Abstract
Background/Aim:
Tumor necrosis factor inhibitors (TNFi) have become the mainstay of treatment in moderate-to-severe cases of inflammatory bowel disease (IBD). Neutropenia has been reported in patients receiving TNFi for IBD and other diseases. In this study, we aimed to ascertain the relationship between the use of TNFi and the development of neutropenia in patients with IBD.
Patients and Methods:
This is a retrospective cohort study including all adult patients with IBD receiving TNFi at a tertiary care center over an 11-year period. The primary outcome was the development of any neutropenic episode after starting a TNFi. For our secondary outcomes, we evaluated the impact of concomitant use of 5-aminosalicylic acid (5-ASA) or an immunomodulator on the risk of developing neutropenia.
Results:
The final analysis included 281 patients. Of those included, 34.2% developed at least one episode of neutropenia while on a TNFi. The majority of these episodes (67.7%) were mild with ANC between 1000 and 1500/mm3. No significant difference was observed in the age, gender, agent used or type of IBD between those who developed neutropenia and those who did not. Concomitant use of azathioprine (OR = 2.32, 95% CI: 1.26–4.28; P = 0.007) or 5-ASA (OR = 3.15, 95% CI: 1.55–6.39; P = 0.001) were significant independent predictors of developing neutropenia.
Conclusions:
In this study, mild neutropenia was common among patients with IBD on TNFi. Future prospective studies are required to further clarify the significance of neutropenia in patients with IBD receiving TNFi.
Keywords: Inflammatory bowel disease, neutropenia, TNF inhibitors
INTRODUCTION
Tumor necrosis factor inhibitors (TNFi) have been widely used for the treatment of immune mediated illnesses such as inflammatory bowel disease (IBD).[1] TNFi are generally well tolerated and result in a significant improvement of patients' reported outcomes.[2] However, a wide range of adverse events have been reported in the literature including hematological, neoplastic, neurological and infectious complications.[3] One of the most commonly reported side effects are infectious events.[4] Bacterial pneumonia and intra-abdominal abscess are the most common reported serious infections among patients with fistulizing Crohn's disease (CD), whereas tuberculosis (TB), histoplasmosis, and pneumocystis are the most common opportunistic infections.[5] Nevertheless, studies showed no significant increase in the rate of serious infections that require intravenous antibiotics and/or hospitalization.[6,7]
Current guidelines do not recommend regular monitoring of complete blood count (CBC) for patients on TNFi as the initial safety studies did not suggest an increase in adverse hematologic events.[8] However, a number of cases of cytopenias have been reported in patients receiving TNFi treatment.[9,10] Some factors were identified to predict the development of neutropenia while on TNFi prior to the initiation of treatment. The most important predictors include history of neutropenia while receiving a previous immunomodulator and a low baseline neutrophil count.[8]
In this study, we aimed to ascertain the relationship between TNFi and the development of neutropenia in patients with IBD. We explored potential risk factors including concomitant use of immunomodulators and low neutrophils count at baseline.
PATIENTS AND METHODS
We conducted a retrospective cohort study including all adult patients with IBD receiving TNFi agents at a tertiary center between the years 2007 and 2018. Information was obtained through a review of the electronic files. Patients who developed neutropenia after starting a TNFi were compared to patients who did not develop neutropenia, with regard to demographics, disease type, and concomitant medications.
We included all patients diagnosed with IBD who were 16 years of age or older and have received at least one dose of TNFi in the time period between 2007 and 2018. Patients with no documented total white blood cell (WBC) count and neutrophil count at baseline were excluded. Also, patients with known hematological malignancies were excluded.
The study protocol was approved by the institutional review board at King Fahad Specialist Hospital - Dammam, Saudi Arabia.
Study endpoints and definitions
Our primary outcome was the development of any episode of neutropenia after starting TNFi including infliximab (IFX), adalimumab (ADL), golimumab (GOL), and certolizumab (CTZ). Secondary outcomes included the effect of the concomitant use of 5-aminosalicylic acid (5-ASA), methotrexate (MTX) or azathioprine (AZA) on the neutrophil count. We also assessed the effect of the baseline neutrophil count and total WBC count on developing neutropenia after starting TNFi and the development of opportunistic infections in patients with severe neutropenia.
Neutropenia was defined as an absolute neutrophil count (ANC) of <1500/mm3. It was classified into mild, moderate, and severe based on an ANC of 1000–1500, 500–1000, and <500/mm3, respectively.[10]
Statistical analysis
Baseline characteristics were summarized and stratified according to the main outcome. Standard Student's t test was used to compare means and Mann–Whitney U test was used to compare medians, where applied. Chi-square and Fisher's exact tests were used to compare frequencies and the Kruskal–Wallis H test to compare multiple groups, where necessary.
Simple and multiple logistic regression analysis was used to study associations between clinical characteristics and the main outcome, where appropriate. Statistical significance was set at a P value of 0.05 and the calculations were made using Statistical Package for the Social Sciences (SPSS) software for Windows.
RESULTS
Baseline characteristics
A total of 292 patients with IBD met the inclusion criteria of which only 281 were analyzed. Eleven patients were excluded for not having a CBC with differential done. Two hundred thirty-five (83.6%) patients in our study were diagnosed with CD and the rest (16.4%) had ulcerative colitis (UC).
Outcomes
Of the 281 patients included in the study, 96 (34.2%) patients developed at least one episode of neutropenia while receiving a TNFi. Table 1 shows a comparison of the demographics of patients with IBD who developed neutropenia after starting a TNFi compared to patients who did not.
Table 1.
Summary of the demographics and concomitant medications used in patients with and without neutropenia receiving TNF inhibitor treatment
| Neutropenia (n=96, 34.2%) | No neutropenia (n=185, 65.8%) | P | |
|---|---|---|---|
| Age (mean) ± SD | 32.90±10.11 | 32.61±9.46 | 0.82 |
| Sex | |||
| Male | 45 (46.9%) | 104 (56.2%) | 0.14 |
| Female | 51 (53.1%) | 81 (43.8%) | |
| Diagnosis | |||
| CD | 75 (78.1%) | 160 (86.5%) | 0.07 |
| UC | 21 (21.9%) | 25 (13.5%) | |
| Concomitant methotrexate | |||
| No | 95 (99%) | 181 (97.8%) | 0.50 |
| Yes | 1 (1%) | 4 (2.2%) | |
| Concomitant azathioprine | |||
| No | 19 (19.8%) | 74 (40%) | 0.001 |
| Yes | 77 (80.2%) | 111 (60%) | |
| Concomitant 5-ASA | |||
| No | 59 (61.5%) | 157 (84.9%) | 0.001 |
| Yes | 37 (38.5%) | 28 (15.1%) | |
| TNFi | |||
| Adalimumab | 58 (60.4%) | 99 (53.5%) | 0.650 |
| Certolizumab | 2 (2.1%) | 4 (2.2%) | |
| Infliximab | 36 (37.5%) | 81 (43.8%) | |
| Golimumab | 0 (0%) | 1 (0.5%) |
Most of the patients who developed neutropenia after starting TNFi had mild neutropenia (67.7%), 30.2% developed moderate neutropenia, and only 2 patients (2.1%) developed severe neutropenia. Both of these patients with severe neutropenia had a low baseline neutrophil count and none of them developed opportunistic infections secondary to neutropenia [Table 2 and Figure 1].
Table 2.
Time-dependent analysis
| T | Hazard ratio | Std error | 95% CI | |
|---|---|---|---|---|
| ASA | 0.7129047 | 0.1807483 | 0.4337302 | 1.171772 |
| Age | 0.9946802 | 0.0114054 | 0.9725753 | 1.017287 |
| Sex | 0.8815616 | 0.1916815 | 0.5756685 | 1.349997 |
| Diagnosis | 1.28217 | 0.3869662 | 0.7096562 | 2.316557 |
| Baseline neutrophil count | 0.9856523 | 0.053551 | 0.8860894 | 1.096402 |
| Previous history of neutropenia | 0.956413 | 0.2212188 | 0.6078021 | 1.504973 |
Figure 1.
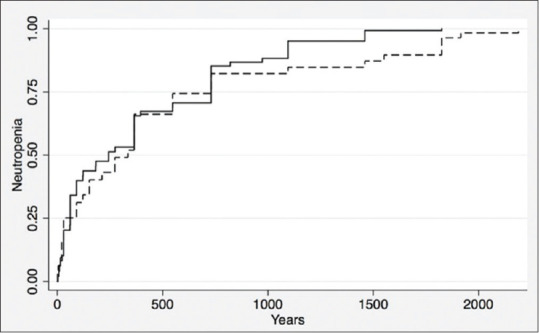
Time to neutropenia according to 5-ASA use
During the follow-up period, 69 patients of 96 patients (72%) had a recurrent episode of neutropenia. The lowest ANC was 280/mm3. The range of time between starting a TNFi agent and developing the lowest neutrophil count was between 1 day and 6 years with a median of 1 year. The mean baseline neutrophil count before starting TNFi in patients who developed neutropenia was 3300/mm3, and only six patients had baseline neutropenia (6.3%). Forty eight percent of patients who developed neutropenia had a previous history of neutropenia at any point in time prior to starting TNFi. A subgroup analysis was done excluding the patients with baseline neutropenia [Table 3] showing the same significant risk factors: concomitant use of 5-ASA and AZA.
Table 3.
Predictors of neutropenia in patients with inflammatory bowel disease treated with TNFi based on simple and multiple logistic regression analysis
| Odds ratio (95% CI) |
||
|---|---|---|
| Simple | Multiple | |
| Age | 1.00 (0.98-1.03, P=0.814) | 0.99 (0.97-1.02, P=0.560) |
| Gender | 1.46 (0.89-2.39, P=0.138) | 1.40 (0.82-2.40, P=0.250) |
| IBD type | 1.79 (0.94-3.40, P=0.075) | 0.92 (0.41-2.09, P=0.960) |
| First anti-TNF agent | 0.91 (0.77-1.08, P=0.279) | 0.84 (0.70-1.02, P=0.070) |
| Concomitant azathioprine | 2.70 (1.51-4.83, P=0.001) | 2.32 (1.26-4.28, P=0.007) |
| Concomitant methotrexate | 0.48 (0.05-4.32, P=0.510) | 0.95 (0.99-9.09, P=0.960) |
| Concomitant 5-ASA | 3.52 (1.98-6.25, P<0.001) | 3.15 (1.55-6.39, P=0.001) |
No significant differences were observed in age, gender, or disease type. There was a significant difference when comparing the use of concomitant medications AZA and 5-ASA but not MTX [Figure 2]. Among those who developed neutropenia, 52 patients (54%) were on ADL, 43 patients (44.8%) were on IFX, 1 patient (1%) was on CTZ; none were on GOL.
Figure 2.
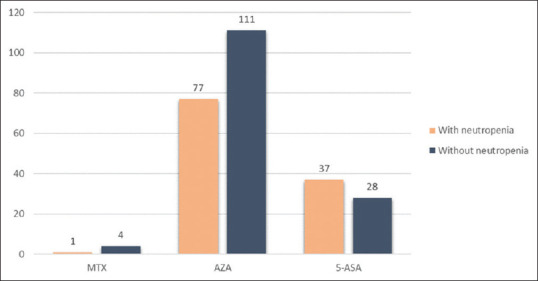
Concomitant medications used in patients who did and did not develop neutropenia while using TNF inhibitors
Predictors of neutropenia
According to logistic regression analysis, concomitant use of AZA (OR = 2.32, 95% CI: 1.26–4.28; P = 0.007) or 5-ASA (OR = 3.15, 95% CI: 1.55–6.39; P = 0.001) were significant independent predictors of neutropenia after commencing TNFi therapy [Table 4].
Table 4.
Summary of the demographics and concomitant medications used in patients with and without neutropenia receiving TNF inhibitor treatment (excluding patients with baseline neutropenia)
| Neutropenia (n=44, 19.2%) | No neutropenia (n=185, 80.8%) | P | |
|---|---|---|---|
| Age | 32.6 | 32.6 | 0.490 |
| Sex | 0.040 | ||
| Male | 17 | 104 | |
| Female | 27 | 81 | |
| Diagnosis | 0.400 | ||
| CD | 36 | 160 | |
| UC | 8 | 25 | |
| Concomitant methotrexate | 0.960 | ||
| No | 43 | 181 | |
| Yes | 1 | 4 | |
| Concomitant azathioprine | 0.007 | ||
| No | 8 | 74 | |
| Yes | 36 | 111 | |
| Concomitant 5-ASA | 0.001 | ||
| No | 28 | 157 | |
| Yes | 16 | 28 |
DISCUSSION
Biologics, in particular TNFi, are widely used for the treatment of IBD.[11] In a cohort of patients with CD managed in a tertiary care center in Saudi Arabia, one-third were on TNFi.[12]
Our study shows that, in real-world practice, the rate of neutropenia among patients with IBD on TNFi is 34.4%. Concurrent therapy with AZA and 5-ASA was the most important predictor of developing neutropenia. Neutropenia was found to be mild in most of these patients, whereas severe neutropenia occurred in two patients, without resulting in serious infections. No difference was observed in the rate of neutropenia between CD and UC patients and the rate of neutropenia was not influenced by TNFi used. To the best of our knowledge, this is the first cohort study looking at the rate of neutropenia exclusively among patients with IBD on TNFi. The previous studies were case series and case reports. A cohort study looking at the rate of neutropenia among inflammatory arthropathy patients including rheumatoid arthritis (RA), ankylosing spondylitis, and psoriatic arthritis patients showed a neutropenia rate of 19%.[8] Another study looking exclusively at patients with RA found the rate to be 14.3%.[9] The difference in the rate between our study and the other two studies might be explained by the longer period of follow-up of our cohort, which was up to 11 years, and the different pharmacological agents used in treating these conditions.
The safety of TNFi has been shown in large cohort studies. The Therapy, Resource, Evaluation, and Assessment Tool (TREAT) registry is a large prospective study that surveyed more than 6000 patients treated with TNFi. It showed that according to multivariate analysis that controlled for factors such as disease duration, severity, and concurrent corticosteroid and immunomodulator use, IFX-treated patients experienced no significant increase in the risk of serious infections.[13]
Overall, the majority of neutropenia cases that were observed in our cohort were mild (67.7%), moderate in around a third, and severe in only two patients (2.1%). It is noteworthy to mention that these two patients had low neutrophil count at baseline. One patient did not recover from that episode until the end of the study, whereas the other patient recovered after 1 month. None of them developed serious infections during follow-up. This is similar to what had been shown by previous rheumatology studies.[8,9]
Our results confirm what was previously reported in rheumatology literature with regard to predictors of TNFi-induced neutropenia. Age, gender, and concomitant use of MTX bore no relevance to the development of neutropenia.[8] Nonetheless, we showed a significant difference when comparing the use of AZA and 5-ASA in conjunction with a TNFi. It is difficult to draw firm conclusions regarding the combination of TNFi with MTX from this cohort as the total number of patients on this medication was only five and only one of them had an episode of neutropenia. Pancytopenia was reported among rheumatology patients who used MTX as monotherapy at a rate of 1.9%.[14] In our population, most subjects had CD (83.6%) but of the patients who developed neutropenia, 78% had CD and 22% had UC. However, no significant statistical difference was observed between the two groups (P = 0.072) that could establish a relationship between the underlying type of IBD and development of neutropenia.
Certain patients are at a higher risk of developing neutropenia than others. In a cohort study reported by Hastings et al.[8] low baseline neutrophil count was associated with a higher risk of developing neutropenia while on TNFi. However, they identified having a previous history of neutropenia as the most important predictor of developing subsequent neutropenic episodes while on a TNFi. Recurrent neutropenia was noticed in 69 of the 96 patients (72%). Some of the patients were on the same TNFi when the second episode of neutropenia occurred, whereas the remaining patients developed the second episode after they switched to another TNFi. The decision to switch was taken due to loss of response rather than due to the neutropenia episode itself. As reported in previous studies, neutropenia is considered a class-related side effect.[8] In our study, no significant difference was observed between the development of neutropenia and the type of TNFi used. Most of our patients 157 patients (55.7%) were on ADL. Only a limited number of patients received CTZ (6 patients [0.02%]) or GOL (1 patient [0.003%]). On the basis of these small numbers, it would be difficult to conclude whether neutropenia is a class-related or agent-related adverse effect. It is however worth noting that etanercept was the most common TNFi associated with neutropenia among patients with RA.[9] This particular TNFi is not approved for use in IBD.
It is not clear what causes neutropenia in patients receiving TNFi. Potential mechanisms include suppression of neutrophil precursors, increased peripheral consumption, and formation of anti-granulocyte antibodies.[15] Current guidelines do not recommend regular monitoring of blood cell count for patients on TNFi. The American Gastroenterological Association (AGA) guidelines 2017 recommend routine CBC monitoring for patients on thiopurine but not for those on TNFi.[16] On the contrary, the British Society of Rheumatology has recently published guidelines on the safety of TNFi. They recommended regular monitoring of blood cell counts in all patients using TNFi every three to 6 months.[17] The Food and Drug Administration (FDA) has warned about the risk of leukopenia, neutropenia, thrombocytopenia, and pancytopenia with TNFi.[18] In a pooled analysis of clinical trials of TNFi in patients with UC, concomitant use of 5-ASA was not associated with higher rates of induction of remission in patients with active disease, or maintenance of remission in patients with quiescent disease.[19] Recent guidelines advise against using concomitant 5-ASA in patients with UC who require TNFi.[20] Similarly, in patients with CD who are started on TNFi, stopping 5-ASA does not increase their risk of adverse clinical events.[21] This observed increase in the risk of neutropenia and no change in risk of developing adverse clinical events warrants further research to decide whether or not 5-ASA derivatives should be continued while on TNFi therapy.
The strength of this study comes from the relatively long follow-up period. This allowed for identifying possible predictors of neutropenia. The sample was relatively large compared to other studies looking at neutropenia in patients with IBD who received TNFi. Nonetheless, the study has several limitations. It is retrospective in design with no control arm of patients with IBD not receiving TNFi to assess the impact of TNFi on neutropenia. Patients were recruited from a single tertiary care center which may have led to including patients who are more likely to have complicated IBD or serious comorbidities.
CONCLUSION
In conclusion, mild neutropenia was common amongst patients with IBD on TNFi. No severe adverse events were seen in this population related to the decrease in neutrophils. Prospective studies are required to further support these findings and help establish guidelines for monitoring neutrophil counts in patients with IBD receiving TNFi.
Financial support and sponsorship
Nil.
Conflicts of interest
DA, MS, and ES declare no conflicts of interest related to this study. MM received consulting and speaker fees from Janseen, Abbvie, and Takeda. TA received consulting and speaker fees from Janseen, Abbvie, and Takeda.
REFERENCES
- 1.Cohen BL, Sachar DB. Update on anti-tumor necrosis factor agents and other new drugs for inflammatory bowel disease. BMJ. 2017;357:j2505. doi: 10.1136/bmj.j2505. [DOI] [PubMed] [Google Scholar]
- 2.Chao CY, Lemieux C, Restellini S, Afif W, Bitton A, Lakatos PL, et al. Maladaptive coping, low self-efficacy and disease activity are associated with poorer patient-reported outcomes in inflammatory bowel disease. Saudi J Gastroenterol. 2019;25:159–66. doi: 10.4103/sjg.SJG_566_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Day R. Adverse reactions to TNF-alpha inhibitors in rheumatoid arthritis. Lancet. 2002;359:540–1. doi: 10.1016/S0140-6736(02)07718-8. [DOI] [PubMed] [Google Scholar]
- 4.Nanau RM, Cohen LE, Neuman MG. Risk of infections of biological therapies with accent on inflammatory bowel disease. J Pharm Pharm Sci. 2014;17:485–531. doi: 10.18433/j3gg6d. [DOI] [PubMed] [Google Scholar]
- 5.Mosli MH, Al-Harbi O, Feagan BG, Almadi MA. A Saudi Gastroenterology association position statement on the use of tumor necrosis factor-alfa antagonists for the treatment of inflammatory bowel disease. Saudi J Gastroenterol. 2015;21:185–97. doi: 10.4103/1319-3767.161635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bonovas S, Fiorino G, Allocca M, Lytras T, Nikolopoulos GK, Peyrin-Biroulet L, et al. Biologic therapies and risk of infection and malignancy in patients with inflammatory bowel disease: A systematic review and network meta-analysis. Clin Gastroenterol Hepatol. 2016;14:1385–97.e10. doi: 10.1016/j.cgh.2016.04.039. [DOI] [PubMed] [Google Scholar]
- 7.Grijalva CG, Chen L, Delzell E, Baddley JW, Beukelman T, Winthrop KL, et al. Initiation of tumor necrosis factor-α antagonists and the risk of hospitalization for infection in patients with autoimmune diseases. JAMA. 2011;306:2331–9. doi: 10.1001/jama.2011.1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hastings R, Ding T, Butt S, Gadsby K, Zhang W, Moots RJ, et al. Neutropenia in patients receiving anti-tumor necrosis factor therapy. Arthritis Care Res (Hoboken) 2010;62:764–9. doi: 10.1002/acr.20037. [DOI] [PubMed] [Google Scholar]
- 9.Rajakulendran S, Gadsby K, Allen D, O'Reilly S, Deighton C. Neutropenia while receiving anti-tumour necrosis factor treatment for rheumatoid arthritis. Ann Rheum Dis. 2006;65:1678–9. doi: 10.1136/ard.2006.056176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bessissow T, Renard M, Hoffman I, Vermeire S, Rutgeerts P, Van Assche G. Review article: Non-malignant haematological complications of anti-tumour necrosis factor alpha therapy. Aliment Pharmacol Ther. 2012;36:312–23. doi: 10.1111/j.1365-2036.2012.05189.x. [DOI] [PubMed] [Google Scholar]
- 11.Rofaiel R, Chande N. Biologic agents in inflammatory bowel disease––quality of internet website information. Saudi J Gastroenterol. 2018;24:336–41. doi: 10.4103/sjg.SJG_55_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aljebreen AM, Alharbi OR, Azzam NA, Almalki AS, Alswat KA, Almadi MA. Clinical epidemiology and phenotypic characteristics of Crohn's disease in the central region of Saudi Arabia. Saudi J Gastroenterol. 2014;20:162–9. doi: 10.4103/1319-3767.132993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lichtenstein GR, Feagan BG, Cohen RD, Salzberg BA, Diamond RH, Chen DM, et al. Serious infections and mortality in association with therapies for Crohn's disease: TREAT registry. Clin Gastroenterol Hepatol. 2006;4:621–30. doi: 10.1016/j.cgh.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 14.Lim AY, Gaffney K, Scott DG. Methotrexate-induced pancytopenia: Serious and under-reported? Our experience of 25 cases in 5 years. Rheumatology (Oxford) 2005;44:1051–5. doi: 10.1093/rheumatology/keh685. [DOI] [PubMed] [Google Scholar]
- 15.Feuerstein JD, Cheifetz AS. Miscellaneous adverse events with biologic agents (excludes infection and malignancy) Gastroenterol Clin North Am. 2014;43:543–63. doi: 10.1016/j.gtc.2014.05.002. [DOI] [PubMed] [Google Scholar]
- 16.Feuerstein JD, Nguyen GC, Kupfer SS, Falck-Ytter Y, Singh S. American Gastroenterological Association Institute Clinical Guidelines Committee. American gastroenterological association institute guideline on therapeutic drug monitoring in inflammatory bowel disease. Gastroenterology. 2017;153:827–34. doi: 10.1053/j.gastro.2017.07.032. [DOI] [PubMed] [Google Scholar]
- 17.Holroyd CR, Seth R, Bukhari M, Malaviya A, Holmes C, Curtis E, et al. The British Society for Rheumatology biologic DMARD safety guidelines in inflammatory arthritis. Rheumatology (Oxford) 2019;58:372. doi: 10.1093/rheumatology/key298. [DOI] [PubMed] [Google Scholar]
- 18.US Food and Drug Administration. Warning: Serious infections and malignancy, Remicade. 2011. [Last accessed on 2019Sep 10]. Available from: https://www.accessdata.fda.g ov/drugsatfda_docs/lab el/2011/103772s5301lbl.pdf .
- 19.Singh S, Proudfoot JA, Dulai PS, Jairath V, Fumery M, Xu R, et al. No benefit of concomitant 5-aminosalicylates in patients with ulcerative colitis escalated to biologic therapy: Pooled analysis of individual participant data from clinical trials. Am J Gastroenterol. 2018;113:1197–205. doi: 10.1038/s41395-018-0144-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rubin DT, Ananthakrishnan AN, Siegel CA, Sauer BG, Long MD. ACG clinical guideline: Ulcerative colitis in adults. Am J Gastroenterol. 2019;114:384–413. doi: 10.14309/ajg.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 21.Ungaro RC, Limketkai BN, Jensen CB, Yzet C, Allin KH, Agrawal M, et al. Stopping 5-aminosalicylate therapy in patients with Crohn's disease starting biologic therapy does not increase risk of adverse outcomes. Clin Gastroenterol Hepatol. 2019 doi: 10.1016/j.cgh.2019.08.012. doi: 10.1016/j.cgh. 2019.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]


