while intra-pVTA infusion of norepinephrine (40 ng/rat) restored ethanol self-administration. To explore whether this noradrenergic modulation of ethanol reinforcement was due to an α1-adrenoceptor-mediated mechanism, Shelker et al. infused various doses of selective α1-adrenoceptor agents into the pVTA. Intra-pVTA administration of 10 and 20 ng prazosin decreased intracranial ethanol self-administration, consistent with its previously described effects on oral alcohol consumption, while the α1-adrenoceptor agonist phenylephrine (5 and 10 ng) produced an opposite effect (Shelkar et al., 2017). This work provides evidence for involvement of α1-adrenoceptors in the pVTA in the reinforcing properties of alcohol. As α1-adrenoceptors are present on both dopaminergic and non-dopaminergic VTA neurons (Mejias-Aponte, 2016; Pradel et al., 2018), future research is required to determine the precise mechanisms by which LC-norepinephrine neuronal innervation of the VTA regulates ethanol-self administration.
4.4.2. Pharmacological effects of alcohol on central norepinephrine signaling
Remarkably little work has attempted to quantify the pharmacological effects of alcohol on norepinephrine neuronal activity and to monitor central norepinephrine activity during ethanol self-administration. In the only study to examine extracellular norepinephrine during operant ethanol self-administration, sweetened ethanol consumption did not evoke a norepinephrine response in the medial prefrontal cortex of experienced, non-dependent rats. In fact, the temporal pattern of norepinephrine activity was similar among separate groups of rodents self-administering ethanol, sucrose, or nothing (Figure 3; Vena et al., 2017). Without additional research, this observation leads to more questions than answers regarding central noradrenergic responses to alcohol during operant self-administration.
Figure 3.
Dialysate norepinephrine (normalized to baseline) in the medial prefrontal cortex of Long Evans rats during operant self-administration session. Three different groups of animals self-administered either 10% ethanol + 10% sucrose (10S10E), 10% sucrose (10S), or nothing (handle; animals exposed to same experimental conditions but did not receive reinforcer access in operant chambers) for six operant sessions. Microdialysis was conducted during the 7th operant session. Basal samples were collected while animals remained in their home cages. The animals were physically transferred (indicated by the arrow) to the operant chambers, initiating the onset of the 30-minute wait period. Upon completion of the response requirement, animals received 30 minutes of access to a sweetened ethanol solution (10S10E), a sucrose solution (10S), or no reinforcer (Handle). Data points represent mean ± SEM; some error bars removed for clarity. Asterisk (*) indicates significance from baseline. Unpublished data from the Gonzales lab (Vena et al., 2017).
Initial evidence from microdialysis studies with experimenter-administered ethanol in rodents suggests that ethanol’s actions are dose-dependent. Intraperitoneal injection of a very low dose of alcohol (0.2 g/kg) increased extracellular norepinephrine in the prefrontal cortex (Rossetti et al., 1992). Similarly, intravenous infusion of an intoxicating dose of ethanol (1.0 g/kg) stimulated a transient, but significant norepinephrine response in the medial prefrontal cortex (Vena et al., 2017). Higher doses (2–2.5 g/kg, intraperitoneal) of alcohol acutely decreased norepinephrine concentrations, particularly in the cortex (Murphy et al., 1983; Rossetti et al., 1992). Across all studies, saline infusions had no effect on extracellular norepinephrine concentrations (Vena et al, 2017; Rossetti et al., 1992), suggesting an alcohol-specific effect. Collectively, these studies indicate that central norepinephrine systems are acutely modulated by systemic ethanol, but the dose-response relationship remains unclear as the extant literature lacks a complete evaluation of ethanol’s acute and chronic pharmacological actions on norepinephrine neurons.
4.4.3. Targeting adrenergic mechanisms in AUD
Recent renewed interest in noradrenergic involvement in alcohol-related behaviors has yielded compelling evidence that noradrenergic mechanisms contribute to alcohol self-administration, likely via mediation of the reinforcing and motivational properties of alcohol. While numerous questions remain, particularly regarding the relative contribution of the central vs peripheral systems, the precise substrates targeted by alcohol, and the consequences of persistent intoxication and withdrawal, these early findings have contributed to emerging hypotheses that have translational significance. For example, if indeed reducing noradrenergic activity enhances sensitivity to the sedative effects of alcohol, currently FDA-approved drugs such as prazosin, doxazosin, clonidine, and guanfacine, may be beneficial for some high-risk drinkers as experiencing greater alcohol stimulation and less alcohol sedation is associated with excessive alcohol consumption (King et al, 2014).
Chronic alcohol consumption and repeated cycles of intoxication and withdrawal induces adaptions in central noradrenergic signaling, which likely to contribute to the progression to AUD. Although these precise adaptations remain largely unexplored in preclinical models, early clinical studies demonstrated enhanced norepinephrine activity during alcohol withdrawal (Linnoila et al., 1987). More recently, adrenergic agents have shown some efficacy as pharmacotherapies for AUD (Fox et al., 2012; Kenna et al., 2016; Simpson et al., 2009), particularly in reducing alcohol craving (Haass-Koffler et al., 2018) and sympathetic overdrive during alcohol withdrawal (Muzyk et al., 2011). In a larger clinical trial with 92 AUD patients (though only 80 were included in the intent-to-treat analyses), Simpson and colleagues reported that prazosin, relative to placebo, reduced the probability of heavy drinking days and the number of drinks per week, but do not affect the number of drinking days per week (Simpson et al. 2018). Although these early studies seem promising, effect sizes have generally been small to moderate so large-scale randomized clinical trials are needed.
4.5. Amino acid involvement
4.5.1. Glutamate
In order to examine extracellular glutamate concentrations during ethanol self-administration, most studies have utilized microdialysis methods. Importantly, the basal glutamate levels measured in these samples often do not fulfill the criteria for neuronal release and likely reflect measurements from an astrocytic glutamate pool (van der Zeyden et al. 2008). Acute ethanol administration is known to produce differential effects on extracellular glutamate concentrations in a biphasic manner. Most studies show lower doses of ethanol increase, while higher doses decrease glutamate levels in the mesolimbic system (Ding et al., 2012; Moghaddam and Bolinao, 1994; Piepponen et al., 2002; Quertemont et al., 2002; Selim and Bradberry, 1996). In separate groups of rats receiving different doses of ethanol (0.5, 1.0, and 2.0 g/kg, i.p.), repeated injections over 7 days increased basal extracellular glutamate concentration and reduced clearance in the VTA regardless of ethanol dose (Ding et al., 2012), consistent with similar reports in the nucleus accumbens (Kapasova and Szumlinski, 2008; Melendez et al., 2005). Voluntary continuous home cage ethanol drinking in alcohol preferring rats also resulted in increased basal extracellular glutamate and reduced clearance (Das et al., 2015b; Ding et al., 2013). These studies and others highlight alterations in glutamate transport as a potential mechanism of increased extracellular glutamate concentration following chronic ethanol exposure (Spencer and Kalivas, 2017; Rao et al., 2015). Indeed, reduced expression of glutamate transporter 1 has been shown following continuous ethanol access (Das et al., 2015b; Sari et al., 2013), however others have observed no changes in protein levels or function after forced or intermittent exposure (Ding et al., 2013; Griffin et al., 2015; Melendez et al., 2005; Stennett et al., 2017). While there appear to be clear differences in the regulation of glutamate depending on ethanol drinking model, future studies are needed to further outline neurochemical mechanisms by measuring extracellular glutamate concentrations during operant ethanol self-administration.
4.5.2. GABA
While there is significant work implicating inhibitory neurotransmission in maladaptive behaviors (Ostroumov and Dani, 2018), considerably less is known about ethanol’s effects on in vivo extracellular GABA concentrations. Potentiation of GABAergic signaling by acute ethanol has been extensively reported in electrophysiology studies (Roberto et al. 2004; Theile et al., 2008; Weiner and Valenzuela 2006; Zuo et al., 2017) although subregional differences have also been described (Guan et al., 2012). Microdialysis experiments have largely found little change following acute ethanol exposure (Fliegel et al., 2013). Initial work found that acute ethanol injections did not alter dialysate GABA concentration in the nucleus accumbens or VTA of naïve rats or those chronically treated with alcohol or morphine (Dahchour et al., 1994; Dahchour et al., 1996; Kemppainen et al., 2010; Ojanen et al., 2007; Yan et al., 2005). Differences in clearance mechanisms and analytical separation conditions may have contributed to variable results (Rea et al., 2005). More recently, acute ethanol injection (2 g/kg, i.p.) was shown to produce an increase in GABA levels in the nucleus accumbens in the first 60 minutes post-injection in both ethanol-naïve mice and mice exposed to intermittent ethanol injections, the latter of which showed reduced basal GABA levels relative to the former (Pavon et al., in press).
Our lab recently reported inhibiting GABA uptake in the VTA significantly reduced in vivo extraction fraction of the probe, underestimating and/or masking potential changes in dialysate concentration (Zandy and Gonzales, 2018). Local morphine delivered through the microdialysis probe has been shown to significantly reduce dialysate GABA concentration in the VTA (Klitenick et al., 1992; Sotomayor et al., 2005). Interestingly, no changes in GABA concentration were measured after systemic morphine administration (Ojanen et al., 2007) but when GABA transporters were blocked a decrease is GABA concentration during intravenous morphine was recorded (Vihavainen et al., 2008). Together, these results suggest that inhibiting uptake prior to ethanol administration may be one strategy to expand the current evidence on neurochemical effects of ethanol on in vivo GABAergic signaling particularly during operant ethanol self-administration.
4.6. Neuropeptide signaling and receptors
4.6.1. Corticotropin-releasing factor
Corticotropin-releasing factor (CRF) is a 41-amino acid neuropeptide that is critically involved in modulating stress responses via stimulation of the hypothalamic-pituitary-adrenal axis. An extensive amount of preclinical work directly implicates extrahypothalamic CRF in various features of alcohol dependence, including excessive alcohol consumption (Valdez et al., 2002), withdrawal-related anxiety and negative affect (Baldwin et al., 1991; Zorrilla et al., 2001; Valdez et al., 2002), and stress-induced reinstatement (Zorrilla et al., 2014; Le et al., 2000). Much of this work focused on the CRF1-receptor as antagonists for this CRF receptor subtype blunted binge-like ethanol consumption in nondependent animals (Lowery-Gionta et al., 2012; Cippitelli et al., 2012), withdrawal-induced increases in ethanol seeking behavior in dependent rats (Valdez et al., 2002; Funk et al., 2007), and stress-induced escalation of alcohol consumption and reinstatement (Marinelli et al., 2007; Le et al., 2000).
Encouraged by these findings in preclinical models, recent translational studies evaluated the efficacy of two different CRF1-receptor antagonists in human alcohol dependence. In both relatively small, randomized clinical studies, the CRF1-receptor antagonist showed no efficacy relative to placebo in reducing subjective craving for alcohol, negative affect, or anxiety, and did not affect neural responses to alcohol-related stimuli (Schwandt et al., 2016; Kwako et al., 2015). These clinical studies are among several in which CRF1-receptor antagonists demonstrated a lack of clinical efficacy in treating psychopathology (Spierling & Zorrilla, 2017). Despite the promising preclinical literature, pharmacological interventions targeting the CRF1-receptor do not appear to be viable treatments for AUD.
4.6.2. Neuropeptide Y
The neuropeptide Y (NPY) system is widely expressed throughout the central nervous system and peripherally. NPY is co-secreted with classic neurotransmitters (i.e., GABA, glutamate, norepinephrine) and interacts with four Gi/o protein-coupled receptors. NPY is involved in regulating a variety of biological functions, with its actions in the hypothalamus regulating feeding behavior, while in the nucleus accumbens and amygdala, it elicits reward behaviors and produces anxiolytic effects, respectively.
Evidence from rodent models suggests an inverse relationship between NPY expression and ethanol intake that is contingent upon genetic background and/or ethanol drinking history (Thiele et al., 1998, 2000; Robinson & Theile, 2017). In rats, basal NPY levels in the hippocampus, amygdala, and frontal cortex are lower in alcohol-preferring strains compared to non-preferring strains (Robinson & Thiele, 2017; Ehlers et al., 1998; Caberlotto et al., 2001). In an ethanol-preferring strain of mice (C57BL/6J), overexpression of NPY results in decreased ethanol consumption, while NPY knockout mice show increased ethanol consumption, increased sensitivity to ethanol-induced locomotion, and reduced sensitivity to the sedative effects of ethanol (Thiele et al., 2000; Thiele et al., 1998). In the same strain of mice, another study demonstrated reduced NPY immunoreactivity in the central amygdala of mice with 1, 3, and 6 weeks of ethanol binge-drinking experience (vs water-drinking controls), with the greatest reductions observed in mice with 3 and 6 weeks of ethanol binge experience (Sparrow et al., 2012).
Pharmacological manipulations of the NPY system may produce differential effects on ethanol seeking and consummatory behaviors depending on genetic background, ethanol drinking history, and the regional target of the manipulation. In non-dependent rodents, central administration of NPY reduces ethanol seeking and self-administration selectively in ethanol-preferring strains, while increasing self-administration or having no effect in non-preferring strains (Badia-elder et al., 2001; Henderson & Czachowski, 2012; Slawecki et al., 2000; Sparrow et al., 2012; Borkar et al., 2016). Interestingly, Y2-receptor antagonism suppresses ethanol intake in both alcohol-preferring and non-preferring rodent strains (Sparrow et al., 2012; Thorsell et al., 2002), with dependent animals demonstrating a sensitized response to the antagonist (Rimondini et al., 2005). Across high and low drinking rodent strains, local infusion of NPY into the hypothalamus consistently increases ethanol self-administration in ethanol-experienced animals (Gilpin et al., 2004; Kelley et al., 2001). However, this effect of NPY on ethanol intake is likely region-specific as NPY exerts its orexigenic actions via hypothalamic nuclei.
In sum, evidence from recent investigations of NPY’s role in alcohol self-administration generally support an inverse relationship between central NPY activity and ethanol self-administration, suggesting that reduced basal NPY expression and activity is a risk factor for increased alcohol consumption. However, as this literature remains preliminary, this interpretation is likely oversimplified and incomplete. For future research, it will be important to evaluate how targeted manipulations of the NPY system affect behavior across a variety of self-administration paradigms and to characterize the adaptations in NPY signaling induced by chronic alcohol consumption.
Although preclinical work has long supported a role of the central NPY system in alcohol responses and related behaviors, translational support is generally lacking. Genetic studies have found polymorphisms in the genes coding for NPY and the Y2-receptor to be strongly associated with alcohol withdrawal severity and alcohol dependence, respectively (Koehnke et al., 2002; Wetherill et al., 2008). Additionally, NPY levels were reduced in postmortem human brain tissue from alcoholics relative to controls (Mayfield et al., 2002), yet whether this was a cause or consequence of alcohol dependence remains unclear. Nevertheless, this observation is intriguing as a recent study in humans demonstrated an inverse relationship between NPY levels (high vs low) and accumbal responses to salient stimuli (Warthen et al., 2019). Extensive research is required to determine whether the NPY system is a viable target for pharmacotherapies for AUD.
4.6.3. Oxytocin
Oxytocin is a peptide hormone synthesized in the paraventricular and supraoptic nuclei of the hypothalamus that mediates sexual, maternal, and pro-social behaviors in mammals, and may have a role in stress and anxiety responses (Bethlehem et al., 2013). Recently, preclinical findings indicate that oxytocin reduces ethanol appetitive and consummatory behaviors across various self-administration paradigms. In mice, relative to vehicle pre-treatment, oxytocin at all tested doses (1, 3, and 10 mg/kg, i.p.) reduced binge-like ethanol intake (an effect that was blocked by an oxytocin receptor antagonist), and during an operant self-administration session, oxytocin (0.1, 0.3, and 1 mg/kg, i.p.) reduced ethanol seeking and consumption (King et al., 2017). This latter finding was specific to ethanol as oxytocin reduced sucrose self-administration only at the highest tested dose (1 mg/kg). Systemic (i.p.) administration of oxytocin (vs vehicle) acutely reduced alcohol consumption in a two-bottle choice paradigm in mice (King et al., 2017) and prairie voles (Stevenson et al, 2017). While vehicle-treated mice consumed ~7 g/kg ethanol in a four-hour period, mice pretreated with 0.3 mg/kg oxytocin consumed ~4 g/kg in the same period and those treated with 1 and 3 mg/kg oxytocin consumed ~3 g/kg. Similarly, oxytocin (1, 3, and 10 mg/kg i.p.) reduced alcohol consumption in male and female prairie voles (Stevenson et al., 2017). Of note, while dose-dependent effects of i.p. oxytocin were observed in mice in the two-bottle choice paradigm, they were not apparent among prairie voles or among mice in the aforementioned binge-drinking and operant paradigms.
An important caveat of these studies, however, is that the extent of brain penetration of systemically administered oxytocin remains unclear. To circumvent this concern, other studies have used intracerebroventricular (i.c.v.) injection of oxytocin. In rats with a prolonged history of intermittent ethanol access, the hormone (1 μg/μl i.c.v.) acutely reduced ethanol, but not water consumption (Peters et al., 2017; Bowen & Neumann, 2017). Compared to vehicle, oxytocin (10 nM i.c.v.) selectively reduced reinstatement of alcohol-seeking behavior in alcohol-dependent rats (Hansson et al., 2018).
Initial evidence suggests that oxytocin attenuates the motivational and reinforcing properties of ethanol, possibly via its interactions with the mesolimbic dopamine system (Bahi, 2015; Peters et al., 2017). The oxytocin analog carbetocin reduced the acquisition and accelerated the extinction of conditioned place preference for ethanol in mice (Bahi, 2015). Similarly, systemic oxytocin reduced the progressive ratio breakpoint ratio for ethanol self-administration (King et al., 2017). Conditioned place preference and progressive ratio models both assay the motivational properties of alcohol, which are attributable, at least in part, to activation of the mesolimbic dopamine system, which is also targeted by oxytocin (Melis, 2007). Systemic administration of oxytocin prior to ethanol consumption blocks the ethanol-induced accumbal dopamine response in rats acutely and chronically exposed to ethanol (Peters et al., 2017). Preliminary findings from animal models indicate that oxytocin acutely reduces ethanol preference, seeking, and consumption via attenuation of the reinforcing properties of ethanol, which may be attributable to oxytocin’s effects on mesolimbic dopamine signaling (Lee and Weerts, 2016).
Few studies have explored the clinical efficacy of oxytocin in reducing ethanol reward and consumption, though initial studies suggest that exogenous oxytocin may have therapeutic benefit specifically in heavy and/or problematic alcohol users. In two small studies, intranasal oxytocin reduced the severity of withdrawal symptoms in AUD patients undergoing acute detoxification and reduced alcohol consumption in heavy drinkers (Pedersen et al., 2013, 2017). In moderate social drinkers, however, a single exposure to intranasal oxytocin did not modulate the subjective reinforcing, behavioral, or psychomotor effects of acute alcohol intoxication (Vena et al., 2018). Translational research on the influence of oxytocin on alcohol responses and self-administration is hampered by the lack of empirical data on the pharmacokinetic profile of exogenous oxytocin in humans, including brain penetrability of intranasally-administered oxytocin (Lee et al., 2016).
4.6.4. Vasopressin
Alcohol is known to acutely activate the hypothalamic-pituitary adrenal axis, and while emphasis has generally been on CRF-mediated mechanisms, vasopressin (AVP) also plays an important role (for review see Harper et al., 2018). In rats, acute administration of a high dose of ethanol (2–3 g/kg, i.p.) increases plasma AVP levels and AVP mRNA expression in the paraventricular nucleus of the hypothalamus (Rivier and Lee, 1996; Colbern et al., 1985; Ogilvie et al., 1997). In contrast, dependence induced by chronic ethanol consumption reduces AVP mRNA levels in several hypothalamic nuclei and in the BNST of mice (Gulya et al., 1991; Ishizawa et al., 1990) and the total number of AVP immunoreactive neurons in the paraventricular nucleus of rats (Silva et al., 2002), suggesting that chronic ethanol exposure interferes with AVP synthesis.
Within the brain, AVP binds to two G protein-coupled receptor subtypes - V1a and V1b – the latter of which is directly implicated in alcohol consumption (Zhou and Kreek, 2018). V1b receptors are most densely expressed in the olfactory bulb, hippocampus, amygdala, and hypothalamus where they contribute to the regulation of stress and anxiety responses (Corbani et al., 2018; Zhou & Kreek, 2018). Systemic intraperitoneal administration of the V1b receptor antagonist SSR149415 reduced ethanol consumption and preference in male Sardinian alcohol-preferring rats and male and female C57Bl/6J mice, without decreasing total fluid intake in a 2-bottle choice paradigm (Zhou et al., 2011, 2018). Similarly, SSR149415 dose-dependently reduced lever press responding in ethanol-dependent rats, but not in non-dependent animals (Edwards et al., 2012).
Based on these promising preclinical findings, a Phase 2 randomized clinical trial recently evaluated the efficacy of another V1b antagonist, ABT-436, for alcohol dependence (Ryan et al., 2017). Although the primary outcome, percentage of heavy drinking days, was similar between the ABT-436 and placebo groups (31.3 and 37.6, respectively), participants receiving the V1b antagonist reported a greater percentage of days abstinent than those receiving placebo (51.2 vs 41.6, respectively). Collectively, these preliminary findings from both clinical and preclinical studies implicate the AVP system, particularly the V1b receptors in alcohol dependence, though additional studies are necessary to determine the viability of V1b antagonists as a pharmacotherapy for AUD.
5. Conclusions and future directions
The present review summarizes the behavioral, neurobiological, and neurochemical mechanisms involved in ethanol self-administration, with an emphasis on recent advances. Decades of animal self-administration models show that prolonged and chronic ethanol consumption, especially in large quantities, can induce neuroadaptations that yield functional consequences, specifically increased ethanol cue reactivity, greater automaticity of ethanol seeking behaviors, and the emergence of compulsive alcohol use behaviors. Similar behavioral phenotypes are observed in human alcohol drinkers, with the exception of habitual alcohol seeking, which has been difficult to capture in clinical research with existing measures and tasks. A goal of future research will be to determine if this indeed occurs in the clinical progression to alcohol use disorder.
With regards to neurobiology and neurochemistry, extensive basic research has focused on the mechanisms of action of ethanol on specific brain targets, though there are gaps, particularly in determining the translatability of preclinical findings, in the literature as noted above. This review has highlighted some of the newer targets that have gained attention by the field (neuroimmune systems, noradrenergic systems; reconsolidation of memories), but research has also continued on some of the originally proposed mechanisms (opioid systems, glutamate, GABA). A major challenge is the continued development of these new findings into potential therapeutic strategies for patients who seek help in reducing ethanol self-administration. With the development of novel techniques to map out microcircuits in the brain, and the functions of these newly identified circuits, the hope is that these new more detailed maps of brain structure and function will also help pave the way to new therapeutic strategies for treatment of AUD in the near future.
Acknowledgments
The authors thank Dr. Vorani Ramachandra and Dr. Catalina Cervantes for excellent editorial assistance.
Funding source
The corresponding author (RAG) receives support from National Institutes of Health Grants R37 AA011852 and R01 AA014874. This grant support was responsible for cited research that was carried out in the corresponding author’s laboratory and partially allowed for the writing of this review. AAV is supported by a training grant from the National Institutes of Health (T32 DA043469). RUC is supported by University of Missouri Department of Psychological Sciences Mission Enhancement Post-Doctoral Fellowship Fund.
Abbreviations:
- AUD
alcohol use disorder
- AVP
vasopressin
- BEC
blood ethanol concentration
- BLA
basolateral complex of the amygdala
- BNST
bed nucleus of the stria terminalis
- CRF
corticotropin releasing factor
- DOR
delta opioid receptor
- GABA
gamma-aminobutyric acid
- i.p.
intraperitoneal
- KOR
kappa opioid receptor
- LC
locus coeruleus
- MCP-1
monocyte chemoattractant protein 1
- MOR
mu opioid receptor
- NPY
neuropeptide Y
- pVTA
posterior ventral tegmental area
- VTA
ventral tegmental area
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement
The authors declare that there are no conflicts of interest.
References
- Adams CD (1982). Variations in the Sensitivity of Instrumental Responding to Reinforcer Devaluation. Quarterly Journal of Experimental Psychology, 77–98. [Google Scholar]
- Adams CD, & Dickinson A (1981). Instrumental responding following reinforcer devaluation. Quarterly Journal of Experimental Psychology, 33B, 109–121. [Google Scholar]
- Akbar M, Egli M, Cho YE, Song BJ, & Noronha A (2018). Medications for alcohol use disorders: An overview. Pharmacology and Therapeutics, 185, 64–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alfonso-Loeches S, Pascual-Lucas M, Blanco AM, Sanchez-Vera I, & Guerri C (2010). Pivotal role of TLR4 receptors in alcohol-induced neuroinflammation and brain damage. Journal of Neuroscience, 30, 8285–8295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alfonso-Loeches S, Urena-Peralta J, Morillo-Bargues MJ, Gomez-Pinedo U, & Guerri C (2016). Ethanol-Induced TLR4/NLRP3 Neuroinflammatory Response in Microglial Cells Promotes Leukocyte Infiltration Across the BBB. Neurochemical Research, 41, 193–209. [DOI] [PubMed] [Google Scholar]
- Alongkronrusmee D, Chiang T, & van Rijn RM (2018). Delta opioid pharmacology in relation to alcohol behaviors In Jutkiewicz EM (Ed.), Delta Opioid Receptor Pharmacology and Therapeutic Applications (Vol. 167, pp. 199–225). Switzerland: Springer International Publishing. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson RI, & Becker HC (2017). Role of the Dynorphin/Kappa Opioid Receptor System in the Motivational Effects of Ethanol. Alcoholism, Clinical and Experimental Research, 41, 1402–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson RI, Lopez MF, & Becker HC (2016). Stress-Induced Enhancement of Ethanol Intake in C57BL/6J Mice with a History of Chronic Ethanol Exposure: Involvement of Kappa Opioid Receptors. Frontiers in Cellular Neuroscience, 10, 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aurelian L, Balan I (2019). GABAAR α2-activated neuroimmune signal controls binge drinking and impulsivity through regulation of the CCL2/CX3CL1 balance. Psychopharmacology (in press). [DOI] [PubMed] [Google Scholar]
- Bach P, Vollstadt-Klein S, Kirsch M, Hoffmann S, Jorde A, Frank J, Charlet K, Beck A, Heinz A, Walter H, Sommer WH, Spanagel R, Rietschel M, & Kiefer F (2015). Increased mesolimbic cue-reactivity in carriers of the mu-opioid-receptor gene OPRM1 A118G polymorphism predicts drinking outcome: A functional imaging study in alcohol dependent subjects. European Neuropsychopharmacology, 25, 1128–1135. [DOI] [PubMed] [Google Scholar]
- Badanich KA, Adler KJ, & Kirstein CL (2006). Adolescents differ from adults in cocaine conditioned place preference and cocaine-induced dopamine in the nucleus accumbens septi. European Journal of Pharmacology, 550, 95–106. [DOI] [PubMed] [Google Scholar]
- Badia-Elder NE, Stewart RB, Powrozek TA, Roy KF, Murphy JM, & Li TK (2001). Effect of neuropeptide Y (NPY) on oral ethanol intake in Wistar, alcohol-preferring (P), and -nonpreferring (NP) rats. Alcoholism, Clinical and Experimental Research, 25, 386–390. [PubMed] [Google Scholar]
- Bahi A (2015). The oxytocin receptor impairs ethanol reward in mice. Physiology and Behavior, 139, 321–327. [DOI] [PubMed] [Google Scholar]
- Baldwin HA, Rassnick S, Rivier J, Koob GF, & Britton KT (1991). CRF antagonist reverses the “anxiogenic” response to ethanol withdrawal in the rat. Psychopharmacology, 103, 227–232. [DOI] [PubMed] [Google Scholar]
- Barkby H, Dickson JM, Roper L, & Field M (2012). To approach or avoid alcohol? Automatic and self-reported motivational tendencies in alcohol dependence. Alcoholism, Clinical and Experimental Research, 36, 361–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker JM, & Taylor JR (2014). Habitual alcohol seeking: modeling the transition from casual drinking to addiction. Neuroscience and Biobehavioral Reviews, 47, 281–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker JM, Torregrossa MM, Arnold AP, & Taylor JR (2010). Dissociation of genetic and hormonal influences on sex differences in alcoholism-related behaviors. Journal of Neuroscience, 30, 9140–9144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker JM, Corbit LH, Robinson DL, Gremel CM, Gonzales RA, & Chandler LJ (2015). Corticostriatal circuitry and habitual ethanol seeking. Alcohol, 49, 817–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bass CE, Grinevich VP, Gioia D, Day-Brown JD, Bonin KD, Stuber GD et al. (2013). Optogenetic stimulation of VTA dopamine neurons reveals that tonic but not phasic patterns of dopamine transmission reduce ethanol self-administration. Frontiers in Behavioral Neuroscience, 7, 173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassareo V, Cucca F, Frau R, & Di Chiara G (2017). Changes in Dopamine Transmission in the Nucleus Accumbens Shell and Core during Ethanol and Sucrose Self-Administration. Frontiers in Behavioral Neuroscience, 11, 71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter-Potter LN, Henricks AM, Berger AL, Bieniasz KV, Lugo JM, & McLaughlin RJ (2017). Alcohol vapor exposure differentially impacts mesocorticolimbic cytokine expression in a sex-, region-, and duration-specific manner. Neuroscience, 346, 238–246. [DOI] [PubMed] [Google Scholar]
- Belknap JK, Crabbe JC, & Young ER (1993). Voluntary consumption of ethanol in 15 inbred mouse strains. Psychopharmacology, 112, 503–510. [DOI] [PubMed] [Google Scholar]
- Bell RL, Rodd ZA, Smith RJ, Toalston JE, Franklin KM, & McBride WJ (2011). Modeling binge-like ethanol drinking by peri-adolescent and adult P rats. Pharmacology, Biochemistry and Behavior, 100, 90–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben Hamida S, Boulos LJ, McNicholas M, Charbogne P, & Kieffer BL (2019). Mu opioid receptors in GABAergic neurons of the forebrain promote alcohol reward and drinking. Addiction Biology, 24, 28–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge CW, & Waterhouse BD (2003). The locus coeruleus-noradrenergic system: modulation of behavioral state and state-dependent cognitive processes. Brain Research: Brain Research Reviews, 42, 33–84. [DOI] [PubMed] [Google Scholar]
- Bethlehem RA, van Honk J, Auyeung B, & Baron-Cohen S (2013). Oxytocin, brain physiology, and functional connectivity: a review of intranasal oxytocin fMRI studies. Psychoneuroendocrinology, 38, 962–974. [DOI] [PubMed] [Google Scholar]
- Bianchi PC, Carneiro de Oliveira PE, Palombo P, Leao RM, Cogo-Moreira H, Planeta CDS, & Cruz FC (2018). Functional inactivation of the orbitofrontal cortex disrupts context-induced reinstatement of alcohol seeking in rats. Drug and Alcohol Dependence, 186, 102–112. [DOI] [PubMed] [Google Scholar]
- Bie B, Zhu W, & Pan ZZ (2009). Ethanol-induced delta-opioid receptor modulation of glutamate synaptic transmission and conditioned place preference in central amygdala. Neuroscience, 160, 348–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blednov YA, Bergeson SE, Walker D, Ferreira VM, Kuziel WA, & Harris RA (2005). Perturbation of chemokine networks by gene deletion alters the reinforcing actions of ethanol. Behavioural Brain Research, 165, 110–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borkar CD, Upadhya MA, Shelkar GP, Subhedar NK, & Kokare DM (2016). Neuropeptide Y system in accumbens shell mediates ethanol self-administration in posterior ventral tegmental area. Addiction Biology, 21, 766–775. [DOI] [PubMed] [Google Scholar]
- Bowen MT, & Neumann ID (2017). Rebalancing the Addicted Brain: Oxytocin Interference with the Neural Substrates of Addiction. Trends in Neurosciences, 40, 691–708. [DOI] [PubMed] [Google Scholar]
- Bragulat V, Dzemidzic M, Talavage T, Davidson D, O’Connor SJ, & Kareken DA (2008). Alcohol sensitizes cerebral responses to the odors of alcoholic drinks: an fMRI study. Alcoholism, Clinical and Experimental Research, 32, 1124–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broadwater M, Varlinskaya EI, & Spear LP (2011). Chronic intermittent ethanol exposure in early adolescent and adult male rats: effects on tolerance, social behavior, and ethanol intake. Alcoholism, Clinical and Experimental Research, 35, 1392–1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burattini C, Gill TM, Aicardi G, & Janak PH (2006). The ethanol self-administration context as a reinstatement cue: acute effects of naltrexone. Neuroscience, 139, 877–887. [DOI] [PubMed] [Google Scholar]
- Burke AR, & Miczek KA (2014). Stress in adolescence and drugs of abuse in rodent models: role of dopamine, CRF, and HPA axis. Psychopharmacology, 231, 1557–1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caberlotto L, Thorsell A, Rimondini R, Sommer W, Hyytia P, & Heilig M (2001). Differential expression of NPY and its receptors in alcohol-preferring AA and alcohol-avoiding ANA rats. Alcoholism, Clinical and Experimental Research, 25, 1564–1569. [PubMed] [Google Scholar]
- Carnicella S, Ron D, & Barak S (2014). Intermittent ethanol access schedule in rats as a preclinical model of alcohol abuse. Alcohol, 48, 243–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrillo J, & Gonzales RA (2011). A single exposure to voluntary ethanol self-administration produces adaptations in ethanol consumption and accumbal dopamine signaling. Alcohol, 45, 559–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrillo J, Howard EC, Moten M, Houck BD, Czachowski CL, & Gonzales RA (2008). A 3-day exposure to 10% ethanol with 10% sucrose successfully initiates ethanol self-administration. Alcohol, 42, 171–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter LP, & Griffiths RR (2009). Principles of laboratory assessment of drug abuse liability and implications for clinical development. Drug and Alcohol Dependence, 105 Suppl 1, S14–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cashman JR, & Azar MR (2014). Potent inhibition of alcohol self-administration in alcohol-preferring rats by a kappa-opioid receptor antagonist. Journal of Pharmacology and Experimental Therapeutics, 350, 171–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang GQ, Karatayev O, Ahsan R, Avena NM, Lee C, Lewis MJ et al. (2007). Effect of ethanol on hypothalamic opioid peptides, enkephalin, and dynorphin: relationship with circulating triglycerides. Alcoholism, Clinical and Experimental Research, 31, 249–259. [DOI] [PubMed] [Google Scholar]
- Chaudhri N, Sahuque LL, & Janak PH (2008). Context-induced relapse of conditioned behavioral responding to ethanol cues in rats. Biological Psychiatry, 64, 203–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhri N, Sahuque LL, Schairer WW, & Janak PH (2010). Separable roles of the nucleus accumbens core and shell in context- and cue-induced alcohol-seeking. Neuropsychopharmacology, 35, 783–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhri N, Woods CA, Sahuque LL, Gill TM, & Janak PH (2013). Unilateral inactivation of the basolateral amygdala attenuates context-induced renewal of Pavlovian-conditioned alcohol-seeking. European Journal of Neuroscience, 38, 2751–2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen F, & Lawrence AJ (2000). Effect of chronic ethanol and withdrawal on the mu-opioid receptor- and 5-Hydroxytryptamine(1A) receptor-stimulated binding of [(35)S]Guanosine-5’-O-(3-thio)triphosphate in the fawn-hooded rat brain: A quantitative autoradiography study. Journal of Pharmacology and Experimental Therapeutics, 293, 159–165. [PubMed] [Google Scholar]
- Ciccocioppo R, Martin-Fardon R, & Weiss F (2002). Effect of selective blockade of mu(1) or delta opioid receptors on reinstatement of alcohol-seeking behavior by drug-associated stimuli in rats. Neuropsychopharmacology, 27, 391–399. [DOI] [PubMed] [Google Scholar]
- Ciccocioppo R, Lin D, Martin-Fardon R, & Weiss F (2003). Reinstatement of ethanol-seeking behavior by drug cues following single versus multiple ethanol intoxication in the rat: effects of naltrexone. Psychopharmacology, 168, 208–215. [DOI] [PubMed] [Google Scholar]
- Cippitelli A, Damadzic R, Singley E, Thorsell A, Ciccocioppo R, Eskay RL et al. (2012). Pharmacological blockade of corticotropin-releasing hormone receptor 1 (CRH1R) reduces voluntary consumption of high alcohol concentrations in non-dependent Wistar rats. Pharmacology, Biochemistry and Behavior, 100, 522–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claus ED, Ewing SW, Filbey FM, Sabbineni A, & Hutchison KE (2011). Identifying neurobiological phenotypes associated with alcohol use disorder severity. Neuropsychopharmacology, 36, 2086–2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cofresi RU, Lewis SM, Chaudhri N, Lee HJ, Monfils MH, & Gonzales RA (2017). Postretrieval Extinction Attenuates Alcohol Cue Reactivity in Rats. Alcoholism, Clinical and Experimental Research, 41, 608–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cofresi RU, Lee HJ, Monfils MH, Chaudhri N, & Gonzales RA (2018). Characterizing conditioned reactivity to sequential alcohol-predictive cues in well-trained rats. Alcohol, 69, 41–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cofresi RU, Grote DJ, Le EVT, Monfils MH, Chaudhri N, Gonzales RA et al. (2019). Alcohol-associated antecedent stimuli elicit alcohol seeking in non-dependent rats and may activate the insula. Alcohol, 76, 91–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colbern DL, ten Haaf J, Tabakoff B, & van Wimersma Greidanus TB (1985). Ethanol increases plasma vasopressin shortly after intraperitoneal injection in rats. Life Sciences, 37, 1029–1032. [DOI] [PubMed] [Google Scholar]
- Coleman LG, & Crews FT (2018). Innate Immune Signaling and Alcohol Use Disorders. Handbook of Experimental Pharmacology, 248, 369–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombo G, Agabio R, Diaz G, Fa M, Lobina C, Reali R et al. (1997). Sardinian alcohol-preferring rats prefer chocolate and sucrose over ethanol. Alcohol, 14, 611–615. [DOI] [PubMed] [Google Scholar]
- Colombo G, Maccioni P, Vargiolu D, Loi B, Lobina C, Zaru A et al. (2014). The dopamine beta-hydroxylase inhibitor, nepicastat, reduces different alcohol-related behaviors in rats. Alcoholism, Clinical and Experimental Research, 38, 2345–2353. [DOI] [PubMed] [Google Scholar]
- Corbani M, Marir R, Trueba M, Chafai M, Vincent A, Borie AM et al. (2018). Neuroanatomical distribution and function of the vasopressin V1B receptor in the rat brain deciphered using specific fluorescent ligands. General and Comparative Endocrinology, 258, 15–32. [DOI] [PubMed] [Google Scholar]
- Corbit LH, & Janak PH (2016). Habitual Alcohol Seeking: Neural Bases and Possible Relations to Alcohol Use Disorders. Alcoholism, Clinical and Experimental Research, 40, 1380–1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbit LH, Nie H, & Janak PH (2012). Habitual alcohol seeking: time course and the contribution of subregions of the dorsal striatum. Biological Psychiatry, 72, 389–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbit LH, Nie H, & Janak PH (2014). Habitual responding for alcohol depends upon both AMPA and D2 receptor signaling in the dorsolateral striatum. Frontiers in Behavioral Neuroscience, 8, 301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowen MS, Rezvani AH, Jarrott B, & Lawrence AJ (1999). Ethanol consumption by Fawn-Hooded rats following abstinence: effect of naltrexone and changes in mu-opioid receptor density. Alcoholism, Clinical and Experimental Research, 23, 1008–1014. [PubMed] [Google Scholar]
- Cox AA, & Mertz JE (1985). Do rats prefer water, near beer, or beer with ethanol? Bulletin of the Psychonomic Society, 23, 335– 338. [Google Scholar]
- Crews FT, & Nixon K (2009). Mechanisms of neurodegeneration and regeneration in alcoholism. Alcohol and Alcoholism, 44, 115–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews FT, Vetreno RP, Broadwater MA, & Robinson DL (2016). Adolescent Alcohol Exposure Persistently Impacts Adult Neurobiology and Behavior. Pharmacological Reviews, 68, 1074–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews FT, Lawrimore CJ, Walter TJ, & Coleman LG Jr. (2017). The role of neuroimmune signaling in alcoholism. Neuropharmacology, 122, 56–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham CL, Fidler TL, & Hill KG (2000). Animal models of alcohol’s motivational effects. Alcohol research and health, 24, 85–92. [PMC free article] [PubMed] [Google Scholar]
- Curry A (2017). A 9,000-year love affair. National Geographic, 231, 31–53. [Google Scholar]
- Czachowski CL, & Samson HH (1999). Breakpoint determination and ethanol self-administration using an across-session progressive ratio procedure in the rat. Alcoholism, Clinical and Experimental Research, 23, 1580–1586. [PubMed] [Google Scholar]
- Dahchour A, Quertemont E, & De Witte P (1994). Acute ethanol increases taurine but neither glutamate nor GABA in the nucleus accumbens of male rats: a microdialysis study. Alcohol and Alcoholism, 29, 485–487. [PubMed] [Google Scholar]
- Dahchour A, Quertemont E, & De Witte P (1996). Taurine increases in the nucleus accumbens microdialysate after acute ethanol administration to naive and chronically alcoholised rats. Brain Research, 735, 9–19. [DOI] [PubMed] [Google Scholar]
- Dai X, Thavundayil J, & Gianoulakis C (2005). Differences in the peripheral levels of beta-endorphin in response to alcohol and stress as a function of alcohol dependence and family history of alcoholism. Alcoholism, Clinical and Experimental Research, 29, 1965–1975. [DOI] [PubMed] [Google Scholar]
- Das RK, Lawn W, & Kamboj SK (2015a). Rewriting the valuation and salience of alcohol-related stimuli via memory reconsolidation. Translational Psychiatry, 5, e645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das SC, Yamamoto BK, Hristov AM, & Sari Y (2015b). Ceftriaxone attenuates ethanol drinking and restores extracellular glutamate concentration through normalization of GLT-1 in nucleus accumbens of male alcohol-preferring rats. Neuropharmacology, 97, 67–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayas CV, Liu X, Simms JA, & Weiss F (2007). Distinct patterns of neural activation associated with ethanol seeking: effects of naltrexone. Biological Psychiatry, 61, 979–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Houwer J, Tanaka A, Moors A, & Tibboel H(2018). Kicking the habit: Why evidence for habits in humans might be overestimated. Motivation Science, .4, 50–59. [Google Scholar]
- de Wit S, Kindt M, Knot SL, Verhoeven AAC, Robbins TW, Gasull-Camos J, Evans M, Mirza H, & Gillan CM (2018). Shifting the balance between goals and habits: Five failures in experimental habit induction. Journal Experimental Psychology General, 147, 1043–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson A (1985). Actions and Habits: The Development of Behavioural Autonomy. Philosophical Transactions of the Royal Society, 67–78. [Google Scholar]
- Dickinson A, & Balleine B (1994). Motivational Control of goal-directed action. Animal Learning and Behavior, 1–18. [Google Scholar]
- Dickinson A, Balleine B, Watt A, Gonzalez F, & Boakes RA (1995). Motivational Control after extended instrumental training. Animal Learning and Behavior, 197–206. [Google Scholar]
- Dickinson A, Wood N, & Smith JW (2002). Alcohol seeking by rats: action or habit? Quarterly Journal of Experimental Psychology. B: Comparative and Physiological Psychology, 55, 331–348. [DOI] [PubMed] [Google Scholar]
- Ding ZM, Engleman EA, Rodd ZA, & McBride WJ (2012). Ethanol increases glutamate neurotransmission in the posterior ventral tegmental area of female wistar rats. Alcoholism, Clinical and Experimental Research, 36, 633–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding ZM, Rodd ZA, Engleman EA, Bailey JA, Lahiri DK, & McBride WJ (2013). Alcohol drinking and deprivation alter basal extracellular glutamate concentrations and clearance in the mesolimbic system of alcohol-preferring (P) rats. Addiction Biology, 18, 297–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty JM, & Gonzales RA (2015). Operant self-administration of sweetened ethanol and time course of blood ethanol levels in adolescent and adult male Long-Evans rats. Alcoholism, Clinical and Experimental Research, 39, 485–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty JM, Schier CJ, Vena AA, Dilly GA, & Gonzales RA (2016). Medial Prefrontal Cortical Dopamine Responses During Operant Self-Administration of Sweetened Ethanol. Alcoholism, Clinical and Experimental Research, 40, 1662–1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doremus TL, Brunell SC, Rajendran P, & Spear LP (2005). Factors influencing elevated ethanol consumption in adolescent relative to adult rats. Alcoholism, Clinical and Experimental Research, 29, 1796–1808. [DOI] [PubMed] [Google Scholar]
- Doremus-Fitzwater TL, & Spear LP (2016). Reward-centricity and attenuated aversions: An adolescent phenotype emerging from studies in laboratory animals. Neuroscience and Biobehavioral Reviews, 70, 121–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doremus-Fitzwater TL, Gano A, Paniccia JE, & Deak T (2015). Male adolescent rats display blunted cytokine responses in the CNS after acute ethanol or lipopolysaccharide exposure. Physiology and Behavior, 148, 131–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowlati Y, Herrmann N, Swardfager W, Liu H, Sham L, Reim EK et al. (2010). A meta-analysis of cytokines in major depression. Biological Psychiatry, 67, 446–457. [DOI] [PubMed] [Google Scholar]
- Doyon WM, York JL, Diaz LM, Samson HH, Czachowski CL, & Gonzales RA (2003). Dopamine activity in the nucleus accumbens during consummatory phases of oral ethanol self-administration. Alcoholism, Clinical and Experimental Research, 27, 1573–1582. [DOI] [PubMed] [Google Scholar]
- Doyon WM, Anders SK, Ramachandra VS, Czachowski CL, & Gonzales RA (2005). Effect of operant self-administration of 10% ethanol plus 10% sucrose on dopamine and ethanol concentrations in the nucleus accumbens. Journal of Neurochemistry, 93, 1469–1481. [DOI] [PubMed] [Google Scholar]
- Edwards S, Guerrero M, Ghoneim OM, Roberts E, & Koob GF (2012). Evidence that vasopressin V1b receptors mediate the transition to excessive drinking in ethanol-dependent rats. Addiction Biology, 17, 76–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers CL, Li TK, Lumeng L, Hwang BH, Somes C, Jimenez P et al. (1998). Neuropeptide Y levels in ethanol-naive alcohol-preferring and nonpreferring rats and in Wistar rats after ethanol exposure. Alcoholism, Clinical and Experimental Research, 22, 1778–1782. [PubMed] [Google Scholar]
- Ellgren M, Artmann A, Tkalych O, Gupta A, Hansen HS, Hansen SH et al. (2008). Dynamic changes of the endogenous cannabinoid and opioid mesocorticolimbic systems during adolescence: THC effects. European Neuropsychopharmacology, 18, 826–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein DH, Preston KL, Stewart J, & Shaham Y (2006). Toward a model of drug relapse: an assessment of the validity of the reinstatement procedure. Psychopharmacology, 189, 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ericson M, Blomqvist O, Engel JA, & Soderpalm B (1998). Voluntary ethanol intake in the rat and the associated accumbal dopamine overflow are blocked by ventral tegmental mecamylamine. European Journal of Pharmacology, 358, 189–196. [DOI] [PubMed] [Google Scholar]
- Espana RA, & Berridge CW (2006). Organization of noradrenergic efferents to arousal-related basal forebrain structures. Journal of Comparative Neurology, 496, 668–683. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, & Robbins TW (2005). Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nature Neuroscience, 8, 1481–1489. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Belin D, Economidou D, Pelloux Y, Dalley JW, & Robbins TW (2008). Review. Neural mechanisms underlying the vulnerability to develop compulsive drug-seeking habits and addiction. Philosophical Transactions of the Royal Society of London. Series B: Biological Sciences, 363, 3125–3135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadardi JS, & Cox WM (2006). Alcohol attentional bias: drinking salience or cognitive impairment? Psychopharmacology, 185, 169–178. [DOI] [PubMed] [Google Scholar]
- Faure A, Haberland U, Conde F, & El Massioui N (2005). Lesion to the nigrostriatal dopamine system disrupts stimulus-response habit formation. Journal of Neuroscience, 25, 2771–2780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Lizarbe S, Montesinos J, & Guerri C (2013). Ethanol induces TLR4/TLR2 association, triggering an inflammatory response in microglial cells. Journal of Neurochemistry, 126, 261–273. [DOI] [PubMed] [Google Scholar]
- Field M, & Cox WM (2008). Attentional bias in addictive behaviors: a review of its development, causes, and consequences. Drug and Alcohol Dependence, 97, 1–20. [DOI] [PubMed] [Google Scholar]
- Field M, Mogg K, Zetteler J, & Bradley BP (2004). Attentional biases for alcohol cues in heavy and light social drinkers: the roles of initial orienting and maintained attention. Psychopharmacology, 176, 88–93. [DOI] [PubMed] [Google Scholar]
- Field M, Mogg K, & Bradley BP (2005). Craving and cognitive biases for alcohol cues in social drinkers. Alcohol and Alcoholism, 40, 504–510. [DOI] [PubMed] [Google Scholar]
- Fields HL, & Margolis EB (2015). Understanding opioid reward. Trends in Neurosciences, 38, 217–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filbey FM, Claus E, Audette AR, Niculescu M, Banich MT, Tanabe J et al. (2008). Exposure to the taste of alcohol elicits activation of the mesocorticolimbic neurocircuitry. Neuropsychopharmacology, 33, 1391–1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorenza AM, Shnitko TA, Sullivan KM, Vemuru SR, Gomez AA, Esaki JY et al. (2018). Ethanol Exposure History and Alcoholic Reward Differentially Alter Dopamine Release in the Nucleus Accumbens to a Reward-Predictive Cue. Alcoholism, Clinical and Experimental Research, 42, 1051–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming KA, & Bartholow BD (2014). Alcohol cues, approach bias, and inhibitory control: applying a dual process model of addiction to alcohol sensitivity. Psychology of Addictive Behaviors, 28, 85–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fliegel S, Brand I, Spanagel R, & Noori HR (2013). Ethanol-induced alterations of amino acids measured by in vivo microdialysis in rats: a meta-analysis. In Silico Pharmacology, 1, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford CP, Mark GP, Williams JT (2006). Properties and Opioid Inhibition of Mesolimbic Dopamine Neurons Vary according to Target Location. Journal of Neuroscience, 26, 2788–2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox HC, Anderson GM, Tuit K, Hansen J, Kimmerling A, Siedlarz KM et al. (2012). Prazosin effects on stress- and cue-induced craving and stress response in alcohol-dependent individuals: preliminary findings. Alcoholism, Clinical and Experimental Research, 36, 351–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredriksson I, Jayaram-Lindstrom N, Wirf M, Nylander E, Nystrom E, Jardemark K et al. (2015). Evaluation of guanfacine as a potential medication for alcohol use disorder in long-term drinking rats: behavioral and electrophysiological findings. Neuropsychopharmacology, 40, 1130–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froehlich JC, Harts J, Lumeng L, & Li TK (1990). Naloxone attenuates voluntary ethanol intake in rats selectively bred for high ethanol preference. Pharmacology, Biochemistry and Behavior, 35, 385–390. [DOI] [PubMed] [Google Scholar]
- Froehlich JC, Zweifel M, Harts J, Lumeng L, & Li TK (1991). Importance of delta opioid receptors in maintaining high alcohol drinking. Psychopharmacology, 103, 467–472. [DOI] [PubMed] [Google Scholar]
- Froehlich JC, Hausauer BJ, Federoff DL, Fischer SM, & Rasmussen DD (2013). Prazosin reduces alcohol drinking throughout prolonged treatment and blocks the initiation of drinking in rats selectively bred for high alcohol intake. Alcoholism, Clinical and Experimental Research, 37, 1552–1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fryer SL, Jorgensen KW, Yetter EJ, Daurignac EC, Watson TD, Shanbhag H, Krystal JH, & Mathalon DH (2013). Differential brain response to alcohol cue distractors across stages of alcohol dependence. Biological Psychology, 92, 282–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuhrmann D, Knoll LJ, & Blakemore SJ (2015). Adolescence as a Sensitive Period of Brain Development. Trends in Cognitive Sciences, 19, 558–566. [DOI] [PubMed] [Google Scholar]
- Funk CK, Zorrilla EP, Lee MJ, Rice KC, & Koob GF (2007). Corticotropin-releasing factor 1 antagonists selectively reduce ethanol self-administration in ethanol-dependent rats. Biological Psychiatry, 61, 78–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funk D, Coen K, Tamadon S, Li Z, Loughlin A, & Le AD (2016). Effects of prazosin and doxazosin on yohimbine-induced reinstatement of alcohol seeking in rats. Psychopharmacology, 233, 2197–2207. [DOI] [PubMed] [Google Scholar]
- Gano A, Vore AS, Sammakia M, & Deak T (in press). Assessment of extracellular cytokines in the hippocampus of the awake behaving rat using large-molecule microdialysis combined with multiplex arrays after acute and chronic ethanol exposure. Alcoholism, Clinical and Experimental Research. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Burgos D, Gonzalez F, Manrique T, & Gallo M (2009). Patterns of ethanol intake in preadolescent, adolescent, and adult Wistar rats under acquisition, maintenance, and relapse-like conditions. Alcoholism, Clinical and Experimental Research, 33, 722–728. [DOI] [PubMed] [Google Scholar]
- Gass JT, & Olive MF (2007). Reinstatement of ethanol-seeking behavior following intravenous self-administration in Wistar rats. Alcoholism, Clinical and Experimental Research, 31, 1441–1445. [DOI] [PubMed] [Google Scholar]
- Gass JT, Sinclair CM, Cleva RM, Widholm JJ, & Olive MF (2011). Alcohol-seeking behavior is associated with increased glutamate transmission in basolateral amygdala and nucleus accumbens as measured by glutamateoxidase-coated biosensors. Addiction Biology, 16, 215–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George SR, Fan T, Xie Z, Tse R, Tam V, Varghese G et al. (2000). Oligomerization of mu- and delta-opioid receptors. Generation of novel functional properties. Journal of Biological Chemistry, 275, 26128–26135. [DOI] [PubMed] [Google Scholar]
- Gilpin NW, Stewart RB, Murphy JM, & Badia-Elder NE (2004). Neuropeptide Y in the paraventricular nucleus of the hypothalamus increases ethanol intake in high- and low-alcohol-drinking rats. Alcoholism, Clinical and Experimental Research, 28, 1492–1498. [DOI] [PubMed] [Google Scholar]
- Gladwin TE, & Wiers RW (2012). Alcohol-related effects on automaticity due to experimentally manipulated conditioning. Alcoholism, Clinical and Experimental Research, 36, 895–899. [DOI] [PubMed] [Google Scholar]
- Gonzales RA, & Weiss F (1998). Suppression of ethanol-reinforced behavior by naltrexone is associated with attenuation of the ethanol-induced increase in dialysate dopamine levels in the nucleus accumbens. Journal of Neuroscience, 18, 10663–10671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzales RA, Job MO, & Doyon WM (2004). The role of mesolimbic dopamine in the development and maintenance of ethanol reinforcement. Pharmacology and Therapeutics, 103, 121–146. [DOI] [PubMed] [Google Scholar]
- Grahame NJ, & Cunningham CL (2002). Intravenous self-administration of ethanol in mice. Current Protocols in Neuroscience, Chapter 9, Unit 9 11. [DOI] [PubMed] [Google Scholar]
- Granholm L, Segerstrom L, & Nylander I (2018). Episodic Ethanol Exposure in Adolescent Rats Causes Residual Alterations in Endogenous Opioid Peptides. Frontiers in Psychiatry, 9, 425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin WC, Ramachandra VS, Knackstedt LA, & Becker HC (2015). Repeated cycles of chronic intermittent ethanol exposure increases basal glutamate in the nucleus accumbens of mice without affecting glutamate transport. Frontiers in Pharmacology, 6, 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grodin EN, Sussman L, Sundby K, Brennan GM, Diazgranados N, Heilig M, & Momenan R (2018). Neural Correlates of Compulsive Alcohol Seeking in Heavy Drinkers. Biological psychiatry. Cognitive Neuroscience and Neuroimaging, 3, 1022–1031. [DOI] [PubMed] [Google Scholar]
- Guan Y, Xiao C, Krnjevic K, Xie G, Zuo W, & Ye JH (2012). GABAergic actions mediate opposite ethanol effects on dopaminergic neurons in the anterior and posterior ventral tegmental area. Journal of Pharmacology and Experimental Therapeutics, 341, 33–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulya K, Dave JR, & Hoffman PL (1991). Chronic ethanol ingestion decreases vasopressin mRNA in hypothalamic and extrahypothalamic nuclei of mouse brain. Brain Research, 557, 129–135. [PubMed] [Google Scholar]
- Haass-Koffler CL, Swift RM, & Leggio L (2018). Noradrenergic targets for the treatment of alcohol use disorder. Psychopharmacology, 235, 1625–1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber SN, Fudge JL, & McFarland NR (2000). Striatonigrostriatal pathways in primates form an ascending spiral from the shell to the dorsolateral striatum. Journal of Neuroscience, 20, 2369–2382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansson AC, Grunder G, Hirth N, Noori HR, Spanagel R, & Sommer WH (2019). Dopamine and opioid systems adaptation in alcoholism revisited: Convergent evidence from positron emission tomography and postmortem studies. Neurosci Biobehav Rev, 106, 141–164. [DOI] [PubMed] [Google Scholar]
- Hansson AC, Koopmann A, Uhrig S, Buhler S, Domi E, Kiessling E, Ciccocioppo R, Froemke RC, Grinevich V, Kiefer F, Sommer WH, Vollstadt-Klein S, & Spanagel R (2018). Oxytocin Reduces Alcohol Cue-Reactivity in Alcohol-Dependent Rats and Humans. Neuropsychopharmacology, 43, 1235–1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper KM, Knapp DJ, Criswell HE, & Breese GR (2018). Vasopressin and alcohol: a multifaceted relationship. Psychopharmacology, 235, 3363–3379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris RA, Bajo M, Bell RL, Blednov YA, Varodayan FP, Truitt JM et al. (2017). Genetic and Pharmacologic Manipulation of TLR4 Has Minimal Impact on Ethanol Consumption in Rodents. Journal of Neuroscience, 37, 1139–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay RA, Jennings JH, Zitzman DL, Hodge CW, & Robinson DL (2013). Specific and nonspecific effects of naltrexone on goal-directed and habitual models of alcohol seeking and drinking. Alcoholism, Clinical and Experimental Research, 37, 1100–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He J, & Crews FT (2008). Increased MCP-1 and microglia in various regions of the human alcoholic brain. Experimental Neurology, 210, 349–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He L, & Whistler JL (2011). Chronic ethanol consumption in rats produces opioid antinociceptive tolerance through inhibition of mu opioid receptor endocytosis. PloS One, 6, e19372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson AN, & Czachowski CL (2012). Neuropeptide Y (NPY) in the central nucleus of the amygdala (CeA) does not affect ethanol-reinforced responding in binge-drinking, nondependent rats. Pharmacology, Biochemistry and Behavior, 101, 8–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson-Redmond A, & Czachowski C (2014). Effects of systemic opioid receptor ligands on ethanol- and sucrose seeking and drinking in alcohol-preferring (P) and Long Evans rats. Psychopharmacology, 231, 4309–4321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirth N, Meinhardt MW, Noori HR, Salgado H, Torres-Ramirez O, Uhrig S, Broccoli L, Vengeliene V, Rossmanith M, Perreau-Lenz S, Kohr G, Sommer WH, Spanagel R, & Hansson AC (2016). Convergent evidence from alcohol-dependent humans and rats for a hyperdopaminergic state in protracted abstinence. Proc Natl Acad Sci U S A, 113, 3024–3029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogarth L, Lam-Cassettari C, Pacitti H, Currah T, Mahlberg J, Hartley L, & Moustafa A (2019). Intact goal-directed control in treatment-seeking drug users indexed by outcome-devaluation and Pavlovian to instrumental transfer: critique of habit theory. European Journal of Neuroscience, 50, 2513–2525. [DOI] [PubMed] [Google Scholar]
- Holgate JY, Shariff M, Mu EWH, Bartlett S (2017). A Rat Drinking in the Dark Model for Studying Ethanol and Sucrose Consumption. Frontiers Behavioral Neuroscience, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollett RC, Stritzke WGK, Edgeworth P, & Weinborn M (2017). Changes in the Relative Balance of Approach and Avoidance Inclinations to Use Alcohol Following Cue Exposure Vary in Low and High Risk Drinkers. Frontiers in Psychology, 8, 645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hon T, Das RK, & Kamboj SK (2016). The effects of cognitive reappraisal following retrieval-procedures designed to destabilize alcohol memories in high-risk drinkers. Psychopharmacology, 233, 851–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopf FW, Chang SJ, Sparta DR, Bowers MS, & Bonci A (2010). Motivation for alcohol becomes resistant to quinine adulteration after 3 to 4 months of intermittent alcohol self-administration. Alcoholism, Clinical and Experimental Research, 34, 1565–1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard EC, Schier CJ, Wetzel JS, & Gonzales RA (2009). The dopamine response in the nucleus accumbens core-shell border differs from that in the core and shell during operant ethanol self-administration. Alcoholism, Clinical and Experimental Research, 33, 1355–1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyytia P (1993). Involvement of mu-opioid receptors in alcohol drinking by alcohol-preferring AA rats. Pharmacology, Biochemistry and Behavior, 45, 697–701. [DOI] [PubMed] [Google Scholar]
- Hyytia P, & Kiianmaa K (2001). Suppression of ethanol responding by centrally administered CTOP and naltrindole in AA and Wistar rats. Alcoholism, Clinical and Experimental Research, 25, 25–33. [DOI] [PubMed] [Google Scholar]
- Ikeda H, Saigusa T, Kamei J, Koshikawa N, & Cools AR (2013). Spiraling dopaminergic circuitry from the ventral striatum to dorsal striatum is an effective feed-forward loop. Neuroscience, 241, 126–134. [DOI] [PubMed] [Google Scholar]
- Imperato A, & Di Chiara G (1986). Preferential stimulation of dopamine release in the nucleus accumbens of freely moving rats by ethanol. Journal of Pharmacology and Experimental Therapeutics, 239, 219–228. [PubMed] [Google Scholar]
- Ingman K, Salvadori S, Lazarus L, Korpi ER, & Honkanen A (2003). Selective delta-opioid receptor antagonist N,N(CH3)2-Dmt-Tic-OH does not reduce ethanol intake in alcohol-preferring AA rats. Addiction Biology, 8, 173–179. [DOI] [PubMed] [Google Scholar]
- Ishizawa H, Dave JR, Liu LI, Tabakoff B, & Hoffman PL (1990). Hypothalamic vasopressin mRNA levels in mice are decreased after chronic ethanol ingestion. European Journal of Pharmacology, 189, 119–127. [DOI] [PubMed] [Google Scholar]
- Jaramillo AA, Randall PA, Frisbee S, & Besheer J (2016). Modulation of sensitivity to alcohol by cortical and thalamic brain regions. European Journal of Neuroscience, 44, 2569–2580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarjour S, Bai L, & Gianoulakis C (2009). Effect of acute ethanol administration on the release of opioid peptides from the midbrain including the ventral tegmental area. Alcoholism, Clinical and Experimental Research, 33, 1033–1043. [DOI] [PubMed] [Google Scholar]
- Jeanblanc J, He DY, Carnicella S, Kharazia V, Janak PH, & Ron D (2009). Endogenous BDNF in the dorsolateral striatum gates alcohol drinking. Journal of Neuroscience, 29, 13494–13502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeanblanc J, Logrip ML, Janak PH, & Ron D (2013). BDNF-mediated regulation of ethanol consumption requires the activation of the MAP kinase pathway and protein synthesis. European Journal of Neuroscience, 37, 607–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juarez B, & Han M (2016). Diversity of dopaminergic neural circuits in response to drug exposure. Neuropsychopharmacology, 41, 2424–2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juarez B, Morel C, Ku SM, Liu Y, Zhang H, Montgomery S, et al. (2017). Midbrain circuit regulation of individual alcohol drinking behaviors in mice. Nature Communications, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- June HL, & Gilpin NW (2010). Operant self-administration models for testing the neuropharmacological basis of ethanol consumption in rats. Current Protocols in Neuroscience, Chapter 9, Unit 9 12 11–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- June HL, Liu J, Warnock KT, Bell KA, Balan I, Bollino D et al. (2015). CRF-amplified neuronal TLR4/MCP-1 signaling regulates alcohol self-administration. Neuropsychopharmacology, 40, 1549–1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jupp B, Krstew E, Dezsi G, & Lawrence AJ (2011). Discrete cue-conditioned alcohol-seeking after protracted abstinence: pattern of neural activation and involvement of orexin(1) receptors. British Journal of Pharmacology, 162, 880–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kambouropoulos N, & Staiger PK (2004). Reactivity to alcohol-related cues: relationship among cue type, motivational processes, and personality. Psychology of Addictive Behaviors, 18, 275–283. [DOI] [PubMed] [Google Scholar]
- Kapasova Z, & Szumlinski KK (2008). Strain differences in alcohol-induced neurochemical plasticity: a role for accumbens glutamate in alcohol intake. Alcoholism, Clinical and Experimental Research, 32, 617–631. [DOI] [PubMed] [Google Scholar]
- Kareken DA, Bragulat V, Dzemidzic M, Cox C, Talavage T, Davidson D et al. (2010). Family history of alcoholism mediates the frontal response to alcoholic drink odors and alcohol in at-risk drinkers. Neuroimage, 50, 267–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karkhanis AN, Rose JH, Weiner JL, & Jones SR (2016). Early-Life Social Isolation Stress Increases Kappa Opioid Receptor Responsiveness and Downregulates the Dopamine System. Neuropsychopharmacology, 41, 2263–2274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katner SN, Magalong JG, & Weiss F (1999). Reinstatement of alcohol-seeking behavior by drug-associated discriminative stimuli after prolonged extinction in the rat. Neuropsychopharmacology, 20, 471–479. [DOI] [PubMed] [Google Scholar]
- Kelley SP, Nannini MA, Bratt AM, & Hodge CW (2001). Neuropeptide-Y in the paraventricular nucleus increases ethanol self-administration. Peptides, 22, 515–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keistler CR, Hammarlund E, Barker JM, Bond CW, DiLeone RJ, Pittenger C, & Taylor JR (2017). Regulation of Alcohol Extinction and Cue-Induced Reinstatement by Specific Projections among Medial Prefrontal Cortex, Nucleus Accumbens, and Basolateral Amygdala. Journal of Neuroscience, 37, 4462–4471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemppainen H, Raivio N, Nurmi H, & Kiianmaa K (2010). GABA and glutamate overflow in the VTA and ventral pallidum of alcohol-preferring AA and alcohol-avoiding ANA rats after ethanol. Alcohol and Alcoholism, 45, 111–118. [DOI] [PubMed] [Google Scholar]
- Kenna GA, Haass-Koffler CL, Zywiak WH, Edwards SM, Brickley MB, Swift RM et al. (2016). Role of the alpha1 blocker doxazosin in alcoholism: a proof-of-concept randomized controlled trial. Addiction Biology, 21, 904–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiefer SW, & Dopp JM (1989). Taste reactivity to alcohol in rats. Behavioral Neuroscience, 103, 1318–1326. [DOI] [PubMed] [Google Scholar]
- Kiefer SW, Bice PJ, Orr MR, & Dopp JM (1990). Similarity of taste reactivity responses to alcohol and sucrose mixtures in rats. Alcohol, 7, 115–120. [DOI] [PubMed] [Google Scholar]
- Kiefer SW, Bice PJ, & Badia-Elder N (1994). Alterations in taste reactivity to alcohol in rats given continuous alcohol access followed by abstinence. Alcoholism, Clinical and Experimental Research, 18, 555–559. [DOI] [PubMed] [Google Scholar]
- Kiefer F, Kirsch M, Bach P, Hoffmann S, Reinhard I, Jorde A et al. (2015). Effects of D-cycloserine on extinction of mesolimbic cue reactivity in alcoholism: a randomized placebo-controlled trial. Psychopharmacology, 232, 2353–2362. [DOI] [PubMed] [Google Scholar]
- King AC, McNamara PJ, Hasin DS, & Cao D (2014). Alcohol challenge responses predict future alcohol use disorder symptoms: a 6-year prospective study. Biological Psychiatry, 75, 798–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King CE, Griffin WC, Luderman LN, Kates MM, McGinty JF, & Becker HC (2017). Oxytocin Reduces Ethanol Self-Administration in Mice. Alcoholism, Clinical and Experimental Research, 41, 955–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kissler JL, Sirohi S, Reis DJ, Jansen HT, Quock RM, Smith DG et al. (2014). The one-two punch of alcoholism: role of central amygdala dynorphins/kappa-opioid receptors. Biological Psychiatry, 75, 774–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klenowski PM (2018). Emerging role for the medial prefrontal cortex in alcohol-seeking behaviors. Addictive Behaviors, 77, 102–106. [DOI] [PubMed] [Google Scholar]
- Klitenick MA, DeWitte P, & Kalivas PW (1992). Regulation of somatodendritic dopamine release in the ventral tegmental area by opioids and GABA: an in vivo microdialysis study. Journal of Neuroscience, 12, 2623–2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koehnke MD, Schick S, Lutz U, Willecke M, Koehnke AM, Kolb W et al. (2002). Severity of alcohol withdrawal symptoms and the T1128C polymorphism of the neuropeptide Y gene. Journal of Neural Transmission, 109, 1423–1429. [DOI] [PubMed] [Google Scholar]
- Kopec AM, Smith CJ, Ayre NR, Sweat SC, & Bilbo SD (2018). Microglial dopamine receptor elimination defines sex-specific nucleus accumbens development and social behavior in adolescent rats. Nature Communications, 9, 3769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krank MD (2003). Pavlovian conditioning with ethanol: sign-tracking (autoshaping), conditioned incentive, and ethanol self-administration. Alcoholism, Clinical and Experimental Research, 27, 1592–1598. [DOI] [PubMed] [Google Scholar]
- Krank MD, O’Neill S, Squarey K, & Jacob J (2008). Goal- and signal-directed incentive: conditioned approach, seeking, and consumption established with unsweetened alcohol in rats. Psychopharmacology, 196, 397–405. [DOI] [PubMed] [Google Scholar]
- Kredlow MA, Unger LD, & Otto MW (2016). Harnessing reconsolidation to weaken fear and appetitive memories: A meta-analysis of post-retrieval extinction effects. Psychological Bulletin, 142, 314–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreusch F, Billieux J, & Quertemont E (2017). Alcohol-cue exposure decreases response inhibition towards alcohol-related stimuli in detoxified alcohol-dependent patients. Psychiatry Research, 249, 232–239. [DOI] [PubMed] [Google Scholar]
- Krishnan-Sarin S, Jing SL, Kurtz DL, Zweifel M, Portoghese PS, Li TK et al. (1995). The delta opioid receptor antagonist naltrindole attenuates both alcohol and saccharin intake in rats selectively bred for alcohol preference. Psychopharmacology, 120, 177–185. [DOI] [PubMed] [Google Scholar]
- Krystal JH, Cramer JA, Krol WF, Kirk GF, Rosenheck RA, & Veterans Affairs Naltrexone Cooperative Study, G. (2001). Naltrexone in the treatment of alcohol dependence. New England Journal of Medicine, 345, 1734–1739. [DOI] [PubMed] [Google Scholar]
- Kuzmin A, Chefer V, Bazov I, Meis J, Ogren SO, Shippenberg T et al. (2013). Upregulated dynorphin opioid peptides mediate alcohol-induced learning and memory impairment. Translational Psychiatry, 3, e310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwako LE, Spagnolo PA, Schwandt ML, Thorsell A, George DT, Momenan R et al. (2015). The corticotropin releasing hormone-1 (CRH1) receptor antagonist pexacerfont in alcohol dependence: a randomized controlled experimental medicine study. Neuropsychopharmacology, 40, 1053–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labots M, Cousijn J, Jolink LA, Kenemans JL, Vanderschuren L, & Lesscher HMB (2018). Age-Related Differences in Alcohol Intake and Control Over Alcohol Seeking in Rats. Frontiers in Psychiatry, 9, 419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam MP, Marinelli PW, Bai L, & Gianoulakis C (2008). Effects of acute ethanol on opioid peptide release in the central amygdala: an in vivo microdialysis study. Psychopharmacology, 201, 261–271. [DOI] [PubMed] [Google Scholar]
- Lammel S, Hetzel A, Häckel O, Jones I, Liss B, & Roeper J (2008). Unique properties of mesoprefrontal neurons within a dual mesocorticolimbic dopamine system. Neuron, 57, 760–773. [DOI] [PubMed] [Google Scholar]
- Lasseter HC, Wells AM, Xie X, & Fuchs RA (2011). Interaction of the basolateral amygdala and orbitofrontal cortex is critical for drug context-induced reinstatement of cocaine-seeking behavior in rats. Neuropsychopharmacology, 36, 711–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le AD, & Kalant H (2017). Intravenous self-administration of alcohol in rats-problems with translation to humans. Addiction Biology, 22, 1665–1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le AD, & Shaham Y (2002). Neurobiology of relapse to alcohol in rats. Pharmacology and Therapeutics, 94, 137–156. [DOI] [PubMed] [Google Scholar]
- Le AD, Quan B, Juzytch W, Fletcher PJ, Joharchi N, & Shaham Y (1998). Reinstatement of alcohol-seeking by priming injections of alcohol and exposure to stress in rats. Psychopharmacology, 135, 169–174. [DOI] [PubMed] [Google Scholar]
- Le AD, Harding S, Juzytsch W, Watchus J, Shalev U, & Shaham Y (2000). The role of corticotrophin-releasing factor in stress-induced relapse to alcohol-seeking behavior in rats. Psychopharmacology, 150, 317–324. [DOI] [PubMed] [Google Scholar]
- Le AD, Funk D, Juzytsch W, Coen K, Navarre BM, Cifani C et al. (2011). Effect of prazosin and guanfacine on stress-induced reinstatement of alcohol and food seeking in rats. Psychopharmacology, 218, 89–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeCocq MR, Lahlou S, Chahine M, Padillo LN, & Chaudhri N (2018). Modeling Relapse to Pavlovian Alcohol-Seeking in Rats Using Reinstatement and Spontaneous Recovery Paradigms. Alcoholism, Clinical and Experimental Research, 42, 1795–1806. [DOI] [PubMed] [Google Scholar]
- Lee HJ, Groshek F, Petrovich GD, Cantalini JP, Gallagher M, & Holland PC (2005). Role of amygdalo-nigral circuitry in conditioning of a visual stimulus paired with food. Journal of Neuroscience, 25, 3881–3888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JLC, Nader K, & Schiller D (2017). An Update on Memory Reconsolidation Updating. Trends in Cognitive Sciences, 21, 531–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MR, & Weerts EM (2016). Oxytocin for the treatment of drug and alcohol use disorders. Behavioural Pharmacology, 27, 640–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MR, Rohn MC, Tanda G, & Leggio L (2016). Targeting the Oxytocin System to Treat Addictive Disorders: Rationale and Progress to Date. CNS Drugs, 30, 109–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leslie JC (2003). A history of reinforcement: the role of reinforcement schedules in behavior pharmacology. The Behavior Analyst Today, 4, 98–108. [Google Scholar]
- Li Q, Zubieta JK, & Kennedy RT (2009). Practical aspects of in vivo detection of neuropeptides by microdialysis coupled off-line to capillary LC with multistage MS. Analytical Chemistry, 81, 2242–2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linnoila M, Mefford I, Nutt D, & Adinoff B (1987). NIH conference. Alcohol withdrawal and noradrenergic function. Annals of Internal Medicine, 107, 875–889. [DOI] [PubMed] [Google Scholar]
- Linseman MA (1987). Alcohol consumption in free-feeding rats: procedural, genetic and pharmacokinetic factors. Psychopharmacology, 92, 254–261. [DOI] [PubMed] [Google Scholar]
- Liu J, Yang AR, Kelly T, Puche A, Esoga C, June HL Jr. et al. (2011). Binge alcohol drinking is associated with GABAA alpha2-regulated Toll-like receptor 4 (TLR4) expression in the central amygdala. Proceedings of the National Academy of Sciences of the United States of America, 108, 4465–4470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logrip ML, Janak PH, & Ron D (2009). Escalating ethanol intake is associated with altered corticostriatal BDNF expression. Journal of Neurochemistry, 109, 1459–1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loi B, Lobina C, Maccioni P, Fantini N, Carai MA, Gessa GL et al. (2010). Increase in alcohol intake, reduced flexibility of alcohol drinking, and evidence of signs of alcohol intoxication in Sardinian alcohol-preferring rats exposed to intermittent access to 20% alcohol. Alcoholism, Clinical and Experimental Research, 34, 2147–2154. [DOI] [PubMed] [Google Scholar]
- Lonergan MH, Olivera-Figueroa LA, Pitman RK, & Brunet A (2013). Propranolol’s effects on the consolidation and reconsolidation of long-term emotional memory in healthy participants: a meta-analysis. Journal of Psychiatry and Neuroscience, 38, 222–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez M, Soto A, & Bura S (2016). Alcohol seeking by rats becomes habitual after prolonged training. Psicothema, 28, 421–427. [DOI] [PubMed] [Google Scholar]
- Lowery-Gionta EG, Navarro M, Li C, Pleil KE, Rinker JA, Cox BR et al. (2012). Corticotropin releasing factor signaling in the central amygdala is recruited during binge-like ethanol consumption in C57BL/6J mice. Journal of Neuroscience, 32, 3405–3413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKillop J, Few LR, Stojek MK, Murphy CM, Malutinok SF, Johnson FT et al. (2015). D-cycloserine to enhance extinction of cue-elicited craving for alcohol: a translational approach. Translational Psychiatry, 5, e544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddux JN, & Chaudhri N (2017). Nicotine-induced enhancement of Pavlovian alcohol-seeking behavior in rats. Psychopharmacology (Berl), 234, 727–738. [DOI] [PubMed] [Google Scholar]
- Manchery L, Yarmush DE, Luehring-Jones P, & Erblich J (2017). Attentional bias to alcohol stimuli predicts elevated cue-induced craving in young adult social drinkers. Addictive Behaviors, 70, 14–17. [DOI] [PubMed] [Google Scholar]
- Mangieri RA, Cofresi RU, & Gonzales RA (2012). Ethanol seeking by Long Evans rats is not always a goal-directed behavior. PloS One, 7, e42886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangieri RA, Cofresi RU, & Gonzales RA (2014). Ethanol exposure interacts with training conditions to influence behavioral adaptation to a negative instrumental contingency. Frontiers in Behavioral Neuroscience, 8, 220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchant NJ, Campbell EJ, & Kaganovsky K (2018). Punishment of alcohol-reinforced responding in alcohol preferring P rats reveals a bimodal population: Implications for models of compulsive drug seeking. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 87, 68–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis EB, Fields HL, Hjelmstad GO, & Mitchell JM (2008). Delta-opioid receptor expression in the ventral tegmental area protects against elevated alcohol consumption. Journal of Neuroscience, 28, 12672–12681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis EB, Mitchell JM, Hjelmstad GO, & Fields HL (2011). A novel opioid receptor-mediated enhancement of GABAA receptor function induced by stress in ventral tegmental area neurons. Journal of Physiology, 589, 4229–4242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinelli M, & McCutcheon JE (2014). Heterogeneity of dopamine neuron activity across traits and states. Neuroscience, 282, 176–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinelli PW, Quirion R, & Gianoulakis C (2003). A microdialysis profile of beta-endorphin and catecholamines in the rat nucleus accumbens following alcohol administration. Psychopharmacology, 169, 60–67. [DOI] [PubMed] [Google Scholar]
- Marinelli PW, Bai L, Quirion R, & Gianoulakis C (2005). A microdialysis profile of Met-enkephalin release in the rat nucleus accumbens following alcohol administration. Alcoholism, Clinical and Experimental Research, 29, 1821–1828. [DOI] [PubMed] [Google Scholar]
- Marinelli PW, Lam M, Bai L, Quirion R, & Gianoulakis C (2006). A microdialysis profile of dynorphin A(1–8) release in the rat nucleus accumbens following alcohol administration. Alcoholism, Clinical and Experimental Research, 30, 982–990. [DOI] [PubMed] [Google Scholar]
- Marinelli PW, Funk D, Juzytsch W, Harding S, Rice KC, Shaham Y et al. (2007). The CRF1 receptor antagonist antalarmin attenuates yohimbine-induced increases in operant alcohol self-administration and reinstatement of alcohol seeking in rats. Psychopharmacology, 195, 345–355. [DOI] [PubMed] [Google Scholar]
- Marinelli PW, Funk D, Harding S, Li Z, Juzytsch W, & Le AD (2009). Roles of opioid receptor subtypes in mediating alcohol-seeking induced by discrete cues and context. European Journal of Neuroscience, 30, 671–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez D, Gil R, Slifstein M, Hwang DR, Huang Y, Perez A, Kegeles L, Talbot P, Evans S, Krystal J, Laruelle M, & Abi-Dargham A (2005). Alcohol dependence is associated with blunted dopamine transmission in the ventral striatum. Biol Psychiatry, 58, 779–786. [DOI] [PubMed] [Google Scholar]
- Matzeu A, Terenius L, & Martin-Fardon R (2018). Exploring Sex Differences in the Attenuation of Ethanol Drinking by Naltrexone in Dependent Rats During Early and Protracted Abstinence. Alcoholism, Clinical and Experimental Research, 42, 2466–2478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayfield RD, Lewohl JM, Dodd PR, Herlihy A, Liu J, & Harris RA (2002). Patterns of gene expression are altered in the frontal and motor cortices of human alcoholics. Journal of Neurochemistry, 81, 802–813. [DOI] [PubMed] [Google Scholar]
- McBride WJ, Murphy JM, & Ikemoto S (1999). Localization of brain reinforcement mechanisms: intracranial self-administration and intracranial place-conditioning studies. Behavioural Brain Research, 101, 129–152. [DOI] [PubMed] [Google Scholar]
- McCutcheon JE, Conrad KL, Carr SB, Ford KA, McGehee DS, & Marinelli M (2012). Dopamine neurons in the ventral tegmental area fire faster in adolescent rats than in adults. Journal of Neurophysiology, 108, 1620–1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mejias-Aponte CA (2016). Specificity and impact of adrenergic projections to the midbrain dopamine system. Brain Research, 1641, 258–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melchior CL, & Myers RD (1976). Genetic differences in ethanol drinking of the rat following injection of 6-OHDA, 5,6-DHT or 5,7-DHT into the cerebral ventricles. Pharmacology, Biochemistry and Behavior, 5, 63–72. [DOI] [PubMed] [Google Scholar]
- Melendez RI, Hicks MP, Cagle SS, & Kalivas PW (2005). Ethanol exposure decreases glutamate uptake in the nucleus accumbens. Alcoholism, Clinical and Experimental Research, 29, 326–333. [DOI] [PubMed] [Google Scholar]
- Melis MR, Melis T, Cocco C, Succu S, Sanna F, Pillolla G et al. (2007). Oxytocin injected into the ventral tegmental area induces penile erection and increases extracellular dopamine in the nucleus accumbens and paraventricular nucleus of the hypothalamus of male rats. European Journal of Neuroscience, 26, 1026–1035. [DOI] [PubMed] [Google Scholar]
- Millan EZ, Reese RM, Grossman CD, Chaudhri N, & Janak PH (2015). Nucleus Accumbens and Posterior Amygdala Mediate Cue-Triggered Alcohol Seeking and Suppress Behavior During the Omission of Alcohol-Predictive Cues. Neuropsychopharmacology, 40, 2555–2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda R Jr., & Treloar H (2016). Emerging Pharmacologic Treatments for Adolescent Substance Use: Challenges and New Directions. Current Addiction Reports, 3, 145–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda R, Ray L, Blanchard A, Reynolds EK, Monti PM, Chun T et al. (2014). Effects of naltrexone on adolescent alcohol cue reactivity and sensitivity: an initial randomized trial. Addiction Biology, 19, 941–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell JM, Bergren LJ, Chen KS, Rowbotham MC, & Fields HL (2009). Naltrexone aversion and treatment efficacy are greatest in humans and rats that actively consume high levels of alcohol. Neurobiology of Disease, 33, 72–80. [DOI] [PubMed] [Google Scholar]
- Mitchell JM, O’Neil JP, Janabi M, Marks SM, Jagust WJ, & Fields HL (2012). Alcohol consumption induces endogenous opioid release in the human orbitofrontal cortex and nucleus accumbens. Science Translational Medicine, 4, 116ra116. [DOI] [PubMed] [Google Scholar]
- Mitchell JM, Margolis EB, Coker AR, Allen DC, & Fields HL (2014). Intra-VTA deltorphin, but not DPDPE, induces place preference in ethanol-drinking rats: distinct DOR-1 and DOR-2 mechanisms control ethanol consumption and reward. Alcoholism, Clinical and Experimental Research, 38, 195–203. [DOI] [PubMed] [Google Scholar]
- Moghaddam B, & Bolinao ML (1994). Biphasic effect of ethanol on extracellular accumulation of glutamate in the hippocampus and the nucleus accumbens. Neuroscience Letters, 178, 99–102. [DOI] [PubMed] [Google Scholar]
- Moorman DE (2018). The role of the orbitofrontal cortex in alcohol use, abuse, and dependence. Progress in Neuro-Psychopharmacology & Biological Psychiatry, 87, 85–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales M, McGinnis MM, & McCool BA (2015). Chronic ethanol exposure increases voluntary home cage intake in adult male, but not female, Long-Evans rats. Pharmacology, Biochemistry and Behavior, 139, 67–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morinville A, Cahill CM, Kieffer B, Collier B, & Beaudet A (2004). Mu-opioid receptor knockout prevents changes in delta-opioid receptor trafficking induced by chronic inflammatory pain. Pain, 109, 266–273. [DOI] [PubMed] [Google Scholar]
- Murphy JM, McBride WJ, Lumeng L, & Li TK (1983). Monoamine and metabolite levels in CNS regions of the P line of alcohol-preferring rats after acute and chronic ethanol treatment. Pharmacology, Biochemistry and Behavior, 19, 849–856. [DOI] [PubMed] [Google Scholar]
- Murray JE, Belin D, & Everitt BJ (2012). Double dissociation of the dorsomedial and dorsolateral striatal control over the acquisition and performance of cocaine seeking. Neuropsychopharmacology, 37, 2456–2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muzyk AJ, Fowler JA, Norwood DK, & Chilipko A (2011). Role of alpha2-agonists in the treatment of acute alcohol withdrawal. Annals of Pharmacotherapy, 45, 649–657. [DOI] [PubMed] [Google Scholar]
- Myrick H, Li X, Randall PK, Henderson S, Voronin K, & Anton RF (2010). The effect of aripiprazole on cue-induced brain activation and drinking parameters in alcoholics. Journal of Clinical Psychopharmacology, 30, 365–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson LH, Saulsbery AI, & Lenz KM (2019). Small cells with big implications: Microglia and sex differences in brain development, plasticity and behavioral health. Progress in Neurobiology, 176, 103–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIAAA. (2001). 10th Special Report to the U.S. Congress on Alcohol and Health: Highlights from Current Research: U.S. Department of Health and Human Services. [Google Scholar]
- Nielsen CK, Simms JA, Bito-Onon JJ, Li R, Ananthan S, & Bartlett SE (2012). The delta opioid receptor antagonist, SoRI-9409, decreases yohimbine stress-induced reinstatement of ethanol-seeking. Addiction Biology, 17, 224–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nutt DJ (2014). The role of the opioid system in alcohol dependence. Journal of psychopharmacology (Oxford, England), 28, 8–22. [DOI] [PubMed] [Google Scholar]
- Oberlin BG, Dzemidzic M, Harezlak J, Kudela MA, Tran SM, Soeurt CM et al. (2016). Corticostriatal and Dopaminergic Response to Beer Flavor with Both fMRI and [(11) C]raclopride Positron Emission Tomography. Alcoholism, Clinical and Experimental Research, 40, 1865–1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien CP, Volpicelli LA, & Volpicelli JR (1996). Naltrexone in the treatment of alcoholism: a clinical review. Alcohol, 13, 35–39. [DOI] [PubMed] [Google Scholar]
- O’Dell LE, Roberts AJ, Smith RT, & Koob GF (2004). Enhanced alcohol self-administration after intermittent versus continuous alcohol vapor exposure. Alcoholism, Clinical and Experimental Research, 28, 1676–1682. [DOI] [PubMed] [Google Scholar]
- Ogilvie KM, Lee S, & Rivier C (1997). Role of arginine vasopressin and corticotropin-releasing factor in mediating alcohol-induced adrenocorticotropin and vasopressin secretion in male rats bearing lesions of the paraventricular nuclei. Brain Research, 744, 83–95. [DOI] [PubMed] [Google Scholar]
- Ojanen SP, Palmen M, Hyytia P, & Kiianmaa K (2007). Extracellular glutamate and GABA in the ventral tegmental area of alcohol-preferring AA and alcohol-avoiding ANA rats treated repeatedly with morphine. European Journal of Pharmacology, 559, 38–45. [DOI] [PubMed] [Google Scholar]
- Olive MF, Koenig HN, Nannini MA, & Hodge CW (2001). Stimulation of endorphin neurotransmission in the nucleus accumbens by ethanol, cocaine, and amphetamine. Journal of Neuroscience, 21, RC184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Malley SS, Corbin WR, Leeman RF, DeMartini KS, Fucito LM, Ikomi J et al. (2015). Reduction of alcohol drinking in young adults by naltrexone: a double-blind, placebo-controlled, randomized clinical trial of efficacy and safety. Journal of Clinical Psychiatry, 76, e207–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neil ML, Beckwith LE, Kincaid CL, & Rasmussen DD (2013). The alpha1-adrenergic receptor antagonist, doxazosin, reduces alcohol drinking in alcohol-preferring (P) Rats. Alcoholism, Clinical and Experimental Research, 37, 202–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostroumov A, & Dani JA (2018). Inhibitory Plasticity of Mesocorticolimbic Circuits in Addiction and Mental Illness. Trends in Neurosciences, 41, 898–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Tousa D, & Grahame N (2014). Habit formation: implications for alcoholism research. Alcohol, 48, 327–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palm S, & Nylander I (2014). Alcohol-induced changes in opioid peptide levels in adolescent rats are dependent on housing conditions. Alcoholism, Clinical and Experimental Research, 38, 2978–2987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palm S, Roman E, & Nylander I (2011). Differences in voluntary ethanol consumption in Wistar rats from five different suppliers. Alcohol, 45, 607–614. [DOI] [PubMed] [Google Scholar]
- Panlilio LV, & Goldberg SR (2007). Self-administration of drugs in animals and humans as a model and an investigative tool. Addiction, 102, 1863–1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papachristou H, Nederkoorn C, Havermans R, van der Horst M, & Jansen A (2012). Can’t stop the craving: the effect of impulsivity on cue-elicited craving for alcohol in heavy and light social drinkers. Psychopharmacology (Berl), 219, 511–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park MS, Sohn JH, Suk JA, Kim SH, Sohn S, & Sparacio R (2007). Brain substrates of craving to alcohol cues in subjects with alcohol use disorder. Alcohol and Alcoholism, 42, 417–422. [DOI] [PubMed] [Google Scholar]
- Pascual M, Blanco AM, Cauli O, Minarro J, & Guerri C (2007). Intermittent ethanol exposure induces inflammatory brain damage and causes long-term behavioural alterations in adolescent rats. European Journal of Neuroscience, 25, 541–550. [DOI] [PubMed] [Google Scholar]
- Pavon FJ, Serrano A, Stouffer DG, Polis I, Roberto M, Cravatt BF et al. (2018). Ethanol-induced alterations in endocannabinoids and relevant neurotransmitters in the nucleus accumbens of fatty acid amide hydrolase knockout mice. Addiction Biology, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen CA (2017). Oxytocin, Tolerance, and the Dark Side of Addiction. International Review of Neurobiology, 136, 239–274. [DOI] [PubMed] [Google Scholar]
- Pedersen CA, Smedley KL, Leserman J, Jarskog LF, Rau SW, Kampov-Polevoi A et al. (2013). Intranasal oxytocin blocks alcohol withdrawal in human subjects. Alcoholism, Clinical and Experimental Research, 37, 484–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters ST, Bowen MT, Bohrer K, McGregor IS, & Neumann ID (2017). Oxytocin inhibits ethanol consumption and ethanol-induced dopamine release in the nucleus accumbens. Addiction Biology, 22, 702–711. [DOI] [PubMed] [Google Scholar]
- Philpot RM, Wecker L, & Kirstein CL (2009). Repeated ethanol exposure during adolescence alters the developmental trajectory of dopaminergic output from the nucleus accumbens septi. International Journal of Developmental Neuroscience, 27, 805–815. [DOI] [PubMed] [Google Scholar]
- Piascik MT, & Perez DM (2001). Alpha1-adrenergic receptors: new insights and directions. Journal of Pharmacology and Experimental Therapeutics, 298, 403–410. [PubMed] [Google Scholar]
- Piepponen TP, Kiianmaa K, & Ahtee L (2002). Effects of ethanol on the accumbal output of dopamine, GABA and glutamate in alcohol-tolerant and alcohol-nontolerant rats. Pharmacology, Biochemistry and Behavior, 74, 21–30. [DOI] [PubMed] [Google Scholar]
- Pradel K, Blasiak T, & Solecki WB (2018). Adrenergic Receptor Agonists’ Modulation of Dopaminergic and Non-dopaminergic Neurons in the Ventral Tegmental Area. Neuroscience, 375, 119–134. [DOI] [PubMed] [Google Scholar]
- Przewlocka B, Turchan J, Lason W, & Przewlocki R (1997). Ethanol withdrawal enhances the prodynorphin system activity in the rat nucleus accumbens. Neuroscience Letters, 238, 13–16. [DOI] [PubMed] [Google Scholar]
- Qin L, He J, Hanes RN, Pluzarev O, Hong JS, & Crews FT (2008). Increased systemic and brain cytokine production and neuroinflammation by endotoxin following ethanol treatment. Journal of Neuroinflammation, 5, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quertemont E, Linotte S, & de Witte P (2002). Differential taurine responsiveness to ethanol in high- and low-alcohol sensitive rats: a brain microdialysis study. European Journal of Pharmacology, 444, 143–150. [DOI] [PubMed] [Google Scholar]
- Qureshi A, Monk RL, Pennington CR, Wilcockson TDW, & Heim D (2019). Alcohol-related attentional bias in a gaze contingency task: Comparing appetitive and non-appetitive cues. Addictive Behaviors, 90, 312–317. [DOI] [PubMed] [Google Scholar]
- Ramos BP, & Arnsten AF (2007). Adrenergic pharmacology and cognition: focus on the prefrontal cortex. Pharmacology and Therapeutics, 113, 523–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao PS, Bell RL, Engleman EA, & Sari Y (2015). Targeting glutamate uptake to treat alcohol use disorders. Frontiers in Neuroscience, 9, 144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen DD, Alexander LL, Raskind MA, & Froehlich JC (2009). The alpha1-adrenergic receptor antagonist, prazosin, reduces alcohol drinking in alcohol-preferring (P) rats. Alcoholism, Clinical and Experimental Research, 33, 264–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen DD, Alexander L, Malone J, Federoff D, & Froehlich JC (2014). The alpha2-adrenergic receptor agonist, clonidine, reduces alcohol drinking in alcohol-preferring (P) rats. Alcohol, 48, 543–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rea K, Cremers TI, & Westerink BH (2005). HPLC conditions are critical for the detection of GABA by microdialysis. Journal of Neurochemistry, 94, 672–679. [DOI] [PubMed] [Google Scholar]
- Rehm J, Taylor B, Mohapatra S, Irving H, Baliunas D, Patra J et al. (2010). Alcohol as a risk factor for liver cirrhosis: a systematic review and meta-analysis. Drug and Alcohol Review, 29, 437–445. [DOI] [PubMed] [Google Scholar]
- Renteria R, Baltz ET, & Gremel CM (2018). Chronic alcohol exposure disrupts top-down control over basal ganglia action selection to produce habits. Nature Communications, 9, 211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds SM, & Zahm DS (2005). Specificity in the projections of prefrontal and insular cortex to ventral striatopallidum and the extended amygdala. Journal of Neuroscience, 25, 11757–11767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riga D, Schmitz LJ, van der Harst JE, van Mourik Y, Hoogendijk WJ, Smit AB et al. (2014). A sustained depressive state promotes a guanfacine reversible susceptibility to alcohol seeking in rats. Neuropsychopharmacology, 39, 1115–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimondini R, Thorsell A, & Heilig M (2005). Suppression of ethanol self-administration by the neuropeptide Y (NPY) Y2 receptor antagonist BIIE0246: evidence for sensitization in rats with a history of dependence. Neuroscience Letters, 375, 129–133. [DOI] [PubMed] [Google Scholar]
- Ripley TL, Sanchez-Roige S, Bullmore ET, Mugnaini M, Maltby K, Miller SR et al. (2015). The novel mu-opioid antagonist, GSK1521498, reduces ethanol consumption in C57BL/6J mice. Psychopharmacology, 232, 3431–3441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivier C, & Lee S (1996). Acute alcohol administration stimulates the activity of hypothalamic neurons that express corticotropin-releasing factor and vasopressin. Brain Research, 726, 1–10. [PubMed] [Google Scholar]
- Roberto M, Madamba SG, Stouffer DG, Parsons LH, & Siggins GR (2004). Increased GABA release in the central amygdala of ethanol-dependent rats. Journal of Neuroscience, 24, 10159–10166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts AJ, McDonald JS, Heyser CJ, Kieffer BL, Matthes HW, Koob GF et al. (2000). mu-Opioid receptor knockout mice do not self-administer alcohol. Journal of Pharmacology and Experimental Therapeutics, 293, 1002–1008. [PubMed] [Google Scholar]
- Roberts AJ, Gold LH, Polis I, McDonald JS, Filliol D, Kieffer BL et al. (2001). Increased ethanol self-administration in delta-opioid receptor knockout mice. Alcoholism, Clinical and Experimental Research, 25, 1249–1256. [PubMed] [Google Scholar]
- Robinson DL, Howard EC, McConnell S, Gonzales RA, & Wightman RM (2009). Disparity between tonic and phasic ethanol-induced dopamine increases in the nucleus accumbens of rats. Alcoholism, Clinical and Experimental Research, 33, 1187–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson S, Rainwater AJ, Hnasko TS, & Palmiter RD (2007). Viral restoration of dopamine signaling to the dorsal striatum restores instrumental conditioning to dopamine-deficient mice. Psychopharmacology, 191, 567–578. [DOI] [PubMed] [Google Scholar]
- Robinson SL, & Thiele TE (2017). The Role of Neuropeptide Y (NPY) in Alcohol and Drug Abuse Disorders. International Review of Neurobiology, 136, 177–197. [DOI] [PubMed] [Google Scholar]
- Rodd ZA, Melendez RI, Bell RL, Kuc KA, Zhang Y, Murphy JM et al. (2004). Intracranial self-administration of ethanol within the ventral tegmental area of male Wistar rats: evidence for involvement of dopamine neurons. Journal of Neuroscience, 24, 1050–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rommelfanger KS, Mitrano DA, Smith Y, & Weinshenker D (2009). Light and electron microscopic localization of alpha-1 adrenergic receptor immunoreactivity in the rat striatum and ventral midbrain. Neuroscience, 158, 1530–1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rorick-Kehn LM, Witkin JM, Statnick MA, Eberle EL, McKinzie JH, Kahl SD et al. (2014). LY2456302 is a novel, potent, orally-bioavailable small molecule kappa-selective antagonist with activity in animal models predictive of efficacy in mood and addictive disorders. Neuropharmacology, 77, 131–144. [DOI] [PubMed] [Google Scholar]
- Rose AK, Brown K, Field M, & Hogarth L (2013). The contributions of value-based decision-making and attentional bias to alcohol-seeking following devaluation. Addiction, 108, 1241–1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose AK, Brown K, MacKillop J, Field M, & Hogarth L (2018). Alcohol devaluation has dissociable effects on distinct components of alcohol behaviour. Psychopharmacology (Berl), 235, 1233–1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose JH, Karkhanis AN, Chen R, Gioia D, Lopez MF, Becker HC et al. (2016). Supersensitive Kappa Opioid Receptors Promotes Ethanol Withdrawal-Related Behaviors and Reduce Dopamine Signaling in the Nucleus Accumbens. International Journal of Neuropsychopharmacology, 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosin A, Lindholm S, Franck J, & Georgieva J (1999). Downregulation of kappa opioid receptor mRNA levels by chronic ethanol and repetitive cocaine in rat ventral tegmentum and nucleus accumbens. Neuroscience Letters, 275, 1–4. [DOI] [PubMed] [Google Scholar]
- Rossetti ZL, Longu G, Mercuro G, Hmaidan Y, & Gessa GL (1992). Biphasic effect of ethanol on noradrenaline release in the frontal cortex of awake rats. Alcohol and Alcoholism, 27, 477–480. [PubMed] [Google Scholar]
- Rostene W, Kitabgi P, & Parsadaniantz SM (2007). Chemokines: a new class of neuromodulator? Nature Reviews: Neuroscience, 8, 895–903. [DOI] [PubMed] [Google Scholar]
- Ryan ML, Falk DE, Fertig JB, Rendenbach-Mueller B, Katz DA, Tracy KA et al. (2017). A Phase 2, Double-Blind, Placebo-Controlled Randomized Trial Assessing the Efficacy of ABT-436, a Novel V1b Receptor Antagonist, for Alcohol Dependence. Neuropsychopharmacology, 42, 1012–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabino V, Kwak J, Rice KC, & Cottone P (2013). Pharmacological characterization of the 20% alcohol intermittent access model in Sardinian alcohol-preferring rats: a model of binge-like drinking. Alcoholism, Clinical and Experimental Research, 37, 635–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sable HJ, Bell RL, Rodd ZA, & McBride WJ (2006). Effects of naltrexone on the acquisition of alcohol intake in male and female periadolescent and adult alcohol-preferring (P) rats. International Journal of Adolescent Medicine and Health, 18, 139–149. [DOI] [PubMed] [Google Scholar]
- Saland LC, Abeyta A, Frausto S, Raymond-Stintz M, Hastings CM, Carta M et al. (2004). Chronic ethanol consumption reduces delta-and mu-opioid receptor-stimulated G-protein coupling in rat brain. Alcoholism, Clinical and Experimental Research, 28, 98–104. [DOI] [PubMed] [Google Scholar]
- Saland LC, Hastings CM, Abeyta A, & Chavez JB (2005). Chronic ethanol modulates delta and mu-opioid receptor expression in rat CNS: immunohistochemical analysis with quantitiative confocal microscopy. Neuroscience Letters, 381, 163–168. [DOI] [PubMed] [Google Scholar]
- Samson HH, & Czachowski CL (2003). Behavioral measures of alcohol self-administration and intake control: rodent models. International Review of Neurobiology, 54, 107–143. [DOI] [PubMed] [Google Scholar]
- Samson HH, & Falk JL (1974). Alteration of fluid preference in ethanol-dependent animals. Journal of Pharmacology and Experimental Therapeutics, 190, 365–376. [PubMed] [Google Scholar]
- Samson HH, Pfeffer AO, & Tolliver GA (1988). Oral ethanol self-administration in rats: models of alcohol-seeking behavior. Alcoholism, Clinical and Experimental Research, 12, 591–598. [DOI] [PubMed] [Google Scholar]
- Samson HH, Slawecki CJ, Sharpe AL, & Chappell A (1998). Appetitive and consummatory behaviors in the control of ethanol consumption: a measure of ethanol seeking behavior. Alcoholism, Clinical and Experimental Research, 22, 1783–1787. [PubMed] [Google Scholar]
- Sara SJ (2009). The locus coeruleus and noradrenergic modulation of cognition. Nature Reviews: Neuroscience, 10, 211–223. [DOI] [PubMed] [Google Scholar]
- Sari Y, Sreemantula SN, Lee MR, & Choi DS (2013). Ceftriaxone treatment affects the levels of GLT1 and ENT1 as well as ethanol intake in alcohol-preferring rats. Journal of Molecular Neuroscience, 51, 779–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacht JP, Anton RF, & Myrick H (2013). Functional neuroimaging studies of alcohol cue reactivity: a quantitative meta-analysis and systematic review. Addiction Biology, 18, 121–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schank JR, Goldstein AL, Rowe KE, King CE, Marusich JA, Wiley JL et al. (2012). The kappa opioid receptor antagonist JDTic attenuates alcohol seeking and withdrawal anxiety. Addiction Biology, 17, 634–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler AG, Tsutsui KT, & Clark JJ (2014). Chronic alcohol intake during adolescence, but not adulthood, promotes persistent deficits in risk-based decision making. Alcoholism, Clinical and Experimental Research, 38, 1622–1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenbaum G, & Shaham Y (2008). The role of orbitofrontal cortex in drug addiction: a review of preclinical studies. Biological Psychiatry, 63, 256–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schramm-Sapyta NL, DiFeliceantonio AG, Foscue E, Glowacz S, Haseeb N, Wang N et al. (2010). Aversive effects of ethanol in adolescent versus adult rats: potential causes and implication for future drinking. Alcoholism, Clinical and Experimental Research, 34, 2061–2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W, Dayan P, & Montague PR (1997). A neural substrate of prediction and reward. Science, 275, 1593–1599. [DOI] [PubMed] [Google Scholar]
- Schwandt ML, Cortes CR, Kwako LE, George DT, Momenan R, Sinha R et al. (2016). The CRF1 Antagonist Verucerfont in Anxious Alcohol-Dependent Women: Translation of Neuroendocrine, But not of Anti-Craving Effects. Neuropsychopharmacology, 41, 2818–2829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz LA, & Luo L (2015). Organization of the locus coeruleus-norepinephrine system. Current Biology, 25, R1051–R1056. [DOI] [PubMed] [Google Scholar]
- Sciascia JM, Reese RM, Janak PH, & Chaudhri N (2015). Alcohol-Seeking Triggered by Discrete Pavlovian Cues is Invigorated by Alcohol Contexts and Mediated by Glutamate Signaling in the Basolateral Amygdala. Neuropsychopharmacology, 40, 2801–2812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebold M, Deserno L, Nebe S, Schad DJ, Garbusow M, Hagele C, Keller J, Junger E, Kathmann N, Smolka MN, Rapp MA, Schlagenhauf F, Heinz A, & Huys QJ (2014). Model-based and model-free decisions in alcohol dependence. Neuropsychobiology, 70, 122–131. [DOI] [PubMed] [Google Scholar]
- Selim M, & Bradberry CW (1996). Effect of ethanol on extracellular 5-HT and glutamate in the nucleus accumbens and prefrontal cortex: comparison between the Lewis and Fischer 344 rat strains. Brain Research, 716, 157–164. [DOI] [PubMed] [Google Scholar]
- Setlow B, Holland PC, & Gallagher M (2002). Disconnection of the basolateral amygdala complex and nucleus accumbens impairs appetitive pavlovian second-order conditioned responses. Behavioral Neuroscience, 116, 267–275. [DOI] [PubMed] [Google Scholar]
- Shelkar GP, Kumar S, Singru PS, Subhedar NK, & Kokare DM (2017). Noradrenergic inputs from locus coeruleus to posterior ventral tegmental area are essential to support ethanol reinforcement. Addiction Biology, 22, 291–302. [DOI] [PubMed] [Google Scholar]
- Shi CJ, & Cassell MD (1998). Cortical, thalamic, and amygdaloid connections of the anterior and posterior insular cortices. Journal of Comparative Neurology, 399, 440–468. [DOI] [PubMed] [Google Scholar]
- Shillinglaw JE, Everitt IK, & Robinson DL (2014). Assessing behavioral control across reinforcer solutions on a fixed-ratio schedule of reinforcement in rats. Alcohol, 48, 337–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shnitko TA, & Robinson DL (2015). Regional variation in phasic dopamine release during alcohol and sucrose self-administration in rats. ACS Chemical Neuroscience, 6, 147–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shnitko TA, Spear LP, & Robinson DL (2016). Adolescent binge-like alcohol alters sensitivity to acute alcohol effects on dopamine release in the nucleus accumbens of adult rats. Psychopharmacology, 233, 361–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siciliano CA, Karkhanis AN, Holleran KM, Melchior JR, & Jones SR (2018). Cross-Species Alterations in Synaptic Dopamine Regulation After Chronic Alcohol Exposure In Grant KA & Lovinger DM (Eds.), The Neuropharmacology of Alcohol (pp. 213–238). Cham: Springer International Publishing. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegmund S, Vengeliene V, Singer MV, & Spanagel R (2005). Influence of age at drinking onset on long-term ethanol self-administration with deprivation and stress phases. Alcoholism, Clinical and Experimental Research, 29, 1139–1145. [DOI] [PubMed] [Google Scholar]
- Silva SM, Madeira MD, Ruela C, & Paula-Barbosa MM (2002). Prolonged alcohol intake leads to irreversible loss of vasopressin and oxytocin neurons in the paraventricular nucleus of the hypothalamus. Brain Research, 925, 76–88. [DOI] [PubMed] [Google Scholar]
- Simms JA, Steensland P, Medina B, Abernathy KE, Chandler LJ, Wise R et al. (2008). Intermittent access to 20% ethanol induces high ethanol consumption in Long-Evans and Wistar rats. Alcoholism, Clinical and Experimental Research, 32, 1816–1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson TL, Saxon AJ, Meredith CW, Malte CA, McBride B, Ferguson LC et al. (2009). A pilot trial of the alpha-1 adrenergic antagonist, prazosin, for alcohol dependence. Alcoholism, Clinical and Experimental Research, 33, 255–263. [DOI] [PubMed] [Google Scholar]
- Sim-Selley LJ, Sharpe AL, Vogt LJ, Brunk LK, Selley DE, & Samson HH (2002). Effect of ethanol self-administration on mu- and delta-opioid receptor-mediated G-protein activity. Alcoholism, Clinical and Experimental Research, 26, 688–694. [PubMed] [Google Scholar]
- Sinclair CM, Cleva RM, Hood LE, Olive MF, & Gass JT (2012). mGluR5 receptors in the basolateral amygdala and nucleus accumbens regulate cue-induced reinstatement of ethanol-seeking behavior. Pharmacology, Biochemistry and Behavior, 101, 329–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjoerds Z, de Wit S, van den Brink W, Robbins TW, Beekman AT, Penninx BW, & Veltman DJ (2013). Behavioral and neuroimaging evidence for overreliance on habit learning in alcohol-dependent patients. Translational Psychiatry, 3, e337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner BF (1938). The Behavior of Organisms: An Experimental Analysis. New York, USA: D. Appleton-Century Company, Inc. [Google Scholar]
- Slawecki CJ, Betancourt M, Walpole T, & Ehlers CL (2000). Increases in sucrose consumption, but not ethanol consumption, following ICV NPY administration. Pharmacology, Biochemistry and Behavior, 66, 591–594. [DOI] [PubMed] [Google Scholar]
- Snelleman M, Schoenmakers TM, & van de Mheen D (2015). Attentional Bias and Approach/Avoidance Tendencies Do Not Predict Relapse or Time to Relapse in Alcohol Dependency. Alcoholism, Clinical and Experimental Research, 39, 1734–1739. [DOI] [PubMed] [Google Scholar]
- Sotomayor R, Forray MI, & Gysling K (2005). Acute morphine administration increases extracellular DA levels in the rat lateral septum by decreasing the GABAergic inhibitory tone in the ventral tegmental area. Journal of Neuroscience Research, 81, 132–139. [DOI] [PubMed] [Google Scholar]
- Sparks LM, Sciascia JM, Ayorech Z, & Chaudhri N (2014). Vendor differences in alcohol consumption and the contribution of dopamine receptors to Pavlovian-conditioned alcohol-seeking in Long-Evans rats. Psychopharmacology, 231, 753–764.24096535 [Google Scholar]
- Sparrow AM, Lowery-Gionta EG, Pleil KE, Li C, Sprow GM, Cox BR et al. (2012). Central neuropeptide Y modulates binge-like ethanol drinking in C57BL/6J mice via Y1 and Y2 receptors. Neuropsychopharmacology, 37, 1409–1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear LP (2018). Effects of adolescent alcohol consumption on the brain and behaviour. Nature Reviews: Neuroscience, 19, 197–214. [DOI] [PubMed] [Google Scholar]
- Spencer S, & Kalivas PW (2017). Glutamate Transport: A New Bench to Bedside Mechanism for Treating Drug Abuse. International Journal of Neuropsychopharmacology, 20, 797–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling RE, Gomes SM, Sypek EI, Carey AN, & McLaughlin JP (2010). Endogenous kappa-opioid mediation of stress-induced potentiation of ethanol-conditioned place preference and self-administration. Psychopharmacology, 210, 199–209. [DOI] [PubMed] [Google Scholar]
- Spierling SR, & Zorrilla EP (2017). Don’t stress about CRF: assessing the translational failures of CRF1antagonists. Psychopharmacology, 234, 1467–1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srey CS, Maddux JM, & Chaudhri N (2015). The attribution of incentive salience to Pavlovian alcohol cues: a shift from goal-tracking to sign-tracking. Frontiers in Behavioral Neuroscience, 9, 54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stennett BA, Frankowski JC, Peris J, & Knackstedt LA (2017). Ceftriaxone reduces alcohol intake in outbred rats while upregulating xCT in the nucleus accumbens core. Pharmacology, Biochemistry and Behavior, 159, 18–23. [DOI] [PubMed] [Google Scholar]
- Stevenson JR, Wenner SM, Freestone DM, Romaine CC, Parian MC, Christian SM et al. (2017). Oxytocin reduces alcohol consumption in prairie voles. Physiology and Behavior, 179, 411–421. [DOI] [PubMed] [Google Scholar]
- Stuber GD, Sparta DR, Stamatakis AM, van Leeuwen WA, Hardjoprajitno JE, Cho S, Tye KM, Kempadoo KA, Zhang F, Deisseroth K, & Bonci A (2011). Excitatory transmission from the amygdala to nucleus accumbens facilitates reward seeking. Nature, 475, 377–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swendsen J, Burstein M, Case B, Conway KP, Dierker L, He J et al. (2012). Use and abuse of alcohol and illicit drugs in US adolescents: results of the National Comorbidity Survey-Adolescent Supplement. Archives of General Psychiatry, 69, 390–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabadi E (2013). Functional neuroanatomy of the central noradrenergic system. Journal of psychopharmacology (Oxford, England), 27, 659–693. [DOI] [PubMed] [Google Scholar]
- Takahashi YK, Roesch MR, Stalnaker TA, Haney RZ, Calu DJ, Taylor AR, Burke KA, & Schoenbaum G (2009). The orbitofrontal cortex and ventral tegmental area are necessary for learning from unexpected outcomes. Neuron, 62, 269–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theile JW, Morikawa H, Gonzales RA, & Morrisett RA (2008). Ethanol enhances GABAergic transmission onto dopamine neurons in the ventral tegmental area of the rat. Alcoholism, Clinical and Experimental Research, 32, 1040–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiele TE, Marsh DJ, Ste Marie L, Bernstein IL, & Palmiter RD (1998). Ethanol consumption and resistance are inversely related to neuropeptide Y levels. Nature, 396, 366–369. [DOI] [PubMed] [Google Scholar]
- Thiele TE, Miura GI, Marsh DJ, Bernstein IL, & Palmiter RD (2000). Neurobiological responses to ethanol in mutant mice lacking neuropeptide Y or the Y5 receptor. Pharmacology, Biochemistry and Behavior, 67, 683–691. [DOI] [PubMed] [Google Scholar]
- Thorsell A, Rimondini R, & Heilig M (2002). Blockade of central neuropeptide Y (NPY) Y2 receptors reduces ethanol self-administration in rats. Neuroscience Letters, 332, 1–4. [DOI] [PubMed] [Google Scholar]
- Tomie A, Festa ED, Sparta DR, & Pohorecky LA (2003). Lever conditioned stimulus-directed autoshaping induced by saccharin-ethanol unconditioned stimulus solution: effects of ethanol concentration and trial spacing. Alcohol, 30, 35–44. [DOI] [PubMed] [Google Scholar]
- Tomie A, Uveges JM, Burger KM, Patterson-Buckendahl P, & Pohorecky LA (2004). Effects of ethanol sipper and social opportunity on ethanol drinking in rats. Alcohol and Alcoholism, 39, 197–202. [DOI] [PubMed] [Google Scholar]
- Tomie A, Hosszu R, Rosenberg RH, Gittleman J, Patterson-Buckendahl P, & Pohorecky LA (2006b). An inter-gender effect on ethanol drinking in rats: proximal females increase ethanol drinking in males. Pharmacology, Biochemistry and Behavior, 83, 307–313. [DOI] [PubMed] [Google Scholar]
- Tomie A, Miller WC, Dranoff E, & Pohorecky LA (2006a). Intermittent presentations of ethanol sipper tube induce ethanol drinking in rats. Alcohol and Alcoholism, 41, 225–230. [DOI] [PubMed] [Google Scholar]
- Tomie A, Lewis K, Curiotto J, & Pohorecky LA (2007). Intermittent exposure to a social stimulus enhances ethanol drinking in rats. Pharmacology, Biochemistry and Behavior, 87, 341–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomie A, Costea NR, Vohra K, & Pohorecky LA (2011). Effects of removing food on maintenance of drinking initiated by pairings of sipper and food. Pharmacology, Biochemistry and Behavior, 98, 462–467. [DOI] [PubMed] [Google Scholar]
- Townshend JM, & Duka T (2001). Attentional bias associated with alcohol cues: differences between heavy and occasional social drinkers. Psychopharmacology (Berl), 157, 67–74. [DOI] [PubMed] [Google Scholar]
- Trantham-Davidson H, Centanni SW, Garr SC, New NN, Mulholland PJ, Gass JT et al. (2017). Binge-Like Alcohol Exposure During Adolescence Disrupts Dopaminergic Neurotransmission in the Adult Prelimbic Cortex. Neuropsychopharmacology, 42, 1024–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng A, Nguyen K, Hamid A, Garg M, Marquez P, & Lutfy K (2013). The role of endogenous beta-endorphin and enkephalins in ethanol reward. Neuropharmacology, 73, 290–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbano-Marquez A, Estruch R, Navarro-Lopez F, Grau JM, Mont L, & Rubin E (1989). The effects of alcoholism on skeletal and cardiac muscle. New England Journal of Medicine, 320, 409–415. [DOI] [PubMed] [Google Scholar]
- Valdez GR, Roberts AJ, Chan K, Davis H, Brennan M, Zorrilla EP et al. (2002). Increased ethanol self-administration and anxiety-like behavior during acute ethanol withdrawal and protracted abstinence: regulation by corticotropin-releasing factor. Alcoholism, Clinical and Experimental Research, 26, 1494–1501. [DOI] [PubMed] [Google Scholar]
- Valenta JP, & Gonzales RA (2016). Chronic Intracerebroventricular Infusion of Monocyte Chemoattractant Protein-1 Leads to a Persistent Increase in Sweetened Ethanol Consumption During Operant Self-Administration But Does Not Influence Sucrose Consumption in Long-Evans Rats. Alcoholism, Clinical and Experimental Research, 40, 187–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenta JP, Job MO, Mangieri RA, Schier CJ, Howard EC, & Gonzales RA (2013). mu-opioid receptors in the stimulation of mesolimbic dopamine activity by ethanol and morphine in Long-Evans rats: a delayed effect of ethanol. Psychopharmacology, 228, 389–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valles SL, Blanco AM, Pascual M, & Guerri C (2004). Chronic ethanol treatment enhances inflammatory mediators and cell death in the brain and in astrocytes. Brain Pathology, 14, 365–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Rijn RM, & Whistler JL (2009). The delta(1) opioid receptor is a heterodimer that opposes the actions of the delta(2) receptor on alcohol intake. Biological Psychiatry, 66, 777–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Rijn RM, Brissett DI, & Whistler JL (2012). Emergence of functional spinal delta opioid receptors after chronic ethanol exposure. Biological Psychiatry, 71, 232–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandaele Y, & Janak PH (2018). Defining the place of habit in substance use disorders. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 87, 22–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veale WL, & Myers RD (1969). Increased alcohol preference in rats following repeated exposures to alcohol. Psychopharmacologia, 15, 361–372. [DOI] [PubMed] [Google Scholar]
- Vena AA, & Gonzales RA (2015). Temporal profiles dissociate regional extracellular ethanol versus dopamine concentrations. ACS Chemical Neuroscience, 6, 37–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vena A, & Gonzales RA (2017). Pharmacological effects of acute ethanol on extracellular norepinephrine in the medial prefrontal cortex. Symposium abstract from the 40th Annual Research Society on Alcoholism Scientific Meeting. [Google Scholar]
- Vena A, King A, Lee R, & de Wit H (2018). Intranasal Oxytocin Does Not Modulate Responses to Alcohol in Social Drinkers. Alcoholism, Clinical and Experimental Research, 42, 1725–1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventura R, De Carolis D, Alcaro A, & Puglisi-Allegra S (2006). Ethanol consumption and reward depend on norepinephrine in the prefrontal cortex. Neuroreport, 17, 1813–1817. [DOI] [PubMed] [Google Scholar]
- Verplaetse TL, Rasmussen DD, Froehlich JC, & Czachowski CL (2012). Effects of prazosin, an alpha1-adrenergic receptor antagonist, on the seeking and intake of alcohol and sucrose in alcohol-preferring (P) rats. Alcoholism, Clinical and Experimental Research, 36, 881–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetter CS, Doremus-Fitzwater TL, & Spear LP (2007). Time course of elevated ethanol intake in adolescent relative to adult rats under continuous, voluntary-access conditions. Alcoholism, Clinical and Experimental Research, 31, 1159–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vihavainen T, Relander TR, Leiviska R, Airavaara M, Tuominen RK, Ahtee L et al. (2008). Chronic nicotine modifies the effects of morphine on extracellular striatal dopamine and ventral tegmental GABA. Journal of Neurochemistry, 107, 844–854. [DOI] [PubMed] [Google Scholar]
- Villaruel FR, & Chaudhri N (2016). Individual Differences in the Attribution of Incentive Salience to a Pavlovian Alcohol Cue. Frontiers in Behavioral Neuroscience, 10, 238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Logan J, Hitzemann R, Ding YS, Pappas N, Shea C, & Piscani K (1996). Decreases in dopamine receptors but not in dopamine transporters in alcoholics. Alcohol Clin Exp Res, 20, 1594–1598. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Telang F, Fowler JS, Logan J, Jayne M, Ma Y, Pradhan K, & Wong C (2007). Profound decreases in dopamine release in striatum in detoxified alcoholics: possible orbitofrontal involvement. J Neurosci, 27, 12700–12706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wiers CE, Shokri-Kojori E, Tomasi D, Wang GJ, & Baler R (2017). Neurochemical and metabolic effects of acute and chronic alcohol in the human brain: Studies with positron emission tomography. Neuropharmacology, 122, 175–188. [DOI] [PubMed] [Google Scholar]
- Vollstadt-Klein S, Loeber S, Richter A, Kirsch M, Bach P, von der Goltz C et al. (2012). Validating incentive salience with functional magnetic resonance imaging: association between mesolimbic cue reactivity and attentional bias in alcohol-dependent patients. Addiction Biology, 17, 807–816. [DOI] [PubMed] [Google Scholar]
- Vollstadt-Klein S, Wichert S, Rabinstein J, Buhler M, Klein O, Ende G, Hermann D, & Mann K (2010). Initial, habitual and compulsive alcohol use is characterized by a shift of cue processing from ventral to dorsal striatum. Addiction, 105, 1741–1749. [DOI] [PubMed] [Google Scholar]
- Walker BM, Rasmussen DD, Raskind MA, & Koob GF (2008). alpha1-noradrenergic receptor antagonism blocks dependence-induced increases in responding for ethanol. Alcohol, 42, 91–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker BM, Zorrilla EP, & Koob GF (2011). Systemic kappa-opioid receptor antagonism by nor-binaltorphimine reduces dependence-induced excessive alcohol self-administration in rats. Addiction Biology, 16, 116–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh KH, Das RK, Saladin ME, & Kamboj SK (2018). Modulation of naturalistic maladaptive memories using behavioural and pharmacological reconsolidation-interfering strategies: a systematic review and meta-analysis of clinical and ‘sub-clinical’ studies. Psychopharmacology, 235, 2507–2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warthen KG, Sanford B, Walker K, Jones KG, Angstadt M, Sripada C et al. (2019). Neuropeptide Y and representation of salience in human nucleus accumbens. Neuropsychopharmacology, 44, 495–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wayner MJ, Greenberg I, Tartaglione R, Nolley D, Fraley S, & Cott A (1972). A new factor affecting the consumption of ethyl alcohol and other sapid fluids. Physiology and Behavior, 8, 345–362. [DOI] [PubMed] [Google Scholar]
- Weiner JL, & Valenzuela CF (2006). Ethanol modulation of GABAergic transmission: the view from the slice. Pharmacology and Therapeutics, 111, 533–554. [DOI] [PubMed] [Google Scholar]
- Weinshenker D, Rust NC, Miller NS, & Palmiter RD (2000). Ethanol-associated behaviors of mice lacking norepinephrine. Journal of Neuroscience, 20, 3157–3164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss F, Lorang MT, Bloom FE, & Koob GF (1993). Oral alcohol self-administration stimulates dopamine release in the rat nucleus accumbens: genetic and motivational determinants. Journal of Pharmacology and Experimental Therapeutics, 267, 250–258. [PubMed] [Google Scholar]
- Wetherill L, Schuckit MA, Hesselbrock V, Xuei X, Liang T, Dick DM et al. (2008). Neuropeptide Y receptor genes are associated with alcohol dependence, alcohol withdrawal phenotypes, and cocaine dependence. Alcoholism, Clinical and Experimental Research, 32, 2031–2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiers CE, Stelzel C, Park SQ, Gawron CK, Ludwig VU, Gutwinski S, Heinz A, Lindenmeyer J, Wiers RW, Walter H, & Bermpohl F (2014). Neural correlates of alcohol-approach bias in alcohol addiction: the spirit is willing but the flesh is weak for spirits. Neuropsychopharmacology, 39, 688–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willuhn I, Burgeno LM, Everitt BJ, & Phillips PE (2012). Hierarchical recruitment of phasic dopamine signaling in the striatum during the progression of cocaine use. Proceedings of the National Academy of Sciences of the United States of America, 109, 20703–20708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windisch KA, Kosobud AE, & Czachowski CL (2014). Intravenous alcohol self-administration in the P rat. Alcohol, 48, 419–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA (1973). Voluntary ethanol intake in rats following exposure to ethanol on various schedules. Psychopharmacologia, 29, 203–210. [DOI] [PubMed] [Google Scholar]
- Witteman J, Post H, Tarvainen M, de Bruijn A, Perna Ede S, Ramaekers JG et al. (2015). Cue reactivity and its relation to craving and relapse in alcohol dependence: a combined laboratory and field study. Psychopharmacology, 232, 3685–3696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witten IB, Steinberg EE, Lee SY, Davidson TJ, Zalocusky KA, Brodsky M et al. (2011). Recombinase-driver rat lines: tools, techniques, and optogenetic application to dopamine-mediated reinforcement. Neuron, 72, 721–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan QS, Zheng SZ, Feng MJ, & Yan SE (2005). Involvement of 5-HT1B receptors within the ventral tegmental area in ethanol-induced increases in mesolimbic dopaminergic transmission. Brain Research, 1060, 126–137. [DOI] [PubMed] [Google Scholar]
- Yin HH, Ostlund SB, Knowlton BJ, & Balleine BW (2005). The role of the dorsomedial striatum in instrumental conditioning. European Journal of Neuroscience, 22, 513–523. [DOI] [PubMed] [Google Scholar]
- Yin HH, Knowlton BJ, & Balleine BW (2006). Inactivation of dorsolateral striatum enhances sensitivity to changes in the action-outcome contingency in instrumental conditioning. Behavioural Brain Research, 166, 189–196. [DOI] [PubMed] [Google Scholar]
- Yin HH, Ostlund SB, & Balleine BW (2008). Reward-guided learning beyond dopamine in the nucleus accumbens: the integrative functions of cortico-basal ganglia networks. European Journal of Neuroscience, 28, 1437–1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoder KK, Albrecht DS, Dzemidzic M, Normandin MD, Federici LM, Graves T, Herring CM, Hile KL, Walters JW, Liang T, Plawecki MH, O’Connor S, & Kareken DA (2016). Differences in IV alcohol-induced dopamine release in the ventral striatum of social drinkers and nontreatment-seeking alcoholics. Drug Alcohol Depend, 160, 163–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoder KK, Morris ED, Constantinescu CC, Cheng TE, Normandin MD, O’Connor SJ et al. (2009). When what you see isn’t what you get: alcohol cues, alcohol administration, prediction error, and human striatal dopamine. Alcoholism, Clinical and Experimental Research, 33, 139–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoneyama N, Crabbe JC, Ford MM, Murillo A, & Finn DA (2008). Voluntary ethanol consumption in 22 inbred mouse strains. Alcohol, 42, 149–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon G, Kim SW, Thuras P, Grant JE, & Westermeyer J (2006). Alcohol craving in outpatients with alcohol dependence: rate and clinical correlates. Journal of Studies on Alcohol, 67, 770–777. [DOI] [PubMed] [Google Scholar]
- Zandy SL, & Gonzales RA (2018). GABA Uptake Inhibition Reduces In Vivo Extraction Fraction in the Ventral Tegmental Area of Long Evans Rats Measured by Quantitative Microdialysis Under Transient Conditions. Neurochemical Research, 43, 306–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zandy SL, Matthews DB, Tokunaga S, Miller A, Blaha CD, & Mittleman G (2015). Reduced dopamine release in the nucleus accumbens core of adult rats following adolescent binge alcohol exposure: age and dose-dependent analysis. Psychopharmacology, 232, 777–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, & Kreek MJ (2018). Involvement of Activated Brain Stress Responsive Systems in Excessive and “Relapse” Alcohol Drinking in Rodent Models: Implications for Therapeutics. Journal of Pharmacology and Experimental Therapeutics, 366, 9–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Colombo G, Carai MA, Ho A, Gessa GL, & Kreek MJ (2011). Involvement of arginine vasopressin and V1b receptor in alcohol drinking in Sardinian alcohol-preferring rats. Alcoholism, Clinical and Experimental Research, 35, 1876–1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Rubinstein M, Low MJ, & Kreek MJ (2018). V1b Receptor Antagonist SSR149415 and Naltrexone Synergistically Decrease Excessive Alcohol Drinking in Male and Female Mice. Alcoholism, Clinical and Experimental Research, 42, 195–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorrilla EP, Valdez GR, & Weiss F (2001). Changes in levels of regional CRF-like-immunoreactivity and plasma corticosterone during protracted drug withdrawal in dependent rats. Psychopharmacology, 158, 374–381. [DOI] [PubMed] [Google Scholar]
- Zorrilla EP, Logrip ML, & Koob GF (2014). Corticotropin releasing factor: a key role in the neurobiology of addiction. Frontiers in Neuroendocrinology, 35, 234–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo W, Wang L, Chen L, Krnjevic K, Fu R, Feng X et al. (2017). Ethanol potentiates both GABAergic and glutamatergic signaling in the lateral habenula. Neuropharmacology, 113, 178–187. [DOI] [PubMed] [Google Scholar]


