ABSTRACT
Background and Objective: China has managed to control the coronavirus disease (COVID-19) with confinement measurements and treatment strategies, while other countries are struggling to contain the spread. This study discusses the guidelines related to COVID-19 in China in order to provide important references for other countries in the fight against COVID-19.
Methods: Chinese guidelines relevant to COVID-19 were systematically searched via the China National Knowledge Infrastructure database, YiMaiTong database, and World Health Organization (WHO) COVID-19 database on March 20th, 2020. Guideline information was extracted, including date of publication, source, objectives and the target population. Guidelines specific to the pharmacological treatment of COVID-19 were further investigated to identify the types of antivirus drugs recommended and to report on how treatment recommendations for COVID-19 have evolved overtime.
Results: A total of 100 guidelines were identified, of which 74 were national guidelines and 26 were regional guidelines. The scope of included guidelines consisted of: the diagnosis and treatment of COVID-19, the management of hospital departments and specific diseases during the outbreak of COVID-19. Fifty-one of the included guidelines targeted overall COVID-19 patients, while the remaining guidelines concentrated on special patient populations (i.e. geriatric population, pediatric population, and pregnant population) or patients with coexisting diseases. Fifteen guidelines focused on the pharmacological treatments for all COVID-19 patients. Interferon, Lopinavir/Ritonavir, Ribavirin, Chloroquine, and Umifenovir represented the most recommended antivirus drugs. Among them, 7 Chinese guidelines have recommended Chloroquine Phosphate or Hydroxychloroquine for the treatment of COVID-19.
Conclusions: China has generated a plethora of guidelines covering almost all aspects of COVID-19. Chloroquine, as one widely affordable treatment, was recommended by Chinese national guidelines and provincial guidelines. Considering the continuous debates around Chloroquine, confirmatory studies with robust methodology are awaited to address the unanswered questions on its potential benefits and risks on COVID-19.
KEYWORDS: Chinese Guidelines, covid-19, pharmaceutical Treatment
Introduction
In late December of 2019, the first pneumonia case with unknown microbial origin was reported in Wuhan, China. A novel coronavirus was subsequently identified as the causative pathogen, provisionally named as the 2019 novel coronavirus (2019-nCoV), and finally named as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), now commonly referred to as COVID-19 [1]. Although the number of newly infected cases per day appeared to decline within China, including the Hubei province [2], the number of COVID-19 cases outside China increased drastically. Concerned by its alarming levels of global spread and severity, COVID-19 was declared a pandemic by the World Health Organization (WHO) on March 11th, 2020 [3].
COVID-19 initially spread quickly to neighboring Asian countries, with South Korea being the most affected [4]. Following this, a sharp growth in confirmed cases was witnessed in Europe, with many European countries reporting nation-wide community transmission; thus, rendering Europe the most affected world region of the pandemic. However, the USA (US) began to report tens of thousands of new cases beginning on March 24th, 2020, eventually exceeding the 123,776 confirmed cases in China recorded on March 29th, 2020, therefore, rendering the US the new center of the pandemic [5].
As of March 28th, 2020, 82,230 patients have been identified as infected by SARS-COV-2 in China, with a total of 3,301 deaths [2]. Based on the data available, as of the 17th of March 2020, the overall fatality rate was 7.2% for confirmed COVID-19 cases in Italy [6], which was much higher than the observed fatality rate of 2.7% in mainland China [7]. This could be due to factors, such as Italy’s high proportion of older patients confirmed with COVID-19 and patients with mild symptoms no longer being tested beginning on the 25th of February 2020 and no longer being accounted for. Although the accurate estimation of mortality rate is unavailable as the denominator is impossible to assess, the absolute number of deaths is relatively high. However, comparisons in mortality rates between countries also depend on how cases are counted, which varies by country.
According to the largest retrospective study consisting of 44, 672 confirmed cases published by the Chinese Center for Disease Control and Prevention, older patients and patients with comorbidities, including cardiovascular disease, diabetes, chronic respiratory disease, hypertension, and cancer, were all associated with an increased risk of death [8]. A high Sequential Organ Failure Assessment (SOFA) score and d-dimer >1 μg/mL could be important indicators for poor prognosis at an early stage [9].
The outbreak seems to be under control in China as several provinces have declared no infected cases remain. However, the rapid expansion of COVID-19 is still ongoing in Europe. The number of COVID-19 infections and deaths in Europe has not yet peaked, despite approximately 16,000 deaths already across Italy, Germany, and France as of the 29th of March 2020 [2].
Therefore, it may be insightful to understand the Chinese experience and what guidelines have been established to control the spread of COVID-19. Guidelines related to patient channeling, the organization of the health care system, patient triage, and patient investigations may not be transferable due to inherently different healthcare systems in different countries. The authors have focused on the specific pharmacological guidelines for the treatment of COVID-19. The objective of this manuscript is to identify and describe the pharmaceutical guidelines developed in China and to analyze adjustments in the treatment of COVID-19 over time based on the increasing experience in treating COVID-19 patients.
Method
Database search
The China National Knowledge Infrastructure (CNKI) database was searched on the 20th of March 2020 to identify guidelines relevant to COVID-19. The following keywords were used: Novel Coronavirus Pneumonia, COVID-19, SARS-CoV-2, expert consensus, guideline, guidance, recommendation, advice, standard pathway, and clinical pathway. Additionally, YiMaiTong database, as one of the most prestigious databases for collecting healthcare information in China, was also complementarily searched in order to identify guidelines potentially published in grey literature. The WHO database of publications on COVID-19 was also searched to ensure all important guidelines were identified, collected, and analyzed. For guidelines with updated versions with the generation of new evidence, all guideline versions were included.
Data extraction and guidelines classification
The following characteristics of included guidelines were extracted: 1) date of publication, 2) source of the guidelines, 3) objectives of the guidelines, and 4) target population of the guidelines.
Guidelines were classified according to the objectives of the guidelines, which were the main aspects relating to COVID-19. These included: 1) prevention, 2) diagnosis, 3) pharmacology treatment, 4) non-pharmacology treatment, 5) management of patients with specific diseases (e.g. myocardial infarction) under the outbreak of COVID-19, 6) management of specific hospital departments (e.g. cardiology department) under the outbreak of COVID-19, and 7) pharmacy service.
The target populations of the COVID-19 guidelines were further categorized: 1) overall patients with COVID-19, 2) patients with a certain degree of severity of COVID-19, including suspected cases and confirmed cases (mild cases, severe cases, and critical cases), 3) special patients, including the geriatric population, pediatric population, and pregnant population, 4) patients with other comorbidities, such as oncology and cerebrovascular disease.
Moreover, pharmacological treatments including Traditional Chinese Medicine (TCM) and Western Medicine (WM), were extracted to investigate what medicines were most recommended in the treatment of COVID-19 patients. Considering the diversity in the names of TCMs, only the TCMs with brand names were investigated in this study.
Diagnosis and Treatment Protocol for Novel Coronavirus Pneumonia (referred to as the Diagnosis and Treatment Protocol), as the most important Chinese national guideline, its different versions the were further analyzed. The publishing date of each protocol and the evolving history in the recommendations on the pharmacology treatments in Protocols were extracted and discussed.
Considering the continuous disputes around Chloroquine and Hydroxychloroquine for the treatment of COVID-19, guidelines recommending or mentioning Chloroquine and Hydroxychloroquine were additionally extracted and evaluated, including the publishing date, guideline name, publishing organization and the key information related to Chloroquine and Hydroxychloroquine.
Result
Description of guidelines
Overview of included guidelines
Among the total of 100 published guidelines identified, 15 of them were published in January of 2020, 58 of them were published in February of 2020, and 27 of them were published in March of 2020. Considering the national and regional source of the included guidelines, 74 of them were national guidelines, which were mainly published by the National Medical Association of China (Figure 1), including the National Health Commission and national associations for varying medical subjects, such as the Respiratory Branch of Chinese Medical Association. Twenty-six of the included guidelines were regional guidelines, which were released by 8 provinces and 2 municipalities. Scope of included guidelines
Figure 1.
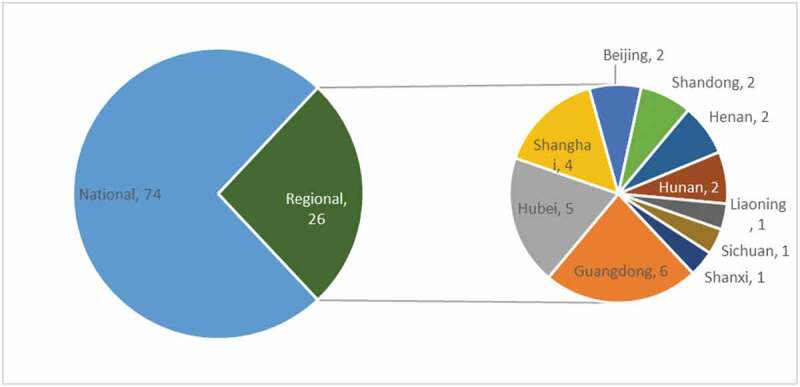
Source of guidelines related to COVID-19 in China.
The diagnosis and treatment of COVID-19 was the most-discussed subject in all of the published guidelines (Figure 2). Management of special hospital departments during hospital outbreaks was paramount, among which, the management of the emergency department, surgery department (e.g, cerebrovascular surgery, colorectal surgery, and articulation surgery), and intensive care units were most targeted. Management of patients with specific diseases was also covered extensively, including the cardiovascular diseases, oncological diseases, Parkinson’s disease, secondary fungal infection, and malaria. Guidelines on providing recommendations for key pharmacy service, such as handling the abundant supply of COVID-19 medicine, the control of donated medicine, the report of adverse effects, and the process for therapeutic drug monitoring, were also available. Non-pharmacology treatment covered nutrition guidelines and guidelines on rehabilitation with TCM. Nutrition guidelines, especially for severe and critical cases, provided important instructions related to energy intake, nutrition regimes, and nutrition evaluation in order to prevent the occurrence of malnutrition in COVID-19 patients. Guidelines on rehabilitation with TCM were developed to standardize its appropriate application and to alleviate the patients’ symptoms, such as Tai Chi, acupuncture, massage, and cupping therapy.
Figure 2.
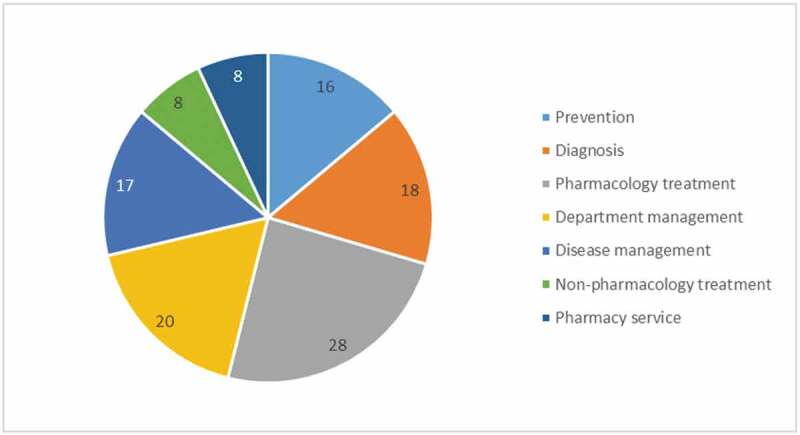
Objective of included guidelines related to COVID-19.
Included guidelines and target patient populations
More than half of the guidelines applied to overall patients affected by COVID-19 (Figure 3). Nine guidelines targeted patients with certain degree of disease severity, 5 applied to severe and critical cases, 2 guidelines applied to suspected cases, and 2 guidelines applied to convalescent cases. Thirteen guidelines focused on special patients: 6 guidelines discussed the prevention and control of COVID-19 in children, from wearing pediatric medical masks, observations of changes in daily activity, to the education on appropriate use of medicines; Six guidelines concentrated on the management of COVID-19 in pregnant women, including psychological counseling, the selection of drugs with lower pregnancy risks, and the assessment of patients’ conditions to terminate the pregnancy. Only one guideline specifically targeted elderly patients. Twenty-seven guidelines focused on COVID-19 patients with coexisting diseases, where patients with cardiovascular diseases and cancer fostered the largest part of discussions.
Figure 3.
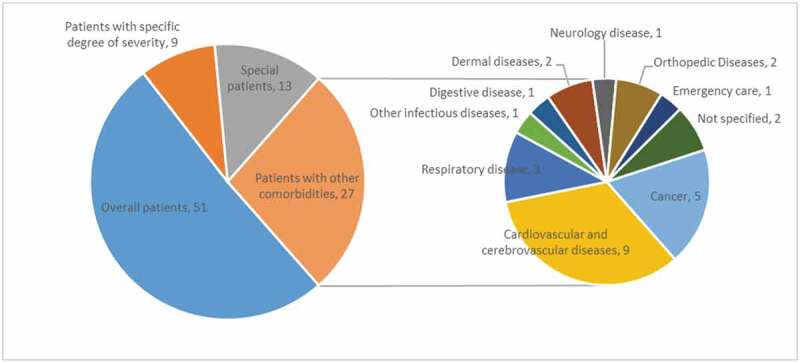
Target patient populations of included guidelines related to COVID-19.
Pharmacological treatments for COVID-19
A total of 15 guidelines focusing on pharmacological treatments for all COVID-19 cases were further investigated (Table 1), including 3 national guidelines: the ‘Diagnosis and Treatment Protocol for Novel Coronavirus Pneumonia’ released by the National Health Commission, the ‘Guideline for the appropriate use of Traditional Chinese Medicine (TCM)’ released by China Association of Chinese Medicine, and ‘Notifications on the dosage adjustment of Chloroquine Phosphate’ released by National Health Commission. Twelve were provincial guidelines, including 4 focused on the diagnosis and treatment released in Beijing, Shanghai, Hubei province, Shandong province, and Guangdong province, 6 guidelines for the appropriate use of TCM in the treatment of COVID-19, and 2 guidelines provided recommendations specific to the optimal use of Chloroquine Phosphate.
Table 1.
Chinese guidelines for the pharmacological treatment of COVID-19.
| Type | Title | Publish Date | TCM | IFN-α | IFN-k | Lopinavir /Ritonavir | Ribavirin | Chloroquine | Hydroxy-chloroquine | Umifenovir | Other antivirus drugs | Tocilizumab |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| National guideline | Notifications on the adjustment of dosage of chloroquine phosphate for the treatment of novel coronavirus pneumonia [21] | 2020–02-28 | NA | NA | NA | NA | NA | Yes | NA | NA | NA | NA |
| Expert consensus for appropriate application of TCM for COVID-19 [22] | 2020–03-01 | Yes | NA | NA | NA | NA | NA | NA | NA | NA | NA | |
| Diagnosis and Treatment Protocol for Novel Coronavirus Pneumonia (7th version) [23] | 2020–03-04 | Yes | Yes | No | Yes | Yes | Yes | No | Yes | No | Yes | |
| Province guidelines-Diagnosis and treatment | Hubei province: A rapid advice guideline for the diagnosis and treatment of 2019 novel coronavirus (2019-nCoV) infected pneumonia (3rd Edition) [24] | 2020–01-28 | Yes | Yes | No | Yes | No | No | No | Yes | Oseltamivir | No |
| Peking Union Medical College Hospital’s proposal for diagnosis and treatment of “novel coronavirus-infected pneumonia” (V2.0) [25] | 2020–01-30 | Yes | No | No | Yes | No | No | No | No | No | No | |
| Hubei province: A rapid advice guideline for diagnosis and treatment of COVID-19 (integrated version) [26] | 2020–02-01 | Yes | Yes | No | Yes | Yes | No | No | No | Remdesivir | No | |
| Shandong expert consensus on the diagnosis and treatment for COVID-19 [27] | 2020–02-20 | Yes | Yes | No | Yes | Yes | No | Yes | Yes | No | No | |
| Province guidelines-TCM | Shanghai expert consensus on the integrated treatment for novel coronavirus pneumonia [28] | 2020–02-20 | Yes | Yes | Yes | No | No | Yes | Yes | Yes | No | No |
| Shanghai: Protocol for the use of traditional Chinese medicine for the diagnosis and treatment of COVID-19 [29] | 2020–02-24 | Yes | NA | NA | NA | NA | NA | NA | NA | NA | NA | |
| Expert Consensus on Rational Drug Use in Clinical Practice for COVID-19 [30] | 2020–02-28 | Yes | NA | NA | NA | NA | NA | NA | NA | NA | NA | |
| Hubei province: Protocol on the use of traditional Chinese medicine for Epidemic prevention and control of COVID-19 [31] | 2020–02-29 | Yes | NA | NA | NA | NA | NA | NA | NA | NA | NA | |
| Shanxi province: Protocol for the use of traditional Chinese medicine of COVID-19 [32] | 2020–03-01 | Yes | No | No | No | No | No | No | No | No | No | |
| Guangdong province: Expert consensus on Chinese integrative medicine for Epidemic prevention and control of COVID-19 [33] | 2020–03-10 | Yes | Yes | No | Yes | Yes | Yes | No | Yes | No | Yes | |
| Province guidelines-Chloroquine phosphate for COVID-19 | Hubei province: Close monitoring the adverse effects of chloroquine phosphate for the treatment of novel coronavirus pneumonia [34] | 2020–02-21 | NA | NA | NA | NA | NA | Yes | NA | NA | NA | NA |
| Guangdong province: Expert consensus on chloroquine phosphate for COVID-19 [35] | 2020–03-04 | NA | NA | NA | NA | NA | Yes | NA | NA | NA | NA |
COVID: Coronavirus Disease; IFN: Interferons; NA: Not applicable; TCM: Traditional Chinese Medicine.
In guidelines discussing WM treatments for COVID-19 (N = 7, excluding guidelines specific for TCM and for Chloroquine Phosphate), antivirus therapies were regarded as the general pharmacological treatment for all COVID-19 patients. However, all 7 guidelines outlined that there were no ‘specific antivirus medicines’ to eradicate the SARS-CoV-2 infection and to show 100% effectiveness in all patients across different disease stages. Interferon (IFN) was recommended in 6 guidelines, with the exception of one Beijing guideline. IFN-α was the only IFN-type medicine recommended in 6 guidelines, except for the Shanghai guideline, which recommended IFN-k as first choice and IFN-α as second choice. Lopinavir/Ritonavir was recommended in 6 guidelines, with the exception of the Shanghai guideline. Ribavirin was recommended in 4 guidelines published after February 1st of 2020, with the exception of the Shanghai guideline. Chloroquine Phosphate was recommended in 3 guidelines published after 19th of February 2020 . The Shanghai guideline recommended both Hydroxychloroquine (first choice) and Chloroquine Phosphate (second choice), the Shandong guideline recommended Hydroxychloroquine only, and 2 guidelines recommended Chloroquine phosphate only. Umifenovir was recommended in the national guideline and 4 provincial guidelines. Other antivirus medicines, Oseltamivir and Remdesivir, were recommended in the rapid advice guidelines published by the Tongji hospital on the 22nd of January 2020 and by the Zhongnan hospital on the 1st of February 2020, respectively.
With regards to TCM therapy for COVID-19 treatment, the Diagnosis and Treatment Protocol for Novel Coronavirus Pneumonia recommended 5 TCM oral preparations during a medical observation period, 5 TCM injections for severe cases, and 7 TCM injections for critical cases. All other included provincial guidelines specific to TCM treatments added several TCM treatments to those recommended by the national Protocol, with the number of additional TCM treatments ranging from 1 (the Shangxi guideline) to 13 (the Beijing guideline). For mild cases, Reyanning injection, Shuanghuanglian oral liquid, and Siji kangbingdu injection were recommended in more than 2 TCM guidelines. For severe cases, Angong niuhuang capsules and Suhexiangwan were recommended in more than 2 TCM guidelines.
Other pharmacological treatments were recommended for use depending on the severity of COVID-19 and other coexisting disorders secondary to COVID-19. These treatments included: 1) short-term corticosteroid therapy recommended for patients who demonstrated progressively deteriorating oxygenation index, rapid imaging progression, and overactive inflammatory responses, 2) antibiotic therapy, with inappropriate use of antibiotic therapy being avoided, and caution taken in combinations with broad-spectrum antibiotics, and 3) Tocilizumab, as an immunosuppressive agent, which was first recommended in the Diagnosis and Treatment Protocol for Novel Coronavirus Pneumonia on the 4th of March 2020, and then included in the Guangdong guideline on the 10th of March 2020.
The description of the diagnosis and treatment protocol
The Diagnosis and Treatment Protocol issued by the National Health Commission of the People’s Republic of China has been updated as additional research evidence and knowledge on COVID-19 have become available. Since the release of the first version of the protocol on January 15th, 2020, 7 versions in total have been produced with 8 updates over a 50-day period. The first and second versions of the Diagnosis and Treatment Protocol are currently not published on the official website, 6 updates are recorded as the fifth version of the protocol underwent 2 updates (Table 2).
Table 2.
Summary of TCMs in the diagnosis and treatment protocol for novel coronavirus pneumonia.
| Protocol Version | Publish date | Basis of TCM Recommendation | TCM injection Recommendations |
|---|---|---|---|
| 3rd [36] | 2020–01–22 | Recommendations for different prescriptions according to the different symptoms (dampness, heat, poison and stasis) of the lung | No recommendation |
| 4th [37] | 2020–01–27 | Recommendations for different prescriptions according to the medical observation period, clinical treatment period (mild, moderate, severe, convalescent period) | Moderate period: Xiyanping injection and Xuebijing injection Severe period: Xuebijing injection, Shenfu injection and Shengmai injection |
| 5th [15] | 2020–02–05 | No changes implemented since previous version | No changes implemented since previous version |
| 5th revised [38] | 2020–02–08 | ||
| 6th [39] | 2020–02–18 | General prescriptions are added during the clinical treatment period; The clinical treatment period is divided into 5 subperiods mild, moderate, severe, critical, and convalescent; Different prescriptions are recommended according to different symptoms of different subperiods | Severe cases: Xiyanping injection, Xuebijing injection, Reduning injection, Tanreqing injection, Xingnaojing injection. Critical cases: Xuebijing injection, Reduning injection, Tanreqing injection, Xingnaojing injection, Shenfu injection, Shengmai injection, Shenmai injection. |
| 7th [23] | 2020–03–04 | No changes implemented since previous version | No changes implemented since previous version |
TCM: Traditional Chinese Medicine.
The evolving history of recommendations in the diagnosis and treatment protocol
TCM recommendations
Beginning with version 3 of the Diagnosis and Treatment Protocol for Novel Coronavirus Pneumonia, the inclusion and elaboration on the use of TCMs in the Diagnosis and Treatment Protocol have been gradually improved. The Diagnosis and Treatment Protocol determined that COVID-19 met the definition of plague caused by the epidemic pathogenic factors from the perspective of TCM. It was stressed in the protocol that TCM treatment regimens for COVID-19 should be adjusted according to the varying local climate characteristics, individual state of illness, and physical conditions. The 3rd version of the protocol states that the choice of TCMs should be made based on patient symptoms, such as dampness, heat, poison, and stasis of the lung, yet no recommendations were given on the specific TCMs to be used.
The 4th protocol version introduced a selection of recommended TCMs to be used either in a medical observation period where patients were only suspected cases of COVID-19 or in a clinical treatment period where patients were confirmed cases of COVID-19. Four kinds of TCM oral preparations were recommended in the medical observation period, including Huoxiangzhengqi (pills, liquid, or oral solution), Jinhuaqinggan (granules), Lianhuaqingwen (granules), and Shufengjiedu (granules). For patients in the clinical treatment period the recommendations for use of TCM were further divided based on clinical manifestations during 4 subperiods. These subperiods included the mild subperiod characterized by cold and damp stagnation lung syndrome, the moderate subperiod presenting plague poison and lung-closing syndrome, the severe subperiod identified by syndrome of inner blocking causing collapse, and the convalescent subperiod showing lung and spleen qi deficiency syndrome. No TCM injection was recommended for the mild subperiod. Two kinds of TCM injections, Xiyanping injection and Xuebijing injection, were recommended for treatment during the moderate subperiod, and three kinds of TCM injections, Xuebijing injection, Shenfu injection, and Shengmai injection, were recommended for treatment during the severe subperiod.
No changes in the recommendations of use and types of TCMs for the treatment of COVID-19 were made in either of the 2 updates of the 5th version of the protocol.
The Diagnosis and Treatment Protocol for Novel Coronavirus Pneumonia’s 6th protocol version added lung cleansing and detoxifying decoction as a general prescription for the clinical treatment period. The 6th protocol version redefined the subperiods characterizing the clinical treatment period and further divided it into 5 subperiods. The five subperiods of the clinical treatment period were based on disease severity and included mild subperiod (cold dampness and stagnation lung syndrome or dampness and heat-accumulation lung syndrome), moderate subperiod (cold dampness lung syndrome or dampness and stagnation lung syndrome), severe subperiod (plague poison and lung-closing syndrome or syndrome of flaring heat in qifen and yingfen), critical subperiod (syndrome of inner blocking causing collapse), and convalescent subperiod (lung and spleen qi deficiency syndrome or Qi and Yin deficiency syndrome). There were several TCM injections newly included for the treatment of COVID-19 patients, which improved the selectivity and precision of medication use. Three more TCM injections, Reduning injection, Tanreqing injection, and Xingnaojing injection, were recommended in the severe subperiod and four more TCM injections, Reduning injection, Tanreqing injection, Xingnaojing injection, and Shenmai injection, were recommended in the critical subperiod.
No changes in the recommendations of use and types of TCMs for the treatment of COVID-19 were made in the 7th version protocol.
Western medicine recommendations
At the beginning of the COVID-19 outbreak, no specific WM with robust evidence was available. Drug repurposing and the combined use of existing broad-spectrum antiviral drugs have constituted the most effective and efficient methods to combat COVID-19. INF-α inhalation and oral Lopinavir/Ritonavir were the only 2 antiviral therapies recommended in the 3rd version the Diagnosis and Treatment Protocol for COVID-19. Ribavirin was then recommended in the 5th version of the protocol, considering its clinical benefits in the treatment of Severe Acute Respiratory Syndrome (SARS). Umifenovir was included in the 6th protocol version, on the basis of in-vitro cell experiments showing effective inhibition of SARS-CoV-2 infection. The large-scale spread of the epidemic has made the quick accumulation of treatment experience possible, as well as open clinical trials allowing for the treatment protocol to be updated and to offer more detailed and practical information regarding the eligibility of patients, antivirus drug dosages, and the observation of adverse events.
Of all the antivirus targeted drugs recommended in the protocol, Chloroquine appears to have the potential to emerge as the standard choice due to promising preclinical evidence, preliminary clinical results and clinical experience unpublished [10,11]. Thus far, results from more than 100 patients have demonstrated that Chloroquine Phosphate is superior to the control treatment in inhibiting the exacerbation of pneumonia, improving lung imaging findings, promoting a virus negative conversion, and shortening the disease course. For example, COVID-19 patients treated with antiviral drugs, Lopinavir/Ritonavir and Umifenovir, showed negative nucleic acid detection on an average of 6 to 7 days of treatment, while the average time for Chloroquine Phosphate treatment to show negative nucleic acid detection was 4.2 days. However, another well conducted study has shown no benefit of Lopinavir/Ritonavir [12]. Despite the limited clinical evidence, experts from health regulation authorities and clinical trial organizers generally supported Chloroquine Phosphate as having a potent impact against SARS-CoV-2. This contributed to the inclusion of Chloroquine Phosphate in the 6th protocol version. As Chloroquine emerged as the preferred treatment option, the recommendations related to the dosage regimen of Chloroquine for treatment of COVID-19 were updated and improved along with the accumulation of research evidence and knowledge on disease progression for COVID-19.
Recommendations for the usage of chloroquine and hydroxychloroquine
Seven guidelines (Table 3) published in China supported the used of Chloroquine Phosphate for the treatment of COVID-19. The dosage of 500 mg Chloroquine Phosphate, twice per day, and for a duration no longer than 10 days was first proposed for the treatment of COVID-19 in the 6th protocol version. The Health Commission of Guangdong Province supported this recommendation, but outlined the importance of monitoring adverse events associated with the Chloroquine Phosphate, such as the risk of prolongation of Q-T interval when combined with macrolide antibiotics (e.g. azithromycin). Afterwards, the Health Commission of Hubei Province warned that Chloroquine Phosphate could cause the occurrence of sudden death at a dosage of 2 ~ 4 g. With the emergence of the safety issues mentioned above, the National Health Commission adjusted the dosage of Chloroquine Phosphate for adults by taking into consideration a patient’s age and body weight. Additionally, the contraindications for the use of Chloroquine Phosphate were also specified, such as in patients with underlying cardiovascular diseases. The new dosage adjustment of the Chloroquine Phosphate was included in the 7th version of the Diagnosis and Treatment Protocol for Novel Coronavirus Pneumonia and the Guangdong expert consensus.
Table 3.
The inclusion of chloroquine phosphate and hydroxychloroquine sulphate in guidelines for the treatment of COVID-19.
| Publish date | Guideline name | Publishing organization | Key information related to chloroquine and Hydroxychloroquine |
|---|---|---|---|
| 2020–02–20 | Expert consensus on chloroquine phosphate for the treatment of novel coronavirus pneumonia [35] | Health Commission of Guangdong Province for chloroquine in the COVID-19 treatment | It recommended chloroquine phosphate tablet, 500 mg bid for 10 days for patients diagnosed as mild, moderate and severe cases of novel coronavirus pneumonia and without contraindications to chloroquine. Contraindicated to use combined with macrolide antibiotics including azithromycin. |
| 2020–02–21 | Close monitoring the adverse effects of chloroquine phosphate for the treatment of novel coronavirus pneumonia [34] | Health Commission of Hubei Province | Chloroquine phosphate can cause acute death. Lethal dose for adults is 2–4 g. |
| 2020–02–28 | Notifications on the adjustment of dosage of chloroquine phosphate for the treatment of novel coronavirus pneumonia [21] | National Health Commission | Chloroquine phosphate (500 mg bid for 7 days for adults aged 18–65 with body weight over 50 kg; 500 mg bid for Days 1–2 and 500 mg qd. for Days 3–7 for adults with body weight below 50 kg) Contraindicated to use combined with macrolide antibiotics including azithromycin. |
| 2020–03–02 | Shanghai expert consensus on the integrated treatment for novel coronavirus pneumonia [28] | Shanghai expert panel on the clinical treatments for COVID-19 | Hydroxychloroquine Sulphate and Chloroquine phosphate were both recommended. |
| 2020–03–04 | The Diagnosis and Treatment Protocol for Novel Coronavirus Pneumonia (7th version) [23] | National Health Commission | Chloroquine phosphate (500 mg bid for 7 days for adults aged 18–65 with body weight over 50 kg; 500 mg bid for Days 1–2 and 500 mg qd. for Days 3–7 for adults with body weight below 50 kg) |
| 2020–03–05 | Guangdong expert consensus on the Chinese integrative medicines for the prevention and treatment of COVID-19 [33] | Guangdong association of integrative medicine | Chloroquine phosphate (500 mg bid for 7 days for adults aged 18–65 with body weight over 50 kg; 500 mg bid for Days 1–2 and 500 mg qd. for Days 3–7 for adults with body weight below 50 kg) |
| 2020–03–15 | Shandong expert consensus on the Diagnosis and Treatment for Novel Coronavirus Pneumonia [27] | Shandong expert panel on the clinical treatments for COVID-19 | Hydroxychloroquine Sulphate (200 mg tid). |
COVID-19: Coronavirus Disease.
Hydroxychloroquine shares similar chemical structures and mechanisms of action with Chloroquine Phosphate, thus it is less surprising that Hydroxychloroquine may also be a candidate to combat the SARS-CoV-2 infection. Encouraging results proved that Hydroxychloroquine also had an in-vitro impact against SARS-CoV-2. On February 15th, 2020, the website of People’s Hospital of Wuhan University released a report titled ‘Hydroxychloroquine showed short-term efficacy in the treatment of Novel Coronavirus Pneumonia’. In this report, researchers found that none of the 80 patients with systemic lupus erythematosus treated in the hospital’s dermatology department were infected by COVID-19 during the period of SARS-CoV-2 transmission in Wuhan. The researchers suspected that Hydroxychloroquine might hold promises to protect the patients from COVID-19. Therefore, Hydroxychloroquine was recommended for the first time in the Shanghai expert consensus for COVID-19. The Shandong expert consensus specified the dosage of Hydroxychloroquine as 200 mg, three times per day.
Discussion
It is evident that China has taken the development of guidelines for the treatment of COVID-19 very seriously with the available guidelines targeting multiple topics and aspects relating to SARS-CoV-2. Interestingly, these guidelines have provided support for most of the issues healthcare professionals and hospital managers may face. Most of the guidelines were developed at the national level, with most of the regional guidelines in line with national guidelines, yet also customized to regions and providing more detailed instructions – with the exception of Shanghai, where guidelines tend to deviate from national guidelines and where, ultimately, the guidelines focused on hydroxychloroquine. All of these provinces, except for Shanxi province and Liaoning province, were severely afflicted areas and were among the top 15 regions with the largest number of infected cases in China. The provinces of Hubei and Guangdong were the regions reporting the largest and second-largest number of confirmed cases and published the greatest number of regional guidelines. Beijing and Shanghai both released their clinical pathways for the diagnosis and treatment of COVID-19 due to their powerful roles in research and academia in China. China is traditionally highly centralized and, at the same time, its decentralized model has proven to be effective in such an outbreak. The development of guidelines has been a very dynamic process with very fast and continuous updating of existing guidelines. Although the guidelines provide limited information on the rational for their updates, it is considered that they were updated based on the development of clinical experience in treating patients and the evidence emerging from the large range of ongoing open studies.
Fifteen guidelines have been targeting the pharmacological management of COVID-19 patients. Initially, Interferon-α and Interferon-K (in Shanghai guideline only), were recommended. Lopinavir/Ritonavir combination was recommended in all guidelines, except in Shanghai. A randomized, controlled, open-label trial involving 199 hospitalized adult patients with severe Covid-19 could provide an answer for the exclusion of Lopinavir/Ritonavir combination in the Shanghai expert consensus, as no benefit was observed with lopinavir–ritonavir treatment beyond the standard of care [13]. Umifenovir was approved in Russia and China, but not approved in Europe and the US. Oseltamivir and Remdesivir, have been initially widely recommended for influenza and other viral diseases, but were recommended to a lesser extent as an antiviral therapy for COVID-19. It does not seem that these products have gained significant interest since. Tocilizumab was just recommended for severe cases in the latest 7th version of the Diagnosis and Treatment Protocol. TCMs were widely used, alone or in combination with other antivirus drugs, in several going clinical trials in China. However, the potential for use of TCMs outside of China remains very limited in the current crisis.
In virology, it is well established that in-vitro results are poorly predictive of clinical outcome even in the case of Chloroquine Phosphate and Hydroxychloroquine for treating a variety of viruses [14]. Researchers in Shanghai showed confidence that Hydroxychloroquine could be a potential choice to reverse this pandemic. This was due to the fact that the number of new severe and critical COVID-19 cases in Shanghai have decreased significantly since Hydroxychloroquine was used for the treatment of COVID-19 since the 5th of February 2020 [15]. The effectiveness of Hydroxychloroquine (combined with basic treatment) was studied in 20 patients with COVID-19 beginning on the 17th of February 2020. After treatment with Hydroxychloroquine, the clinical symptoms of these 20 patients were significantly improved in 1 ~ 2 days. Chen et al. conducted a randomized study including 62 patients to investigate the efficacy of Hydroxychloroquine, which suggested a significant shorter time to clinical recovery, temperature recovery, and cough remission in patients receiving Hydroxychloroquine compared with those in control group [12]. Gautret et al. also reported that Hydroxychloroquine alone or in combination with Azithromycin reduced the detection of SARS-CoV-2 RNA in upper respiratory tract specimens compared with the control group in an open label, non-randomized clinical trial [16] and showed a rapid decline in the detection of SARS-CoV-2 RNA in upper respiratory tract specimens and length of stay in highly contagious wards in his later observational study including 80 patients [11]. However, one small pilot study including 30 patients investigating the standard dose of Hydroxychloroquine (400 mg, once per day) in the treatment of patients with COVID-19 did not show significant clinical benefits compared with the standard of care (other antiviral therapies) in the negative conversion rate of COVID-19 nucleic acid [17]. Several factors may explain these differences in the clinical results between the two studies. When patients are treated at an early stage of disease with a relatively lower risk of further progression, they will likely resolve the disease spontaneously, thus bringing the response rate very high and being unable to detect the differences between study groups. However, when patients are treated too late, the acute inflammation related to cytokines blast [18] will prevent treatments to reach their target and make the patients unresponsive to potentially effective drugs.
The effectiveness of Chloroquine Phosphate and Hydroxychloroquine still remains to be examined in rigorous, comparative studies before any firm conclusions may be drawn. Furthermore, the adverse effects of Chloroquine must be closely monitored to minimize its potential harms in already vulnerable COVID-19 patients.
It remains unfortunate that more knowledge has not yet been shared at this point at the time of publication when the pandemic may severely target Africa. In Africa, the healthcare infrastructure will be unable to absorb the consequences of a pandemic that may affect up to one-third or more of the population. Physical distancing is unlikely feasible due to the extreme poverty and cultural standards and habits. People are living together in large numbers, with as many as 20 persons in a single large room. Houses in large cities and suburbs with different families are all built one next to the other, with no clear dividers distinguishing properties and where who lives exactly. It is also common, cultural practice that when someone gets sick, all their relatives and close friends visit to check on the ill person and to spend time with them, thus increasing contact with ill people. Most people live off of informal, noncontractual work. They are, therefore, paid every day and with these low wages they must manage to feed their family. A pandemic, such as COVID-19, may force people in such situations to choose between either respecting confinement and losing their jobs and daily wages, therefore being unable to feed their families, or they will leave home in order to work and disseminate the virus, overwhelming their ill-prepared healthcare system. In Africa, they are recommended to wash their hands frequently, yet a vast majority do not have access to water and even when they do, they must carry it by hand over long distances. Moreover, they have little access to soap and cleansing products. They are recommended to blow their nose in disposable handkerchiefs, which they cannot afford, and are recommended to sneeze in their elbows, while in this season will find many dressed in short sleeves. It is apparent that without an effective, affordable pharmaceutical, such as Hydroxychloroquine or Chloroquine Phosphate, or any other potentially effective product, a pandemic disaster is bound to impact Africa.
Many of the obstacles faced by Africa are currently being seen in India as well. The 21-day nationwide lockdown plan implemented by Prime Minister Narendra Modi’s, might only work for India’s middle and upper classes, who have less difficulties to meet their basic life needs and who may even work from home, using modern technology. However, social distancing is impractical for the approximate 74 million people living in the country’s slums, which are known for their extreme poverty, unsanitary conditions, and inaccessibility to bathrooms and clean water [19]. As of the 1st of April 2020, India has recorded 1466 COVID-19 cases and 38 deaths [20]. The actual number of patients affected by COVID-19 in India could be underestimated due to their limited detection capacity. It is concerning that a catastrophe will occur in this country, home to the world’s second largest population. Other developing countries generally share the common features and face the same threats as those in India. The lack of clear WHO recommendations for using this ammunition considered by the Chinese as one of the ultimate options to control the pandemic may have long-term consequences on the WHO’s credibility in developing countries.
Conclusion
China has generated and continues to generate a massive source of information with several guidelines addressing almost all aspects of COVID-19 management. Guidelines were mainly from the central government and national associations, however, customization of guidelines was also allowed at the regional level. Only the Shanghai province deviated from central government guidelines, with Hydroxychloroquine and Chloroquine Phosphate being the most preferred treatments. These guidelines were likely based on evidence that is critical for defeating the COVID-19 pandemic. It would be of significant importance that Chinese scientists could share their valuable knowledge and insights on COVID-19 timely with global scientific communities. As the pandemic continues to unfold, additional clinical evidence for the treatment of COVID-19 will continue to emerge from China as well as other countries.
Funding Statement
The authors received no financial support for the research, authorship and/or publication of this article.
Disclosure statement
The authors declare that they have no conflicts of interest.
References
- [1].Lu R, Zhao X, Li J, et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395(10224):565–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].World Health Organization . Coronavirus disease (COVID-2019) situation reports. 2020. [cited 2020 March 29]. Available from: https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200328-sitrep-68-covid-19.pdf?sfvrsn=384bc74c_2.
- [3].World Health Organization . WHO director-general’s opening remarks at the media briefing on COVID-19-11 March 2020 2020. [cited 2020 April 05]. Available from: https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19—11-march-2020.
- [4].Shim E, Tariq A, Choi W, et al. Transmission potential and severity of COVID-19 in South Korea. IJID. 2020;93:339–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Worldometer . Number of COVID-19 cases in USA. 2020. [cited 2020 March 29]. Available from: https://www.worldometers.info/coronavirus/country/us/.
- [6].Grasselli G, Pesenti A, Cecconi M, et al. Critical care utilization for the COVID-19 outbreak in Lombardy, Italy: early experience and forecast during an emergency response. JAMA. 2020;323(16):1545–1546. [DOI] [PubMed] [Google Scholar]
- [7].Zhang Z, Yao W, Wang Y, et al. Wuhan and Hubei COVID-19 mortality analysis reveals the critical role of timely supply of medical resources. J Infect. 2020;81(1):147–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Jordan RE, Adab P, Cheng KK, et al. Covid-19: risk factors for severe disease and death. BMJ. 2020;368:m1198. [DOI] [PubMed] [Google Scholar]
- [10].Liu J, Cao RY, Xu MY, et al. Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS-CoV-2 infection in vitro. Cell Discov. 2020;6(1):1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Yao XT, Ye F, Zhang M, et al. In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Clin Infect Dis. 2020;71(15):732–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Chen ZW, Hu JJ, Zhang ZW, et al. Efficacy of hydroxychloroquine in patients with COVID-19: results of a randomized clinical trial. MedRxiv. 2020. [Google Scholar]
- [13].Cao B, Wang YM, Wen DN, et al. A trial of lopinavir–ritonavir in adults hospitalized with severe Covid-19. N Engl J Med. 2020;382(19):1787–1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Touret F, de Lamballerie X.. Of chloroquine and COVID-19. Antiviral Res. 2020;177:104762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].National Health Commission . Diagnosis and treatment protocol for novel coronavirus pneumonia (5th trial version). [cited 2020 March 25]. Available from: http://www.chinacdc.cn/jkzt/crb/xcrxjb/202002/W020200205535799210271.pdf.
- [16].Gautret P, Lagier JC, Parola P, et al. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int J Antimicrob Agents. 2020;56(1):105949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Chen J, Liu DP, Li L, et al. A pilot study of hydroxychloroquine in treatment of patients with common coronavirus disease-19 (COVID-19). J Zhejiang Univ Med sci. 2020. 49(2):215–219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Conti P, Ronconi G, Gallenga C, et al. Induction of pro-inflammatory cytokines (IL-1 and IL-6) and lung inflammation by Coronavirus-19 (COVI-19 or SARS-CoV-2): anti-inflammatory strategies. J Biol Regul Homeost Agents. 2020;34(2):327–331. [DOI] [PubMed] [Google Scholar]
- [19].Priyali Sur EM. Social distancing is a privilege of the middle class. For India’s slum dwellers, it will be impossible. 2020. [cited 2020 April 1]. Available from: https://edition.cnn.com/2020/03/30/india/india-coronavirus-social-distancing-intl-hnk/index.html.
- [20].Ministry of Health and Family Welfare-Government of India . Latest updates on the COVID-19 in India. 2020. [cited 2020 April 1]. Available from: https://webcache.googleusercontent.com/search?q=cache:Q7S18eyhhpgJ:https://www.mohfw.gov.in/.
- [21].Notifications on the adjustment of dosage of chloroquine phosphate for the treatment of novel coronavirus pneumonia . [cited 2020 March 25]. Available from: http://www.nhc.gov.cn/yzygj/s7653p/202002/0293d017621941f6b2a4890035243730.shtml.
- [22].Branch of hospital medicine in China Association of Chinese Medicine . Expert consensus for appropriate application of TCM for COVID-19. Beijing JTCM. 2020;7:657–664. [Google Scholar]
- [23].National Health Commission . Diagnosis and treatment protocol for novel coronavirus pneumonia (7th trial version). [cited 2020 March 25]. Available from: http://www.nhc.gov.cn/yzygj/s7653p/202003/46c9294a7dfe4cef80dc7f5912eb1989/files/ce3e6945832a438eaae415350a8ce964.pdf.
- [24].Tongji Hospital of Huazhong University of Science and Technology . A rapid advice guideline for the diagnosis and treatment of 2019 novel coronavirus (2019-nCoV) infected pneumonia (3rd Edition). [cited 2020 March 25]. Available from: https://www.tjh.com.cn/html/2020/0128/28713.shtml#title. [DOI] [PMC free article] [PubMed]
- [25].Peking Union Medical College Hospital . Peking union Medical College Hospital’s proposal for diagnosis and treatment of “novel coronavirus-infected pneumonia” (V2.0). Med J PUMCH. 2020;11:1–4. [Google Scholar]
- [26].Zhongnan hosptial of Wuhan University . A rapid advice guideline for the diagnosis and treatment of 2019 novel coronavirus (2019-nCoV) infected pneumonia (full version). New Med. 2020;30(1):35–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Shandong medical aid expert group for novel coronavirus pneumonia . Shandong expert consensus on the diagnosis and treatment for novel coronavirus pneumonia. J Shandong Med. 2020;60(7):1–5. [Google Scholar]
- [28].Shanghai medical aid expert group for novel coronavirus pneumonia . Shanghai expert consensus on the integrated treatment for novel coronavirus pneumonia. Chin J Infect Dis. 2020;38. 38(3):134–138. [Google Scholar]
- [29].Shanghai Health Commission . Shanghai: protocal for the use of traditional Chinese medicine for the diagnosis and treatment of COVID-19 2020 [cited 2020 March 25]. Available from: http://www.shanghai.gov.cn/nw2/nw2314/nw2319/nw12344/u26aw64089.html.
- [30].Hospital Pharmacy Professional Committee of Chinese Pharmaceutical Association . Expert consensus on rational drug use in clinical practice for COVID-19. Chin J Hospital Pharm. 2020;6:593–605. [Google Scholar]
- [31].Ba YM, Li XD, Min XJ, et al. HuBei provence: protocal on the use of traditional Chinese medicine for epidemic prevention and control of COVID-19. J Hubei Chin Med. 2020;2:7–8. [Google Scholar]
- [32].Ma ZP, Yin ZM, Wei GS, et al. Shanxi province: protocal for the use of traditional Chinese medicine of COVID-19 Shanxi Chinese Medicine. J Tradit Chin Med Shanxi. 2020;41(3):275–277. [Google Scholar]
- [33].Guangdong expert group of integrated Chinese Medicine . Guangdong expert consensus on the Chinese integrative medicines for the prevention and treatment of novel coronavirus pneumonia. WJTCM. 2020;5:805–812. [Google Scholar]
- [34].Hubei Health Commission . Close monitoring the adverse effects of chloroquine phosphate for the treatment of novel coronavirus pneumonia. [cited 2020 March 25]. Available from: http://wjw.hubei.gov.cn/.
- [35].Health Commission of Guangdong Province . Expert consensus on chloroquine phosphate for the treatment of novel coronavirus pneumonia. CJTRD. 2020;43(3):185–188. [DOI] [PubMed] [Google Scholar]
- [36].National Health Commission . Diagnosis and treatment protocol for novel coronavirus pneumonia (3rd trial version). [cited 2020 March 25]. Available from: http://www.gov.cn/zhengce/zhengceku/2020-01/23/5471832/files/106d59e45ac948ceb3cb12d400b8053c.pdf.
- [37].National Health Commission . Diagnosis and treatment protocol for novel coronavirus pneumonia (4th trial version). [cited 2020 March 25]. Available from: http://www.nhc.gov.cn/yzygj/s7653p/202001/4294563ed35b43209b31739bd0785e67/files/7a9309111267475a99d4306962c8bf78.pdf.
- [38].National Health Commission . Diagnosis and treatment protocol for novel coronavirus pneumonia (5th trial revised version). [cited 2020 March 25]. Available from: http://www.gov.cn/zhengce/zhengceku/2020-02/09/5476407/files/765d1e65b7d1443081053c29ad37fb07.pdf.
- [39].National Health Commission . Diagnosis and treatment protocol for novel coronavirus pneumonia (6th trial version). [cited 2020 March 25]. Available from: http://www.nhc.gov.cn/yzygj/s7653p/202002/8334a8326dd94d329df351d7da8aefc2/files/b218cfeb1bc54639af227f922bf6b817.pdf.


