Abstract
Background/Aims:
The purpose of this study is to explore the expression characteristics of lncRNA NEAT1 in hepatocellular carcinoma (HCC) and the molecular mechanism of its regulation on sorafenib resistance.
Materials and Methods:
This experimental study was performed from June 2013 to June 2019. The level of NEAT1 was determined using RT-PCR in HCC and matched adjacent tissues from 79 HCC patients in Linyi central hospital. The patients were divided into two groups to compare their prognosis based on the median NEAT1 expressions as a cutoff value. HCC cell line HepG2 negative control (HepG2-NC), sorafenib-resistant HepG2 cells (HepG2-SR) were transfected with or without NEAT1 siRNA, followed by subsequent molecular analysis, to determine the function of NEAT1 on sorafenib resistance in HCC cells. The cell transcripts were determined by RNA-sequencing analysis. The binding site of the NEAT1 and microRNA-149-5p (miR-149-5p) was verified by luciferase assay.
Results:
We found that NEAT1 was significantly increased in HCC tissues. Furthermore, NEAT1 expressions were significantly associated with HCC prognosis and chemoresistance patterns against sorafenib. Subsequently, the sorafenib-resistant HCC cell lines, together with the controls, were used to determine the regulatory effect of NEAT1 on HCC cells' progression and sorafenib resistance. NEAT1 targets the miR-149-5p, and therefore, decrease the activity of sorafenib against HCC cells. NEAT1 functions were demonstrated to be triggered by the regulation of miR-149-5p/AKT1 axis.
Conclusions:
NEAT1/miR-149-5p/AKT1 pathway-based therapy might be a potential clinical application for HCC patients.
Keywords: Hepatocellular carcinoma, lncRNA, microRNA, prognosis, sorafenib resistance
INTRODUCTION
Hepatocellular carcinoma (HCC) is the fifth most common cancer worldwide and the second leading cause of cancer-related death in China.[1] Traditional treatment includes resection and subsequent chemotherapy.[2] However, during treatment, cancer cells develop resistance toward functionally and structurally different anticancer drugs via either acquired (due to host factors) or intrinsic (due to genetic or epigenetic) mechanisms.[3] Sorafenib, an oral multitarget tyrosine kinase inhibitor, targets various molecular mechanisms, including tumor growth and angiogenesis.[4] It is one of the few systemic chemotherapy drugs for HCC that is approved by the US Food and Drug Administration and as such has been extensively applied for treating HCC patients.[5] However, the clinical efficacy of sorafenib is limited, and the prognosis of HCC patients is poor because of sorafenib resistance.[6] The biological processes involved in tumor microenvironment, inflammation, fibrosis, angiogenesis, hypoxia, autophagy, viral reactivation, and oxidative stress may all contribute to sorafenib resistance.[7] However, the resistance mechanisms of sorafenib remain unclear, and novel research may provide insight into the discovery of an effective treatment or personalized therapy for advanced HCC.
In recent years, emerging evidence has regarded noncoding RNAs, including lncRNA and miRNAs, as major regulators of various types of diseases, including infections,[8] autoimmune diseases,[9] and cancer.[10] The newly identified lncRNA NEAT1 has been found to act as a promoter in the progression of HCC[11,12] and many other tumor types.[13,14,15] In addition, the role of NEAT1 in cancer resistance to chemotherapy has also been widely reported.[16,17,18] However, little is known regarding its expression pattern, biological function, and potential mechanism used in HCC to cause resistance to sorafenib.
In the current study, we aim to explore the expression characteristics of lncRNA NEAT1 in HCC and the molecular mechanism of its regulation on sorafenib resistance.
MATERIALS AND METHODS
Cell line culture
HCC cell lines HepG was included in this study. RPMI-1640 Dulbecco's modified Eagle's medium was used to culture the cells and supplemented with 10% fetal bovine serum, together with a 1% antibiotic mixture of penicillin–streptomycin. The cells were grown in the desired culture medium and then incubated.
Establishment of sorafenib-resistant cells
Establishment of sorafenib-resistant cells was performed in accordance with a previous report.[19] The half maximal inhibitory concentration (IC50) of HCC cells to sorafenib was initially determined by incubating cells with different concentrations of sorafenib in 96-well plates, and cell viability was measured 3 days later as described below. The cells were cultured in 6-well plates at 1 × 104 cells/well and incubated with sorafenib at a concentration just below their respective IC50. The concentration of sorafenib was slowly increased by 0.25 μM per week. After 6 months, the sorafenib-resistant cell lines were obtained and termed HepG2-SR, together with wide type HepG2 cell lines termed HepG2-NC, and the two cell lines were continuously maintained by culturing them in the presence of sorafenib.
Clinical samples
A total of 79 HCC patients were included in this study. All HCC patients underwent liver resection at Linyi Central Hospital. HCC tissue samples were obtained from all included patients. The use of HCC patient samples was approved by the ethics committee of Linyi Central Hospital. Informed consent was obtained from all patients. All experiments were conducted in accordance with the Declaration of Helsinki.
Primary culture of HCCs
HCC was identified by histological testing according to the Edmondson's grading criteria. HCC matched adjacent benign liver tissues were obtained 3 cm away from HCC tissues. Isolation of the HCC or tumor-adjacent primary cells was performed as per the previous description.[20] Briefly, tumor tissues were immediately immersed in Hank's balanced salt solution (HBSS; Gibco) after hepatectomy, and transported to the laboratory. After removal of blood, the liver sample was cut into small fragments, gently dispersed, and placed in HBSS containing 0.03% pronase (Gibco), 0.05% type IV collagenase (Gibco), and 0.01% deoxyribonuclease (DNase, from bovine pancreas, Gibco) for 20 min at 37°C. The resultant suspension was filtered through a 100-μm-nylon filter (BD Falcon, Franklin Lakes, NJ, USA) and centrifuged at 50 ×g for 2 min at 4°C to obtain hepatocytes. The pellet was washed twice in HBSS containing 0.005% DNase. The final cell suspensions were cultured in collagen-coated T25 flasks (BD Falcon) in hepatocyte basal medium (HBM) (Lonza, Basel, Switzerland) supplemented with 10% heat-inactivated FBS, 1 ng/mL hepatocyte growth factor (HGF, Prospec, Rehovot, Israel), and 1× antibiotic-antimycotic (Gibco) as HBM at 37°C in a humidified incubator with 5% CO2. The medium was changed 24 h after seeding, to remove dead cells and debris. When cells reached 70–80% confluence, the cells were replated in HBM with supplements. Confluent cells were trypsinized, counted, and diluted 1:3–1:5 at every passage. Once cells were maintained for more than 30 passages, the cells were collected and stored in liquid nitrogen.
MTT assay
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay was employed to determine the cell proliferation of all types of tumor cells. Each well of a 96-well plate was seeded with about 10,000 cells and then incubated for 24 h. Tested agents at specific concentrations were used for cell treatment and then incubated for 24 h. The cells were ultimately treated with the MTT solution and incubated for 3 h, followed by the addition of DMSO and further incubated for 15 min. An ELISA microplate reader (DYNEX, USA) was used to measure the absorbance at 570 nm (optical density [OD] value).
RT-PCR assay
Total RNA was extracted from cells using TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.) according to the manufacturer's protocol. Medium without cells served as a negative control for this experiment. RT-qPCR for mRNA was carried out with the PrimeScript RT-PCR kit (Takara, Bio, Inc., Shiga, Japan), using β-actin as an internal control, in the Eppendorf Realplex4 machine (cat. no. X222687G; Hamburg, Germany). The following parameters were used for the reverse transcription reaction: 65°C for 5 min, 37°C for 15 min, and finally 98°C for 5 min. The following parameters were used for the subsequent PCR reaction: 95°C for 30 s, then 40 cycles of 95°C for 5 s, 60°C for 5 s, and finally, 72°C for 30 s. The primers used in this study were synthesized by Invitrogen and presented as follows: NEAT1: 5′-ATGCCACAACGCAGATTGAT-3′ (for ward) and 5′-CGAGAAACGCACAAGAAGG-3′ (reverse); β-actin: 5'-AAGGAAGCTTGGCGTTGTGA-3' (for ward) and 5'-GAGAGGTGAGGAGTCTTATG-3' (reverse); AKT1: 5'-TCCTCCTCAAGAATGATGGCA-3' (for ward) and 5'- GTGCGTTCGATGACAGTGGT-3' (reverse).
qRT-PCR for microRNAs was performed using TaqMan miRNA assays (Ambion; Thermo Fisher Scientific, Inc.) and has-miR-16 RT-PCR primer set (Abbexa, Inc., Cambridge, UK). Reverse transcription reactions were performed using TAQMAN® microRNA RT kit (Ambion; Thermo Fisher Scientific, Inc.) under the following conditions: 16°C for 30 min, 42°C for 30 min, and 84°C for 5 min. PCR reactions were performed using the following conditions: 95°C for 2 min followed by 40 cycles of 95°C for 15 s, and 60°C for 30 s. U6 small nuclear RNA was used as an endogenous control for data normalization.
Western blotting assay
Cells were washed three times with cold phosphate buffer saline (PBS) and then lysed with protein lysate (Pierce, Rockford, IL, USA). The supernatant of the mixture of lysate and cell components was centrifuged at 4°C for 15 min at 5000 g. Then, the protein concentration was measured by Pierce kit for bicinchoninic acid protein determination (Pierce, Rockford, IL, USA). The purified protein was separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and transferred onto a polyvinylidene difluoride membrane. The membrane was sealed with 5% skimmed milk powder containing 0.05% Tween-20 Tris buffer saline (pH 7.4) and incubated with the primary antibody (Santa Cruz, Delaware Avenue, CA, USA) at 1:200 followed by secondary antibody (Santa Cruz, Delaware Avenue, CA, USA) at 1:5000. The target protein was detected by enhanced chemiluminescence (ECL) and film exposure.
Plasmid construction and transfection
We synthesized human NEAT1 cDNA and subsequently cloned it into pcDNA3.1 vector (Thermo Fisher, USA) and termed it as pcDNA3.1-NEAT1. The sequence for NEAT1 siRNAs was: 5-GCCATCAGCTTTGAATAAA -3'. All microRNA oligonucleotides were synthesized by Shanghai GenePharma Co., Ltd. (Shanghai, China). Transfections were performed using Lipofectamine® 2000 (Invitrogen; Thermo Fisher Scientific, Inc.), according to the manufacturer's protocol. Cells were transfected with 50 nM miR-149a-5p mimics, inhibitors, or scrambled miR-control (negative control) for 24 h prior to subsequent experiments.
Luciferase activity
Cells were cultured overnight after being seeded into a 24-well plate, co-transfected with the wide type-NEAT1 or mutant-NEAT1 reporter gene plasmid containing a 5-bp mutation in the predicted binding site of miR-149a-5p and miR-149a-5p mimics or miR-125b inhibitor. Forty eight h after transfection, Dual Luciferase Reporter Assay System (Promega, U.S.A.) was used to perform the luciferase assays.
Statistical analyses
Data are represented as the mean ± standard deviation from ≥3 separate experiments performed in triplicate. The differences between groups were determined using two-tailed student's t-test with SPSS 16.0 software (SPSS, Inc., Chicago, IL, USA). P < 0.05 was considered as statistically significant.
RESULTS
Expression of NEAT1 in HCC tissues was correlated with cancer progression in HCC patients
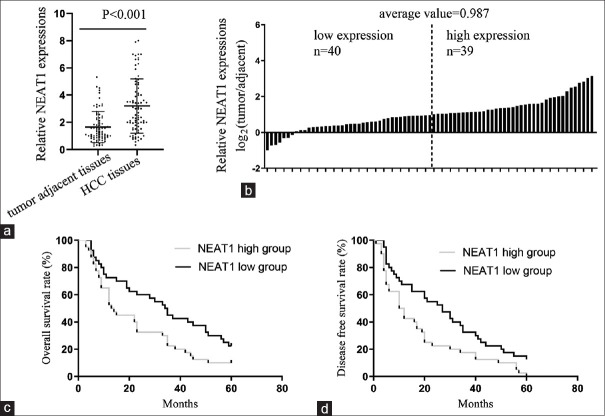
HCC tissues and matched adjacent benign liver tissues from 79 patients were collected. It was determined that the expression of NEAT1 significantly increased in HCC tissues compared with the adjacent tissues [Figure 1a]. In 50.6% (40/79) of HCC tissues, NEAT1 underwent a fold-change of more than 2 (log2(fold change) >1), compared with the adjacent tissues, and the mean log2(fold change) =0.987 [Figure 1b]. All HCC subjects were subsequently assigned to the low-NEAT1 (below the mean NEAT1 expression, n = 39) or high-NEAT1 group (above the mean NEAT1 expression, n = 40), and the relationship between the NEAT1 level and clinicopathological features was then analyzed. We found that the NEAT1 level was closely associated with tumor stage, lymphatic metastasis, and sorafenib resistance, while the expression level of NEAT1 had no significant correlation with other parameters including age, gender, tumor size, distant metastasis, or cirrhosis [Table 1]. Kaplan-Meier analysis indicated that patients in the low-NEAT1 group exhibited a longer overall survival (OS) time and disease-free survival (DSF) time than those in the high-NEAT1 group [Figures 1c and 1d.
Figure 1.
NEAT1 expressions in HCC patients. (a) qPCR results in comparing NEAT1 levels in hepatocellular carcinoma (HCC) tissues and adjacent tissues. (b) Fold changes of NEAT1 expression was exhibited as log2(tumor/adjacent) for each HCC patient. (c and d) Kaplan-Meier curves for time to (c) OS and (d) DFS of patients according to different NEAT1 expressions
Table 1.
Clinicopathological features of the hepatocellular carcinoma patients and relationship with NEAT1 expressions
| Total patients number (n=79) | Patients with NEAT1 expressions |
||||
|---|---|---|---|---|---|
| Low group (n=39) | High group (n=40) | P | OR (95% CI) | ||
| Age | |||||
| ≤55 | 45 | 24 | 21 | 0.417 | 1.448 (0.591-3.543) |
| >55 | 34 | 15 | 19 | ||
| Gender | |||||
| Female | 32 | 17 | 15 | 0.581 | 1.288 (0.542-3.168) |
| Male | 47 | 22 | 25 | ||
| Stage | |||||
| I or II | 51 | 30 | 21 | 0.023 | 3.016 (1.144-7.952) |
| III or IV | 28 | 9 | 19 | ||
| Lymphatic metastasis | |||||
| No | 46 | 31 | 15 | 0.001 | 6.458 (2.360-17.677) |
| Yes | 33 | 8 | 25 | ||
| Distant metastasis | |||||
| No | 49 | 27 | 22 | 0.193 | 1.841 (0.732-4.629) |
| Yes | 30 | 12 | 18 | ||
| Tumor size | |||||
| ≤5 | 31 | 19 | 12 | 0.088 | 2.217 (0.881-5.578) |
| >5 | 48 | 20 | 28 | ||
| Cirrhosis | |||||
| No | 32 | 13 | 19 | 0.200 | 0.553 (0.222-1.373) |
| Yes | 47 | 26 | 21 | ||
| Sorafenib resistance | |||||
| No | 54 | 32 | 22 | 0.010 | 3.740 (1.338-10.456) |
| Yes | 25 | 7 | 18 | ||
High levels NEAT1 in sorafenib-resistant HCC cells influence the effects of sorafenib on HCC cells
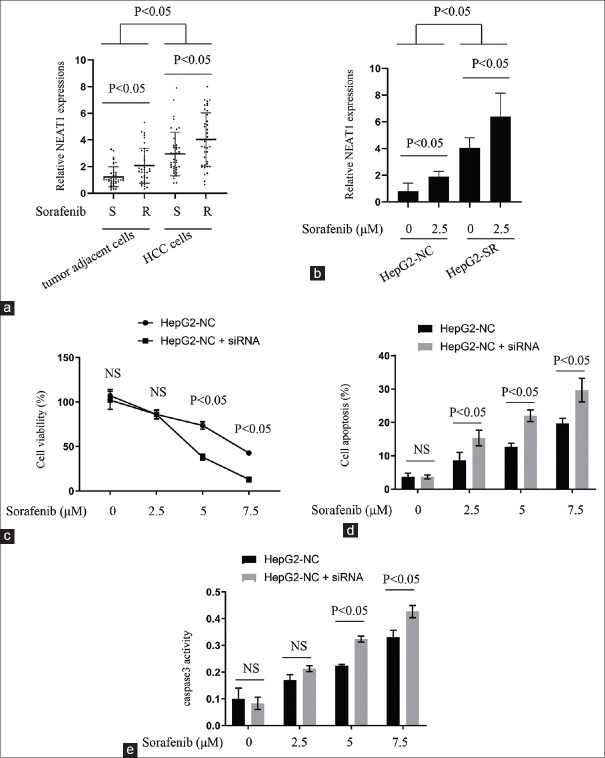
Next, the relationship between NEAT1 expression and HCC progression in vitro was determined. Primary cells were isolated from the 79 HCC tissue samples and the matched adjacent tissues, counted manually using hemocytometer, and their NEAT1 expressions. The data indicated that the NEAT1 level was increased in HCC cells, compared with the adjacent cases. Furthermore, sorafenib-resistant HCC cells exhibited significantly higher NEAT1 expression than sorafenib nonresistant cases [Figure 2a]. To confirm the above results, we incubated HepG2-NC and HepG2-SR cells with a lower concentration of sorafenib (2.5 μM) for 96 h. We found that exposure of either parental or sorafenib-resistant HCC cells to the lower concentration of sorafenib, upregulated the NEAT1 expression, although sorafenib-resistant cells expressed higher NEAT1 levels than their parental cells, in the presence or absence of sorafenib [Figure 2b].
Figure 2.
Relationship between NEAT1 expressions and sorafenib resistance patterns. (a) qPCR results comparing NEAT1 levels between sorafenib sensitivity and resistance patterns in HCC tissues and adjacent tissues. (b) qPCR results comparing NEAT1 levels between sorafenib sensitivity and resistance patterns in HCC cell lines. (c-e) HepG2-NC cells transfected with NEAT1 siRNA or negative control, together with sorafenib treatment for 24 hours. (c) The cell viability of each group determined; (d) apoptosis rate of each group determined; (e) caspase-3 activity of each group determined
We next examined whether NEAT1 could influence sorafenib's effects on the viability and apoptosis of HCC cells. Transfection of NEAT1 siRNA enhanced the inhibitory effects of sorafenib on the viability of HepG2-NC cells [Figure 2c]. Furthermore, interfering with NEAT1 enhanced the pro-apoptotic activity of sorafenib in HepG2-NC cells Figure 2d. Caspase-3 activation, readout of apopotosis, was also significantly enhanced under NEAT1 siRNA treatment [Figure 2e]. Collectively, the above results demonstrated that highly expressed NEAT1 in sorafenib-resistant HCC cells was correlated with resistance to sorafenib in benign and malignant hepatocytes.
Global changes of the expression of genes in HepG2-NC and HepG2-SR cells during interference of NEAT1
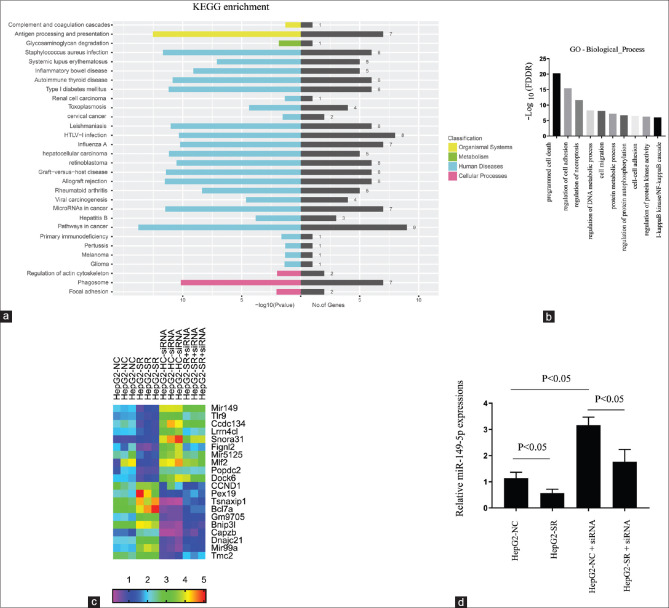
Further, HepG2-NC and HepG2-SR cells transfected with NEAT1 siRNA were used to investigate global changes in gene expression by RNA-seq assay. A sorafenib-resistant pattern was associated with numerous dysregulated protein-coding and noncoding genes (HepG2-SR vs HepG2-NC), which could be reversed by NEAT1 siRNA transfection. Kyotoe encyclopedia of genes and genomes (KEGG) pathway enrichment analyses revealed that many of the NEAT1-regulated genes were involved in the cancer process [Figure 3a]. Gene ontology (GO) enrichment demonstrated that NEAT1 siRNA could regulate many genes related to various types of cell death [Figure 3b]. The heatmap cluster described the top 10 upregulated genes, and the top 10 downregulated genes in NEAT1 siRNA cells as shown in Figure 3c. The most upregulated target in the NEAT1 siRNA group was miR-149-5p, which is a reported microRNA that plays an important role in tumor progression and chemoresistance.[21] This miR-149-5p upregulation by NEAT1 siRNA was subsequently confirmed by qRT-PCR assay [Figure 3d].
Figure 3.
Genome-wide sequencing together with qRT-PCR analysis identifies miR-149-5p in NEAT1 knockdown HepG2 cells. (a) the HepG2-NC and HepG2-SR cells transfected with NEAT1 siRNA for 24 h. The total RNA samples extracted and used to perform the RNA-seq experiment. KEGG bioinformatics analysis determined the pathways related to the differentially expressed genes among the groups. (b) GO bioinformatics analysis enriched the biological functions related to the differentially expressed genes among the groups. (c) Heatmap analysis displaying the deregulated targets among the groups. (d) Of the deregulated genes found in (c), miR-149-5p was selected for qRT-PCR verification
NEAT1 acts as a ceRNA for miR-149-5p to facilitate the death of sorafenib-resistant HCC cells by regulating AKT1 expression
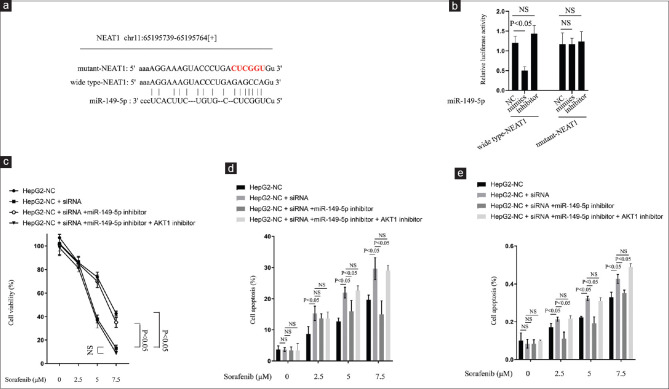
Recent studies have proposed that as a lncRNA, NEAT1 may participate in the ceRNA regulatory network.[11,12,13] By using the online software program Starbase v2.0 (http://starbase.sysu.edu.cn/), it was found that lncRNA NEAT1 formed complementary base pairing with miR-149-5p [Figure 4a]. To confirm the interaction between NEAT1 and miR-149-5p, a wild-type NEAT1 luciferase reporter gene vector, as well as a mutant NEAT1 luciferase reporter gene vector containing a mutation at the putative binding site of miR-149-5p [Figure 4a] was constructed. The indicated vectors were co-transfected into HepG2-NC cells with miR-149-5p mimics, inhibitor, or NC, and the luciferase activity was then monitored using dual luciferase assays. The results showed that the luciferase activity of the wild-type NEAT1 luciferase reporter vector was notably suppressed in response to miR-149-5p, whereas mutant NEAT1 luciferase reporter vector had no significant changes [Figure 4b], indicating that miR-149-5p was a direct target of NEAT1. AKT1 is a proto-oncogene that is overactive in many cancer cells,[15] and a previous report indicated that it decreased the sensitivity of cancer cells to sorafenib.[19] It has been reported that AKT1 is a direct miR-149-5p target, and miR-149-5p can inhibit cell proliferation, invasion and migration in tumor cells through targeting AKT1.[22] These existing reports, together with our findings, make us suspect that NEAT1 may act by regulating mir-149-5p/AKT1 axis. In this study, we found that the function of NEAT1 siRNA on sorafenib activity could be reversed by miR-149-5p inhibitor treatment. Meanwhile, there was no significant change in the effect of NEAT1 siRNA on sorafenib activity when it was simultaneously treated with miR-149-5p inhibitor and AKT1 inhibitor (MK-2206 2HCl) [Figure 4c-e]. Collectively, the above results demonstrated that NEAT1 liberated AKT1 by competitively binding to miR-149-5p and there by facilitating the death of sorafenib-resistant HCC cells by regulating AKT1.
Figure 4.
NEAT1 regulated miR-149-5p by directly targeting in HCC cells. (a). Wide type-NEAT1 and mutant-NEAT1 luciferase reporter gene vectors constructed by mutating the putative binding site of miR-149-5p in NEAT'. (b) The indicated vectors co-transfected into the HepG2-NC cells with miR-149-5p mimics, inhibitor or NC. The luciferase activity in each group determined using dual-luciferase assays. (c-e) HepG2-NC cells co-transfected with or without NEAT1 siRNA, with or without miR-149-5p inhibitor, with or without AKT1 inhibitor, together with sorafenib treatment for 24 h. (c) cell viability of each group determined; (d) apoptosis rate of each group determined; (e) caspase-3 activity of each group determined
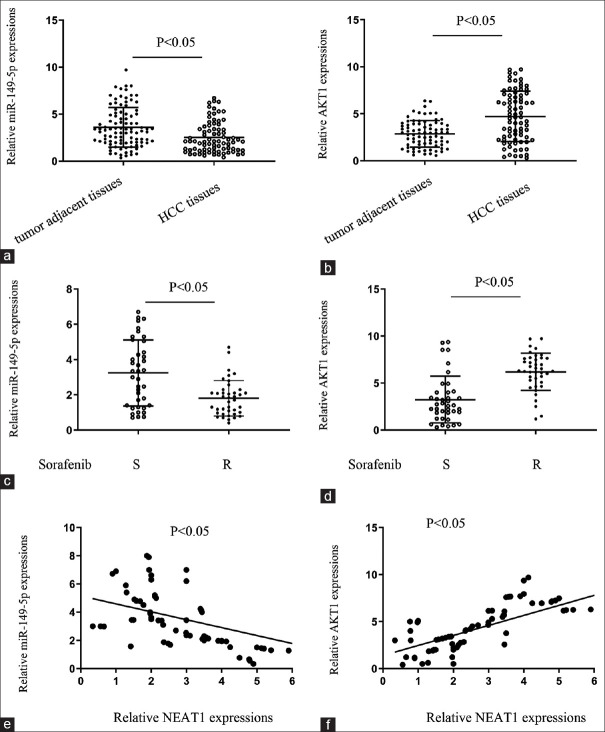
The expression levels and correlations of NEAT1, miR-149-5p, and AKT1 in HCC and adjacent tissues
Finally, to further confirm the above findings, the expression of NEAT1, miR-149-5p, and AKT1 in HCC and adjacent tissues was monitored using real-time PCR assays. The results showed that in HCC tissues, miR-149-5p was decreased, and AKT1 was upregulated as compared to adjacent tissues [Figure 5a and b]. Further, miR-149-5p was decreased, and AKT1 was upregulated in sorafenib resistant HCC tissues, as compared to sorafenib sensitive cases [Figure 5c and d]. Spearman's rank correlation coefficient was obtained to analyze the correlation between NEAT1 and AKT1 and between miR-149-5p and NEAT1. The results showed that miR-149-5p was inversely correlated with NEAT1 and AKT1 was positively correlated with NEAT1 [Figure 5e and f]. Taken together, these data suggest that NEAT1 can inhibit miR-149-5p expression by directly binding to miR-149-5p and subsequently upregulating the expression of AKT1 in HCC tissues to facilitate sorafenib resistance in HCC cells.
Figure 5.
The expression levels and correlations of NEAT1, miR-149-5p, and AKT1 in HCC patients. (a and b) qPCR results comparing (a) miR-149-5p and (b) AKT1 expressions in HCC tissues and matched tumor-adjacent tissues. (c and d) qPCR results demonstrating the relationship between (c) miR-149-5p and (d) AKT1 expressions in HCC tissues and sorafenib resistance patterns. (e and f) qPCR results demonstrating the correlation between NEAT1 and (e) miR-149-5p and (f) AKT1 mRNA expressions analyzed using Spearman's rank correlation analysis
DISCUSSION
Due to the high morbidity and mortality associated with HCC worldwide, new diagnostic and prognostic markers of this disease are urgently needed. lncRNA NEAT1 expression has been reported to be associated with an unfavorable prognosis for HCC.[12,23] Our clinical data partially confirms these earlier reports. We found that there was an inverse association of NEAT1 with miR-149-5p expression. Patients with highly expressed NEAT1 had worse overall survival and disease-free survival rates. In particular, we found a positive correlation between NEAT1 expression and sorafenib resistance patterns.
Sorafenib is a multikinase inhibitor that blocks tumor cell proliferation by inhibiting the serine/threonine kinase isoforms of Raf, Raf-1, and B-Raf, leading to the inhibition of mitogen-activated protein kinase/extracellular signal-regulated kinase (ERK) signaling pathways, decreased expression of cyclin D1, and cell cycle arrest.[24] Although some patients are initially resistant to sorafenib because of HCC heterogeneity, in most cases, the resistance is acquired after long-term exposure to the drug. Several mechanisms are implicated in the reduction of tumor cell sensitivity to sorafenib, such as loops of the phosphatidylinositol-3-kinase (PI3K)/protein kinase B (Akt) and Janus tyrosine kinase (JAK)/signal transducer and activator of transcription (STAT) pathways, epithelial–mesenchymal transition (EMT), or hypoxia-inducible response.[25,26]
The role of dysregulated lncRNAs in the chemoresistance of many cancers has garnered increased scientific interest in recent years. Accumulating evidence confirms that lncRNAs can affect the sensitivity of cancer cells to chemotherapy, including sorafenib. For example, lncRNA SNHG1 has been reported to contribute to sorafenib resistance by activating the Akt pathway.[27] It was demonstrated that HOXA13, a well-known lncRNA, correlated with poorly differentiated HCCs and modulated the sorafenib response.[28]
It was recently discovered that the interaction between lncRNAs and miRNAs affects post-transcriptional regulation by inhibiting the available miRNA activity. According to previous studies, lncRNA can act as a specific “sponge” for miRNAs to attenuate their regulatory effect on mRNAs.[29] In the current study, we found that miR-149-5p could be directly targeted and downregulated by NEAT1. Furthermore, knockdown of NEAT1 with siRNA can upregulate the activity of sorafenib in HCC cells, while the miR-149-5p inhibitor can reverse this effect, indicating that NEAT1 may regulate the sensitivity of HCC cells to sorafenib by regulating the miR-149-5p-involved pathway.
Many studies have reported that miR-149-5p is one of the most important miRNAs in tumor pathogenesis as it plays a part in a number of diverse crucial cellular pathways.[30] It is generally believed that miR-149-5p is an epigenetically silenced tumor-suppressive microRNA that can inhibit the proliferation of glioma cells through directly targeting and inhibiting many key proteins, including AKT1.[31] Herein, we found that the miR-149-5p/AKT1 axis plays a key role in mediating the ability of NEAT1 to alter the chemosensitivity of HCC cells against sorafenib.
Additionally, we confirmed the correlation between NEAT1, miR-149-5p, and AKT1 in clinical samples of HCC patients. The expression of miR-149-5p was downregulated, and NEAT1 and AKT1 were upregulated in HCC samples. Furthermore, it was found that NEAT1 was negatively correlated with miR149-5p and positively correlated with AKT1 expression, suggesting that mir149-5p could decrease the expression of NEAT1 and AKT1, thus enhancing the chemosensitivity of HCC cells against sorafenib. Intervention in the NEAT1/miR-149-5p/AKT1 pathway may be a promising strategy to increase the effectiveness of sorafenib. We expect that further studies of the ideal regimen (dose, frequency, delivery method) of NEAT1/miR-149-5p/AKT1 pathway-based therapy will be the next key steps toward the eventual clinical application of these basic findings.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet. 2012;379:1245–55. doi: 10.1016/S0140-6736(11)61347-0. [DOI] [PubMed] [Google Scholar]
- 3.Awan FM, Naz A, Obaid A, Ikram A, Ali A, Ahmad J, et al. MicroRNA pharmacogenomics based integrated model of miR-17-92 cluster in sorafenib resistant HCC cells reveals a strategy to forestall drug resistance. Sci Rep. 2017;7:11448. doi: 10.1038/s41598-017-11943-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Le Grazie M, Biagini MR, Tarocchi M, Polvani S, Galli A. Chemotherapy for hepatocellular carcinoma: The present and the future. World J Hepatol. 2017;9:907–20. doi: 10.4254/wjh.v9.i21.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tovar V, Cornella H, Moeini A, Vidal S, Hoshida Y, Sia D, et al. Tumour initiating cells and IGF/FGF signalling contribute to sorafenib resistance in hepatocellular carcinoma. Gut. 2017;66:530–40. doi: 10.1136/gutjnl-2015-309501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang D, Yuan W, Li H, Li S, Chen Z, Yang H. Identification of key pathways and biomarkers in sorafenib-resistant hepatocellular carcinoma using bioinformatics analysis. Exp Ther Med. 2018;16:1850–8. doi: 10.3892/etm.2018.6427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen J, Jin R, Zhao J, Liu J, Ying H, Yan H, et al. Potential molecular, cellular and microenvironmental mechanism of sorafenib resistance in hepatocellular carcinoma. Cancer Lett. 2015;367:1–11. doi: 10.1016/j.canlet.2015.06.019. [DOI] [PubMed] [Google Scholar]
- 8.Chen W, Yang X, Zhao Y, Xu S, Wang D. lncRNA-cox2 enhance the intracellular killing ability against mycobacterial tuberculosis via up-regulating macrophage M1 polarization/nitric oxide production. Int J Clin Exp Med. 2019;12:2402–10. [Google Scholar]
- 9.Wu GC, Pan HF, Leng RX, Wang DG, Li XP, Li XM, et al. Emerging role of long noncoding RNAs in autoimmune diseases. Autoimmun Rev. 2015;14:798–805. doi: 10.1016/j.autrev.2015.05.004. [DOI] [PubMed] [Google Scholar]
- 10.Bhan A, Soleimani M, Mandal SS. Long noncoding RNA and cancer: A new paradigm. Cancer Res. 2017;77:3965–81. doi: 10.1158/0008-5472.CAN-16-2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang Z, Zou Q, Song M, Chen J. NEAT1 promotes cell proliferation and invasion in hepatocellular carcinoma by negative regulating miR-613 expression. Biomed Pharmacother. 2017;94:612–8. doi: 10.1016/j.biopha.2017.07.111. [DOI] [PubMed] [Google Scholar]
- 12.Mang Y, Li L, Ran J, Zhang S, Liu J, Li L, et al. Long noncoding RNA NEAT1 promotes cell proliferation and invasion by regulating hnRNP A2 expression in hepatocellular carcinoma cells. Onco Targets Ther. 2017;10:1003–16. doi: 10.2147/OTT.S116319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li X, Wang S, Li Z, Long X, Guo Z, Zhang G, et al. The lncRNA NEAT1 facilitates cell growth and invasion via the miR-211/HMGA2 axis in breast cancer. Int J Biol Macromol. 2017;105:346–53. doi: 10.1016/j.ijbiomac.2017.07.053. [DOI] [PubMed] [Google Scholar]
- 14.Jen J, Tang YA, Lu YH, Lin CC, Lai WW, Wang YC. Oct4 transcriptionally regulates the expression of long non-coding RNAs NEAT1 and MALAT1 to promote lung cancer progression. Mol Cancer. 2017;16:104. doi: 10.1186/s12943-017-0674-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peng W, Wang Z, Fan H. LncRNA NEAT1 impacts cell proliferation and apoptosis of colorectal cancer via regulation of Akt Signaling. Pathol Oncol Res. 2017;23:651–6. doi: 10.1007/s12253-016-0172-4. [DOI] [PubMed] [Google Scholar]
- 16.Guo Y, Zhang H, Xie D, Hu X, Song R, Zhu L. Non-coding RNA NEAT1/miR-214-3p contribute to doxorubicin resistance of urothelial bladder cancer preliminary through the Wnt/β-catenin pathway. Cancer Manag Res. 2018;10:4371–80. doi: 10.2147/CMAR.S171126. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 17.Liu F, Chen N, Gong Y, Xiao R, Wang W, Pan Z. The long non-coding RNA NEAT1 enhances epithelial-to-mesenchymal transition and chemoresistance via the miR-34a/c-Met axis in renal cell carcinoma. Oncotarget. 2017;8:62927–38. doi: 10.18632/oncotarget.17757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shin VY, Chen J, Cheuk IW, Siu MT, Ho CW, Wang X, et al. Long non-coding RNA NEAT1 confers oncogenic role in triple-negative breast cancer through modulating chemoresistance and cancer stemness. Cell Death Dis. 2019;10:270. doi: 10.1038/s41419-019-1513-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.He C, Dong X, Zhai B, Jiang X, Dong D, Li B, et al. MiR-21 mediates sorafenib resistance of hepatocellular carcinoma cells by inhibiting autophagy via the PTEN/Akt pathway. Oncotarget. 2015;6:28867–81. doi: 10.18632/oncotarget.4814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Song Y, Kim JS, Kim SH, Park YK, Yu E, Kim KH, et al. Patient-derived multicellular tumor spheroids towards optimized treatment for patients with hepatocellular carcinoma. J Exp Clin Cancer Res. 2018;37:109. doi: 10.1186/s13046-018-0752-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xu M, Xiao J, Chen M, Yuan L, Li J, Shen H, et al. miR-149-5p promotes chemotherapeutic resistance in ovarian cancer via the inactivation of the Hippo signaling pathway. Int J Oncol. 2018;52:815–27. doi: 10.3892/ijo.2018.4252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xue L, Wang Y, Yue S, Zhang J. Low MiR-149 expression is associated with unfavorable prognosis and enhanced Akt/mTOR signaling in glioma. Int J Clin Exp Pathol. 2015;8:11178–84. [PMC free article] [PubMed] [Google Scholar]
- 23.Guo S, Chen W, Luo Y, Ren F, Zhong T, Rong M, et al. Clinical implication of long non-coding RNA NEAT1 expression in hepatocellular carcinoma patients. Int J Clin Exp Pathol. 2015;8:5395–402. [PMC free article] [PubMed] [Google Scholar]
- 24.Méndez-Blanco C, Fondevila F, García-Palomo A, González-Gallego J, Mauriz JL. Sorafenib resistance in hepatocarcinoma: Role of hypoxia-inducible factors. Exp Mol Med. 2018;50:134. doi: 10.1038/s12276-018-0159-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhai B, Sun XY. Mechanisms of resistance to sorafenib and the corresponding strategies in hepatocellular carcinoma. World J Hepatol. 2013;5:345–52. doi: 10.4254/wjh.v5.i7.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhu Y, Zheng B, Wang H, Chen L. New knowledge of the mechanisms of sorafenib resistance in liver cancer. Acta Pharmacol Sin. 2017;38:614–22. doi: 10.1038/aps.2017.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li W, Dong X, He C, Tan G, Li Z, Zhai B, et al. LncRNA SNHG1 contributes to sorafenib resistance by activating the Akt pathway and is positively regulated by miR-21 in hepatocellular carcinoma cells. J Exp Clin Cancer Res. 2019;38:183. doi: 10.1186/s13046-019-1177-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Quagliata L, Quintavalle C, Lanzafame M, Matter MS, Novello C, di Tommaso L, et al. High expression of HOXA13 correlates with poorly differentiated hepatocellular carcinomas and modulates sorafenib response in in vitro models. Lab Invest. 2018;98:95–105. doi: 10.1038/labinvest.2017.107. [DOI] [PubMed] [Google Scholar]
- 29.Du P, Zhao H, Peng R, Liu Q, Yuan J, Peng G, et al. LncRNA-XIST interacts with miR-29c to modulate the chemoresistance of glioma cell to TMZ through DNA mismatch repair pathway. Biosci Rep. 2017;37:pii: BSR20170696. doi: 10.1042/BSR20170696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gao F, Zhu Y. Association between miRNA polymorphisms and susceptibility to brain tumors: A meta-analysis. Medicine (Baltimore) 2019;98:e16933. doi: 10.1097/MD.0000000000016933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pan SJ, Zhan SK, Pei BG, Sun QF, Bian LG, Sun BM. MicroRNA-149 inhibits proliferation and invasion of glioma cells via blockade of AKT1 signaling. Int J Immunopathol Pharmacol. 2012;25:871–81. doi: 10.1177/039463201202500405. [DOI] [PubMed] [Google Scholar]