ABSTRACT
Community health workers (CHWs) can participate in the cascade of hypertension and diabetes management in low and middle-income countries (LMICs). Their services may be enhanced with mobile health (mHealth) tools. In this operational research study, we describe the AFYACHAT mHealth-assisted cardiovascular health screening program in rural Kenya. In this study, A CHW screened a convenience sample of adults ≥ 40 years old in rural Kenya for cardiovascular disease (CVD) risk using the two-way AFYACHAT mHealth instrument. AFYACHAT analyzes a patient’s age, sex, smoking, diabetes and systolic blood pressure and provides a four-tiered 10-year CVD risk score. User acceptability was assessed by an end-of-study interview with the CWH. Automated error logs were analyzed. Patient satisfaction was measured with a six-question satisfaction questionnaire. Screened participants with high CVD risk were followed-up via telephone to explore any actions taken following screening. In 24 months, one CHW screened 1650 participants using AFYACHAT. The 10-year risk of CVD was <10% for 1611 (98%) patients, 10 to <20% for 26 (1.6%), 20 to <30% in 12 (0.7%), and ≥30% for 1 (0.1%). The point prevalence of hypertension and diabetes was 27% and 1.9%, respectively. Seventy-five percent of participants with elevated CVD risk sought further medical care. There was high acceptability, a 15% miscode error rate, and high participant satisfaction with the screening program. Our operational research outlines how AFYACHAT mHealth tool can assist CHW perform rapid CVD screening; this provides a model framework for non-communicable disease screening in LMICs.
KEYWORDS: Cardiovascular diseases, public Health, non-communicable diseases, telemedicine
INTRODUCTION
Non-communicable diseases (NCDs), including cardiovascular disease (CVD), have steadily increased globally and have become a growing health concern for low and middle-income countries (LMICs) [1]. CVD refers to a collection of pathologies pertaining to narrowed or blocked blood vessels resulting in heart dysfunction (e.g., myocardial infarction) or coagulopathies (e.g., stroke). CVD accounts for most NCD deaths [2] and an estimated 80% of CVD deaths occur in LMICs [3]. Hypertension and diabetes are amongst the most important risk factors for the development of CVD. In Sub-Saharan Africa (SSA) the number of persons with hypertension and diabetes is expected to double between 2000 and 2030 [4,5]. Unfortunately, primary data reporting on NCD in LMICs are scarce, and consequently disease burden is likely underreported [1]. In SSA, it is estimated that 34% of persons with hypertension and 15% with diabetes are aware of their health condition, and only 15% receive adequate treatment [6].
Despite representing 11% of the global population and experiencing 24% of the global burden of disease, SSA hosts only 3% of the world’s health workers [7]. Consequently, employing Community Health Workers (CHWs) is a promising health care solution [8]. CHWs are lay persons with minimal formal medical training and provide basic health care including: social support, health teaching, resource sharing, as well as diagnostic and case management services [9]. They can be trained quickly at low cost and their community knowledge allows them to navigate culture norms and barriers [10]. International initiatives have demonstrated that CHWs are able to screen and identify persons at risk for CVD and diabetes [10,11].
Mobile health (mHealth) is an electronic health tool that uses mobile technologies, chiefly cellular phones, to provide health services and information [12]. It is estimated 83% of Kenyans own a cellular device [13]; as such, mHealth has emerged as a potentially powerful instrument for data collection, health monitoring and disease surveillance, especially in rural communities with limited access to healthcare services [14]. There is evidence to suggest that mHealth tools are well perceived by rural communities, improve patient medication compliance and appointment attendance [15–17]. Compared to hand-written reporting, electronic data collection minimizes data loss and enables rapid information analysis [18]. Bernabe-Ortiz found survey workers made less errors (5%) and collected information faster when using an electronic tool compared to traditional paper surveys [19]. Similarly, a study comparing a mobile phone mHealth application for CVD screening to paper-charts found CHWs required less training and made no CVD-risk miscalculations [14].
Our group has developed a two-way mHealth tool, AFYACHAT, which processes short message system (SMS) text messages from CHW reported data on a patient’s age, sex, smoking, diabetes and systolic blood pressure (SBP) and replies via SMS text message with an estimate of the patient’s 10-year risk of developing an overt CVD event. This allows CHWs to rapidly stratify participants and assign them a CVD risk. We have previously demonstrated the feasibility of this screening tool in aiding CHWs efficiently screen large numbers of patients [20]. Here, we report of the implementation of the screening program in rural Kenya. In this operational research study, our objectives were to assess replicability of screening results, ease of use of AFYACHAT, CHW feedback, participant perceptions of the screening program, and actions taken by participants in response to screening results.
METHODS
Setting
The study was conducted in Isiolo County (estimated population 142,000) in central Kenya, which consists largely of rural farming communities. We partnered with the Lewa Wildlife Conservancy, which runs a community engagement program including a healthcare initiative with a central health clinic (Lewa Medical Clinic) and outreach to nearby communities. Kenya has a population of 51.4 million and is ranked 147 out of 189 in terms of human development index [21]. An estimated 38.7% of its population lives in multidimensional poverty. The life expectancy at birth is 66.3 years [21].
AFYACHAT screening program
A CHW was trained by Canadian-certified nurses and physicians in a single half-day session to [1]: operate an automated blood pressure monitor and glucometer [2]; input and interpret data from the two-way AFYACHAT SMS system; and [3] counsel patients based on screening results.
The CHW screened a convenience sample of community members who wished to have their blood pressure and glucose level checked. The CHW traveled on a weekly basis to communities within the geographical catchment area of the Lewa Medical Clinic. In group settings (e.g., faith-based gatherings and marketplaces), she made an announcement that she would be screening for blood pressure and glucose for anyone interested in participating, explaining that screening was free of charge but that written informed consent would be required. Willing participants then approached her if they wished to participate in the screening. Additionally, the CHW traveled door-to-door to solicit potential participants; again, participation was free and voluntary. An age of 40 years or older was required to be eligible for screening. Age was assessed by national identification card; if no card was available, the CHW relied on the reported age of the participant.
The CHW asked participants basic health questions including: their age, sex, smoking status and diabetes history. Additionally, the CHW was trained to take SBP and blood sugar levels using an automated blood pressure monitor and glucometer, respectively. These data were entered on the CHWs cellular device and sent as a single SMS message to the AFYACHAT tool. Immediately, a CVD risk response was sent back to the CHW, which they would relay to the participant. If the risk level was greater than 10%, the CHW provided a referral for follow-up at the Lewa Medical Clinic for further treatment considerations.
AFYACHAT mHealth tool
AFYACHAT is a customized two-way electronic data program which performs algorithmic CVD-risk stratification based on SMS text input. The system was built using RapidSMS, a free, open-source framework built with Python and Django. We have previously described the design of AFYACHAT [20]. Users (CHWs) enter patient data as a single SMS text message to a local (Kenya Safaricom) telephone number, where the information is captured in a central database. The following patient data is captured: name, age, sex, tobacco use, glucometer reading and SBP. AFYACHAT then returns an SMS text message with the CVD risk profile, coded as ‘GREEN’ (<10% 10-year risk of cardiovascular event), ‘YELLOW’ (from 10% to <20%), ‘ORANGE’ (from 20% to <30%) or ‘RED’ (≥30%). The AFYACHAT system derives its CVD risk estimate based on the WHO/ISH risk prediction chart (AFR E region, no cholesterol measurement) [22–24]. The interface allows for a CHW to input patient data and rapidly receive a CVD risk assessment, which they may then relay to the patient. In contrast to our previous project [20], the current iteration of AFYACHAT used random blood glucose level to screen for diabetes, using a spot-check glucometer reading. Hypertension was operationally defined as SBP ≥140 mmHg [22] (single measurement) and diabetic status was ascribed if the participant had a random blood glucose ≥11.1 mmol/L [25] (single measurement).
AFYACHAT evaluation
An operational research approach was used for the evaluation of the AFYACHAT screening program. Operational research uses qualitative and quantitative data to produce practically usable knowledge for the advancement of program implementation in the real world [26]. This is meaningful in LMICs where there is often a disconnect between proposed programs and the practical implementation of suggested initiatives [27].
We determined the sample size for the AFYACHAT evaluation as follows. Based on pilot data, we estimated that the prevalence of elevated CVD risk (>10% 10-year risk) would be 2.2%. In order to estimate the prevalence with a precision of ±1% at the 95% confidence interval, standard sample size calculations indicated that 1180 participants needed to be enrolled.
Error log analysis
The AFYACHAT tool requires the user to input subject demographics in a specific coding format to process a participant’s CVD risk. The frequency of CHW data input error was recorded as a means to measure the ease of use.
Participant satisfaction survey
In order to evaluate the reception of the CVD screening program by the community, a six-item satisfaction questionnaire was provided to a subset of participants using non-probability convenience sampling (see supplementary materials). The questionnaire was developed using the net promoter score (NPS) model [28]. The NPS proposes that client satisfaction can be accurately assessed with a single question: would you recommend our service to a friend [28]? Beyond traditional business models, the NPS has been used to quantify patient satisfaction of healthcare services and electronic health tools [29,30]. Participants were classified as ‘promoters’ if they answered ‘yes’ or ‘yes, definitely’ to the question ‘If a friend were in need of knowing his/her CVD risk status, would you recommend our program to him/her?’ A standard sample size calculation showed that 89 patients would be required to estimate the proportion of participants who were satisfied with the program, to within 10 percentage points, at the 95% confidence level, assuming a point estimate of 75%. All participants were asked if they would be willing to complete the satisfaction survey. Once a sufficient sample size was obtained, no further satisfaction surveys were collected.
User feedback
At the end of the study, the CHW was interviewed to solicit feedback on her experience using the AFYACHAT tool and screening patients. The objective of the post-study interview was to assess user experience with the AFYACHAT mHealth interface.
High-risk patient follow-up
Patients who had an elevated risk for a 10-year CVD (≥10%) were contacted via telephone interview 6 or more months after their screening visit to inquire about any actions taken to respond to their CVD-risk.
Ethics and knowledge translation
This study was approved by the Kenya Medical Research Institute (protocol no. KEMRI/SERU/CGHR/0060/3920). Participants provided written, informed consent. After the completion of the study, an interactive public meeting was held to disseminate our findings to participants and local leaders
Statistical Analysis
Duplicates, defined as persons with the same full name of the same gender who have a reported age within one year of each other, were removed for analysis. Non-parametric methods were used for descriptive and comparative statistics: Chi-squared test for dichotomous variables and Kruskall–Wallis U-test for continuous variables, and Spearman’s rank correlation coefficient (ρ) for correlations. Statistical analysis was performed using PRISM (GraphPad Software Inc., La Jolla, CA), version 7 or SPSS Statistics 23 (SPSS Inc., Chicago, IL). Statistical significance was considered p < 0.05 for a two-sided test.
RESULTS
Between January 2017 and January 2019, a total of 1650 unique individuals were screened by a single CHW. The characteristics of the screened participants are summarized in Table 1. The majority of participants were female (63%) and the median age was 50 years old. The 10-year risk of CVD was <10% for 1611 (98%) patients, 10 to <20% for 26 (1.6%) patients, 20 to <30% in 12 (0.7%) patients, and ≥ 30% for 1 (0.1%) patient. Males were more likely to smoke than females (odds ratio (OR) = 17, 95% confidence interval (95% CI) 9.0–30, p < 0.0001). Participants who were 65 years of age or older had a higher prevalence of systolic hypertension (SBP>140 mmHg, 41% vs. 25%, OR 2.1 (95%CI 1.6–2.9), p < 0.0001) and self-reported diabetes (4.3% vs. 1.7%, OR 2.5 (95%CI 1.2–5.4), p = 0.01). Amongst patients with self-reported diabetes, the prevalence of systolic hypertension was higher (52.9% vs 26.4%, OR 3.1 (95%CI 1.6–6.1), p = 0.0005). Among participants who did not report a history of diabetes, none had a random blood glucose ≥11.1 mmol/L (no new cases of diabetes were detected by the screening program). Following correlational analyses of major CVD risk factors, age was found to be positively correlated with SBP (ρ = 0.16; p < 0.0001) (Table 2).
Table 1.
Characteristics of 1650 participants screened for CVD risk with AFYACHAT mHealth tool.
| Predicted 10-year CVD Risk | ||||||
| Overall (N = 1650) |
<10% GREEN (N = 1611) |
10% to <20% YELLOW (N = 26) |
20% to <30% ORANGE (N = 12) |
> 30% RED (N = 1) |
P-value | |
| Age, median (IQR) | 50 (43–59) | 49 (43–58) | 57 (50–69) | 62 (67–79) | 55 | <0.0001 |
| Sex, n(%) | 0.046 | |||||
| Male | 618 [38] | 596 (96) | 16 (2.6) | 6 (0.97) | 0 | |
| Female | 1032 (63) | 1015 (98) | 10 (0.97) | 6 (0.58) | 1 (0.1) | |
| Tobacco, n (%) | 0.0042 | |||||
| User | 105 (6.4) | 99 (94) | 6 (5.7) | 0 (0) | 0 (0) | |
| Non-User | 1545 (94) | 1512 (98) | 20 (1.3) | 12 (0.78) | 1 (0.06) | |
| Diabetes, n(%) | <0.0001 | |||||
| Known Diabetic | 34 (2.1) | 28 (82) | 2 (5.9) | 3 (8.8) | 1 (2.9) | |
| Not Known to be Diabetic | 1616 (98) | 1583 (98) | 24 (1.5) | 9 (0.56) | 0 (0) | |
| Blood Sugar, median (IQR) | 4.6 (3.9–5.9) | 4.6 (3.9–5.8) | 4.7 (3.9–7.1) | 6.7 (4.3–7.9) | 9.1 | 0.0031 |
| Current Diabetes, n(%) | 0.053 | |||||
| Blood Glucose ≥11.1 | 31 (1.9) | 28 (90) | 2 (6.5) | 1 (3.2) | 0 (0) | |
| Blood Glucose <11.1 | 1619 (98) | 1583 (98) | 24 (1.5) | 11 (0.68) | 1 (0.06) | |
| Systolic blood pressure (mmHg), median (IQR) | 129 (117–141) | 128 (117–140) | 172 (164–188) | 186 (164–200) | 193 | <0.0001 |
| Elevated Blood Pressure, n(%) | <0.0001 | |||||
| SBP (≥140 mmHg) | 444 [27] | 407 (92) | 26 (5.9) | 10 (2.3) | 1 (0.22) | |
| SBP (<140 mmHg) | 1206 (73) | 1204 (99.8) | 0 (0) | 2 (0.16) | 0 (0) | |
Data presented as median and interquartile range (IQR) or n (percentage). Chi-squared tests were performed for dichotomous variables and Kruskall–Wallis U-test for continuous variables.
Table 2.
Patient CVD risk factors: Correlation Matrix.
| Sex | Age | Smoker | History of Diabetes | Systolic Blood Pressure | Blood Glucose level | |
| Sex | ||||||
| Age | 0.0897** | |||||
| Smoker | 0.2805*** | 0.0375 | ||||
| History of Diabetes | −0.0153 | 0.0780** | −0.0029 | |||
| Systolic Blood Pressure | 0.0422 | 0.1644*** | 0.0648* | 0.0935*** | ||
| Blood Glucose level | 0.0379 | 0.0272 | −0.0501* | 0.1864*** | 0.0325 |
Values represent the Spearman rank correlation coefficient between continuous variables (ρ)
Statistical significance indicated by asterisks: *p < 0.05; **p < 0.001; ***p < 0.0001.
Error log analysis
A total of 304 SMS message submission errors were captured, which represents a 15% formatting error submission rate. Of the 1726 patients recorded in the AFYACHAT database; 79 (4.6%) were duplicates.
Participant satisfaction questionnaire
The participant satisfaction questionnaire was administered to a subset of screened participants (n = 124). Overall, participants responded positively to the program (Figure 1). Favorable or very favorable responses ranged from 86 to 99% for all questions. Specifically, 98% would recommend AFYACHAT to a friend (promoter). Questionnaire items, graded on a four-point scale, showed statistically significant correlation with each other (Table 3). In particular, the NPS was positively correlated with the responses to all other items, which suggests that we were measuring a single unified latent construct, representing participant satisfaction (Table 3).
Figure 1.
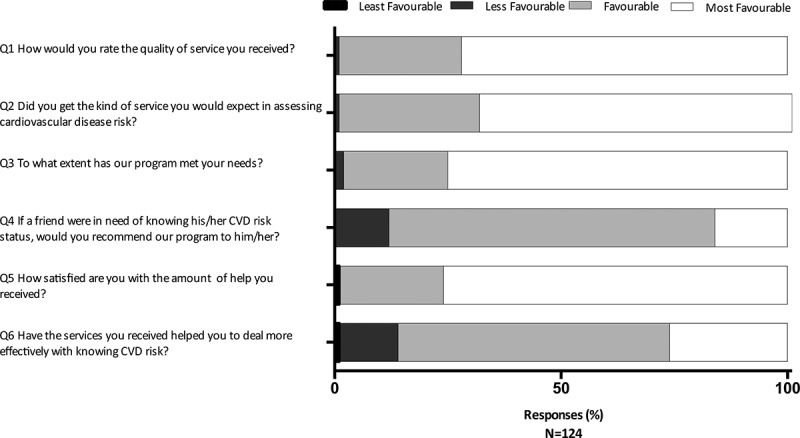
Patient Satisfaction Questionnaire. A convenience sample of participants (n = 124) completed a six-item satisfaction questionnaire, with responses graded on a four-point scale. The responses to the questions from least favorable to most favorable are as follows for the respective questions: Q1 (Poor, Fair, Good, Excellent); Q2 (Definitely not, Not really, Yes Generally, Definitely); Q3 (None of my needs have been met, Only a few of my needs have been met, Most of my needs have been met, Almost all of my needs have been met); Q4 (Definitely not, I don’t think so, I think so, Definitely); Q5 (Quite dissatisfied, Indifferent or mildly dissatisfied, Mostly satisfied, Very satisfied); Q6 (They seemed to get worse, They really didn’t help, they helped somewhat, they helped a great deal).
Table 3.
Satisfaction Questionnaire: Correlation Matrix.
| Q1 | Q2 | Q3 | Q4 | Q5 | Q6 | |
| Q1 | ||||||
| Q2 | 0.0486 | |||||
| Q3 | 0.1021 | 0.0057 | ||||
| Q4 | 0.3554*** | 0.048 | 0.3821*** | |||
| Q5 | 0.4934*** | 0.2962*** | 0.3367** | 0.6203*** | ||
| Q6 | 0.378*** | 0.1046 | 0.2784** | 0.6043*** | 0.5656*** |
Values represent the Spearman rank correlation coefficient between continuous variables (ρ)
Statistical significance indicated by asterisks: **p < 0.001; ***p < 0.0001
User feedback
Informal feedback was solicited from the CHW at the end of the program (Supplementary Table 1). The CHW indicated that AFYACHAT was user-friendly. The main limitation was occasional poor network responsiveness. Screening was well received by the community but uptake by males was suboptimal. Some participants were deterred from participating in the study because of the requirement to sign an informed consent form: ‘[they] did not know how to sign [or] were not sure what they were getting into by signing the consent form.’ The CHW found her work empowering: ‘[I] felt like I was doing an important thing and most people too liked me when I shared with them the test results.’
High-risk patient follow-up
Of the 39 screened patients with elevated (≥10%) CVD risk, we were able to contact 17 (44%) by telephone for follow-up. Of these, 13 (76%) sought further medical consultation and all those who attended a medical institution received medication to control for blood sugar or hypertension (Table 4). The cost of transportation and distance of travel were cited as barriers to follow-up.
Table 4.
Follow-up interviews of patients screened and found to be at increased risk for CVD (YELLOW – RED).
| High-Risk Patients | ||
| Post-Screening Outcome |
YELLOW, 10 to <20% (n = 9) |
ORANGE & RED, ≥20% (n = 8) |
| Follow-up at Lewa Health Clinic | 3 [33] | 1 [13] |
| Follow-up at another Health Facility | 4 [44] | 5 (63) |
| Medication Prescribed | 3 [33] | 7 (88) |
| Lifestyle Changes | 4 [44] | 1 [13] |
| Deceased | 0 | 1 [13] |
| Information Not Available (n)* | 17 | 5 |
*Reasons for missing information were: patient did not provide a telephone number at the time of screening; the number provided was not in service at the time of follow-up; or the patient had moved away and could not be reached at the number provided
DISCUSSION
We have demonstrated the successful use of AFYACHAT, a two-way CVD risk screening mHealth tool, in rural Kenya. Over the course of 24 months, one CHW assessed 1650 unique community members. Overall, participants were very satisfied with our service as nearly all screened individuals indicated that they would recommend the screening program to a friend. The CHW reported the AFYACHAT system to be largely user friendly. The main limitation was the occasionally slow and non-responsive network, which delayed or prevented patients from receiving their CVD risk assessment.
The primary purpose of AFYACHAT-assisted screening was to provide an actionable 10-year CVD risk assessment to individual participants so that they could respond by modifying lifestyle risk factors or by seeking medical care. In aggregate, the tool may also provide useful point prevalence data on the CVD risk profile, smoking, hypertension, and diabetes in an understudied population, although the external validity of the sample warrants examination. In our sample, the risk of CVD was <10%, 10 to <20%, 20 to <30%, and ≥ 30% in 98%, 1.6%, 0.7% and 0.1% of participants, respectively. In another study in rural India which also used WHO/ISH risk assessment tables, the comparable prevalence was 75%, 14%, 7.6%, and 3.8%, respectively. Lower CVD risk in our sample may relate to genetic or environmental differences in the population, or sampling bias which may have preferentially selected healthier voluntary participants in the screening program. In our sample, 27% of adults aged 40 years or older had systolic hypertension (≥140 mmHg) and 1.9% were diabetic (blood glucose ≥11.1 mmol/L). These results are comparable to the 23% and 3.2% prevalence for hypertension and diabetes, respectively, that we reported previously [20], and to a national NCD survey, which indicated that the age-standardized prevalence of hypertension and diabetes in Kenya were 24.5% and 2.4% respectively [31]. In contrast, data collected by the National, Heart, Lung and Blood Institute (NHLBI)/United Health Group Centers of Excellence estimated that the prevalence of diabetes amongst Kenyans aged 35–74 was higher (5.1%) [32] and the prevalence of diabetes is estimated at 4.7% in the Africa Region [33]. Similarly, the mean SBP was 135.4 ± 30.1 [32] compared to median 129 mmHg (117–141) in our study. Again, genetic or environmental differences in the population may explain these differences, or these may reflect a bias in our sample toward healthier voluntary participants. It is estimated 13.5% of adult Kenyans (21–69 years old) are current smokers, of which the majority are male [34]. Similarly, in this study, 15% and 1.1% of our male and female participants self-identified as current smokers. Taken together, these aggregate point prevalence data suggest that our CHW-mediated screening program can be used to gather important public health data on cardiovascular risk factors but may underestimate the risk profile in the population because of sampling bias due to voluntary participation in the program.
In this study we included a random blood sugar test in our screening process, which increased screening costs (an estimated USD$0.74 per individual screened) and adds logistical difficulty to the CHW’s screening process. The added value of hyperglycemia screening was not demonstrated in this study as all persons with hyperglycemia (≥11.1 mmol/L) also had a self-reported history of diabetes. However, this finding should be extrapolated with caution since participants in this project lived in proximity to a medical facility and may have higher awareness of their diabetes status than other rural populations in SSA. Alternatively, the program may have missed undiagnosed diabetics due to sampling bias which may have selected for health-aware voluntary participants. Further investigation in different settings would be required to assess the general utility of glucose measurement versus self-reported diabetes status for CVD-risk screening. Measuring random glucose levels at a single point in time is imperfectly sensitive and specific for the diagnosis of diabetes mellitus. Alternative diagnostic modalities include hemoglobin A1c (HbA1c), which provides an estimate of chronic hyperglycemia. Future iterations of AFYACHAT may incorporate HbA1c testing for self-reported diabetics and those with elevated random glucose levels to more accurately determine their long-term glycemic control.
Of the subset of persons who participated in the satisfaction questionnaire (n = 124), the majority responded favorably or very favorably (86–99%) to the service provided by the CHW. Specifically, 98% would recommend the service to a friend; similar to the NPS [28], these ‘promoters’ likely have a high level of satisfaction. Of note, correlation between this and other items on the satisfaction questionnaire (Table 3) suggest convergence validity and internal consistency of our satisfaction questionnaire. Informal feedback from the CHW corroborated high levels of participant satisfaction. In follow-up, many high-risk respondants indicated that they sought additional medical consultion or implemented lifestyle changes following their AFYACHAT CVD risk assessment. This may demonstrate the positive behavioral impact of the CHW-lead screening program. Studies conducted in rural China have found CHWs are accepted as legitimate providers of healthcare service [35]. Likewise, it is believed CHWs increase participation rates in health initiatives because of their understanding of the local culture and the prexisting connections they have within the community [9].
The principal limitation of AFYACHAT, as highlighted by feedback from the CHW, was periods of poor network responsiveness. Currently, we are working to improve central database hardware and power connection to reduce service interruptions. While intended to be fully electronic, the CHW also used ledger books to record all data. This was a backup database for when the system was non-responsive and may reflect her comfort level with traditional hand-written data collection. We propose using a bridging paper-phone screening method to accommodate areas with poor cellular service and aid CHWs familiarize themselves with the AFYACHAT tool. In our study, formatting errors occurred relatively frequently, at 15% while using predominately basic cell phones. Small phone keyboards have previously been identified as possible causes of data miscoding [36], as noted by our CHW in her closing interview. By upgrading to smart phones for our CHWs, we may reduce submission errors and increase screening speed.
Another challenge of our program was duplication of patients in the database. To ensure patient confidentiality and protection, all data was stored in a central SMS repository and the CHW deleted their SMS history at the end of every working day. This posed challenges for the CHW to quickly identify persons already screened. Likewise, many community members did not come to outreach initiatives with identification cards, which may have compounded this issue. The issue of minimizing duplicate patients is an issue shared amongst similar mHealth studies conducted in LMICs [36]. Moving forward, giving CHWs remote access to the central databank may facilitate participant identification and mitigate duplications.
Cardiovascular risk stratification by AFYACHAT is based on the WHO/ISH risk prediction charts [23,37]. The risk prediction charts were developed using a modeling approach using data derived from WHO Comparative Risk Assessment Study [38]. There is limited information about the accuracy of the WHO/ISH risk prediction charts in the medical literature [24,39]. Other authors have pointed out that the charts were not developed using prospective or out-of-sample test data and the methods employed differ from other risk estimation functions [39]. Further validation of this highly practical tool is therefore needed. In another study in rural India, which used the WHO/ISH risk prediction chart with and without cholesterol measurement, the proportion of 1066 screened individuals who were misclassified by the algorithm was 15% [39]. Alternative risk stratification instruments are available which, like the WHO/ISH risk prediction chart, do not require laboratory measurements but have been more rigorously validated (e.g., the Harvard National Health and Nutrition Examination Survey (NHANES) Cardiovascular Risk Score) [11,40,41]. Future studies should compare the accuracy of the WHO/ISH risk prediction charts to other validated tools. Of note, the versatile AFYACHAT electronic platform, together with CHW implementation at the village level, could be adapted to use alternative risk stratification tools.
This study has several limitations. We employed one CHW to screen patients, which does not allow us to generalize her experience to other CHWs who would use the AFYACHAT tool. With respect to the prevalence of CVD risk factors, the non-probability sample in our study may not be representative of the Kenyan population at large. Our sampling technique may have been subject to ‘voluntary response sampling’ bias [42]. Our sample had a disproportionate representation of women (63% female) similar to another study which compared voluntary to mandatory sampling strategies [42]. Voluntary samples may also be enriched in participants with higher education level, lower smoking rate, less alcohol consumption, and better subjective health status [42]. Thus, AFYACHAT may have missed community members who were at higher risk of adverse cardiovascular health but who chose not participate in the voluntary program. Feedback from the CHW indicated that, in addition to males, a number of community members were deterred from participating in the screening program because of the written informed consent process. Participants with limited literacy skills may therefore have been under-represented in our study. As a result, the prevalence of other demographic and health characteristics, including hypertension, diabetes and smoking in our study should be generalized with caution to the rural Kenyan population at large. Additional participation incentives for men, those with lower education, and lower health-seeking behavior should be considered in order to reach these groups. A simplified consent process that does not dissuade participants who are unable to read and write those should be considered in future projects. Likewise, non-probability convenience sampling was used for the satisfaction survey and results may not be representative of all study participants. Furthermore, to validate the accuracy of the AFYACHAT mHealth screening service, the platform should be compared to a gold standard (e.g. physician diagnosis).
The AFYACHAT-assisted screening program functions using minimal resources. Some of the costs include the CHW salary ($100/month), travel costs ($20/month), blood pressure monitor ($30-50), glucometer ($30-50), and glucose test strips ($0.74/test). These represent modest expenses that should be balanced with the benefits of early identification of CVD risk. If adequately identified and subsequently treated, the management CVD risk factors such as hypertension is substantially cheaper than treatments for CVD episodes. In LMICs, treatments per CVD episode can exceed $USD 5,000, notwithstanding the economic losses associated with reduced productivity and supportive care [43]. While screening does not explicitly prevent CVD episodes, it can lead to earlier treatment interventions and lifestyle changes that can mitigate such events [43]. In the future, a formal cost effectiveness assessment of the screening program would be of interest to ensure the project achieves its objectives while maintaining practical applicability and utility. Although costs were modest, our program only identified 39 people with an elevated 10-year CVD risk out of 1650 screened. This raises the question if screening could be performed more efficiently. To increase the yield of the screening program, several strategies can be contemplated: restricting screening to older community members with higher baseline CVD risk; encouraging the participation of high-risk individuals through targeted screening; or the use of a different risk stratification tool with higher sensitivity.
With respect to dissemination of our research findings, we held a community forum in which our CHW and study investigators invited community members and study participants to discuss our findings (16 January 2019). Anecdotally, attendees (n = 55) actively and enthusiastically participated in the information session, were positively engaged with the research team, and were motivated to actively manage their own cardiovascular health. Researchers have an ethical responsibility to share new knowledge, especially when dealing with underprivileged communities [44]. Through bilateral knowledge translation and exchange with community leaders, we can partner with affected communities in the co-creation of knowledge and shared-decision making, which may lead to more impactful initiatives and improved health outcomes [37].
Moving forward, we hope to introduce AFYACHAT screening to more communities throughout Kenya and other LMICs, employing more CHWs, and screening more participants to assess the scalability and replicability of this mHealth screening service. A larger sample size and demographic diversity will also allow us to more thoroughly evaluate the generalizability of the AFYACHAT screening tool. AFYACHAT represents a powerful tool to preemptively mitigate NCD risk; however, its clinical impact is restricted unless additional healthcare resources are deployed to support those at moderate to high risk. Knowing that access to healthcare in rural communities in SSA is limited, it is important to develop a comprehensive strategy to encourage follow-up after CVD screening and ensure access and adherence to treatment regimens to diminish CVD.
CONCLUSION
Here we demonstrate that the AFYACHAT mHealth-assisted CHW program can be used for screening of CVD risk in rural Kenya. This screening strategy benefits from the use of low-cost CHWs and a scalable technology, adapted from the WHO risk stratification tool. The mHealth platform allows for a CHW to perform risk assessments with minimal training. Patients were chiefly satisfied with the service provided and were open to participating in the screening program. Further evidence is required to evaluate the accuracy of the AFYACHAT tool and complementary programs are needed to manage and treat those at-risk.
Acknowledgments
We would like to thank our CHW, Emily Marete, for her efforts attending outreach initiatives to screen all of the study participants. Likewise, we want to thank the Lewa Wildlife Conservatory and Medcan clinic for providing the human, technical and capital resources required for the preparation and execution of this project.
Funding Statement
This project was supported by Medcan clinic. The funder had no role in study design, data collection and interpretation, or the decision to submit the work for publication;Medcan Corporation.
Disclosure statement
The authors have no conflicts of interests to declare.
References
- [1].Haregu TN, Oti S, Egondi T, et al. Co-occurrence of behavioral risk factors of common non-communicable diseases among urban slum dwellers in Nairobi, Kenya. Glob Health Action. 2015;8(1):28697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Dahlöf B. Cardiovascular disease risk factors: epidemiology and risk assessment. Am J Cardiol. 2010;105(1):3A–9A. [DOI] [PubMed] [Google Scholar]
- [3].Strong K, Mathers C, Leeder S, et al. Preventing chronic diseases: how many lives can we save? Lancet. 2016;16(1):1578–1582. [DOI] [PubMed] [Google Scholar]
- [4].Rathmann W, Giani G.. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030: response to Wild et al. Diabetes Care. 2004;27(10):2568–2569. author reply 9.. [DOI] [PubMed] [Google Scholar]
- [5].Adeloye D, Basquill C, Schnabel RB. Estimating the prevalence and awareness rates of hypertension in Africa: a systematic analysis. PLoS One. 2014;9(8):e104300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Mathenge W, Foster A, Kuper H. Urbanization, ethnicity and cardiovascular risk in a population in transition in Nakuru, Kenya: a population-based survey. BMC Public Health. 2010;10(1):569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Tsolekile LP, Abrahams-Gessel S, Puoane T. Healthcare professional shortage and task-shifting to prevent cardiovascular disease: implications for low- and middle-income countries. Curr Cardiol Rep. 2015;17(12):115. [DOI] [PubMed] [Google Scholar]
- [8].Kok MC, Dieleman M, Taegtmeyer M, et al. Which intervention design factors influence performance of community health workers in low- and middle-income countries? A systematic review. Health Policy Plan. 2015;30(9):1207–1227. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Fernandes R, Braun KL, Spinner JR, et al. Healthy heart, healthy family: A NHLBI/HRSA collaborative employing community health workers to improve heart health. J Health Care Poor Underserved. 2012;23(3):988–999. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Balcázar HG, de Heer HD. Community health workers as partners in the management of non-communicable diseases. Lancet Glob Health. 2015;3(9):e508–9. [DOI] [PubMed] [Google Scholar]
- [11].Gaziano TA, Abrahams-Gessel S, Denman CA, et al. An assessment of community health workers‘ ability to screen for cardiovascular disease risk with a simple, non-invasive risk assessment instrument in Bangladesh, Guatemala, Mexico, and South Africa: an observational study. Lancet Glob Health. 2015;3(9):e556–63. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Aranda-Jan CB, Mohutsiwa-Dibe N, Loukanova S. Systematic review on what works, what does not work and why of implementation of mobile health (mHealth) projects in Africa. BMC Public Health. 2014;14(1):188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Union IT . CT Facts & Figures 2015 International Telecommunication Union 2015 [Available from: http://www.itu.int/en/ITU-D/Statistics/Pages/facts/default.aspx
- [14].Surka S, Edirippulige S, Steyn K, et al. Evaluating the use of mobile phone technology to enhance cardiovascular disease screening by community health workers. Int J Med Inform. 2014;83(9):648–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Stephani V, Opoku D, Quentin W. A systematic review of randomized controlled trials of mHealth interventions against non-communicable diseases in developing countries. BMC Public Health. 2016;16(1):572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Kazi AM, Carmichael J-L, Hapanna GW, et al. Assessing mobile phone access and perceptions for texting-based mhealth interventions among expectant mothers and child caregivers in remote regions of northern kenya: a survey-based descriptive study. JMIR Public Health and Surveillance. 2017;3(1):e5. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Hall CS, Fottrell E, Wilkinson S, et al. Assessing the impact of mHealth interventions in low- and middle-income countries – what has been shown to work? Glob Health Action. 2014;7(1):25606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Braun R, Catalani C, Wimbush J, et al. Community health workers and mobile technology: a systematic review of the literature. PLoS One. 2013;8(6):e65772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Bernabe-Ortiz A, Curioso WH, Gonzales MA, et al. Handheld computers for self-administered sensitive data collection: a comparative study in Peru. BMC Med Inform Decis Mak. 2008;8(1):11. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Mannik J, Figol A, Churchill V, et al. Community-based screening for cardiovascular risk using a novel mHealth tool in rural Kenya. Journal of Innovation in Health informatics. 2018;25(3):176–182. . [DOI] [PubMed] [Google Scholar]
- [21].Inequalities in Human Development in the 21st CenturyBriefing note for countries on the 2019 Human Development Report - Kenya. United Nations; 2019
- [22].WHO. World Health Organization . WHO/ISH cardiovascular risk prediction charts. Available at: https://www.who.int/cardiovascular_diseases/guidelines/Chart_predictions/en/. Accessed 2020 Aug 11; 2007
- [23].Mendis S, Lindholm LH, Mancia G, et al. World Health Organization (WHO) and International Society of Hypertension (ISH) risk prediction charts: assessment of cardiovascular risk for prevention and control of cardiovascular disease in low and middle-income countries. J Hypertens. 2007;25(8):1578–1582. . [DOI] [PubMed] [Google Scholar]
- [24].Lindholm LH, Mendis S. Prevention of cardiovascular disease in developing countries. Lancet. 2007;370(9589):720–722. [DOI] [PubMed] [Google Scholar]
- [25].WHO. World Health Organisation and International Diabetes Federation . Definition and diagnosis of diabetes mellitus and intermediate hyperglycemia: report of a WHO/IDF consultation. Available at: https://www.who.int/diabetes/publications/Definition%20and%20diagnosis%20of%20diabetes_new.pdf Accessed 2020 Jul 6
- [26].Malhotra S, Zodpey SP. Operations research in public health. Indian J Public Health. 2010;54(3):145–150. [DOI] [PubMed] [Google Scholar]
- [27].Quaglio G, Ramsay A, Harries AD, et al. Calling on Europe to support operational research in low-income and middle-income countries. Lancet Glob Health. 2014;2(6):e308–10. . [DOI] [PubMed] [Google Scholar]
- [28].Reichheld F. The ultimate question. Boston, MA, USA: Harvard Business School Press; 2006. [Google Scholar]
- [29].Duman-Lubberding S, van Uden-kraan CF, Jansen F, et al. Feasibility of an eHealth application “OncoKompas” to improve personalized survivorship cancer care. Support Care Cancer. 2016;24(5):2163–2171. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Koladycz R, Fernandez G, Gray K, et al. The net promoter score (NPS) for insight into client experiences in sexual and reproductive health clinics. Glob Health Sci Pract. 2018;6(3):413–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Wamai RG, Kengne AP, Levitt N. Non-communicable diseases surveillance: overview of magnitude and determinants in Kenya from STEPwise approach survey of 2015. BMC Public Health. 2018;18(Suppl S3):1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Gaziano TA, Abrahams-Gessel S, Alam S, et al. Comparison of nonblood-based and blood-based total cv risk scores in global populations. Global Heart. 2016;11(1):37–46 e2. . [DOI] [PubMed] [Google Scholar]
- [33].Saeedi P, Petersohn I, Salpea P, et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: results from the international diabetes federation diabetes atlas, 9th edition. Diabetes Res Clin Pract. 2019;157:107843. [DOI] [PubMed] [Google Scholar]
- [34].Ngaruiya C, Abubakar H, Kiptui D, et al. Tobacco use and its determinants in the 2015 Kenya WHO STEPS survey. BMC Public Health. 2018;18(Suppl S3):1223. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Long H, Huang W, Zheng P, et al. Barriers and facilitators of engaging community health workers in non-communicable disease (NCD) Prevention and Control in China: A Systematic Review (2006–2016). Int J Environ Res Public Health. 2018;15(11):11. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].White A, Thomas DSK, Ezeanochie N, et al. Health worker mhealth utilization: a systematic review. Comput Inform Nurs. 2016;34(5):206–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].WHO . Prevention of cardiovascular disease: pocket guidelines for assessment and management of cardiovascular risk: (WHO/ISH cardiovascular risk prediction charts for the African Region). Available at: https://apps.who.int/iris/bitstream/handle/10665/43787/9789241547260_eng.pdf?sequence=1&isAllowed=y Accessed 2020 Aug 11. 2007
- [38].Ezzati M, Hoorn SV, Rodgers A, et al. Estimates of global and regional potentil health gains from reducing muliple major risk factors. Lancet. 2003;362(9380):271–280. . [DOI] [PubMed] [Google Scholar]
- [39].Raghu A, Praveen D, Peiris D, et al. Implications of cardiovascular disease risk assessment using the WHO/ISH risk prediction charts in rural India. PLoS One. 2015;10(8):e0133618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Gaziano TA, Young CR, Fitzmaurice G, et al. Laboratory-based versus non-laboratory-based method for assessment of cardiovascular disease risk: the NHANES I Follow-up Study cohort. Lancet. 2008;371(9616):923–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Pandya A, Weinstein MC, Gaziano TA. A comparative assessment of non-laboratory-based versus commonly used laboratory-based cardiovascular disease risk scores in the NHANES III population. PLoS One. 2011;6(5):e20416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Cheung KL, Ten Klooster PM, Smit C, et al. The impact of non-response bias due to sampling in public health studies: A comparison of voluntary versus mandatory recruitment in a Dutch national survey on adolescent health. BMC Public Health. 2017;17(1):276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Gheorghe A, Griffiths U, Murphy A, et al. The economic burden of cardiovascular disease and hypertension in low- and middle-income countries: a systematic review. BMC Public Health. 2018;18(1):975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].George AS, Mehra V, Scott K, et al. Community participation in health systems research: a systematic review assessing the state of research, the nature of interventions involved and the features of engagement with communities. PLoS One. 2015;10(10):e0141091. [DOI] [PMC free article] [PubMed] [Google Scholar]


