Abstract
Background:
Surface detail reproduction (SDR) and dimensional stability of elastomeric impression material play a crucial role in fixed prosthodontics.
Aim and Objectives:
The aim of this study was to compare the effect of chemical disinfection on SDR and dimensional stability of polyvinyl siloxane (PVS) and polyether (PE) with a new vinyl polyether silicone (VPES) elastomeric impression material.
Materials and Methods:
A stainless steel mold was made to fabricate the study specimens for nonaqueous PVS, PE, and VPES elastic dental impression materials. Single mix impression techniques with light- and heavy-body consistency of all three materials were used to prepare the test specimens. The specimens were immersed in glutaraldehyde (Cidex) 2.45% and sodium hypochlorite (Hypo) 3.0% disinfection for 15 min (T1) and 12 h (T2) immersion after which dimensional stability and SDR were recorded using stereomicroscope and digital Vernier caliper.
Statistical Analysis:
The data were analyzed using the one-way ANOVA, paired t-test, Kruskal–Wallis test, and Wilcoxon signed-rank test.
Results:
Dimensional stability at the T2 time interval showed a highly significant difference for control and glutaraldehyde groups (P < 0.001), whereas a statistically significant difference for Hypo group (P < 0.05). SDR of the three materials when compared at T1 time interval showed a statistically significant difference (P = 0.015). A comparison between two disinfectants at T1 time interval revealed a highly significant difference (P < 0.001), while at T2 interval significant difference was obtained (P = 0.009).
Conclusion:
VPES impressions display acceptable dimensional stability and SDR for clinical use with immersion disinfection. Although some statistically significant differences in linear dimensional stability and semidefinite programming were observed among VPES, PE, and PVS, but the clinical impact of these differences is minor considering the overall accuracy of casts which was high.
Keywords: Glutaraldehyde, immersion disinfection, polyether, polyvinyl siloxane, sodium hypochlorite, vinyl polyether silicone
Introduction
Contamination of the working atmosphere by microorganisms from oral flora in dental clinic offers constant risks to the health professionals. There are evidence regarding the pathogenesis and intensity of viruses of hepatitis B, herpes, tuberculosis, and AIDS in dentistry.[1,2,3] Impression material exposure to saliva and blood provides a significant source for cross-contamination. Impression materials disinfected by immersion, however, may be subjected to dimensional changes, which may hamper the accuracy of the restoration. The duration and mode of applying the disinfectants depends on the potential of the impression material to absorb water and the time that has transpired since the impression was made. After disinfection, the impression should be removed, rinsed, and poured with the gypsum product as soon as possible.[2] Elastomeric impression materials have always been the material of choice for definitive impressions in fixed prosthodontics due to inherent properties such as reduced marginal voids and less distortion in the impressions, resulting in improved quality of gypsum dies.[3]
Amongst the available elastomeric materials, polyvinyl siloxane (PVS) and polyether (PE) are used most frequently. Advances in elastomeric chemistries have led to the invention of a new generation of impression materials which is the combination of PVS and PE called “Vinyl Polyether Silicone” (VPES).[4] The surface detail reproduction (SDR) and dimensional stability of vinyl polysiloxane (VPS) and PE are well documented. This material has been proposed by the manufacturer to possess good mechanical and flow properties, along with excellent wetting characteristics in the unset condition when applied to the prepared tooth and also in the set condition. Enhancement of hydrophilicity may influence the accuracy of impressions and can result in improved flow and finer detail of impressions made on moist dentinal surfaces and in the area of the gingival sulcus. The effect of disinfection on the new material must also be established.[5]
The purpose of this study was to evaluate and compare the dimensional stability and SDR of VPS, PE, and new VPES elastomeric impression materials with and without disinfection after 15 min and 12 h of immersion. The null hypothesis stated that there was no significant difference in both physical properties of the impression materials after disinfection at two-time intervals.
Materials and Methods
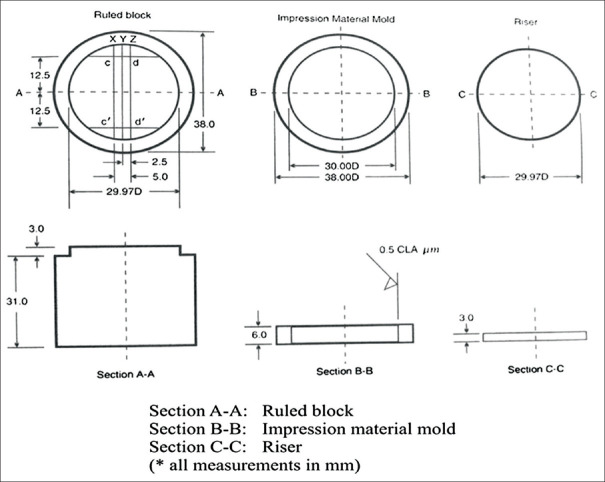
A standardized stainless steel (SS) mold customized as per revised American Dental Association (ADA) specification no. 19 was used for testing nonaqueous elastic dental impression materials [Figure 1]. The mold comprised three sections: section A-A was a block with scribed lines, which is the base; section B-B having impression material mold, which is a SS ring of thickness 3 mm and 29.97 mm; and section C-C having riser which is perforated plate to apply uniform pressure over impression material, mechanical retention, and escape of excess material [Figure 2 and 3].[6,7,8]
Figure 1.
Schematic diagram showing American Dental Association No. 19 for elastomers to test the dimensional stability and surface detail reproduction
Figure 2.

Stainless steel metal die
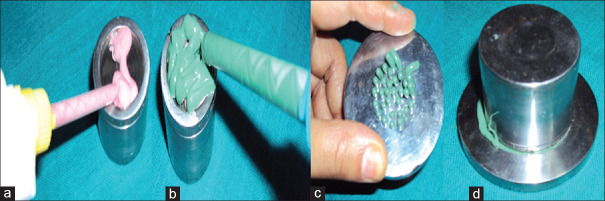
Figure 3.
Procedure for making specimens (a-injection of light body, b-loading of heavy body, c-placing perforated tray, d-entire assembly upside down)
The impression materials used in the study were PVS (Flexceed [GC Dental products Corp, Japan]), PE (Impregum™ Soft [3M ESPE, Deutschland GmbH, Germany]), and PVES (EXA'lence™ [GC Dental products Corp, Japan]) in heavy- and light-body consistencies. A total of thirty specimens of each test material were made (n = 90). Specimens having bubbles and irregularities were discarded.[9,10]
The disinfectants used in the study were glutaraldehyde (2.45%) (Cidex [Johnson and Johnson, India]) and sodium hypochlorite (Hypo) (3.0%) (NaOCl [Vishal, India]). All the specimens were immersed in both the disinfectants for 10 min and tested at two-time intervals T1 (15 min) and T2 (12 h) after fabrication [Figure 4]. The study groups of the specimens were the control group (n = 10) which were not disinfected (CT1 and CT2), glutaraldehyde group (n = 10) immersed in glutaraldehyde (GT1 and GT2), and NaOCl group (n = 10) immersed in NaOCl (ST1 and ST2). Two physical properties assessed were linear dimensional stability (LDS) and SDR at T1 and T2 for all the specimens.
Figure 4.
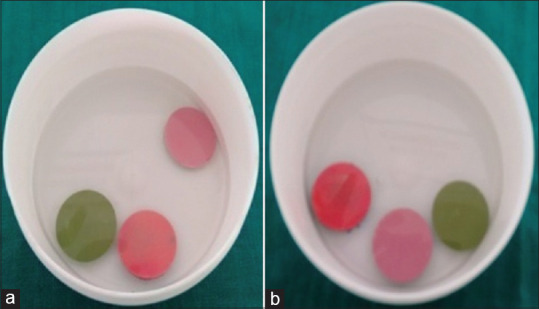
Disinfection of specimens: (a) glutaraldehyde (2.45%), and (b) sodium hypochlorite
LDS for the control group specimens was assessed by measuring the distance between the cross hatches of the upper horizontal line four and five with the help of digital Vernier caliper under a stereomicroscope. LDS for test groups (GT1, GT2, ST1, and ST2) was measured by retrieving the specimens from respective group disinfectants for 10 min and tested at two-time intervals T1 (15 min) and T2 (12 h) after fabrication. Statistical analysis for LDS measurement was performed using the one-way ANOVA (intergroup comparison) followed by Tukey's test. Comparison between the time intervals was done using the paired t-test.[9]
SDR for the control group was done by recording the reproduction of 50 μ line on to elastomeric impressions. Outcomes were recorded as (a) line reproduced completely and (b) line not completely reproduced. This was used as a quality-control measure. The entire length of the 50 μ was observed at ×10 magnification and given an ordinal score as follows:
(a) Score 1: well defined, sharp, continuous lines; (b) Score 2: continuous line but with some loss of sharpness; (c) Score 3: significant deterioration of edge detail or loss of continuity of the line; and (d) Score 4: Failure to reproduce the line.
SDR for specimens was done after 10 min immersion followed by recording the SDR according to ordinal scales. Statistical analysis for SDR was performed using the Wilcoxon signed-rank test, and comparison between the time intervals was done using the Kruskal–Wallis test.[11]
Results
LDS for control group and test group specimens of the three materials at T1 time interval showed no statistically significant difference, whereas at T2 showed statistically significant (P ≤ 0.001) findings between control group and immersion disinfection [Graph 1]. SDR for VPES test impression materials at T1 and T2 time intervals showed a statistically significant difference (P = 0.009) at T1 and statistically highly significant difference at T2 interval [Table 1].
Graph 1.
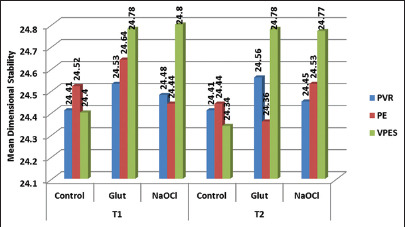
Comparison of linear dimensional stability between polyvinyl siloxane, polyether, and vinyl polyether silicone based on disinfectants at T1 and T2 time interval
Table 1.
Comparison of surface detail reproduction between glutaraldehyde and sodium hypochlorite at T1 and T2 time intervals
| Time interval | Groups | P | ||
|---|---|---|---|---|
| PVS | PE | VPES | ||
| T1 | Control | 0.001** | 0.001** | 0.009* |
| Glut | ||||
| NaOCl | ||||
| T2 | Control | 0.001** | 0.001** | 0.001** |
| Glut | ||||
| NaOCl | ||||
Kruskal–Wallis test: **P≤0.001 (highly significant); *P=0.009 (significant). PVS: Polyvinyl siloxane; PE: Polyether; VPES: Vinyl PE silicone
One-way ANOVA (intragroup comparison) followed by (LSD post hoc) Tukey's test was applied. Comparison between the time intervals was done using the paired t-test [*P ≤ 0.05 (significance); **P ≤ 0.001 (highly significant)].
Discussion
Dental practitioners encounter potentially harmful microorganisms, and patients are the most common source of microorganisms.[12] Studies indicate that the surface of impressions taken out of the mouth is polluted with bacteria.[13,14,15,16] As impressions and occlusal records cannot be sterilized by heat, chemical disinfection is the common practicable method to eradicate microorganisms.[17,18] The ADA recommends soaking impression materials in disinfectant solutions for <30 min.[19] Soaking method is applied in 63% of alginate impressions and in 73% of silicone impressions in European schools of dentistry.[17] Furthermore, the approximate time of disinfection was 10.3 ± 6.3 min. In order to take advantage of the properties of both PVS and PE impression materials, a new generation of impression material, called VPES has being developed. The potential advantages of VPES include intrinsic hydrophilicity without using surfactants, handling characteristics similar to PVS, high tear strength with flexibility, predictable subgingival flow ability, and a mild mint taste. This study evaluated and compared the dimensional changes of three elastomers after impression disinfection by immersion. Impressions were subjected to two different procedures commonly employed in dental practice, immediate disinfection (T1), disinfection after 12 h (T2), to assess the effect of 2.45% glutaraldehyde and 3% Hypo (test specimens). The later would simulate the disinfection performed by the dental technician when they receive an impression previously disinfected by the dental office personnel. For comparative purposes, the dimensional changes were also assessed when the impression did not undergo any disinfection (control specimens).
In the present study, PVS disinfected with glutaraldehyde and NaOCl at T1 and T2 time intervals showed no significant difference in dimensional stability [Table 1]. This result was in accordance with the studies conducted by Carvalhal et al.[20] and Ahila and Thulasingam et al.[21,22] This was due to the hydrophobic nature of silicones, which made the surface of these impressions highly resistant to the attack by the aqueous disinfectants regardless of their types or the length of exposure period.[21] PE when disinfected with glutaraldehyde and NaOCl at T1 and T2 time interval showed a significant difference in dimensional stability. NaOCl causes more significant difference as compared to glutaraldehyde when compared to the control group at T2 time interval. These findings were similar to the studies conducted by Herrera and Merchant,[23] Rios et al.,[24] and Johansen and Stackhouse et al.[25] This may be due to the inherent hydrophilicity, lesser contact angle, more wettability in aqueous environment, and lesser filler content implies more water absorption by the material.[26,27] VPES, when disinfected with glutaraldehyde and NaOCl at T1 and T2 time interval, showed a significant difference in dimensional stability in the present study at T2 time interval. This conclusion is in accordance with the studies conducted by Stober et al.[5] This could be due to water absorption with consequential swelling or chemical interactions between the impression material and disinfection solution.
According to ADA Specification no. 19, elastomeric impression materials used to fabricate precision castings must be able to reproduce fine detail to a level of 20 μm or less. In this study, a modified ISO standard metal die was employed to reduce the variables associated with the uncontrollable factors; thus, the ability of the impression material to reproduce surface detail was assessed in an approach that was more precise and comparable. The results showed no statistically significant difference observed at T1 and T2 time intervals without disinfection and after disinfection with glutaraldehyde and NaOCl for PVS, PE, and VPES, all three materials produced good SDR according to scores when observed separately. This result was in accordance with a study conducted by Jagger et al.[27]
The limitations of the present study were that the impressions were made of standardized SS dies which do not resemble the behavior of the oral tissues, and it was an in vitro study. In order to determine the appropriate disinfectant for VPES material, a clinical investigation should be undertaken with different concentrations and types of chemical disinfectants.
Conclusion
Considering the limitations of this study, it was observed that vinyl PE silicone impressions display acceptable dimensional stability and SDR for clinical use with immersion disinfection. Although some statistically significant differences in LDS and semidefinite programming were observed among VPES, PE, and PVS, the clinical impact of these differences is minor considering the overall accuracy of casts which was high.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Runnells RR. An overview of infection control in dental practice. J Prosthet Dent. 1988;59:625–9. doi: 10.1016/0022-3913(88)90083-2. [DOI] [PubMed] [Google Scholar]
- 2.Shen C. Impression materials. In: Anusavice KJ, editor. Phillip's Science of Dental Materials. 11th ed. St. Louis: Elsevier; 2003. p. 225. [Google Scholar]
- 3.Rubel BS. Impression materials: A comparative review of impression materials most commonly used in restorative dentistry. Dent Clin North Am. 2007;51:629–42. doi: 10.1016/j.cden.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 4.Shen C. Impression materials. In: Anusavice KJ, editor. Philips' Science of Dental Materials. 11th ed. St Louis: Elsevier; 2003. pp. 212–6. [Google Scholar]
- 5.Stober T, Johnson GH, Schmitter M. Accuracy of the newly formulated vinyl siloxanether elastomeric impression material. J Prosthet Dent. 2010;103:228–39. doi: 10.1016/S0022-3913(10)60035-2. [DOI] [PubMed] [Google Scholar]
- 6.Kumari N, Nandeeshwar DB. The dimensional accuracy of polyvinyl siloxane impression materials using two different impression techniques: An in vitro study. J Indian Prosthodont Soc. 2015;15:211–7. doi: 10.4103/0972-4052.158074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Revised American Dental Association Specification no. 19 for non-aqueous, elastomeric dental impression materials. J Am Dent Assoc. 1977;94:733–41. doi: 10.14219/jada.archive.1977.0334. [DOI] [PubMed] [Google Scholar]
- 8.Surendra GP, Anjum A, Satish Babu CL, Shetty S. Evaluation of dimensional stability of autoclavable elastomeric impression material. J Indian Prosthodont Soc. 2011;11:63–6. doi: 10.1007/s13191-011-0061-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vadenal LH, Carvalho V, Goncalves MC, Pereira DA. Evaluation of dimensional stability of impression materials immersed in disinfectant solutions using a metal tray. Rev Odonto Cienc Fac Odonto. 2005;20:319–23. [Google Scholar]
- 10.Melilli D, Rallo A, Cassaro A, Pizzo G. The effect of immersion disinfection procedures on dimensional stability of two elastomeric impression materials. J Oral Sci. 2008;50:441–6. doi: 10.2334/josnusd.50.441. [DOI] [PubMed] [Google Scholar]
- 11.Puttaiah R, Griggs JA, Kanabar J, Coon D. Effects of an Immersion Disinfectant and a Surface Disinfectant on Three Elastomeric Impression Materials Dallas. USA: Texas A and M University System Health Science Centre; 2014. [Google Scholar]
- 12.Szymańska J. Microbiological risk factors in dentistry. Current status of knowledge. Ann Agric Environ Med. 2005;12:157–63. [PubMed] [Google Scholar]
- 13.Rowe AH, Forrest JO. Dental impressions. The probability of contamination and a method of disinfection. Br Dent J. 1978;145:184–6. doi: 10.1038/sj.bdj.4804140. [DOI] [PubMed] [Google Scholar]
- 14.Samaranayake LP, Hunjan M, Jennings KJ. Carriage of oral flora on irreversible hydrocolloid and elastomeric impression materials. J Prosthet Dent. 1991;65:244–9. doi: 10.1016/0022-3913(91)90169-w. [DOI] [PubMed] [Google Scholar]
- 15.Hudson-Davies SC, Jones JH, Sarll DW. Cross-infection control in general dental practice: Dentists' behaviour compared with their knowledge and opinions. Br Dent J. 1995;178:365–9. doi: 10.1038/sj.bdj.4808775. [DOI] [PubMed] [Google Scholar]
- 16.Jennings KJ, Samaranayake LP. The persistence of microorganisms on impression materials following disinfection. Int J Prosthodont. 1991;4:382–7. [PubMed] [Google Scholar]
- 17.Muller-Bolla M, Lupi-Pégurier L, Velly AM, Bolla M. A survey of disinfection of irreversible hydrocolloid and silicone impressions in European Union dental schools: Epidemiologic study. Int J Prosthodont. 2004;17:165–71. [PubMed] [Google Scholar]
- 18.Infection control recommendations for the dental office and the dental laboratory. ADA Council on Scientific Affairs and ADA Council on Dental Practice. J Am Dent Assoc. 1996;127:672–80. doi: 10.14219/jada.archive.1996.0280. [DOI] [PubMed] [Google Scholar]
- 19.Egusa H, Soysa NS, Ellepola AN, Yatani H, Samaranayake LP. Oral candidosis in HIV-infected patients. Curr HIV Res. 2008;6:485–99. doi: 10.2174/157016208786501445. [DOI] [PubMed] [Google Scholar]
- 20.Carvalhal CI, Mello JA, Sobrinho LC, Correr AB, Sinhoreti MA. Dimensional change of elastomeric materials after immersion in disinfectant solutions for different times. J Contemp Dent Pract. 2011;12:252–8. doi: 10.5005/jp-journals-10024-1043. [DOI] [PubMed] [Google Scholar]
- 21.Ahila SC, Thulasingam C. Effect of disinfection on gypsum casts retrieved from addition and condensation silicone impressions disinfected by immersion and spray methods. SRM J Res Dent Sci. 2015;5:163–9. [Google Scholar]
- 22.Ahila SC, Subramaniam E. Comparative evaluation of dimensional stability and surface quality of gypsum casts retrieved from disinfected addition silicone impressions at various time intervals: An in vitro study. J Dent Oral Hygien. 2012;4:34–43. [Google Scholar]
- 23.Herrera SP, Merchant VA. Dimensional stability of dental impressions after immersion disinfection. J Am Dent Assoc. 1986;113:419–22. doi: 10.14219/jada.archive.1986.0214. [DOI] [PubMed] [Google Scholar]
- 24.Rios MP, Morgano SM, Stein RS, Rose L. Effects of chemical disinfectant solutions on the stability and accuracy of the dental impression complex. J Prosthet Dent. 1996;76:356–62. doi: 10.1016/s0022-3913(96)90538-7. [DOI] [PubMed] [Google Scholar]
- 25.Johansen RE, Stackhouse JA., Jr Dimensional changes of elastomers during cold sterilization. J Prosthet Dent. 1987;57:233–6. doi: 10.1016/0022-3913(87)90152-1. [DOI] [PubMed] [Google Scholar]
- 26.Duseja S, Shah RJ, Shah DS, Duseja S. Dimensional measurement accuracy of recent polyether and addition silicone monophase impression materials after immersion in various disinfectants: An in vitro study. Int J Health Biomed Res. 2014;2:87–7. [Google Scholar]
- 27.Jagger DC, Vowles RW, McNally L, Davis F, O'Sullivan DJ. The effect of a range of disinfectants on the dimensional accuracy and stability of some impression materials. Eur J Prosthodont Restor Dent. 2007;15:23–8. [PubMed] [Google Scholar]