ABSTRACT
Background: Many trials in actinic keratoses (AK) use complete clearance rate (100% reduction in number of lesions) as the primary endpoint. We explore limitations (predominantly baseline factors) associated with this outcome.
Objective: This analysis assessed the effect of baseline lesion count on complete clearance rate using randomized controlled trials (RCTs) that evaluated 5-fluorouracil (5-FU) formulations, alone or with 10% salicylic acid solution, in patients with AK.
Methodology: Correlation between baseline lesion count and complete clearance rate at week 8 was assessed using Pearson’s coefficient.
Results: Five RCTs assessing 5-FU (4%, 5%, or 0.5% in 10% salicylic acid solution) in 1,080 patients with AK were included. Mean lesion count at baseline ranged from 8.1 to 21.2 lesions per patient. Complete clearance rate was negatively associated with number of lesions at baseline. Correlation between mean number of lesions at baseline and complete clearance rate was strong (r2 = 0.94) and statistically significant (p < 0.001).
Conclusion: This analysis showed that, in a homogenous set of trials, complete clearance rates achieved with 5-FU interventions are inversely related to number of lesions at baseline. These findings highlight the limits of restricting treatment evaluation to complete clearance rate and the relevance of alternative measures.
KEYWORDS: Actinic keratosis, systematic literature review, complete clearance rate, clinical trial endpoints, 5-fluorouracil
Introduction
Actinic keratoses (AK) are common epithelial lesions characterized by the formation of keratotic macules, papules, or plaques with superficial scaly growth, most commonly due to intense exposure to ultraviolet radiation [1–4]. Prompt diagnosis and therapy are important because AK is recognized as one of the earliest clinical events that may lead to the development of invasive squamous cell carcinoma (SCC) [3,5,6]. Importantly, while it has traditionally been believed that invasive SCC arises through a linear progression from grade I to III lesions, recent data indicate that grade I lesions can evolve directly into invasive SCC [5,7,8].
The primary goal of AK therapy is to achieve clearance of clinical and subclinical lesions in order to prevent their progression to invasive SCC; a secondary aim may be to improve the skin’s appearance and the patient’s quality of life [3,5]. To date, the majority of clinical trials in patients with AK have used the complete clearance rate, defined as the proportion of patients with no clinically visible lesions (i.e., a 100% reduction in the number of lesions) as the primary efficacy endpoint, and this is commonly required for regulatory purposes [3,6]. However, complete clearance rate is more difficult to achieve when several areas are treated simultaneously; success may also depend on the anatomical site of the lesions and the number of lesions at baseline [3].
Alternative measures of efficacy have consequently been suggested and include partial clearance rate as well as percent reduction of AK lesions. Partial clearance rate is defined as the proportion of patients with at least a 75% reduction in the number of baseline AK lesions in the selected treatment area at the post-treatment evaluation visit and at any follow-up visits; therapy responders are defined as those with a partial clearance of ≥75% and non-responders as those with a partial clearance of <75% [9]. The percent reduction of AK lesions is also a relevant endpoint as it takes into account the number of lesions at baseline, a factor that is known to influence the complete clearance rate [10,11]. However, it is not clearly shown how the number of lesions at baseline can influence the achievement of clearance.
The present analysis used data from a previously reported systematic literature review (SLR) (Ezzedine et al., forthcoming manuscript, 2020) to assess the effect of baseline lesion count on the complete clearance rate achieved with different 5-fluorouracil (5-FU) formulations, alone or with 10% salicylic acid solution.
Materials and methods
The conduct of the systematic review has been reported in detail elsewhere (Ezzedine K, et al., forthcoming manuscript, 2020). In brief, published reports of randomized controlled trials (RCTs) in adult patients (≥18 years) with AK affecting the face, ears, or scalp and receiving field-directed therapy (5-FU, diclofenac sodium [DFC], imiquimod [IMQ], ingenol mebutate [Ing. Meb] or photodynamic therapy [PDT]), lesion-directed therapy (cryosurgery/cryotherapy, laser therapy, curettage, excision, or shave biopsy) or other interventions (e.g., chemical peeling, adapalene gel, vitamin D, or colchicine) were identified by searches of the MEDLINE®, MEDLINE® In-Process and Embase® databases, and the Cochrane Central Register of Clinical Trials. All searches covered the period from inception to 29 November 2017 and were confined to English-language publications. Further potential trials were identified by manual searching of relevant conference proceedings, bibliographies of included publications and ClinicalTrials.gov. In addition, information on four pivotal trials with 5-FU 4%, two of which have been reported previously [12], was provided by the marketing authorization holder.
Trial selection process
A homogenous set of trials evaluating approved field-directed interventions administered according to their Summary of Product Characteristics (SmPC) criteria were identified from the SLR. The trials were then refined according to the feasibility criteria shown in Table 1 (Ezzedine K, et al., forthcoming manuscript, 2020). Eventually, the RCTs identified from the homogenous set were included in the regression analysis if they met the following criteria: (1) assessed 5-FU interventions used to treat patients with AK over 4 weeks, consistent with the recommended duration in the SmPC; (2) reported mean number of lesions at baseline; and (3) reported complete clearance at the recommended timepoint of 4 weeks after the end of treatment for efficacy assessment.
Table 1.
Feasibility assessment network for quantitative comparative analyses of 5-FU: exclusion criteria of trials identified from the SLR.
|
aTrials included if one or more arms did not have prior treatment.
Interventions
As interventions in AK differ in terms of treatment modalities, a pool of interventions with comparable treatment duration and time for efficacy assessment were selected. The interventions of interest included 5-FU at different dosages: 4%, 5%, and 0.5% with potentially the addition of 10% salicylic acid as investigational or comparator arm in selected trials.
Data extraction
Trial characteristics (design, number of arms, sample size), participant characteristics (age, sex), intervention, and comparator details (dosage and type of intervention, duration of treatment), as well as aggregated data on complete clearance rates, partial clearance rates, and number of lesions in the intention-to-treat group were extracted from the included trials by two independent reviewers. Any discrepancies were resolved by a third reviewer.
Analytical approach
The correlation between complete clearance rate and number of lesions at baseline for 5-FU interventions was assessed using the Pearson correlation formula. A linear regression was fitted through the points representing treatment effect according to baseline lesion count and the strength of association between baseline lesion count and complete clearance rate was quantified. The Pearson’s correlation coefficient was calculated using this formula in which mx and my represent the means of x = mean number of lesions at baseline and y = the complete clearance rate:
A Pearson’s correlation coefficient value varies between – 1 (a perfect negative and linear relationship) and +1 (a perfect positive and linear relationship).
The correlation was then tested with the corresponding degree of freedom (df = n-2) where n is the number of observations in x and y variables to assess if the correlation between the mean number of lesions at baseline and complete clearance rate was significant. Only 5-FU interventions were considered for inclusion in the regression analysis, other treatment arms and placebo were excluded.
To evaluate the robustness of the findings, a sensitivity analysis was conducted using other active treatment arms of included studies. The correlation coefficient was recalculated by including other active treatment arms from the subset of trials used in the correlation analysis.
All analyses were performed using Microsoft® Excel®.
Results
Trial characteristics
Seventy-five eligible RCTs reported in 151 publications were included in the SLR, of which 31 met the feasibility criteria for inclusion in quantitative comparisons of 5-FU, and 12 assessed 5-FU (4%, 5%, and 0.5%) interventions in AK (Figure 1). Nine of the 12 trials included data for baseline lesion count and complete clearance rate, and five of these trials met the criteria in terms of evaluation of treatment effect at 4 weeks off treatment (Figure 2) [13–17]. A summary of these trials is shown in Table 2. The five trials were phase 2 or 3 trials with either a single- or double-blind design. Treatments included 5-FU 4% or 5% versus placebo [15,17], 5-FU 4% versus placebo [16], 5-FU 5% versus IMQ [13], and 5-FU 0.5% versus placebo [14]. Total trial sample size (based on treatment arms included in the SmPC) ranged from 50 to 772 patients.
Figure 1.
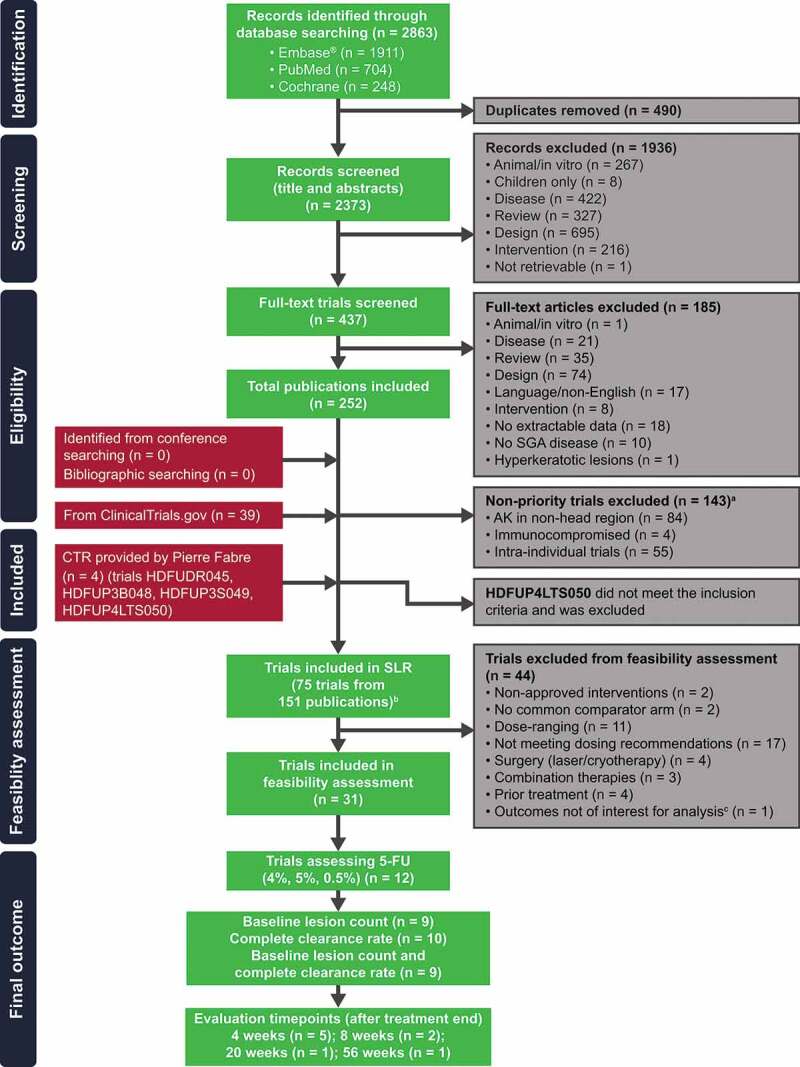
Trial selection flowchart.
a128 non-priority trials from database searching and 15 trials from ClinicalTrials.gov that were included, but not extracted. bHDFUDR045 and HDFUP3S049 results were reported by Dohil et al. [12] and not discussed separately. cHDFUP4LTS050 was an uncontrolled, single-group trial that assessed patients from previous trials (HDFUP3B048 and HDFUP3S049). CTR = clinical trial report; SGA = subgroup analysis.
Figure 2.
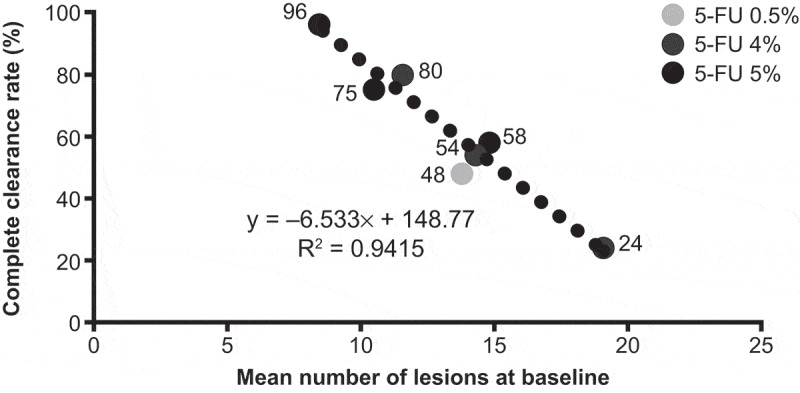
Correlation between complete clearance rate at 8 weeks and baseline lesion count in trials with 5-FU 4%, 5%, or 0.5% [13–17].
Table 2.
Trial and participant characteristics of trials included in the correlation analysis.
| Trial | Trial phase | Blinding status | Treatments | Sample size, n | Age (SD) | Male, % | Baseline lesion count (SD) | Mean baseline lesion count across treatment arms |
|---|---|---|---|---|---|---|---|---|
| HDFUDR045 [15] | 2 | Single | 5-FU 4% (4 weeks; OD) | 20 | 65.9 (8.3) | 85 | 11.6 (4.2) | 10.6 |
| 5-FU 5% (4 weeks; BID) | 20 | 65.2 (11.3) | 95 | 10.5 (3.5) | ||||
| Placebo (4 weeks BID) | 20 | 65 (11.8) | 90 | 9.7 (2.2) | ||||
| HDFUP3B048 [17] | 3 | Single | 5-FU 4% (4 weeks; OD) | 353 | 67.7 (9.8) | 81 | 14.4 (10.8) | 15.1 |
| 5-FU 5% (4 weeks; BID) | 349 | 67.4 (10) | 81 | 14.8 (10.6) | ||||
| Placebo (4 weeks; OD) | 70 | 68 (10.1) | 83 | 16.2 (15.1) | ||||
| HDFUP3S049 [16] | 3 | Double | 5-FU 4% (4 weeks; OD) | 50 | 67.9 (11.7) | 78 | 19.2 (15.0) | 21.2 |
| Placebo (4 weeks; OD) | 50 | 66.9 (11.7) | 92 | 23.2 (18.5) | ||||
| Krawtchenko 2007 [13] | Unclear | Unclear | 5-FU 5% (4 weeks; BID) | 24 | 70.4 (6.7) | 79 | 8.3 (NR) | 8.1 |
| IMQ (4 weeks; TIW) | 26 | 70.8 (5.6) | 85 | 7.9 (NR) | ||||
| Weiss 2002 [14] | 3 | Double | 5-FU 0.5% (4 weeks; OD) | 40 | 62.7 (NR) | 75 | 14.1 (8.2) | 15.3 |
| Placebo (4 weeks; OD) | 58 | 63.6 (NR) | 95 | 16.4 (11.1) |
BID = twice daily; NR = not reported; OD = once daily; SD = standard deviation; TIW = three times a week.
Patient and disease characteristics
A summary of key disease characteristics is shown in Table 2. Across the trials, the patient age ranged from 62.7 to 70.8 years, and the majority were male (75–95%). The mean lesion count at baseline ranged from 8.1 to 21.2 lesions per patient. In general, the trials with 5-FU 4% [15–17] were among the trials in the feasibility assessment that tended to include patients with greater numbers of lesions at baseline.
Correlation analysis
Complete clearance rates at 8 weeks (4 weeks after the end of treatment) ranged from 24% to 80% in patients receiving 5-FU 4%, from 58% to 96% in those receiving 5-FU 5%, and was 48% in those receiving 5-FU 0.5% (Table 3). Partial clearance rates were reported in four of the five trials and were greater than the complete clearance rates; rates ranged from 74% to 100% for 5-FU 4%, from 80% to 95% for 5-FU 5%, and was 89% for 5-FU 0.5%.
Table 3.
Complete and partial clearance rates in relation to mean number of lesions at baseline in clinical trials with 5-FU.
| Trial | Treatments | Baseline lesion count (SD) | Complete clearance rate at week 8 (except IMQ: at week 24), % | Partial clearance rate at week 8, % |
|---|---|---|---|---|
| HDFUDR045 [15] | 5-FU 4% (4 weeks; OD) | 11.6 (4.2) | 80 | 100 |
| 5-FU 5% (4 weeks; BID) | 10.5 (3.5) | 75 | 95 | |
| HDFUP3B048 [17] | 5-FU 4% (4 weeks; OD) | 14.4 (10.8) | 54 | 81 |
| 5-FU 5% (4 weeks; BID) | 14.8 (10.6) | 58 | 80 | |
| HDFUP3S049 [16] | 5-FU 4% (4 weeks; OD) | 19.2 (15) | 24 | 74 |
| Krawtchenko 2007 [13] | 5-FU 5% (4 weeks; BID) | 8.3 (NR) | 96 | NR |
| IMQ (4 weeks; TIW) | 7.9 (NR) | 85 | NR | |
| Weiss 2002 [14] | 5-FU 0.5% (4 weeks; OD) | 14.1 (8.2) | 48 | NR |
Complete clearance rate was negatively correlated to the number of lesions at baseline (r = – 0.97). The correlation was strong (r2 = 0.94) between the mean number of lesions at baseline and the complete clearance rate (Figure 2), as described by the equation y = – 6.533x + 148.77. Moreover, the corresponding P value showed that the correlation was statistically significant (p < 0.001).
The sensitivity analysis confirmed the robustness of the findings; when including IMQ in the correlation analysis, the correlation coefficient was still highly significant (r2 = 0.92). The relationship between lesion count at baseline and complete clearance was described by the equation y = – 5.9556x + 140.02.
Discussion
The present analysis has shown that it is feasible to identify a homogenous set of trials, from the SLR reported elsewhere (Ezzedine et al., forthcoming manuscript, 2020), evaluating 5-FU interventions and enabling quantitative comparisons between treatments. In this set, there was a strong correlation between AK disease severity, as reflected in the baseline lesion count, and clinical efficacy (complete clearance rate). The inverse relationship between mean number of lesions at baseline and complete clearance was confirmed in the sensitivity analysis.
This finding is consistent with a published review of six trials with Ing. Meb., where the complete clearance rate decreased with increasing baseline lesion count [10]. It should be noted that some investigators have challenged the clinical relevance of complete clearance rate as a trial endpoint, and its relevance in a daily real-world setting, as this is rarely attained in practice and does not capture the full benefits of field-directed therapies [3,5,6]. Furthermore, AK typically follows a fluctuating course, and lesions (particularly grade I/II) may regress and relapse over time [1]. Complete clearance rate may not be a suitable endpoint for a disease with a fluctuating course and can be influenced by several factors, including the number of baseline lesions and the size of treatment area [3,6,10]. Such differences make comparisons of efficacy across trials problematic [6].
For these reasons, complete clearance rate, although informative, should not be the only efficacy criterion for AK therapies. Other endpoints, such as partial clearance, percent reduction in AK lesion count or lesion response rate, are also clinically relevant [3,5,10]. In particular, percent reduction in AK count, as opposed to complete clearance rate, is unaffected by the number of baseline lesions [10]. It is noteworthy that there are currently no regulatory guidelines presenting a rationale for the use of complete clearance rate as the primary endpoint [10]. An international core outcome set for all clinical trials on AK treatment has been recently published by the Core Outcome Measures in Effectiveness Trials (COMET) Initiative and the Cochrane Skin Group Core Outcomes Set Initiative (CSG-COUSIN). Core outcome set parameters included percentage of AKs cleared, complete clearance of AKs, severity of adverse events, patient perspective on effectiveness, patient-reported future treatment preference, and rate of occurrence [18]. The international experts and patients recognized the importance of including the efficacy endpoint ‘percentage of AKs cleared’ in their core outcome set. However, treatment adherence was not included in this set. This outcome was mentioned as critically important by 79% of participating experts, and 54% of participating patients considered dosage adherence as critically important. Topical dermatology treatments are often challenging due to prolonged and complex treatment regimens and can often lead to treatment nonadherence [19,20]. Therefore, efforts to maximize treatment compliance should be encouraged [19].
The Harmonisation of Outcome Parameters and Evaluation (HOPE) program is currently underway, with the aim of developing a further set of core outcomes for the treatment of AK [21]. It is hoped that this set will refine existing outcomes and address treatment/dosage adherence.
Multivariate analyses on another set of studies capturing additional factors that may influence the effect of interventions would be of interest for future investigations.
In conclusion, this analysis has shown that, in a homogenous set of trials, the complete clearance rates achieved with 5-FU interventions is inversely related to the number of lesions at baseline. This highlights the importance of having an efficacy outcome that is applicable to the real-world setting and that minimizes the underestimation of treatment benefit.
Acknowledgments
Editorial and writing supports were provided by Christina Campbell of Parexel International and funded by Pierre Fabre.
Funding Statement
This analysis was sponsored by Pierre Fabre.
Disclosure of interest
Khaled Ezzedine is a consultant for Pierre Fabre.
Caroline Painchault is an external consultant contracted by Pierre Fabre.
Melanie Brignone is an employee of Pierre Fabre and has nothing to disclose.
Disclosure statement
Melanie Brignone is an employee of Pierre Fabre and has nothing to disclose.
Data availability statement
Data derived from public domain resources for published trials. Data available on request from the authors for unpublished trials.
References
- [1].de Berker D, McGregor JM, Mohd Mustapa MF, et al. British Association of Dermatologists’ guidelines for the care of patients with actinic keratosis 2017. Br J Dermatol. 2017;176(1):20–6. . [DOI] [PubMed] [Google Scholar]
- [2].Stockfleth E, Kerl H, Zwingers T, et al. Low-dose 5-fluorouracil in combination with salicylic acid as a new lesion-directed option to treat topically actinic keratoses: histological and clinical study results. Br J Dermatol. 2011;165(5):1101–1108. . [DOI] [PubMed] [Google Scholar]
- [3].Szeimies RM, Atanasov P, Bissonnette R.. Use of lesion response rate in actinic keratosis trials. Dermatol Ther (Heidelb). 2016;6(4):461–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Traianou A, Ulrich M, Apalla Z, et al. Risk factors for actinic keratosis in eight European centres: a case-control study. Br J Dermatol. 2012;167(Suppl2):36–42. . [DOI] [PubMed] [Google Scholar]
- [5].Dirschka T, Gupta G, Micali G, et al. Real-world approach to actinic keratosis management: practical treatment algorithm for office-based dermatology. J Dermatolog Treat. 2017;28(5):431–442. . [DOI] [PubMed] [Google Scholar]
- [6].Wolf JE Jr., Rigel DS.. Understanding efficacy end-points in studies of field-directed therapy for actinic keratosis. Int J Dermatol. 2013;52(9):1063–1070. [DOI] [PubMed] [Google Scholar]
- [7].Fernandez-Figueras MT, Carrato C, Saenz X, et al. Actinic keratosis with atypical basal cells (AK I) is the most common lesion associated with invasive squamous cell carcinoma of the skin. J Eur Acad Dermatol Venereol. 2015;29(5):991–997. . [DOI] [PubMed] [Google Scholar]
- [8].Stockfleth E, von Kiedrowski R, Dominicus R, et al. Efficacy and safety of 5-fluorouracil 0.5%/salicylic acid 10% in the field-directed treatment of actinic keratosis: a phase III, randomized, double-blind, vehicle-controlled trial. Dermatol Ther (Heidelb). 2017;7(1):81–96. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Huyke C, Reuter J, Rodig M, et al. Treatment of actinic keratoses with a novel betulin-based oleogel. A prospective, randomized, comparative pilot study. J Dtsch Dermatol Ges. 2009;7(2):128–133. [DOI] [PubMed] [Google Scholar]
- [10].Skov T, Stockfleth E, Szeimies RM, et al. Efficacy endpoints in clinical trials in actinic keratosis. Dermatol Ther (Heidelb). 2018;8(3):425–433. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Stockfleth E, Ulrich C, Lange-Asschenfeldt B, et al. Treatment of multiple, multiform actinic keratoses on the head with imiquimod 5% cream. Eur J Dermatol. 2009;19(4):355–359. . [DOI] [PubMed] [Google Scholar]
- [12].Dohil MA. Efficacy, safety, and tolerability of 4% 5-fluorouracil cream in a novel patented aqueous cream containing peanut oil once daily compared with 5% 5-fluorouracil cream twice daily: meeting the challenge in the treatment of actinic keratosis. J Drugs Dermatol. 2016;15(10):1218–1224. [PubMed] [Google Scholar]
- [13].Krawtchenko N, Roewert-Huber J, Ulrich M, et al. A randomised study of topical 5% imiquimod vs. topical 5-fluorouracil vs. cryosurgery in immunocompetent patients with actinic keratoses: a comparison of clinical and histological outcomes including 1-year follow-up. Br J Dermatol. 2007;157(Suppl 2):34–40. . [DOI] [PubMed] [Google Scholar]
- [14].Weiss J, Menter A, Hevia O, et al. Effective treatment of actinic keratosis with 0.5% fluorouracil cream for 1, 2, or 4 weeks. Cutis. 2002;70(2Suppl):22–29. [PubMed] [Google Scholar]
- [15].Pierre Fabre data on file . HD-FUDR-045: A Randomized, Evaluator Blinded, Vehicle Controlled, Parallel Group, Dose Ranging Study of the Safety and Efficacy of 4% 5-FU Cream Versus its Vehicle Cream Versus Efudex in the Treatment of Actinic Keratosis. 2005.
- [16].Pierre Fabre data on file . HD-FUP3S-049: A Randomized, Double-Blind, Vehicle-Controlled Multi-Center Study of the Safety and Efficacy of 4% 5-FU Cream Versus its Vehicle Cream in the Treatment of Actinic Keratosis. 2007.
- [17].Pierre Fabre data on file . HD-FUP3B-048: A Randomized, Evaluator Blinded, Vehicle-Controlled Multi-Center Study of the Safety and Efficacy of 4% 5-FU Cream Versus its Vehicle Cream Versus Efudex Cream in the Treatment of Actinic Keratosis. 2007.
- [18].Reynolds KA, Schlessinger DI, Vasic J, et al. Core outcome set for actinic keratosis clinical trials. JAMA Dermatology. 2020. January 15;156(3):326. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- [19].Foley P, Stockfleth E, Peris K, et al. Adherence to topical therapies in actinic keratosis: a literature review. J Dermatolog Treat. 2016;27(6):538–545. . [DOI] [PubMed] [Google Scholar]
- [20].Norrlid H, Norlin JM, Holmstrup H, et al. Patient-reported outcomes in topical field treatment of actinic keratosis in Swedish and Danish patients. J Dermatological Treat. 2018;29(1):68–73. . [DOI] [PubMed] [Google Scholar]
- [21].Heppt MV, Steeb T, Schmitz L, et al. Harmonisation of Outcome Parameters and Evaluation (HOPE) for actinic keratosis: protocol for the development of a core outcome set. Trials. 2019;20(1):589. . [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data derived from public domain resources for published trials. Data available on request from the authors for unpublished trials.


